Abstract
Excess enzyme-mediated protein O-GlcNAcylation is known to occur with diabetes mellitus. A characteristic of diabetic cardiomyopathy is the development of myocardial fibrosis. The role that enhanced protein O-GlcNAcylation plays in modulating the phenotype of cardiac fibroblasts (CF) is unknown. To address this issue, rat CF were cultured in normal glucose (NG; 5 mM glucose) or high-glucose (HG; 25 mM) media for 48 h. Results demonstrate that CF cultured in HG have higher levels (∼50%) of overall protein O-GlcNAcylation vs. NG cells. Key regulators of collagen synthesis such as transforming-growth factor-β1 (TGF-β1), SMADs 2/3, and SMAD 7 protein levels, including those of arginase I and II, were altered, leading to increases in collagen levels. The nuclear transcription factor Sp1 and arginase II evidence excess O-GlcNAcylation in HG cells. Expression in CF of an adenovirus coding for the enzyme N-acetylglucosaminidase, which removes O-GlcNAc moieties from proteins, decreased Sp1 and arginase II O-GlcNAcylation and restored HG-induced perturbations in CF back to NG levels. These findings may have important pathophysiological implications for the development of diabetes-induced cardiac fibrosis.
Keywords: cardiomyopathy, diabetes, fibroblast, fibrosis, glycosylation
long-standing hyperglycemia such as observed in patients with type 2 diabetes mellitus (DM) can induce heart failure in the absence of ischemic heart disease or hypertension, a condition known as diabetic cardiomyopathy (DC) (8, 35). Notable modifications in the structure of the diabetic heart include excess deposition of extracellular matrix proteins (i.e., fibrosis), which can contribute to impaired diastolic function (2, 3). Hyperglycemia promotes the modifications of molecules via the addition of glucose adducts through nonenzymatic (glycation) or enzymatic (O-GlcNAcylation) processes (17, 27). The nonenzymatic process occurs slowly and leads to the production of advanced glycation end products (AGE). AGE can activate signaling pathways associated with adverse outcomes via RAGE receptors or lead to cross-linking of adjoining molecules (40). The activation of RAGE and collagen cross-linking on cardiac fibroblasts (CF) are suspected to contribute to the development of cardiac fibrosis (40).
O-GlcNAcylation is an enzymatic process that results in a β-N-acetylglucosamine (O-GlcNAc) molecule being attached to proteins via O-linkage by the N-acetylglucosamine transferase to serine and threonine residues (23). Enhanced protein O-GlcNAcylation has been reported with DM and can impair cardiac myocyte function (5). However, the role that O-GlcNAcylation plays in the regulation of CF phenotype or in tissue fibrosis is poorly understood. Sp1 is a nuclear transcription factor that is known to activate the type I collagen alpha-1 promoter and whose activity can be modified via O-GlcNAcylation (22). Little is known about the role of Sp1 O-GlcNAcylation in cardiac fibrosis. However, in diabetic nephropathy the stimulation of collagen production is associated with increases in Sp1 O-GlcNAcylation levels (14). In a reverse reaction, O-GlcNAc moieties can be enzymatically detached from proteins as a direct action of N-acetylglucosaminidase (GCA) (38). The overexpression of GCA has been shown to restore normal function in nonfibrotic diabetic myocardium via the removal of excess protein O-GlcNAcylation suggesting an important role in the modulation of contractile function (19). However, no studies have examined the effects of GCA overexpression on hyperglycemia-induced fibrosis like responses and downstream signaling mediators.
Central to the development of fibrosis is the role played by stimulatory humoral factors in particular, transforming-growth factor-β1 (TGF-β1). TGF-β1 is produced by most cell types and is known to enhance CF proliferation and the synthesis of extracellular matrix proteins (7). The SMAD signaling pathway acts as downstream mediators of TGF-β1 action (7). SMADs 2/3 translocate into the nucleus accompanied by SMAD 4. In the nucleus, this protein complex acts in conjunction with Sp1 and enhances the transcriptional activity of several genes including type I collagen (7, 12, 15). The inhibitory SMADs 6 and 7 block TGF-β signaling by antagonizing its receptor-mediated activity and regulating the phosphorylation of SMADs 2 and 3 (12).
Fibrosis results from the synthesis of collagen, which in turns depends on the bioavailability of proline. Proline is a product of the arginine/arginase pathway (20), and a dysregulation of arginine metabolism has been implicated in the pathophysiology of DM (20). Arginase converts l-arginine to urea and ornithine. Decreased availability of l-arginine via increases in arginase activity can reduce nitric oxide levels leading to impaired vascular function as seen with DM (29, 30). On the other hand, increases in ornithine levels lead to enhanced polyamine and proline production. There are two isoforms of arginase that are ubiquitously expressed in most tissues and organs and whose activity can also be detected in blood (42). Both isoforms are observed in many cell types including fibroblasts and can be induced by proinflammatory cytokines (25). Arginase I is mainly cytoplasmic and II mitochondrial (29). Increases in arginase(s) activity correlate positively with the level of glycemic control and tissue fibrosis in DM patients (20, 29, 30). The role of arginases in regulating CF collagen synthesis in the setting of DM is yet to be explored.
This study aims to understand the role that enhanced protein O-GlcNAcylation (as stimulated by high glucose) has on promoting a profibrotic CF phenotype. We specifically hypothesize that the overexpression of GCA in CF exposed to high glucose will decrease Sp1 and arginase protein O-GlcNAcylation and restore CF functions to normal.
MATERIALS AND METHODS
Materials.
Reagents were obtained as follows: [3H]proline from Perkin Elmer; collagenase from Worthington Biomedical; cell culture media, trypsin, and antibiotics from GIBCO-BRL Invitrogen. Fetal bovine serum (FBS) was from Omega Scientific, and bovine serum albumin (BSA), α-isonitrosopropiophenone, Na3VO4, NaF, Tween-20, and d-glucose were from Sigma. polyvinylidene difluoride (PVDF) membranes were from Immobilon, and Bradford assay kits were from Bio-Rad and scintillation fluid from ICN Biomedicals. Antibodies RL2 and tata binding protein (TBP) were from Abcam; S6RP, SMAD 2/3, Sp1, GAPDH, and IgG anti-rabbit control were from Cell Signaling; SMAD 7, TGF-β, and O-GlycNAcase were from Santa Cruz; and collagen I and collagen III were from GenTex. Protein G-Sepharose/agarose was from Santa Cruz. The GCA inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino N-phenylcarbamate (PugNac) was purchased from Toronto Research Chemicals.
Cell culture.
All procedures were approved by University of California, San Diego Institutional Animal Care and Use Committee and conform to published National Institutes of Health guidelines for animal research. Male Sprague-Dawley rats (250–275 g) were obtained from Harlan Laboratories. Primary CF cultures were generated from adult rat ventricular tissues. Briefly, animals were injected with sodium heparin (400 IU/kg). After 15 min, rats were anesthetized with 80 mg/kg ketamine and 8 mg/kg xylazine. Hearts were removed under sterile conditions and perfused with a BSA (1 mg/ml for 8 min) containing buffer solution via the aorta to flush the heart clean of remaining blood. Hearts were perfused (1 ml/min for 30 min.) with collagenase solution (100 U/ml collagenase plus 0.5 mg/ml BSA) at 37°C. Partially digested ventricular tissue was isolated, minced, and digested for 3 min in a dish with fresh collagenase. The tissue suspension was pooled together and centrifuged at 300 rpm for 3 min at room temperature. The supernatant was recovered and centrifuged at 1,000 rpm for 10 min. The pellet was resuspended in growth media (DMEM: 5.5 mM d-glucose, 10% FBS, and 1% penicillin/streptomycin/fungizone pH 7.4). The cell suspension was plated in tissue-culture dishes for 30 min. Nonadherent cells were washed away, and the medium was replaced. CF were maintained in growth media and incubated in humidified atmosphere of 5% CO2 at 37°C until fully confluent. All studies were performed with cells at passages 2–3 and cultured in normal glucose (NG; 5 mM d-glucose) or high glucose (HG; 25 mM d-glucose). For experimental purposes during treatment, growth media used 0.5% BSA (insulin free).
Generation of GCA adenovirus.
To study the effects of GCA expression, we cloned the human gene coding for GCA into a replication-deficient adenoviral vector (Adv-GCA) under the control of the promoter-enhancer region of the human cytomegalovirus as previously described (5). An empty adenovirus without transgene (Adv-SR-) was used as an infection control.
CF adenovirus infection.
CF were plated at a density of 2.5 × 106 cells in 10-cm dishes with growth media. Six hours later, CF were starved for 24 h in NG media. CF were infected at a multiplicity of infection of 20 viral particles/cell with Adv-GCA or Adv SR- viruses for an additional 24 h. CF were then treated for 48 h with NG or HG media.
Arginase activity assay.
