Abstract
Background
The role of systemic chemotherapy (SC) in conjunction with cytoreductive surgery (CS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in appendiceal mucinous carcinoma peritonei (MCP) is unknown.
Methods
A retrospective review (1999–2011) of MCP patients who had undergone CS/HIPEC with or without perioperative SC.
Results
Twenty-two low-grade MCP patients treated with CS/HIPEC and SC were matched to patients who received CS/HIPEC alone. Median overall survival (OS) was 107 months for patients treated with perioperative SC compared to 72 without (P = 0.46). CS/HIPEC was performed on 109 patients with high-grade MCP: 70 were treated with perioperative SC, while 39 were not. Median OS (22.1 vs. 19.6 months, P = 0.74) and progression-free survival (PFS) (10.9 vs. 7.0 months, P = 0.47) were similar in patients treated with SC compared to CS/HIPEC alone. Progression while on pre-operative SC was seen in eight patients (17%), while four (8%) had a partial response. Treatment with postoperative SC was associated with longer PFS (13.6 months) compared to pre-operative SC (6.8 months, P < 0.01) and CS/HIPEC alone (7.0 months, P = 0.03).
Conclusions
Post-operative SC appears to improve PFS in patients with high-grade appendiceal MCP treated with CS/HIPEC. In contrast, there is no evidence to support the routine use of perioperative SC in low-grade disease.
Keywords: appendiceal epithelial neoplasm, disseminated peritoneal adenomucinosis, peritoneal mucinous carcinomatosis, overall survival
Introduction
Appendiceal epithelial neoplasms (AENs) are rare malignancies which have an annual incidence of 9 per million people [1].Most AENs are found incidentally and removed during appendectomy; however, some patients develop peritoneal dissemination, also known as mucinous carcinoma peritonei (MCP). As MCP progresses, it can cause massive ascites, anorexia, abdominal pain, bowel obstruction, and death. AENs rarely metastasize beyond the peritoneum [2]. Consequently, locoregional therapy in the form of cytoreductive surgery (CS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has become a recommended therapy for appendiceal MCP with 10-year survival rates as high as 63% [3].
While the efficacy of CS/HIPEC for appendiceal MCP is well established, the role of systemic chemotherapy (SC) is not. Due to the rarity of the disease, there are no prospective trials to guide clinicians and the decision to use SC is based on multidisciplinary discussion and clinical judgment. Early series showed little benefit to SC in patients with metastatic AEN. Smith et al. [4] found no survival difference with the use of SC in 34 patients with MCP who underwent cytoreduction. Similarly, Gough et al. [5] analyzed 56 patients treated with debulking surgery, radiation, and/or chemotherapy and found SC to be a negative predictor of survival. In contrast, Shapiro et al. [6] recently reported a disease control rate of 56% using modern SC regimens in surgically unresectable patients with a median overall survival (OS) of 56 months. Finally, Farquharson et al. [7] reported that 38% of patients with unresectable disease benefited from SC by reducing ascites or stabilizing disease.
While some of these studies suggest that SC may benefit patients with unresectable MCP, several studies [3,8] have linked treatment with SC prior to CS/HIPEC with poorer outcomes. In a recent multi-international CS/HIPEC registry study [3] of 2,298 patients with appendiceal MCP, treatment with any prior chemotherapy was independently associated with poorer progression-free survival (PFS) and OS. Yet, 16 to 24% of patients with appendiceal MCP receive SC in conjunction with CS/HIPEC [3,9] without clearly defined benefit.
Histologic grade in appendiceal MCP has consistently been one of the most important prognostic factors [3,8–13]. In practice, SC is often given to patients with poor prognostic factors (incomplete cytoreduction, high-grade histology, lymph node involvement), although the efficacy of SC in these settings is unknown. In general, appendiceal MCP is an indolent disease with median OS as high as 196 months following CS/HIPEC [3]. Given this favorable prognosis, it is imperative to identify patients who will benefit from SC and those who can be spared the attendant toxicity. The purpose of the current study is to explore the efficacy of SC in the pre- and post-operative setting, in conjunction with CS/HIPEC, for the treatment of appendiceal MCP.
