Abstract
Pseudomonas aeruginosa secretes N-(3-oxododecanoyl)-homoserine lactone (C12) as a quorum-sensing molecule to regulate gene expression. Micromolar concentrations are found in the airway surface liquid of infected lungs. Exposure of the airway surface to C12 caused a loss of transepithelial resistance within 1 h that was accompanied by disassembly of tight junctions, as indicated by relocation of the tight junction protein zonula occludens 1 from the apical to the basolateral pole and into the cytosol of polarized human airway epithelial cell cultures (Calu-3 and primary tracheal epithelial cells). These effects were blocked by carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone, a pan-caspase blocker, indicating that tight junction disassembly was an early event in C12-triggered apoptosis. Short-duration (10 min) pretreatment of airway epithelial (Calu-3 and JME) cells with 1 μM thapsigargin (Tg), an inhibitor of Ca2+ uptake into the endoplasmic reticulum (ER), was found to be protective against the C12-induced airway epithelial barrier breakdown and also against other apoptosis-related effects, including shrinkage and fragmentation of nuclei, activation of caspase 3/7 (the executioner caspase in apoptosis), release of ER-targeted redox-sensitive green fluorescent protein into the cytosol, and depolarization of mitochondrial membrane potential. Pretreatment of Calu-3 airway cell monolayers with BAPTA-AM [to buffer cytosolic Ca2+ concentration (Cacyto)] or Ca2+-free solution + BAPTA-AM reduced C12 activation of apoptotic events, suggesting that C12-triggered apoptosis may involve Ca2+. Because C12 and Tg reduced Ca2+ concentration in the ER and increased Cacyto, while Tg increased mitochondrial Ca2+ concentration (Camito) and C12 reduced Camito, it is proposed that Tg may reduce C12-induced apoptosis in host cells not by raising Cacyto, but by preventing C12-induced decreases in Camito.
Keywords: innate immunity, calcium, tight junctions, zonula occludens 1, caspase, mitochondria, endoplasmic reticulum
pseudomonas aeruginosa are opportunistic bacteria that accumulate and form biofilms in the lungs of cystic fibrosis patients. P. aeruginosa uses N-(3-oxododecanoyl)-homoserine lactone (C12) as a quorum-sensing molecule to control bacterial gene expression, including production of biofilms and virulence factors (9, 24, 26). C12 elicits multiple effects, including activation of inflammatory, apoptotic, and Ca2+ and/or cAMP signaling in leukocytes, fibroblasts, and/or epithelial cells, in host cells that are likely to be exposed to C12 in lungs from cystic fibrosis patients (1, 3, 10, 11, 13, 14, 16, 17, 27, 28, 30, 34–37).
In our previous study (27) of C12 activation of apoptosis in airway epithelial cell lines (Calu-3 and JME cells), 10 μM was the C12 threshold concentration and 50–100 μM C12 caused quite similar responses. These responses included 1) activation of caspases 3/7, 8, and 9 within 1–2 h, 2) depolarization of mitochondrial membrane potential (Δψmito), beginning within 5 min and reaching a maximum within 30 min, followed by release of cytochrome c into the cytosol after 1 h, and 3) release of endoplasmic reticulum (ER)-targeted, redox-sensitive green fluorescent protein (roGFP) from the lumen of the ER into the cytosol and nucleus beginning within ∼20 min and becoming complete within 45 min. Recent work on fibroblasts has shown that C12-triggered apoptosis, including release of cytochrome c from mitochondria, occurs independent of the proapoptotic mediators Bak and Bax (28). Importantly, P. aeruginosa biofilms elicited lasI-dependent (lasI is the enzyme responsible for synthesis in P. aeruginosa) increases in cytosolic Ca2+ concentration (Cacyto) and depolarization of Δψmito, indicating that P. aeruginosa biofilms produce sufficient C12 to induce the observed proapoptotic responses (27). It might be expected that biofilm formation and C12-induced host cell apoptosis might be beneficial to the bacteria, in that barrier function may be compromised to permit bacterial access to the body.