As a means to quantitate arginase activity, the reaction by-product urea was measured according to previously reported methodology with some modifications (34). Briefly, 80–85% confluent CF cultures were lysed with 25 mM Tris·HCl, 0.1% Triton X-100, 5 mM phenylmethanesulfonylfluoride, and 0.025 μM PugNAc pH 7.4. Homogenates were sonicated for 15 min at 4°C and centrifuged at 12,000 g for 10 min at 4°C. The supernatant was collected, and total protein concentrations were determined by Bradford assays. A total of 25 μl of supernatant was mixed with 25 μl of reaction buffer reaction (25 mM Tris·HCl and 5 mM MnCl2 pH 7.4). Arginase was activated by heating the mixture at 56°C for 10 min. l-Arginine hydrolysis was evaluated by the addition of 100 μl of 0.5 M l-arginine and incubated at 37°C for 60 min. The reaction was stopped by addition of 200 μl of an acid solution (H2SO4, H3PO4, and H2O 1:3:7 vol/vol). Urea concentrations were measured at 545 nm after the addition of 25 μM of isonitrosopropiophenone in 100% ethanol and heating of the samples at 100°C for 45 min.
Western blots.
CF grown in 10-cm dishes were homogenized in 50 μl lysis buffer (1% Triton X-100, 20 mmol/l Tris, 140 mmol/l NaCl, 2 mmol/l EDTA, 0.1% SDS in mmol/l, 10 μM Tris, 250 μM sucrose, and 0.025 μM PugNAc) with protease and phosphatase inhibitor cocktails supplemented with 0.15 mmol/l phenylmethanesulfonylfluoride, 5 mmol/l Na3VO4, and 1 mmol/l NaF pH 7.5. Homogenates were sonicated for 15 min at 4°C and centrifuged (12,000 g) for 15 min at 4°C. After centrifugation, the supernatant was collected and total protein concentrations were measured by Bradford assays. A total of 40 μg of protein was loaded onto a 4–15% precast SDS gels and electrophoresis applied at 100 mV for 3 h. Proteins were electrotransferred to PVDF membranes using a semidry transfer system, incubated for 1 h in TBS-Tween (T) blocking solution (5% nonfat dry milk in TBS plus 0.1% Tween 20), and followed by either 3 h incubation at room temperature or overnight incubation at 4°C with primary antibodies. Primary antibodies were typically diluted 1:1,000 or 1:2,000 in TBS-T plus 5% bovine serum albumin. RL2 antibody was used to detect O-GlcNAc modifications. Membranes were washed (3 × 5 min) in TBS-T and incubated for 1 h at room temperature in the presence of horseradish peroxidase-conjugated secondary antibodies diluted 1:10,000 in blocking solution. Membranes were washed in TBS-T, and Western blots were developed using an enhanced chemiluminescence detection kit. Band intensities were digitally quantified using ImageJ software (National Institutes of Health). To normalize for loading differences, we measured S6RP protein levels.
Nuclear Sp1 isolation.
The nuclear transcription factor Sp1 was isolated from CF using Nuclear Protein Isolation-Translocation Assay Kit (Fivephoton Biochemicals). Briefly, 80% confluent CF plated on 10-cm dishes were rinsed with phosphate-buffered saline at 25°C. The dish was placed on a bed of ice and incubated 5 min with 500 μl of cytoplasmic isolation reagent with protease and phosphatase inhibitors including 0.025 μM PugNAc. Cells were scraped and centrifuged 2,500 rpm for 3 min at 4°C. Supernatant (cytoplasmic fraction) was removed and stored for subsequent analyses. The pellet was resuspended in 500 μl of cytoplasmic isolation reagent and incubated 5 min on ice and then centrifuged at 2,500 rpm for 3 min at 4°C. The supernatant was discarded, and 60 μl of cold nuclear isolation reagent supplemented with protease inhibitors and 0.025 μM PugNAc were added and then vortexed for 1 min and incubated on ice for 10 min. Samples were centrifuged at 14,000 rpm for 10 min at 4°, and supernatant that corresponded to the nuclear fraction was collected. Purity of isolated fractions was assessed by Westerns blot using TBP as a nuclear marker and GAPDH as a cytoplasmic marker. Nuclear fractions were also used for Sp1 immunoprecipitation (IP) purposes as described below.
Immunoprecipitation.
IP were performed as previously described (5). CF were lysed with 50 μl of nondenaturing extraction buffer (0.5%, Triton X-100, 50 mmol/l Tris·HCl, pH 7.4, 0.15 mol/l NaCl, 0.1 mmol/l EDTA, and 0.025 μM PugNAc pH 7.4) and supplemented with protease and phosphatase inhibitor cocktail, plus 0.15 mmol/l PMSF, 5 mmol/l Na3VO4, and 1 mmol/l NaF. Homogenates were incubated on ice for 10 min and passed through an insulin syringe three times. The homogenate was incubated on ice with shaking for 10 min and centrifuged for 10 min at 12,000 g at 4°C. A total of 0.5 mg protein was diluted in binding buffer (10 mM Tris·HCl, 2 mM MgCl2, 0.15 mM NaCl, 10% glycerol, and 0.15 mM PMSF, supplemented with protease and phosphatase inhibitor cocktails, plus 0.15 mmol/l PMSF, 5 mmol/l Na3VO4, and 1 mmol/l NaF pH 7.9) to a final concentration of 1 μg/μl. Subsequently, samples were precleared by addition of 1 μg of normal rabbit IgG control and 20 μl protein-G-agarose with mixing for 30 min (4°C) and centrifuged at 12,000 g for 10 min at 4°C. The supernatant was recovered and incubated for 3 h at 4°C using mild agitation with 3 μg of an arginase II antibody. Although a similar approach was implemented using an arginase I antibody, no successful IP was achieved. For Sp1 IP, nuclear fractions were incubated as above using Sp1 antibody. Twenty microliters of protein G-Sepharose were added, and the mixture was incubated overnight at 4°C with shaking. The IP mixture was centrifuged at 3,500 g for 4 min at 4°C, and the supernatant was recovered and stored at 4°C. The pellet was washed with binding buffer for 15 min at 4°C with shaking and centrifuged at 3,500 g for 4 min at 4°C. Washes were repeated thrice. The IP proteins in the pellet and those remaining in the supernatant were applied to a 4–15% gradient gel for Western blot as described above.
Proline incorporation.
[3H]proline incorporation was used as an indicator of total collagen synthesis. CF were plated in 24-well tissue culture dishes and grown to 80–85% confluence. Cells were incubated with either NG or HG (with Adv-GCA or Adv-SR-) for 48 h. During the last 36 h of treatment, CF were pulsed with [3H]proline (1 μCi/ml). To terminate the experiment, each well was washed twice with cold PBS solution followed by the addition of cold 10% trichloroacetic acid (TCA; 500 μl/well) to lyse the cells and precipitate cellular proteins. Wells were washed three times with TCA. NaOH (250 μl) of 1 N was added to each well to solubilize proteins. Samples were neutralized with 250 μl of 1 N HCl for 30 min and radioactivity counted after the addition of scintillation fluid.
Chromatin immunoprecipitation and PCR assays.
Chromatin immunoprecipitation (ChIP) assays were performed using the Chromatin IP Kit (Agarose Beads) from Cell Signaling. DNA-protein interactions were cross-linked using 1% (final concentration) of formaldehyde, isolated and digested using micrococcal nuclease. Digested chromatin was subjected to IP with anti-Sp1 (ChIP grade from Abcam) antibody and ChIP grade Protein G agarose beads. Cross-linking was reversed by eluting chromatin from the Sp1 antibody/protein G beads, and DNA was purified using MiniElute Spin Columns (Qiagen) and used as template for PCR assays using the following primers: collagen I alpha-1 promoter (COL1A1) consensus sequence for Sp1 binding: forward 5′-CAGAGCTGCGAAGAGGGGA-3′ and reverse, 5′-AGACTCTTTGTGGCTGGGGAG-3′. Amplification resulted in a 300-bp amplicon (−200/+100 bp) of the promoter. Amplification condition was 93°C for 30 s, 58°C for 60 s, and 72°C for 30 s for 28 cycles. The PCR products were analyzed in 2% agarose gel electrophoresis and stained with ethidium bromide. As a negative control, a parallel ChIP assay was implemented using normal rabbit IgG. As a DNA loading control, the RPL30 sequence was amplified using primers provided by the ChIP assay kit. As a negative control for the PCR assays, a 147-bp DNA fragment of the sarcospan gene was included using the following primers: forward 5′-CTAGTCAGGGACACTCCATT-3′ and reverse 5′-GGCACTCAGCAGAAAGTATAA-3′.
Statistical analysis.
Data are presented as means ± SE. Comparisons were made using t-tests or ANOVA using Bonferroni corrections. All experiments were performed three times each, in triplicates. P < 0.05 was considered statistically significant.
RESULTS
O-GlcNAcylation of CF proteins.