Methods
Patients who underwent CS/HIPEC for appendiceal MCP between January 1997 and January 2011 at two high volume academic cancer centers were identified from prospectively maintained databases. Perioperative SC was arbitrarily defined as any systemic therapy received within 3 months of CS/HIPEC. Patients were stratified by the type of perioperative SC used (none, pre-operative, post-operative, or both) and by histologic grade as described below. Details regarding chemotherapy regimens, duration, and response were gathered retrospectively. In most cases, the decision to administer SC and the specific regimen was determined by the referring oncologist.
Tumor grade was defined as low-grade (MCP-L) or high-grade (MCP-H) according to the Bradley classification system [14]. While data regarding tumor histology and grade were collected prospectively, some of the pathology reports did not conform to the Bradley classification. Pathology slides from reports that classified tumors as moderate grade or did not designate a grade were reviewed by two independent pathologists and were assigned either MCP-L or MCP-H. The presence of signet ring cells in any specimen was assigned MCP-H.
Due to the paucity of MCP-L patients who were treated with SC, a control group was selected by matching them to patients with MCP-L who did not received SC using known prognostic factors [3,9–10]. Patients were first matched by resection status, followed by age (±5 years) and then lymph node status. Resection status and age have consistently been associated with OS in patients with MCP-L. Lymph node metastasis is relatively rare in low-grade disease, however, a significant number of our patients with node positive MCP-L received SC. As node positivity likely influenced the oncologists' decision to administer SC, we chose to include lymph node status as matching criteria in attempt to reduce this bias.
Our technique of CS/HIPEC has been previously described [10]. Briefly, aggressive cytoreduction is performed to remove as much macroscopic disease as possible. Two inflow and two outflow catheters are placed in the abdomen. After the abdominal skin is temporarily closed, mitomycin (40 mg/m2) or oxaliplatin (200 mg/m2) is warmed to an inflow temperature of 40 to 42°C and circulated through the peritoneal cavity for 60 to 120 min. Resection status was defined by the operating surgeon at the end of the case to quantify the amount of residual disease according to AJCC staging guidelines [15]: R0/1— complete cytoreduction of all visible disease; R2a—minimal residual disease (tumor nodules <0.5cm); R2b—gross residual disease (between 0.5 and 2.0 cm); and R2c—extensive residual disease (>2 cm nodules). Patients were followed every 6 to 12 months post-operatively with physical exam, tumor markers when applicable and CT imaging to determine disease recurrence or progression.
Response to SC was defined as partial response, stable disease, or progressive disease after reviewing radiologic imaging, tumor markers (CEA, CA 19–9), and clinic notes. RECIST criteria [16] were applied when pre-SC and post-SC imaging were available which was possible in 27 of 57 patients (47%) in our cohort who received pre-operative SC. When imaging was not available, treatment response was assigned based on radiology reports and overall clinical benefit as determined by clinic notes, tumor markers, and the improvement or progression of ascites.
Descriptive statistics were generated for all continuous and categorical data. Fisher's exact tests were used to test for differences in categorical variables and Wilcox on rank sum tests were used to test for group differences. PFS and OS were calculated from the date of CS/HIPEC to the date of last follow-up or the date of progression/recurrence or death. Patients were considered lost to follow up if no data could be attained within 3 years of the analysis. Estimates of survival were calculated by using the Kaplan–Meier method. Statistical significance was defined as a P-value <0.05. This study was approved by the institutional review boards of both participating institutions.
Results
Between January 1997 and January 2011, 393 patients were identified who underwent CS/HIPEC for appendiceal MCP. MCP-L was found in 284 patients, while 109 had MCP-H. Median follow-up was 55 months for MCP-L patients and 40 months for MCP-H patients.