It has been reported that C12 (20–200 μM) also causes altered morphology and integrity of tight junctions and loss of barrier function in the intestinal cell line Caco-2 and that this response is blocked when cells have been pretreated with the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) blocker thapsigargin (Tg) (39). Since C12 caused apoptosis in the airway epithelial cell line Calu-3 (27), we hypothesized that C12-induced breakdown of tight junctions in airway epithelia was part of the C12-induced apoptosis cascade and, furthermore, that Tg might prevent apoptosis-related degradation of tight junctions through its role in altering Cacyto or mitochondrial Ca2+ concentration (Camito). We first tested whether C12-induced activation of apoptosis included breakdown of barrier function and tight junctions in Calu-3 cells by pretreating Calu-3 cells with the selective pan-caspase blocker carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (ZVAD-fmk) to test whether blockade of caspases would prevent the effects of C12 on barrier function and tight junctions of Calu-3 cells. We then tested whether Tg prevented C12's effects on barrier function, as well as other previously identified aspects of the apoptosis cascade in airway epithelia, including activation of caspase 3/7 (the executioner caspase), release of GFP and oxidizing ER contents into the cytosol, and depolarization of Δψmito (27). Some experiments were performed in another human airway epithelial cell line (JME cells), which are easier to transfect than Calu-3 cells and are also flatter and better for imaging. We also tested for effects in mouse embryonic fibroblasts (MEFs) to test cell specificity of Tg's effects. Tg prevented all C12-induced proapoptotic effects. We used Ca2+-free solution and BAPTA-AM (which buffers Cacyto) to test whether C12's proapoptotic effects or Tg's protective effects were mediated through alterations in Ca2+. C12 and Tg raise Cacyto, so it was expected that if increases in Cacyto were involved in response to C12 or Tg, then Ca2+-free solution + BAPTA-AM might alter the response, e.g., exacerbating C12's proapoptotic effects or preventing C12's protective effects. However, Ca2+-free solution + BAPTA-AM inhibited C12's proapoptotic effects, similar to Tg, indicating that increases in Cacyto may not have been involved in the proapoptotic effects of C12 or the protective effects of Tg. Finally, we tested whether Tg reduced C12-induced apoptosis by raising Camito.
MATERIALS AND METHODS
Reagents.
Unless otherwise specified, reagents and chemicals were obtained from Sigma (St. Louis, MO). C12 (Cayman Chemical, Ann Arbor MI) was dissolved in DMSO, frozen in separate vials, and then thawed for single experiments. Preliminary experiments showed that C12 lost potency with repeated thaw-freeze-thaw cycles. The Ca2+-ATPase blockers Tg and cyclopiazonic acid (CPA) (2) were prepared as 1 mM stock solutions in DMSO and used at 1–10 μM. The CFTR blocker CFTRinh 172 was obtained from Alan Verkman (University of California San Francisco), dissolved in DMSO, and added at 1:1,000 dilution to a final concentration of 10 μM.
Tissue culture and transfections.
Calu-3 cells, a human airway glandlike epithelial cell line expressing CFTR (33), were used for experiments in which tight junctions and barrier function were tested. Calu-3 cells were cultured in DMEM or Eagle's MEM supplemented with 10% FBS, 2 mM l-glutamine, and 1% penicillin-streptomycin. For electrophysiological experiments, Calu-3 cells were plated on Snapwell inserts (polyester membrane, 1.1 cm2, 0.4-μm pore size; Snapwell no. 3801, Corning Costar, Lowell, MA) and grown until cells formed confluent monolayers. Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2-95% air. Calu-3 cells routinely had transepithelial resistance (RT) >400 Ω·cm2 and exhibited polarized responses consistent with previous studies (5, 33).
JME cells, a continuous SV40 large-T antigen-transformed human nasal epithelial cell line homozygous for ΔF508 CFTR, were used for many imaging experiments, because they exhibit a higher transfection efficiency than Calu-3 cells and have excellent imaging properties (27–29). JME cells were cultured in DMEM-Ham's F-12 medium supplemented with 10% FBS, 2 mM l-glutamine, 1% penicillin-streptomycin, 10 ng/ml EGF, 1 μM hydrocortisone, 5 μg/ml insulin, 5 μg/ml transferrin, 30 nM triiodothyronine, 180 μM adenine, and 5.5 μM epinephrine.
Mouse embryonic fibroblasts (a wild-type cell line obtained from Chi Li, University of Louisville) were cultured in DMEM-high-glucose medium (Mediatech, Manassas, VA) with 10% FBS (Gemini, West Sacramento, CA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Mediatech). Cells were grown in a humidified 95% air-5% CO2 incubator at 37°C and seeded on tissue culture plates (BD Falcon, Bedford, MA) to assay caspase activity.
Human primary tracheal epithelial cell cultures were obtained through Dr. Finkbeiner at the University of California San Francisco. Use of human cultures was reviewed and approved by the Institutional Review Board at Children's Hospital Oakland. Cultures were maintained in Gray's medium on Transwell polycarbonate inserts (Costar, Corning, NY), as previously described (25). Because the supply of primary cell cultures was limited, these preparations were used only for experiments comparing control with C12 treatment.