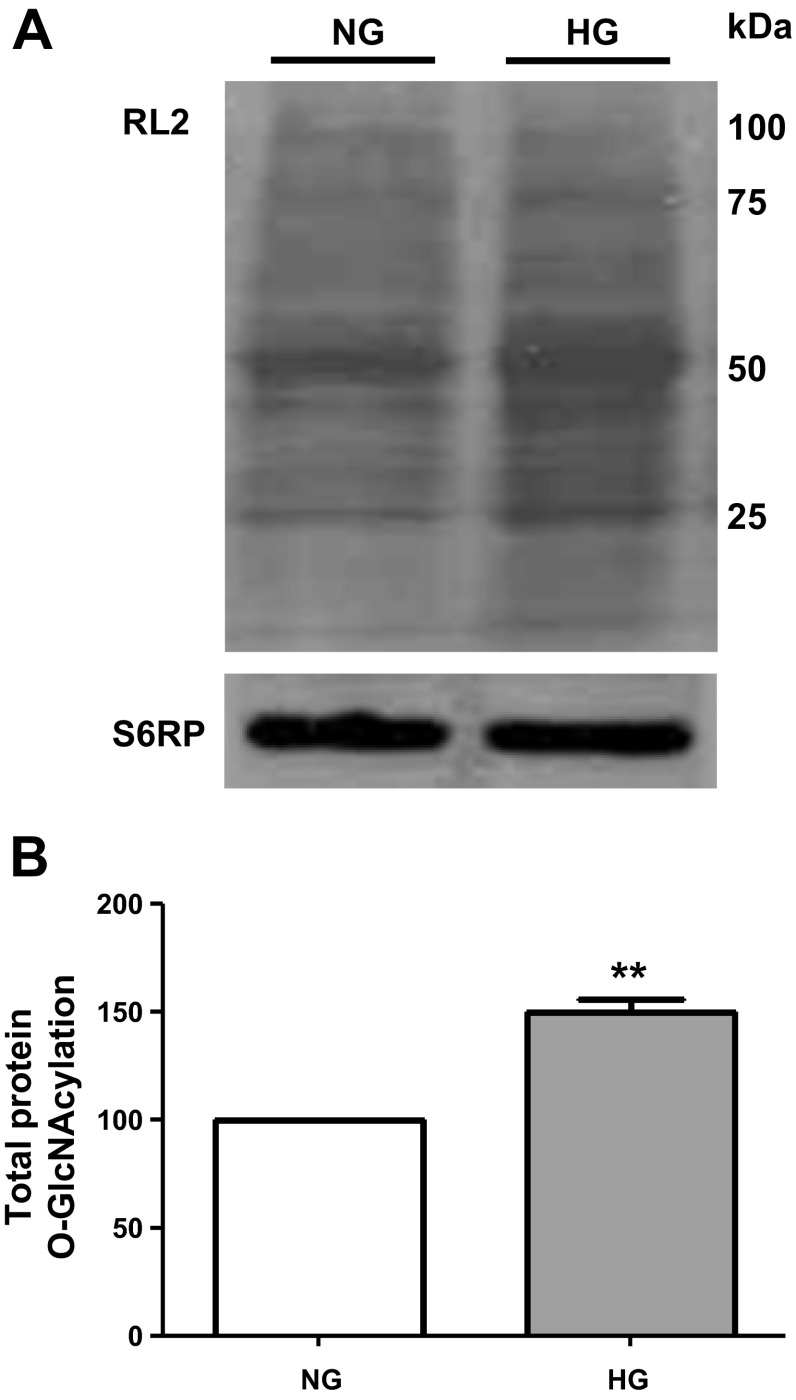
CF were cultured for 48 h in either NG or HG media, and cell lysates were collected to assess for changes in protein O-GlcNAcylation levels using the RL2 antibody. Figure 1A illustrates a representative image obtained from total cell lysates probed with the antibody where multiple bands were detected and used to quantify protein O-GlcNAcylation. Figure 1B plots the average values obtained, demonstrating significant increases of ∼50% in total protein O-GlcNAcylation levels in CF cultured in HG compared with NG.
Fig. 1.
Protein O-GlcNAcylation levels in cardiac fibroblasts. A: representative image of a Western blot for total protein O-GlcNAcylation levels using the RL2 O-GlcNAc-specific antibody in cardiac fibroblasts (CF) cultured in normal glucose (NG; 5.5 mM dextrose) or high-glucose (HG; 25 mM dextrose) media. B: graphical representation of average data (n = 3; **P < 0.01).
TGF-β1 signaling mediators and Sp1.
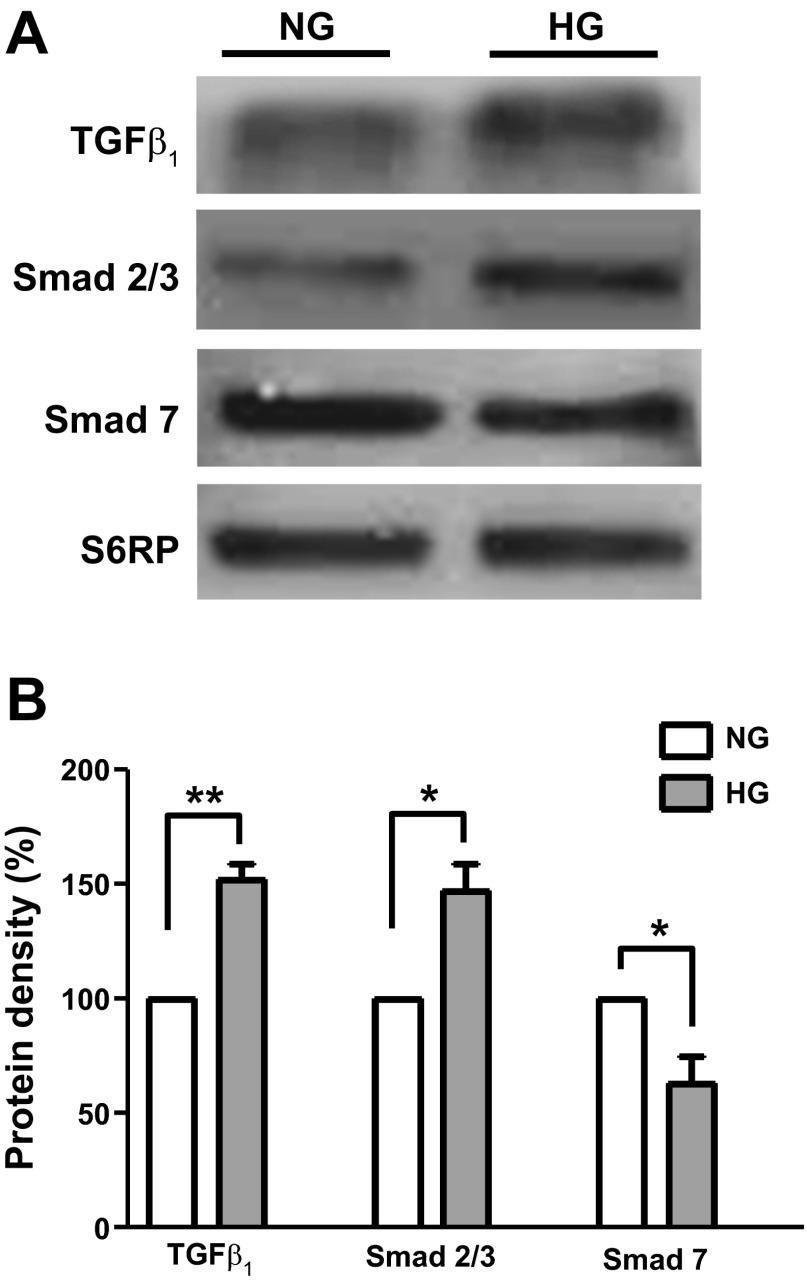
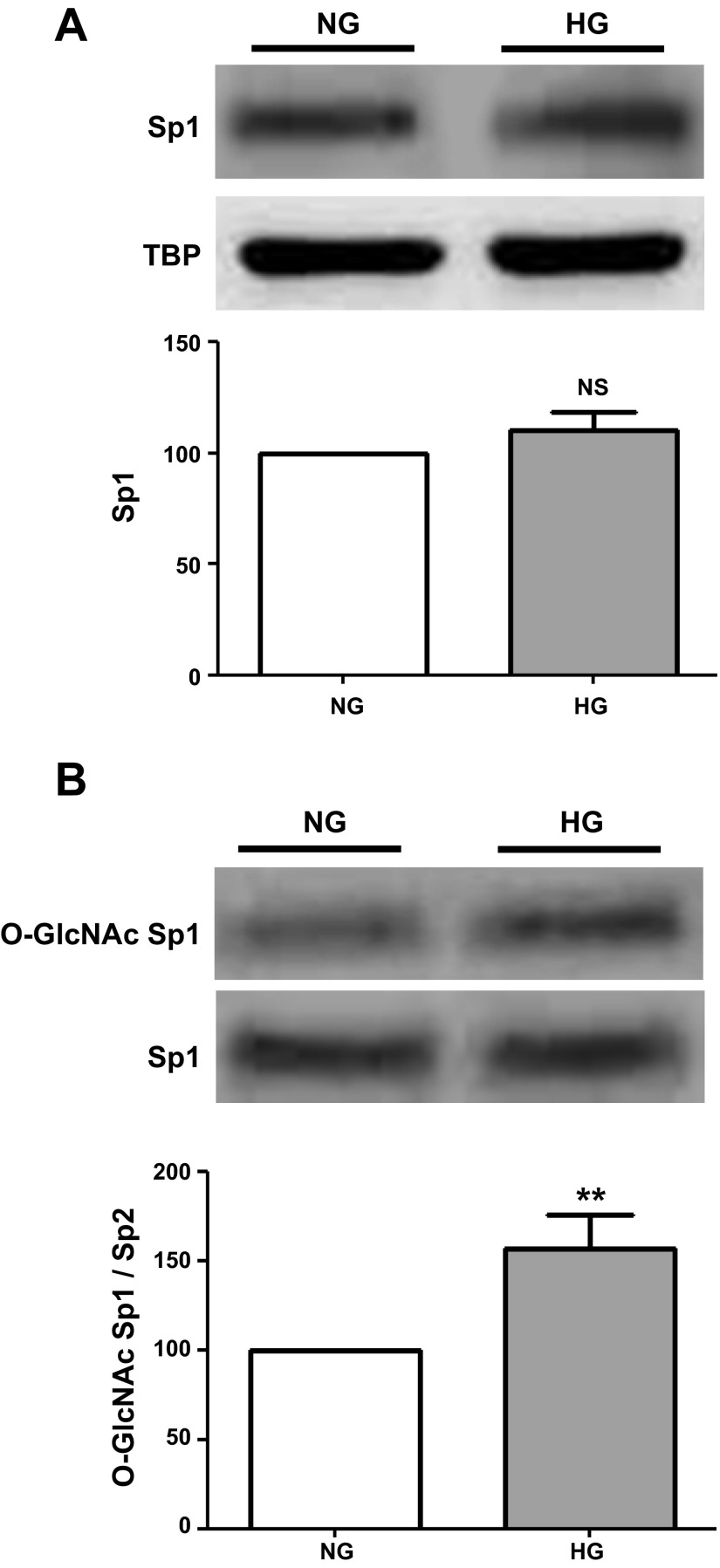
Cell lysates from NG- and HG-cultured CF were used to probe for changes in TGF-β1 and related signaling molecules (SMAD 2/3 and SMAD 7). A representative Western blot image is shown in Fig. 2A, and average results were plotted in Fig. 2B. As can be observed, culturing of CF in HG media for 48 h led to significant increases in TGF-β1 and SMAD 2/3, which were accompanied by decreases in the inhibitory SMAD 7 protein. Figure 3A shows a Western blot image of Sp1 protein levels, and Fig. 3B summarizes these results. Exposure of CF to HG did not yield changes in Sp1 protein levels. However, the analysis O-GlcNAcylation of Sp1 levels (normalized to total Sp1 levels) led to a significant ∼50% increase as shown in Fig. 3B.
Fig. 2.
Transforming-growth factor-β1 (TGF-β1) and SMAD protein levels in cardiac fibroblasts. A: Western blots for TGF-β1, SMAD 2/3, and SMAD 7 protein levels of CF cultured in NG (5.5 mM dextrose) or HG (25 mM dextrose) media. B: graphical representation of average data (n = 3; *P < 0.05; **P < 0.01).