Low-Grade Appendiceal MCP
Of the 284MCP-L patients, 22 were treated with perioperative SC; 13 received pre-operative SC, while 9 patients were treated with postoperative SC. The specific SC regimens used are summarized in Table I. The median duration of pre-operative SC was 4.5 months (range 1.5– 18); while the median post-operative SC duration was 4.0 months (range 3–6.5). There were no responders and one patient had clinical progression while receiving pre-operative FOLFOX. Twenty-two patients with MCP-L treated without SC were selected as controls based on similar resection status, age, and lymph node status. As shown in Table II, there were no significant differences between these groups. Four patients in the study groups were lost to follow up.
Table I. Perioperative Chemotherapy Regimens in Patients with Low- and High-Grade Appendiceal Mucinous Carcinoma Peritonei.
| Low-grade | Pre-op | Adjuvant | Pre-op + adjuvant |
|---|---|---|---|
|
|
|
|
|
| (n = 13) | (n = 9) | (n = 0) | |
| Fluorpyrimidine alone | 4 (31%) | 2 (22%) | |
| Fluorpyrimidine + oxaliplatin | 5 (39%) | 4 (44%) | |
| Fluorpyrimidine + irinotecan | 2 (15%) | 1 (11%) | |
| Other or unknown | 2 (15%) | 2 (22%) | |
| Addition of bevacizamab | 7 (54%) | 2 (22%) | |
| Addition of cetuximab | 1 (7%) | 0 |
| High-grade | n = 37 | n = 22 | n = 11 | |
|---|---|---|---|---|
|
| ||||
| Pre-op | Adjuvant | |||
| Fluorpyrimidine alone | 3 (8%) | 1 (4%) | 0 | 4 (36%) |
| Fluorpyrimidine + oxaliplatin | 28 (74%) | 16 (73%) | 9 (82%) | 4 (36%) |
| Fluorpyrimidine + irinotecan | 3 (8%) | 3 (14%) | 1 (9%) | 2 (18%) |
| Other or unknown | 3 (8%) | 2 (9%) | 1 (9%) | 1 (9%) |
| Addition of bevacizamab | 19 (50%) | 5 (23%) | 7 (64%) | 5 (45%) |
| Addition of cetuximab/panitumumab | 0 | 1 (5%) | 1 (9%) | 1 (9%) |
Table II. Demographics of Patients with Appendiceal Mucinous Carcinoma Peritonei, Stratified by Histologic Grade, Who with Treated with CS/HIPEC with or Without Perioperative Systemic Chemotherapy (SC).
| Variable | Low-grade | High-grade | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SC (%) | No SC (%) | P-value | SC (%) | No SC (%) | P-value | |
| Number of patients | 22 | 22 | 70 | 39 | ||
| Age (median, years) | 56.0 | 53.9 | 0.46 | 54.4 | 54.1 | 0.71 |
| Gender | 1.00 | 0.32 | ||||
| Male | 14 (64) | 13 (59) | 37 (53) | 16 (41) | ||
| Female | 8 (36) | 9 (41) | 33 (47) | 23 (59) | ||
| Race | 0.41 | 0.27 | ||||
| White | 20 (91) | 17 (77) | 58 (83) | 34 (87) | ||
| Black | 2 (9) | 4 (18) | 7 (10) | 5 (13) | ||
| Other | 0 | 1 (5) | 5 (7) | 0 | ||
| BMI (mean) | 26.7 | 28.7 | 0.59 | 27.0 | 27.1 | 0.94 |
| Performance status (ECOG) | 0.21 | 0.11 | ||||
| 0 | 11 (79) | 10 (50) | 27 (44) | 22 (56) | ||
| 1 | 3 (21) | 9 (45) | 26 (43) | 8 (21) | ||
| 2 | 0 | 1 (5) | 6 (10) | 7 (18) | ||