Electrophysiology.
For measurements of transepithelial Cl− current (ICl), Calu-3 cell monolayers were grown on Snapwell inserts, washed in PBS, mounted into water-jacketed (37°C) EasyMount Ussing chambers (Physiologic Instruments, San Diego, CA), and used for electrophysiological studies, as described previously (8, 30). Transepithelial voltage (VT) was clamped to 0 mV, and RT and short-circuit current (ICl under the conditions of these experiments) were measured using a four-electrode voltage clamp (VCC MC6 multichannel voltage clamp, Physiologic Instruments), with Ag-AgCl electrodes (World Precision Instruments, Sarasota, FL) connected to the solutions through agar bridges containing 1 M KCl. VT was briefly clamped from 0 to 1 mV and RT was calculated from the corresponding current deflection using Ohm's law. Positive currents were defined as anion movements from serosa to mucosa.
In the Ussing chambers, bicarbonate-buffered solutions (5 ml) recirculated on the apical and basolateral sides separately. A serosal-to-mucosal Cl− gradient was used to increase the electrochemical driving force for Cl− secretion across the apical membrane. The basolateral solution contained (in mM) 120 NaCl, 25 NaHCO3, 5 KCl, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, and 1.2 MgCl2. The mucosal Cl−-free solution contained (in mM) 120 Na-gluconate, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 Ca(gluconate)2, and 1.2 MgSO4. Previous experiments have shown that the free Ca2+ concentration is 0.5 mM in this apical Cl−-free solution (15), sufficient to sustain normal barrier function of the tight junctions, which become unstable at ≤10 μM Ca2+ in apical and basolateral solutions (31). Composition of the Cl−-free + Ca2+-free solution was the same as that of the Cl−-free solution, but Ca2+ was omitted. In some experiments, cells were incubated with Ca2+-containing basolateral solution and Ca2+-free apical solution, so that BAPTA-AM could be added to buffer Cacyto. Although free Ca2+ concentration in the apical solution was likely to be <50 μM in these experiments (15), the presence of 1 mM Ca2+ in the basolateral solution was sufficient to maintain barrier function of the monolayers, because RT measured under these conditions was 404 ± 112 Ω·cm2. All solutions were gassed with 5% CO2-95% air, resulting in pH 7.4.
Caspase activation.
Caspase 3/7 activity was measured by cell-based homogenous luminescent assay (Caspase-Glo, Promega, Madison, WI), in which a specific substrate that contains the tetrapeptide DEVD was cleaved by the activated caspase from the cells to release aminoluciferin, reacting with the luciferase and resulting in the production of light. Calu-3 cells, JME cells, or MEFs were plated on a clear-bottom, white 96-well plate in 100 μl of medium per well for 4–5 days until they were confluent. During the experiment, the cells were treated with compounds in the 37°C incubator for 0.5–4 h or were left untreated as controls. On the same plate, some wells without cells but 100 μl of the same medium served as blanks. After treatment, 100 μl of reagent were added to each well with cells (treated or controls) and their medium or blank (media only). The plate was incubated at room temperature for 1 h on a shaker, and the end-point luminescence was measured in a plate-reading luminometer (LmaxII 384, Molecular Devices, Sunnyvale, CA). Data were background (blank)-subtracted and averaged and are reported as relative light units of activity.
Confocal immunomicroscopy: zonula occludens 1 and Hoechst 33342.
Calu-3 cells grown on Snapwell inserts were mounted in the Ussing chambers incubated under conditions used for the electrophysiological experiments and left untreated or incubated with C12 (50–100 μM), ZVAD, Tg, or Ca2+-free solution + BAPTA-AM. Primary cultures of human airway epithelial cells grown on Snapwell inserts were incubated with Ringer solution and left untreated or incubated with C12 (100 μM). After experimental treatments, Calu-3 and primary human cell cultures were rinsed three times with PBS, fixed for 5 min with 3.7% formaldehyde in PBS, rinsed with PBS, and permeabilized with 0.5% Triton X-100 (in PBS) for 15 min. After the cells were blocked with 1% BSA-5% goat serum-PBS for 20 min, they were incubated for 1 h at room temperature with monoclonal mouse anti-zonula occludens 1 (ZO-1) antibody (BD Transduction Laboratories, San Jose, CA) and then thoroughly rinsed with 1% BSA-PBS and 0.1% BSA-PBS. Finally, cells were incubated for 1 h with an Alexa 546 goat anti-mouse IgG secondary antibody (Molecular Probes, Eugene, OR) and for 10 min with 1 μM Hoechst 33342. Images were obtained on a laser scanning confocal microscope (model LSM710) using a ×63/1.4 numerical aperture oil objective (Carl Zeiss, Thornwood, NY). Samples were excited with 405- and 561-nm laser light. Confocal image stacks were collected by stepping the image plane along the optical (z) axis through the sample, where the lateral (xy) dimensions are orthogonal to z. Image stacks are displayed as compressed projections as viewed from the apical or the basolateral side or as a three-dimensional perspective view. Orthogonal cuts along the z-axis are displayed as an average of a 3-μm-thick (in the y direction) section. All image stacks were cropped to 100 × 100 μm base dimensions. For final display, all images were lightened by ∼20%. Image handling was performed using Imaris version 7.3.1 (Bitplane Scientific Software, South Windsor, CT).