Fig. 3.
Sp1 protein and O-GlcNAcylation levels in cardiac fibroblasts. A: Western blot of Sp1 protein levels in CF cultured in NG (5.5 mM dextrose) or HG (25 mM dextrose) media with average data plotted below. B: Western blot images of O-GlcNAc Sp1 levels and corresponding plots (n = 3; **P < 0.01).
Collagen synthesis and arginases.
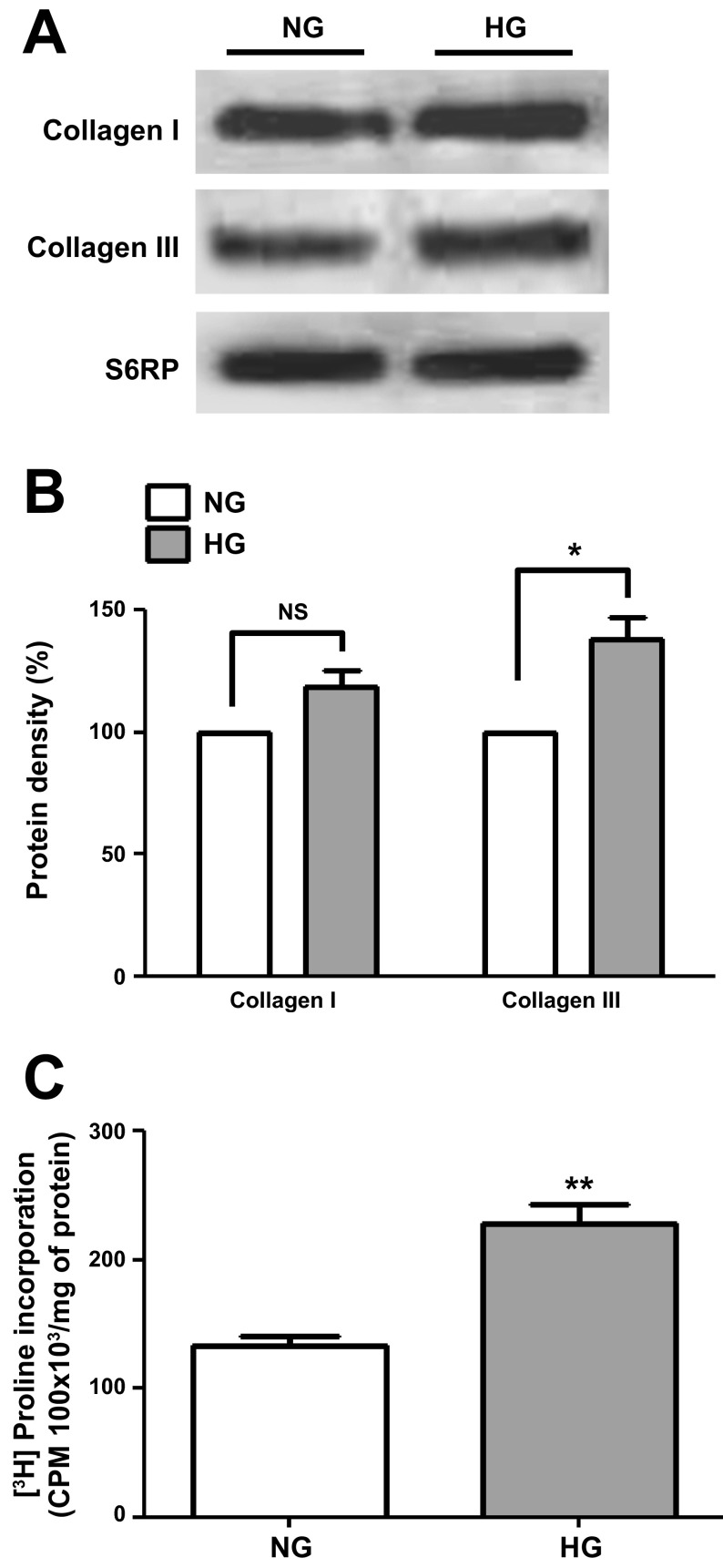
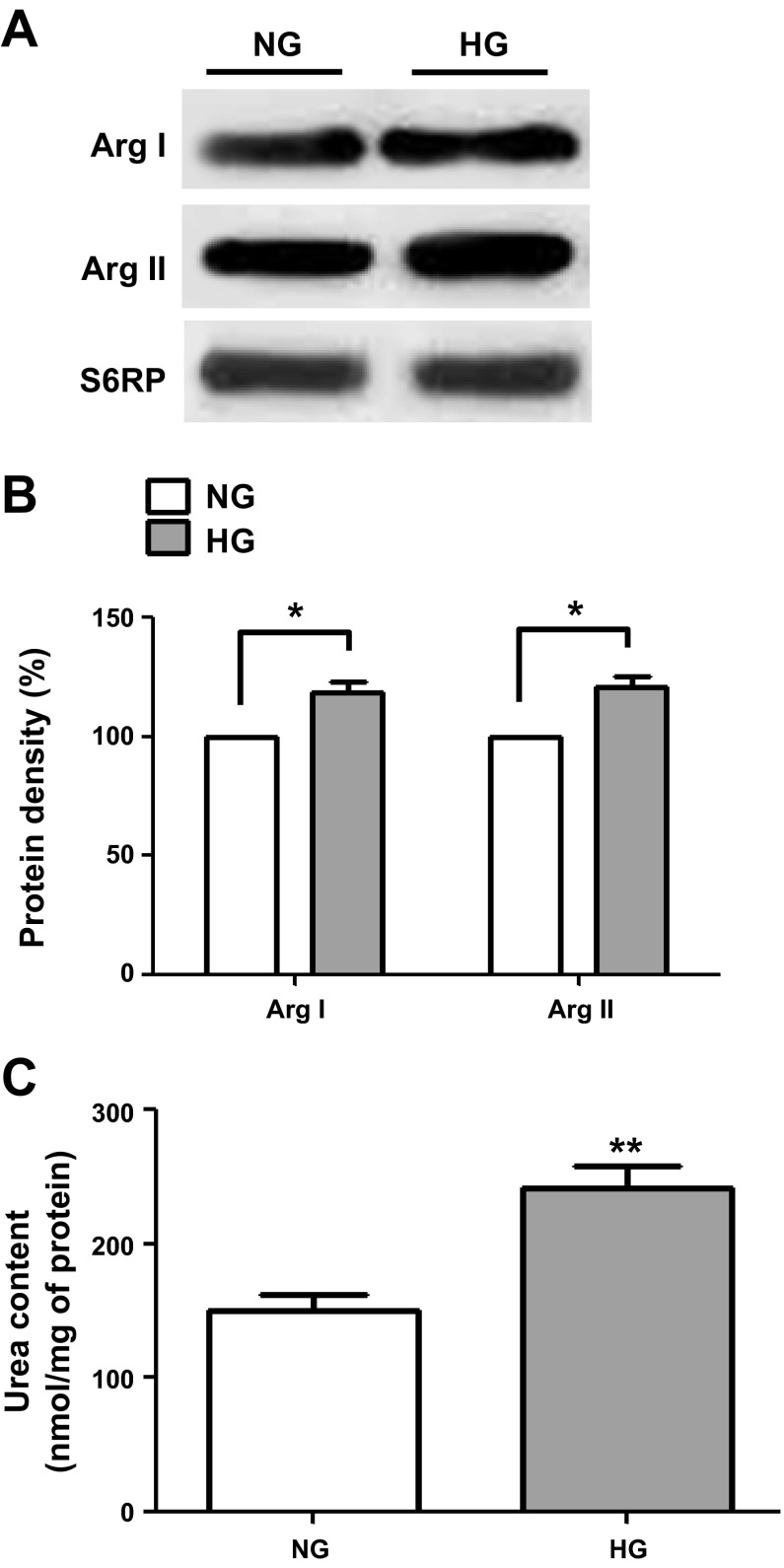
Cell lysates from NG- and HG-cultured CF were analyzed by Western blot for changes in collagen type I and III protein levels (Fig. 4A), and the results are summarized in Fig. 4B. HG-induced significant increases of ∼40% in type III collagen protein levels (normalized vs. S6RP) while a nonsignificant increase (P = 0.06) was noted for type I collagen. Increases in collagen protein levels were further validated by the significant increases noted in HG-cultured CF [3H]proline incorporation levels (Fig. 4C). The total levels of arginase I and II were also analyzed using Western blots (values normalized vs. S6RP) and results are shown in Fig. 5, A and B. CF cultured in HG demonstrated significant increases of ∼30% in the total levels of arginase I and II. Increases in protein levels for the enzyme also correlated with a significant increase of ∼75% in overall arginase activity as shown in Fig. 5C.
Fig. 4.
Collagen type I and III protein levels in cardiac fibroblasts. A: Western blots for collagen type I and III protein levels in CF cultured in NG (5.5 mM dextrose) or HG (25 mM dextrose) media. B: graphical representations of collagen type I and III data. C: [3H]proline incorporation levels observed in both conditions (n = 3; *P < 0.05; **P < 0.01).
Fig. 5.
Arginase (Arg) I and II protein and activity levels in cardiac fibroblasts. A: Western blots of arginase I and II protein levels in CF cultured in NG (5.5 mM dextrose) or HG (25 mM dextrose) media. B: graphical representation of arginase I and II protein levels. C: arginase activity as measured by urea determinations (n = 3; *P < 0.05; **P < 0.01).