| 3 | 0 | 0 | 2 (3) | 2 (5) | ||
| Lymph nodes status | 0.40 | 1.00 | ||||
| Positive | 5 (42) | 3 (21) | 34 (56) | 17 (56) | ||
| Negative | 7 (58) | 11 (79) | 27 (44) | 13 (44) | ||
| Prior debulking | 0.76 | 0.68 | ||||
| Yes | 11 (50) | 13 (59) | 26 (38) | 13 (33) | ||
| No | 11 (50) | 9 (41) | 42 (62) | 26 (67) | ||
| Presence of signet ring cells | 1.00 | 0.22 | ||||
| Yes | 0 | 0 | 31 (44) | 12 (31) | ||
| No | 22 (100) | 22 (100) | 39 (56) | 27 (69) | ||
| Resection status | 1.00 | 0.94 | ||||
| R0/1 | 11 (50) | 11 (50) | 28 (40) | 14 (36) | ||
| R2a | 6 (27) | 6 (27) | 19 (27) | 10 (26) | ||
| R2b | 3 (14) | 3 (14) | 16 (23) | 11 (28) | ||
| R2c | 2 (9) | 2 (9) | 7 (10) | 4 (10) | ||
Median PFS was 29.5 months for MCP-L patients treated with CS/HIPEC and perioperative SC compared to 37.0 months with CS/HIPEC alone (P = 0.18). Patients treated with perioperative SC had a 3- and 5-year OS of 90% and 62%, compared to 70% and 64% in patient not treated with SC. Median OS was 107 months compared to 72 months, with and without perioperative SC, respectively (P = 0.46). Kaplan– Meier curves for PFS and OS are shown in Figure 1A and B. The use of perioperative SC was not associated with increased survival in any subgroup including patients with incomplete resections or nodal metastases.
Fig. 1.
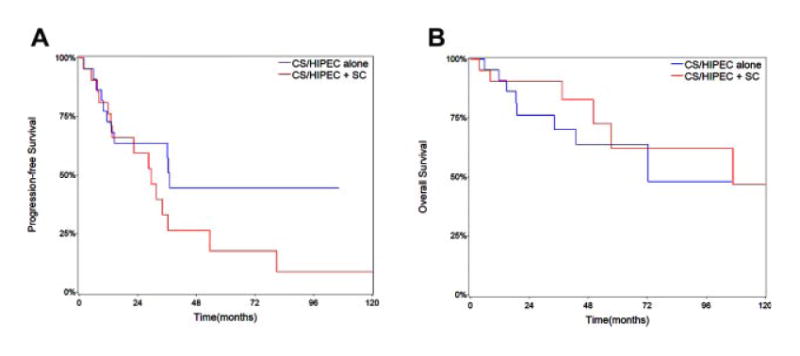
Kaplan–Meier curves for progression-free survival (A) and overall survival (B) in patients with low-grade appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery (CS) and hyperthermic intraperitoneal chemotherapy (HIPEC) stratified by the use of perioperative systemic chemotherapy (SC).
High-Grade Appendiceal MCP
The 109 patients with MCP-H were divided into two groups according to perioperative SC administration: 70 patients received SC and 39 patients did not. One patient was lost to follow up. As shown in Table II, there were no significant differences identified between the two groups.
Of the 70 patients with MCP-H treated with perioperative SC, 37 received pre-operative SC, 22 received post-operative SC, and 11 received both pre-operative and post-operative SC. Table I outlines the SC regimens administered in each group. The median duration of preoperative SC was 4.0 months (range 1.5–16); while the median postoperative SC duration was 6.0 months (range 1.5–17). Among the 48 patients with MCP-H who received pre-operative SC, 75% demonstrated stable disease. Eight patients (17%) progressed on preoperative SC; while four patients (8%) had a partial clinical response— decreased ascites (2), decreased tumor markers (1), and undefined (1). No patient met RECIST criteria for treatment response. Additionally, eight patients (24%) who received post-operative SC developed recurrence/progression of disease while on therapy.