Effects of GCA overexpression.
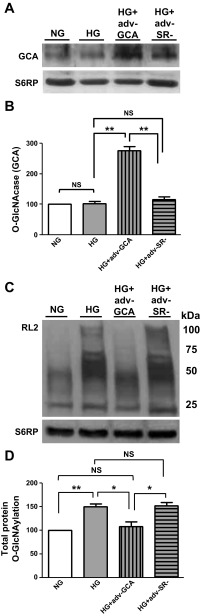
To ascertain the role that enhanced O-GlcNAcylation of proteins plays in determining a profibrotic phenotype in HG-cultured CF, cells were transduced with adenovirus to overexpress GCA (Adv-GCA). Data generated from cells transduced with an empty adenovirus (Adv-SR-) were used for control purposes. Figure 6, A and B, shows the effects that the GCA transgene expression had on CF enzyme protein levels (values normalized vs. S6RP). Overexpression led to significant increases in GCA enzyme levels of ∼2.5-fold vs. nontransduced cells or cells transduced with the empty adenovirus. Figure 6, C and D, demonstrates the normalizing effects that overexpression of GCA had on total protein O-GlcNAcylation levels in CF cultured in HG media. Figure 7A is a representative Western blot image of the effect of the overexpression of GCA on TGF-β1, SMAD 2/3, and SMAD 7 protein levels in cultured CF. The overexpression of GCA in CF cultured in HG restored proteins to levels comparable to those cultured in NG. The corresponding bar graphs for the different proteins are presented in Figs. 7B.
Fig. 6.
O-GlcNAcase effects on cardiac fibroblast O-GlcNAcylation protein levels. A: Western blot of N-acetylglucosaminidase (GCA) protein levels in CF cultured in NG (5.5 mM dextrose), HG (25 mM dextrose), HG + GCA (Adv-GCA), or HG + SR- [empty adenovirus without transgene (Adv-SR-)]. B: graphical representation of data. C: CF protein O-GlcNAcylation as detected using the RL2 O-GlcNAc specific antibody. D: graphical representation of data (n = 3; *P < 0.05; **P < 0.01).
Fig. 7.
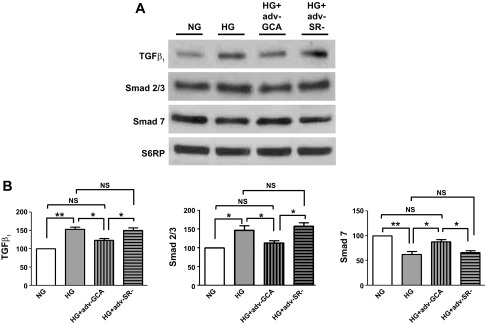
O-GlcNAcase effects on the TGF-β1 signaling pathway. A: Western blots of TGF-β1 and signaling molecules SMAD 2/3 and SMAD 7 proteins in CF cultured in NG (5.5 mM dextrose), HG (25 mM dextrose), HG + GCA (Adv-GCA), or HG + SR- (Adv-SR-). B: Graphical representation of data (n = 3; *P < 0.05; **P < 0.01).
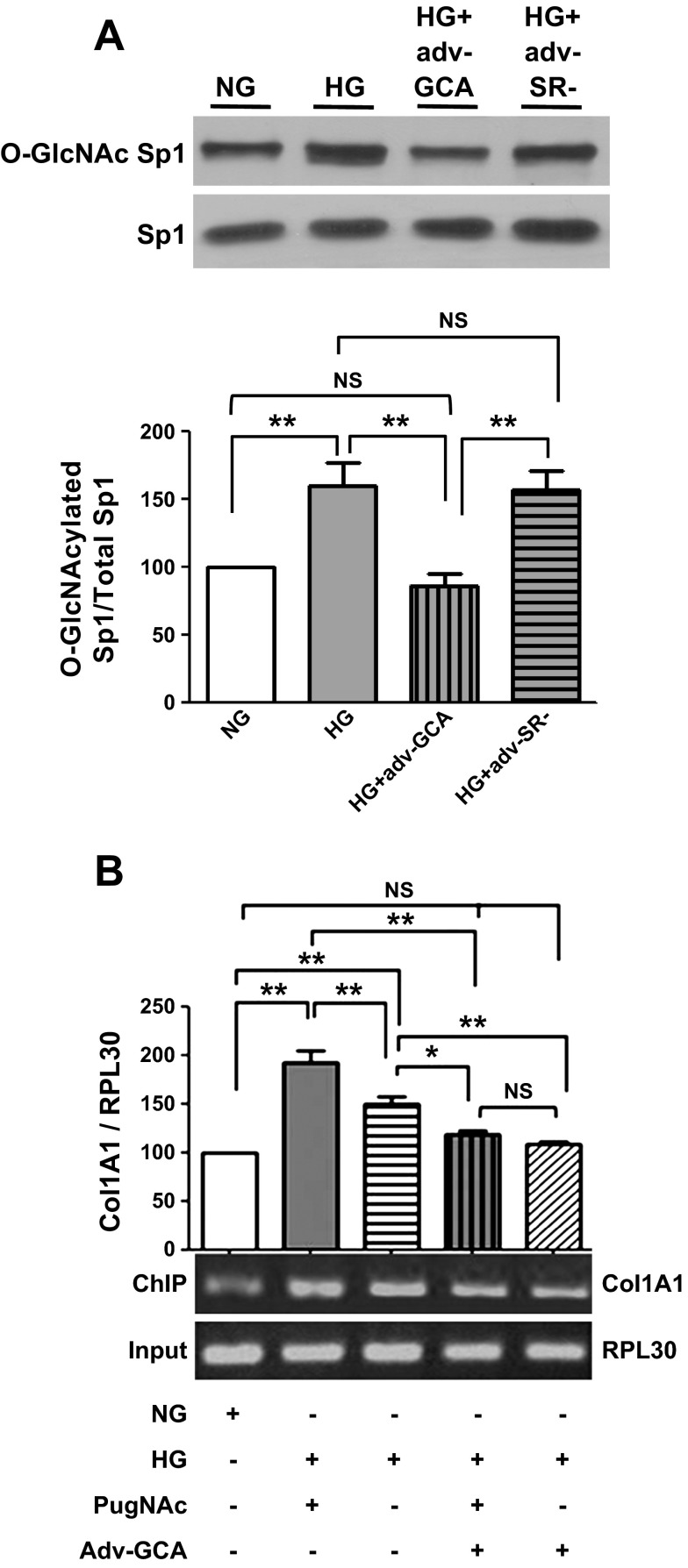
Figure 8A illustrates the effects that the overexpression of GCA levels had on the O-GlcNAc modification of Sp1 in CF exposed to HG. As noted, GCA overexpression normalized O-GlcNAcylation levels while the empty vector (control virus) failed to exert such an effect. To determine the mechanism underlying these effects, a ChIP assay was performed. Results shown in Fig. 8B, denote the greater levels of Sp1 bound to the type I collagen promoter when cells were incubated with HG with or without PugNAc and the corresponding reversal with GCA overexpression.
Fig. 8.
O-GlcNAcase effects on Sp1 O-GlcNAcylation levels and modulation of nuclear events associated with Sp1. A: Western blots and graphical representation of total Sp1 and Sp1 O-GlcNAcylation levels from CF cultured in NG (5.5 mM glucose), HG (25 mM glucose), HG + GCA (Adv-GCA), or HG + SR- (Adv-SR-). B: PCR amplified DNA levels derived from the chromatin immunoprecipitation (ChIP) assay evidencing the binding of the Sp1 transcription factor to the COL1A1 promoter (as documented by ethidium bromide stained gel electrophoresis) (n = 3; *P < 0.05; **P < 0.01).
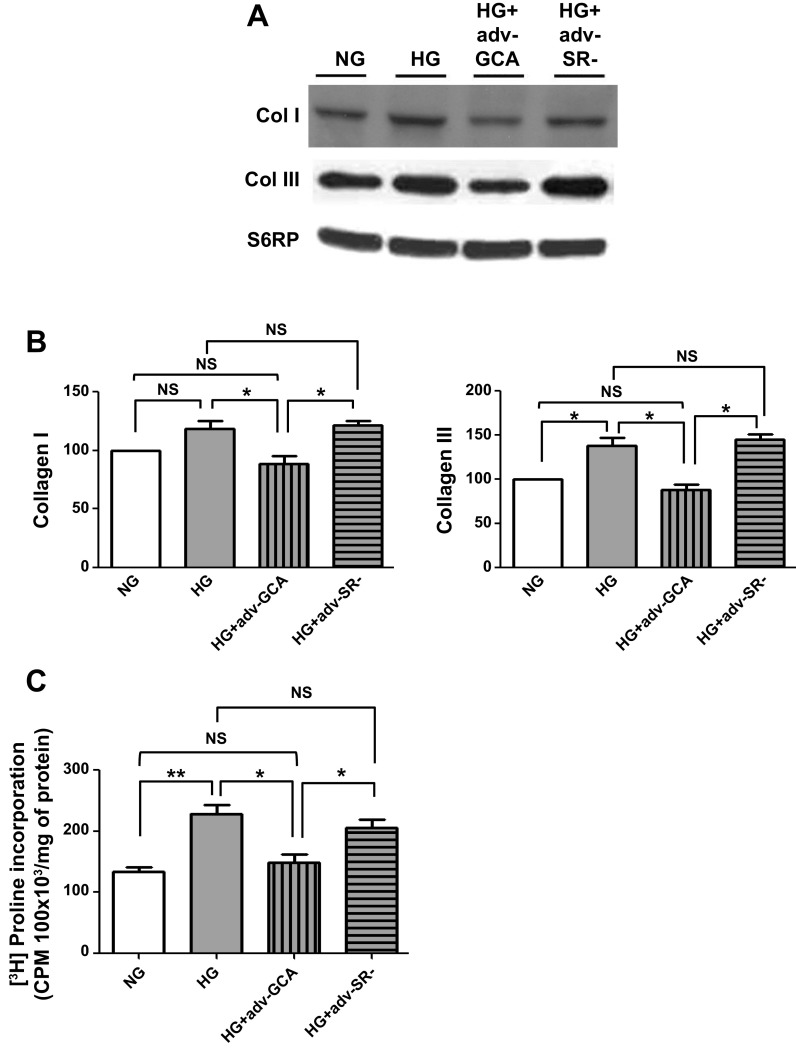
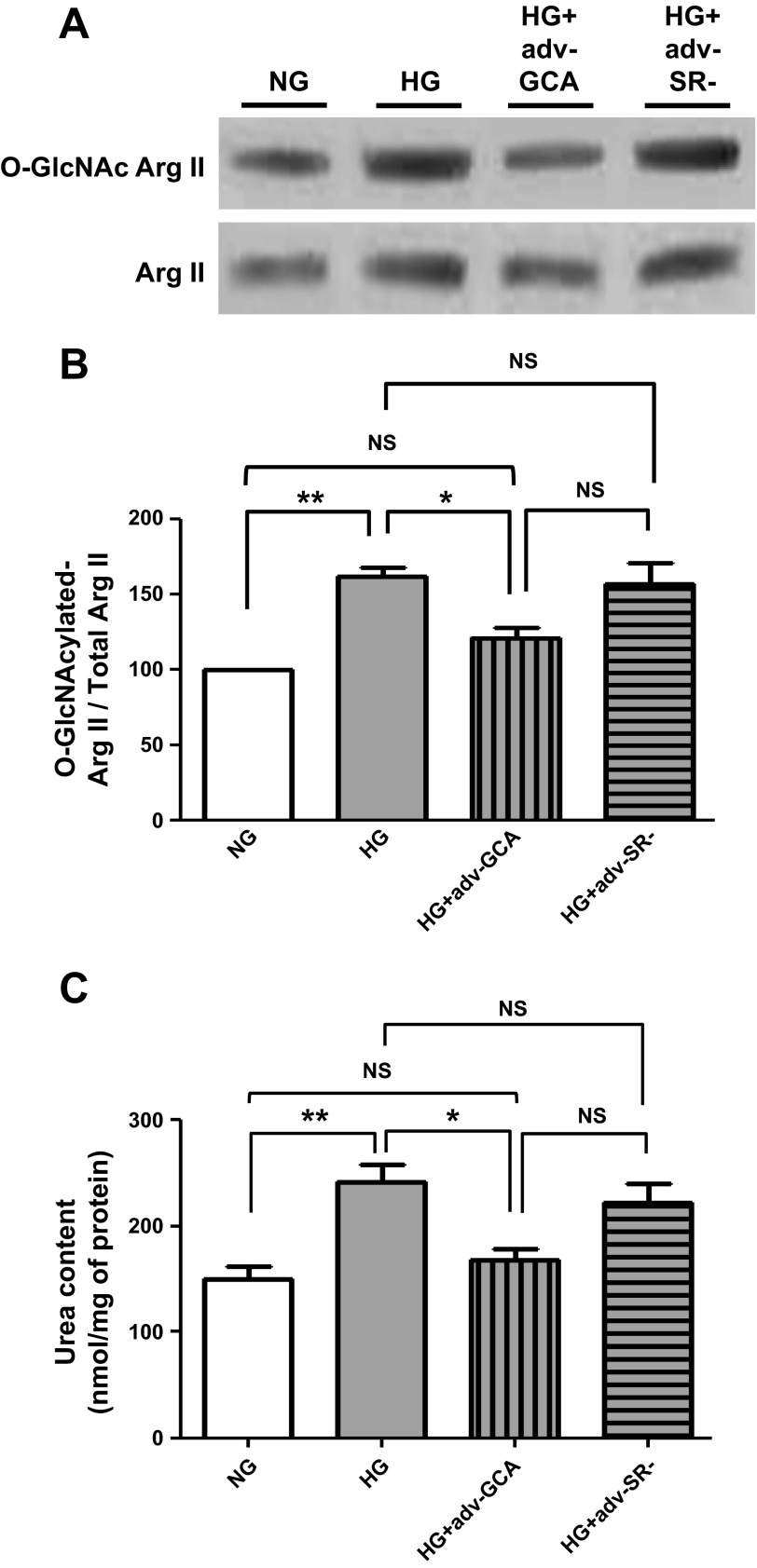
The effects of GCA overexpression on type I and type II collagen protein levels were also evaluated. As shown in Fig. 9, A and B, CF cultured in HG and treated with adenovirus expressing GCA had collagen protein levels restored to values comparable to NG-cultured cells. Similar normalizing effects of GCA were evidenced in proline incorporation (Fig. 9C). The effects that GCA overexpression had on HG-cultured CF arginase II O-GlcNAcylation levels were evaluated as well. Results indicate a normalization of arginase O-GlcNAcylation levels to those comparable of NG cells and a corresponding restoration of total arginase activity, as measured by urea production, to values observed in NG-cultured cells. The use of the empty vector virus had no effects (Fig. 10, A–C).