Comparing patients who received perioperative SC to those treated with CS/HIPEC alone, there was no difference in median PFS (10.9 vs. 7.0 months, P = 0.47) or median OS (22.1 vs. 19.6 months, P = 0.74). Eight of 22 patients (36.4%) who developed recurrence/progression following CS/HIPEC alone underwent repeat CS ± HIPEC; while six patients (27.3%) were treated with second/third line SC. Of the 51 patients treated with perioperative SC and had recurrence/progression, 11 (21.6%) underwent repeat CS ± HIPEC and 22 (43.1%) received further systemic therapy.
Patients with MCP-H were also evaluated based on timing of SC (Table III and Fig. 2). The use post-operative SC was associated with longer median PFS (13.6 months) compared to pre-operative SC (6.8 months, P < 0.01) and HIPEC alone (7.0 months, P = 0.03). Similarly, there was a trend toward improved OS for patients treated with post-operative SC (36.4 months) compared to pre-operative SC (16.0 months, P = 0.07) or HIPEC alone (19.6 months, P = 0.14). Patients treated with both pre-operative and post-operative SC had similar PFS compared to patients treated with post-operative SC (12.9 vs. 13.6 months, P = 0.24) and similar OS to patients who received pre-operative SC (17.8 vs. 16.0 months, P = 0.76).
Table III. Progresssion-Free Survival and Overall Survival in Patients with High-Grade Appendiceal Mucinous Carcinoma Peritonei Treated with HIPEC with or Without Perioperative Chemotherapy.
| HIPEC alone (n = 39) | Post-SC (n = 22) | Pre-SC (n = 37) | Both (n = 11) | |
|---|---|---|---|---|
| Progression-free survival | ||||
| 1-year % (SE) | 27 (7) | 63 (10) | 30 (8) | 55 (15) |
| 2-year % (SE) | 14 (6) | 24 (9) | 4 (4) | 9 (9) |
| Median (months) | 7.0 | 13.6 | 6.8 | 12.9 |
| P-value vs. post-SC | 0.03 | — | <0.01 | 0.24 |
| Overall survival | ||||
| 1-year % (SE) | 65 (8) | 90 (6) | 59 (8) | 73 (13) |
| 3-year % (SE) | 28 (8) | 53 (12) | 25 (10) | 18 (12) |
| Median (months) | 19.6 | 36.4 | 16.0 | 17.8 |
| P-value vs. post-SC | 0.14 | — | 0.07 | 0.17 |
HIPEC, hyperthermic intraperitoneal chemotherapy; Post-SC, post-operative systemic chemotherapy; Pre-SC, pre-operative systemic chemotherapy; SE, standard error.
Fig. 2.
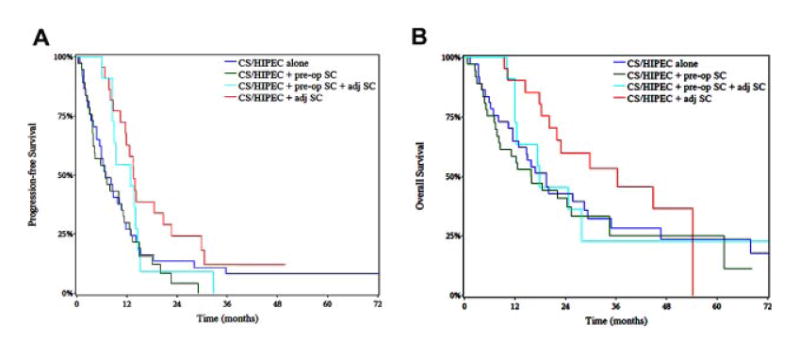
Kaplan–Meier curves for progression-free survival (A) and overall survival (B) in patients with high-grade appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery (CS) and hyperthermic intraperitoneal chemotherapy (HIPEC) stratified by timing of perioperative systemic chemotherapy (SC).