Fig. 9.
O-GlcNAcase effects on cardiac fibroblast collagen synthesis. A: Western blot of collagen I and collagen III protein levels in CF cultured in NG (5.5 mM dextrose), HG (25 mM dextrose), HG + GCA (Adv-GCA), or HG + SR- (Adv-SR-). B: graphical representation collagen I and III data. C: graphical representation of effects on CF [3H]proline incorporation (n = 3; *P < 0.05; **P < 0.01).
Fig. 10.
O-GlcNAcase effects on cardiac fibroblast arginase O-GlcNAcylation levels and activity. A: Western blots of arginase II O-GlcNAcylation levels. B: graphical representation of arginase II protein O-GlcNAcylation normalized to total arginase II in CF cultured in NG (5.5 mM dextrose), HG (25 mM dextrose), HG + GCA (Adv-GCA), or HG + SR- (Adv-SR-). C: graphical representation for changes in arginase activity levels (n = 3; *P < 0.05; **P < 0.01).
DISCUSSION
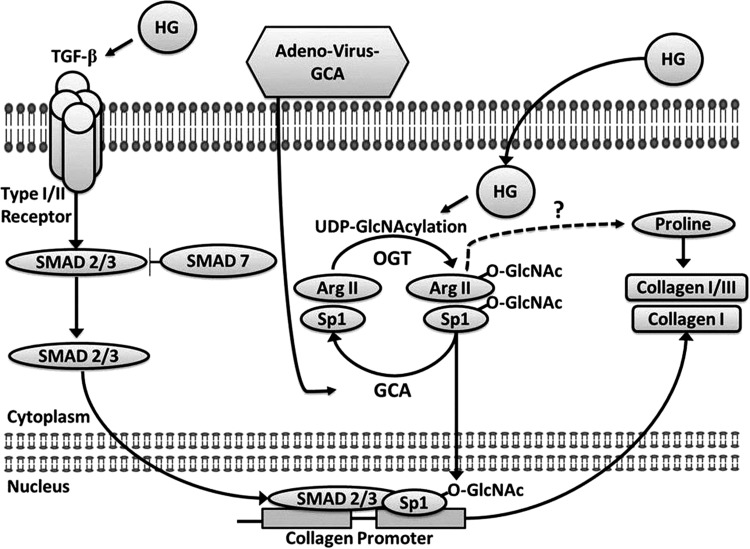
As summarized in Fig. 11, results demonstrate that HG activates CF profibrotic pathways and induces increases in collagen synthesis. HG also leads to enhanced protein O-GlcNAcylation including that of the nuclear transcription factor Sp1 and arginase II. Increases in protein O-GlcNAcylation appear to stimulate collagen synthesis as adenovirus-mediated overexpression of GCA can revert the profibrotic effects of HG. These results may have major implications for the pathophysiology of cardiac fibrosis as seen with DM.
Fig. 11.
Schematic summarizing the main findings of the study and linkage between documented events leading to modified CF phenotypes.
Under HG conditions as observed with DM, glucose flux through the hexosamine biosynthetic pathway increases, resulting in enhanced protein O-GlcNAc modification (22). Elevated O-GlcNAcylation of proteins has been reported in endothelial, cardiomyocyte, and macrophage cells exposed to HG (13, 19, 22). Notably, O-GlcNAcylation has been increasingly recognized as a means to alter protein function (6, 16, 18). For example, O-GlcNac posttranslational modification of cardiac myofilament proteins affects calcium sensitivity and may contribute to altered contractile function (28). Cardiac fibrosis is an important component of DC and can contribute to the development of diastolic dysfunction. In this study, we aimed to understand the role played by protein O-GlcNAcylation in defining a profibrotic phenotype in CF. Results indicate that following incubation in HG media “total” CF protein O-GlcNAcylation levels were significantly increased. This is the first study to report such effects in CF. However, our results contrast with those of Kohda et. al. (21) where they failed to evidence increased O-GlcNAcylation in CF cultured in HG but did observe such effects when cells were incubated with glucosamine. Failure to reveal HG-induced increased protein O-GlcNAcylation may be due to the absence of PugNAc in the lysis buffer, which can prevent O-GlcNAc removal by O-GlcNAcase. We have also reported on the effects of excess O-GlcNAcylation in cultured neonatal cardiac myocytes and also in diabetic mice which contribute to altered contractile function (5, 10).
We previously reported that culturing of CF in 25 mM glucose stimulates proline incorporation (1). In the current study, we replicate our previous findings and also document higher protein levels for type III collagen and a trend for similar effects for type I collagen (1). Cell culture results are also consistent with clinical findings from Shimizu et al. (32), where they reported significant increases in myocardial fibrosis and type III collagen levels (with a trend toward increased collagen type I) in biopsy samples of DM patients. TGF-β1 is recognized as a major stimulator of fibrous tissue deposition in the heart and can induce a profibrotic phenotype in CF (3). We demonstrate that HG increases TGF-β1 protein levels in CF. These results are consistent with those reported by Singh et. al. (33) where neonatal rat CF cultured in 25 mM glucose showed increased TGF-β1 protein levels. Multiple pathways can be activated by TGF-β1. However, the SMAD 2/3 protein complex is recognized as a key mediator of TGF-β1 profibrotic effects as the complex can translocate into the nucleus where it binds to gene promoter regions (37). SMAD 7 can inhibit the translocation of SMAD 2/3 by competing for the TGF-β1 receptor binding site of SMAD 2/3 and also enhancing receptor degradation (24). In the current study, we report for the first time that SMAD 2/3 protein levels increase while SMAD 7 levels are suppressed in CF cultured in HG, which may facilitate the development of a profibrotic phenotype. These observations are in agreement with those of Wu and Derynck (39) who used fluorescence microscopy to evidence HG-induced enhanced nuclear SMAD 2/3 translocation in embryonic mouse fibroblasts and rat kidney epithelial cells.
The O-GlcNAcylation of transcription factors has long been recognized and can lead to their altered activity (22, 36). Sp1 is a transcription factor that is known to participate in the activation of multiple promoters including that of the alpha-1 chain of type I collagen (22). However, less is known about the regulation of type III collagen expression by Sp1. Sp1 has several sites that can be subjected to O-GlcNAc modification (41), and its O-GlcNAcylation has been associated with stimulating the transcriptional activity of genes known to participate in the development of kidney fibrosis (14). In this study, ChIP assays were implemented to gain insight into the role that Sp1 O-GlcNAcylation may have in promoting increases in collagen production in CF cultured in HG media. Our results indicate that greater levels of O-GlcNAcated Sp1 were bound to the COL1A1 promoter region in response to HG conditions and that the inclusion of PugNAc exacerbated the effect whereas GCA overexpression prevented such increases. The increased amplification of Sp1 bound collagen promoter DNA fragments observed with HG with or without PugNAc evidenced the increased activity of the transcriptional factor and was also blocked by GCA overexpression. Of interest is that studies performed in bovine aortic endothelial cells cultured in HG also revealed that increased Sp1 O-GlcNAc modification stimulated TGF-β1 expression (9). In our study, we demonstrate that O-GlcNAc modification of Sp1 in CF was increased with HG without altering Sp1 protein levels. Thus the enhanced O-GlcNAcylation of Sp1 may partly account for the increases observed in TGF-β1 levels, a humoral factor, which is also known to stimulate arginase activity (4).
Plasma arginase activity positively correlates with the severity of hyperglycemia in type 2 DM patients (20). In experimental DM animal models, aortic fibrosis is associated with increased arginase I and II protein levels and activity (29, 30). Increases in arginase II activity are also linked to diabetic renal injury (26). In the current study, we demonstrate that HG increases CF arginase I and II protein levels and total enzyme activity. As the development of tissue fibrosis in DM patients is well recognized (3), our results suggest that the development of cardiac fibrosis may be due in part to HG-induced increases in CF arginase activity leading to enhanced proline availability and thus collagen synthesis.