Among patients who underwent an R0/1 resection, there was no discernible benefit in PFS (13.6 vs. 13.6 months, P = 0.59) or OS (34.6 vs. 29.3 months, P = 0.98) with the use of perioperative SC compared to CS/HIPEC alone. Likewise, perioperative SC was not associated with improved survival in patients who underwent R2a/b/c resections (15.9 vs. 14.7 months, P = 0.58), had positive lymph nodes (18.5 vs. 16.9, P = 0.64) or had signet ring cells (20.2 vs.15.0 months, P = 0.17). The use of perioperative SC trended toward improved PFS in patients with incomplete cytoreductions (8.6 vs. 4.8 months, P = 0.05) or had signet ring cells (10.9 vs.4.8 months, P = 0.06).
Discussion
Over the past 15 years, CS/HIPEC has become an effective treatment for patients with appendiceal MCP; however, defining the role of SC in the setting of CS/HIPEC has received little attention. The present study represents the largest experience and most in-depth analysis to date using SC in conjunction with CS/HIPEC for appendiceal MCP. Modern chemotherapy in the treatment of metastatic colorectal cancer continues to evolve and given the paucity of data heretofore, treatment guidelines for metastatic colorectal cancer have been extrapolated to appendiceal MCP. However, clinical outcomes in patients treated with CS/HIPEC for appendiceal MCP are consistently superior to colorectal peritoneal carcinomotosis [17–19]. Furthermore, distinctly unique genetic expressions exist between appendiceal and colorectal peritoneal disease [20]. These observations call into question the use of colorectal treatment strategies when managing appendiceal cancers.
Another challenge in developing treatment regimens for appendiceal MCP is the grading classification is not universal. As a consequence, significant confusion exists regarding the diagnosis, prognosis and treatment strategies of AENs. The original three-tier system was proposed in 1995 by Ronnett et al. [21]. Disseminated AENs were classified using the terms disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and PMCA with discordant or intermediate features (PMCA-I). More recently, our group [14] analyzed survival following CS/HIPEC in patients stratified by the three-tier classification and found no difference in survival between patients with DPAM or PMCA-I. A pattern of similar outcomes in DPAM and PMCA-I patients has been seen in other large series [3,9– 10]. Based on these data, we have adopted a simplified two-tier system (low-grade vs. high-grade) to prognosticate patients at our institution. We've reported 5-year OS of 63% for appendiceal MCP-L compared to 38% in MCP-H [14].
In the current study, less than 8% of patients with MCP-L received perioperative SC. By comparing outcomes in these patients to a matched cohort, we found no evidence that SC is beneficial for patients with low-grade appendiceal MCP. These data support the bias of most oncology providers and suggests that the treatment for recurrent or progressive disease is repeat CS/HIPEC when possible rather than systemic therapy.
Few studies [22,23] have evaluated the role of perioperative SC in the setting of CS/HIPEC for patients with high-grade appendiceal MCP. Lieu et al. [22]reported a radiographic response rate of 44% in 78 patients with poorly-differentiated or signet ring cell appendiceal MCP who received SC as first line treatment. Median PFS and OS were 6.9 months and 1.7 years, respectively. Response to chemotherapy was not independently associated with longer survival. They also analyzed 26 patients who underwent complete CS with (35%) or without (65%) HIPEC. This cohort had median PFS and OS of 1.2 and 4.2 years, respectively. The use of perioperative SC in this small cohort was of borderline significance for PFS (HR = 0.22, 95% CI 0.04–1.25) and OS (HR = 0.12, 95% CI 0.01–1.59) on multivariate analysis.
Our results are the first to suggest that perioperative SC may benefit patients with high-grade appendiceal MCP. Compared to CS/HIPEC alone, post-operative SC was associated with better PFS and trended toward improved OS. Surprisingly, there was no such advantage observed in the patients treated with pre-operative SC. These results differ from Bijelic et al. [23] who compared 34 patients with PMCA who received SC prior to CS/HIPEC to 24 patients who were treated with CS/HIPEC alone. While there was no difference in OS between the groups, they noted improved survival in the 10 patients who responded to preoperative SC. Their response rate was 29%—much higher than in our dataset (8%), but could be explained by the different definitions used for response. While 10 patients in their series responded to therapy, 17 patients (50%) were noted to have progressive disease at the time of CS/HIPEC. More patients progressed on pre-operative chemotherapy than responded in our cohort as well. Without known factors to predict response, there is currently no way to select MCP-H patients accurately for pre-operative SC.