The role of excess O-GlcNAcylation in determining the profibrotic CF phenotype was examined by overexpression of GCA. Prior studies performed by our group demonstrated that increased expression of GCA by adenovirus in neonatal cardiac myocytes cultured in HG decreases total protein O-GlcNAcylation and restores contractile function (19). Similarly, in a recent study using GCA-inducible transgenic diabetic mice, we show that overexpression of GCA restored systolic function (10). In this study, we document that CF overexpressing GCA show decreased total protein O-GlcNAcylation. In addition, the overexpression of GCA reduced TGF-β1 and SMAD 2/3 and restored SMAD 7 protein levels. Interestingly, these changes correlated with a decrease in Sp1 O-GlcNAc modification along with normalization of proline incorporation and collagen I and III protein levels. We also document that arginase II is O-GlcNAcylated and that removal of O-GlcNAc by GCA decreases the level of O-GlcNAcylation and normalizes overall arginase activity. Interestingly, there is precedent for posttranslational modifications of arginases (by S-nitrosylation) leading to the enhancement of their activity (31). Our results suggest that O-GlcNAcylation of arginases may also enhance their activity. Future studies would need to be performed to identify the specific amino acid residue(s) in arginases modified by O-GlcNAcylation and to relate this to altered enzymatic activity and what are the consequences of blocking such events.
Noteworthy is that although GCA protein levels increased 2.5-fold, the levels of O-GlcNAcylation were simply reduced to NG levels, thus suggesting a complex regulatory control system. Altogether, given the scope of normalizing effects exerted by the overexpression of GCA results suggests that O-GlcNAcylation may play a key role in regulating complex pathways such as those associated with tissue fibrosis.
To our knowledge this is the first study to demonstrate that GCA overexpression can readily revert a HG-induced profibrotic phenotype back to normal. These findings have important implications, as the development of fibrosis in patients with DM is known to adversely affect multiple organ function such as that of kidneys and blood vessels. A similar paradigm occurs with aging where excess O-GlcNAcylation is also observed as well as multiple organ fibrosis and loss of function (11). Our results suggest that excess O-GlcNAcylation may represent a viable target for preventive therapeutic antifibrotic interventions.
GRANTS
The study was supported by National Institutes of Health Grants R24-DK-092154, R01-HL-43617, and P60-MD-00220 (to F. Villarreal) and the P. Robert Majumder Charitable Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.A., E.F., S.I., M.S., L.M.-R., G.C., W.H.D., F.V., and I.R.-S. conception and design of research; H.A., E.F., S.I., M.S., L.M.-R., N.A., G.C., W.H.D., F.V., and I.R.-S. performed experiments; H.A., E.F., S.I., M.S., L.M.-R., G.C., W.H.D., F.V., and I.R.-S. analyzed data; H.A., E.F., S.I., M.S., L.M.-R., G.C., W.H.D., F.V., and I.R.-S. interpreted results of experiments; H.A., E.F., S.I., M.S., L.M.-R., G.C., W.H.D., F.V., and I.R.-S. prepared figures; H.A., E.F., S.I., M.S., L.M.-R., G.C., W.H.D., F.V., and I.R.-S. drafted manuscript; H.A., E.F., S.I., M.S., L.M.-R., G.C., W.H.D., F.V., and I.R.-S. edited and revised manuscript; H.A., E.F., S.I., M.S., L.M.-R., G.C., W.H.D., F.V., and I.R.-S. approved final version of manuscript.
REFERENCES
- 1.Asbun J, Manso AM, Villarreal FJ. Profibrotic influence of high glucose concentration on cardiac fibroblast functions: effects of losartan and vitamin E. Am J Physiol Heart Circ Physiol 288: H227–H234, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol 47: 693–700, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11: 31–39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutard V, Havouis R, Fouqueray B, Philippe C, Moulinoux JP, Baud L. Transforming growth factor-beta stimulates arginase activity in macrophages. Implications for the regulation of macrophage cytotoxicity. J Immunol 155: 2077–2084, 1995 [PubMed] [Google Scholar]
- 5.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 278: 44230–44237, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Copeland RJ, Han G, Hart GW. O-GlcNAcomics-revealing roles of O-GlcNAcylation in disease mechanisms and cevelopment of potential diagnostics. Proteomics Clin Appl 2013. May 3 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutroneo KR. TGF-beta-induced fibrosis and SMAD signaling: oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen 15, Suppl 1: S54–60, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40: 239–247, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 97: 12222–12226, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fricovsky ES, Suarez J, Ihm SH, Scott BT, Suarez-Ramirez JA, Banerjee I, Torres-Gonzalez M, Wang H, Ellrott I, Maya-Ramos L, Villarreal F, Dillmann WH. Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol 303: R689–R699, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulop N, Feng W, Xing D, He K, Not LG, Brocks CA, Marchase RB, Miller AP, Chatham JC. Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats. Biogerontology 9: 139–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 227: 301–314, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg H, Whiteside C, Fantus IG. O-linked beta-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am J Physiol Endocrinol Metab 301: E713–E726, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg HJ, Whiteside CI, Hart GW, Fantus IG. Posttranslational, reversible O-glycosylation is stimulated by high glucose and mediates plasminogen activator inhibitor-1 gene expression and Sp1 transcriptional activity in glomerular mesangial cells. Endocrinology 147: 222–231, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Gressner OA. Less Smad2 is good for you! A scientific update on coffee's liver benefits. Hepatology 50: 970–978, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Groves JA, Lee A, Yildirir G, Zachara NE. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones 18, 535–558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart GW. How sugar tunes your clock. Cell Metab 17: 155–156, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 80: 825–858, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 96: 1006–1013, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kashyap SR, Lara A, Zhang R, Park YM, DeFronzo RA. Insulin reduces plasma arginase activity in type 2 diabetic patients. Diabetes Care 31: 134–139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohda Y, Kanematsu M, Kono T, Terasaki F, Tanaka T. Protein O-glycosylation induces collagen expression and contributes to diabetic cardiomyopathy in rat cardiac fibroblasts. J Pharm Sci 111: 446–450, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, Chen YF, Darley-Usmar V, Oparil S, Chatham JC. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol 296: H13–H28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazarus BD, Love DC, Hanover JA. Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology 16: 415–421, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res 74: 207–212, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Maarsingh H, Pera T, Meurs H. Arginase and pulmonary diseases. Naunyn Schmiedebergs Arch Pharmacol 378: 171–184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris SM, Jr, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 mediates diabetic renal injury. Diabetes 60: 3015–3022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann NY Acad Sci 1243: 88–102, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez-Correa GA, Ferrando IM, Hart G, Murphy A. Detection of O-GlcNAc modifications on cardiac myofilament proteins. Methods Mol Biol 1005: 157–168, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Romero MJ, Iddings JA, Platt DH, Ali MI, Cederbaum SD, Stepp DW, Caldwell RB, Caldwell RW. Diabetes-induced vascular dysfunction involves arginase I. Am J Physiol Heart Circ Physiol 302: H159–H166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 102: 95–102, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santhanam L, Christianson DW, Nyhan D, Berkowitz DE. Arginase and vascular aging. J Appl Physiol 105: 1632–1642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, Okada Y, Nakanishi I. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol 46: 32–36, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol 294: H1675–H1684, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Spector EB, Kiernan M, Bernard B, Cederbaum SD. Properties of fetal and adult red blood cell arginase: a possible prenatal diagnostic test for arginase deficiency. Am J Hum Genet 32: 79–87, 1980 [PMC free article] [PubMed] [Google Scholar]
- 35.Spector KS. Diabetic cardiomyopathy. Clin Cardiol 21: 885–887, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan NY, Khachigian LM. Sp1 phosphorylation and its regulation of gene transcription. Mol Cell Biol 29: 2483–2488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, Omar A, Angelovska T, Drobic V, Rattan SG, Jones SC, Dixon IM. Regulation of collagen synthesis by inhibitory Smad7 in cardiac myofibroblasts. Am J Physiol Heart Circ Physiol 293: H1282–H1290, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal overexpression and O-GlcNAc. Science 291: 2376–2378, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Wu L, Derynck R. Essential role of TGF-beta signaling in glucose-induced cell hypertrophy. Dev Cell 17: 35–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamazaki KG, Gonzalez E, Zambon AC. Crosstalk between the renin-angiotensin system and the advance glycation end product axis in the heart: role of the cardiac fibroblast. J Cardiovasc Transl Res 5: 805–813, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci USA 98: 6611–6616, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu H, Yoo PK, Aguirre CC, Tsoa RW, Kern RM, Grody WW, Cederbaum SD, Iyer RK. Widespread expression of arginase I in mouse tissues. Biochemical and physiological implications. J Histochem Cytochem 51: 1151–1160, 2003 [DOI] [PubMed] [Google Scholar]