As large referral centers, many of our patients receive some amount of tumor debulking and begin SC before being referred for CS/HIPEC. Some studies have found prior surgery [3,10] and prior chemotherapy [3,8] to be negative predictive factors in patient undergoing CS/HIPEC; possibly by delaying definitive sursgery, degrading performance status or making complete CS more difficult. Another explanation of why preoperative SC may be less effective than post-operative SC is the abundant intra-abdominal mucin present prior to cytoreduction may essentially block the chemotherapy agent from diffusing from the vasculature to the malignant cells. Based on these observations and our data, we recommend that all patients with appendiceal MCP be referred for CS/HIPEC as soon as they are diagnosed without extensive tumor debulking and prior to receiving SC.
The current study is limited in that it is retrospective in nature. In addition, due to the rarity of appendiceal MCP, the number of patients in our study is small, despite pooling data from two large centers. Certainly there is the possibility of bias due to lack of power. This is especially true for our MCP-L analysis. We attempted to overcome this possibility by selecting patients with similar clinical features as controls.
Other factors, beyond histologic grade, could select patients for perioperative SC; although, we were unable to detect any. We observed a trend toward improved PFS with the use of SC in patients with incomplete cytoreductions or signet ring cell pathology, suggesting that SC may have an effect in other patient subgroups. However, we found no difference in OS among these subgroups. Further analyses are needed before other factors are definitely used to select patients for perioperative SC.
Another limitation to the present study is the decision to initiate SC and the specific regimens were not standardized and were determined almost exclusively by the referring oncologist. Many of our patients were referred for CS/HIPEC only after failing systemic therapy. The observation that one patient received SC for 18 months prior to being referred for CS/HIPEC highlights the lack of accepted guidelines in treating appendiceal MCP and the hesitancy of some oncologists to refer patients for surgical assessment. In addition, postoperative chemotherapy may have been withheld in some patients due to poor performance status. Finally, given the time period of the study, some included patients were treated before combination SC and biologic agents were available. Unpublished data from our institutions suggests that survival in patients with unresectable appendiceal MCP is improved with the use of combination SC and biologics agents. Unfortunately, the present study does not have enough patients to detect a different in outcomes following CS/HIPEC based on specific SC regimens. These variables introduce a number of confounding effects and treatment bias into our analysis. Multicenter, randomized trials with standardized treatment regimens are needed to eliminate such bias and explore the most efficacious perioperative SC regimen. However, the rarity of the disease makes the completion of such trials unlikely.
In conclusion, our results suggest that the role for perioperative SC in low-grade appendiceal MCP treated with CS/HIPEC is limited. In contrast, multidisciplinary management of patients with high-grade MCP is essential as these patients appear to benefit from perioperative SC. Our data suggests that these patients benefit most from post-operative SC and that pre-operative SC should be reserved for patients who are borderline or non-resectable. Until prospective, randomized studies are available to validate our findings, patients who are diagnosed with appendiceal MCP should be referred for CS/HIPEC prior to receiving SC. The use of post-operative SC can then be tailored based on operative finding and pathologic grade.
Abbreviations
- AEN
appendiceal epithelial neoplasm
- CS
cytoreductive surgery
- HIPEC
hyperthermic intraperitoneal chemotherapy
- MCP-L
low-grade mucinous carcinoma peritonei
- MCP-H
high-grade mucinous carcinoma peritonei
- OS
overall survival
- PFS
progression-free survival
- SC
systemic chemotherapy
References
- 1.Smeenk RM, van Velthuysen ML, Verwaal VJ, et al. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur J Surg Oncol. 2008;34:196–201. doi: 10.1016/j.ejso.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Geisinger K, Levine E, Shen P, et al. Pleuropulmonary involvement in pseudomyxoma peritonei: Morphologic assessment and literature review. Am J Clin Pathol. 2007;127:135–143. doi: 10.1309/601K2L2T7CR5U7G1. [DOI] [PubMed] [Google Scholar]
- 3.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-termoutcomedata of patients with pseudomyxomaperitonei from appendicealorigintreated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 4.Smith JW, Kemeny N, Caldwell C, et al. Pseudomyxoma peritonei of appendiceal origin. The Memorial Sloan-Kettering Cancer Center experience. Cancer. 1992;70:396–401. doi: 10.1002/1097-0142(19920715)70:2<396::aid-cncr2820700205>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei: Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112–119. doi: 10.1097/00000658-199402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro JF, Chase JL, Wolff RA, et al. Modernsystemicchemotherapy in surgicallyunresectableneoplasms of appendicealorigin: A single-institution experience. Cancer. 2010;116:316–322. doi: 10.1002/cncr.24715. [DOI] [PubMed] [Google Scholar]
- 7.Farquharson AL, Pranesh N, Witham G, et al. A phase II studyevaluating the use of concurrentmitomycin C and capecitabine in patients with advanced unresectable pseudomyxoma peritonei. Br J Cancer. 2008;99:591–596. doi: 10.1038/sj.bjc.6604522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baratti D, Kusamura S, Nonaka D, et al. Pseudomyxoma peritonei: Clinicalpathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2008;15:526–534. doi: 10.1245/s10434-007-9691-2. [DOI] [PubMed] [Google Scholar]
- 9.Elias D, Gilly F, Quenet F, et al. Pseudomyxomaperitonei: A Frenchmulticentricstudy of 301patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2010;36:456–462. doi: 10.1016/j.ejso.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JH, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: Outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13:624–634. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 11.González-Moreno S, Sugarbaker PH. Righthemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91:304–311. doi: 10.1002/bjs.4393. [DOI] [PubMed] [Google Scholar]
- 12.Smeenk RM, Verwaal VJ, Antonini N, et al. Survival analysis of pseudomyxoma peritoneipatients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104–109. doi: 10.1097/01.sla.0000231705.40081.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan TD, Links M, Xu ZY, et al. Cytoreductivesurgery and perioperativeintraperitonealchemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg. 2006;93:1270–1276. doi: 10.1002/bjs.5427. [DOI] [PubMed] [Google Scholar]
- 14.Bradley RF, Stewart JH, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: A clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551–559. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 7th. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Levine EA, Stewart JH, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: Experience with 501procedures. J Am Coll Surg. 2007;204:943–953. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 18.Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with Perioperative intraperitoneal chemotherapy: A multi-institutional study of 1290 patients. Cancer. 2010;116:5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 19.Elias D, Glehen O, Pocard M, et al. A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal Dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann Surg. 2010;251:896–901. doi: 10.1097/SLA.0b013e3181d9765d. [DOI] [PubMed] [Google Scholar]
- 20.Levine EA, Blazer DG, Kim MK, et al. Gene expression profiling of peritonealmetastases from appendiceal and colon cancer demonstrates unique biologic signatures and predicts patient outcomes. J Am Coll Surg. 2012;214:599–606. doi: 10.1016/j.jamcollsurg.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19:1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Lieu CH, Lambert LA, Wolff RA, et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann Oncol. 2012;23:652–658. doi: 10.1093/annonc/mdr279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bijelic L, Kumar AS, Stuart OA, et al. Systemic chemotherapy prior to cytoreductive surgery and HIPEC for carcinomatosis from appendix cancer: Impact on perioperative outcomes and short-term survival. Gastroenterol Res Pract. 2012;2012:163284. doi: 10.1155/2012/163284. [DOI] [PMC free article] [PubMed] [Google Scholar]