Imaging measurements of redox potential in the ER, Δψmito, and Camito.
For measurements of redox potential in the ER (redoxer), JME cells were transfected with a plasmid encoding roGFP targeted to the ER lumen (ER-roGFP). This probe has previously been shown to target correctly and to measure oxidized redox potentials characteristic of the ER (27). Cells were alternately excited at 385 ± 5 and 474 ± 5 nm, and emission (>510 nm) images were collected and analyzed. Images were background-subtracted, and normalized data were calibrated at the end of experiments by recording the 385 nm-to-474 nm ratios during maximal oxidation (10 mM H2O2) and maximal reduction (10 mM DTT). Fluorescence ratios were calibrated as relative levels of oxidation, with the ratio in the presence of DTT designated 0% and the ratio in the presence of H2O2 designated 100% (29).
For measurement of Δψmito, JME or Calu-3 cells were incubated with medium containing the Δψmito probe JC-1 (10 μM) for 10 min at room temperature and then washed three times with Ringer solution. Dye-loaded cells were mounted onto a chamber on the stage of a wide-field or a confocal imaging microscope and maintained at room temperature. Treatment consisted of diluting stock solutions into Ringer solution. Control experiments showed that equivalent amounts of DMSO (0.1%) used to dissolve C12 and Tg did not affect the JC-1 signal. Real-time imaging measurements of Δψmito were performed using equipment and methods that have been reported previously (4, 7, 28, 29). Briefly, a Nikon Diaphot inverted microscope with a ×40 Neofluar objective (1.4 numerical aperture) was used. A charge-coupled device camera collected JC-1 emission images (510–540 nm) during excitation at 490 ± 5 nm using a filter wheel (Lambda-10, Sutter Instruments, Novato, CA). Axon Imaging Workbench 4.0 (Axon Instruments, Foster City, CA) controlled filters and collection of data. Images were corrected for background (region without cells). Corresponding confocal images where collected with a confocal microscope (model LSM710, Zeiss) using laser excitation at 488 nm. Emission was collected at 510–545 nm to observe green fluorescence and at 580–620 nm to observe red fluorescence. Under control conditions, mitochondria exhibited red and green fluorescence of JC-1. C12 and the protonophore FCCP (10 μM) caused reductions of JC-1 red fluorescence and increases in JC-1 green fluorescence consistent with depolarization of mitochondria (27). When cells were treated with FCCP to elicit maximal depolarization of Δψmito, JC-1 red fluorescence decreased to very low levels, and JC-1 green fluorescence also decreased as the dye was released from mitochondria into the cytosol and then into the bathing solution (27). Quantitative data are reported as fluorescence intensities (recorded at 510–545 nm, where changes were most dramatic) normalized by setting the minimum of JC-1 green fluorescence as the starting value in control cells and the maximum JC-1 green intensities at the end of the experiment during treatment of cells with 10 μM FCCP to completely depolarize Δψmito.
For measurements of Camito, JME cells were transfected with a plasmid encoding the Ca2+-sensitive fluorescence resonance energy transfer probe pericam targeted to the mitochondrial matrix (20). Ratiometric imaging of pericam was performed using the Nikon Diaphot inverted microscope, charge-coupled device camera, filter wheel, and Axon Imaging Workbench 4.0, as described above. Cells were alternately excited at 410 ± 5 and 474 ± 5 nm, and emission (510–540 nm) images were collected, background-subtracted, and analyzed. At the end of experiments, 410 nm-to-474 nm ratios were normalized by exposure of cells to solutions with 0 mM Ca2+ and then with 20 mM Ca2+ in the presence of ionomycin. The 410 nm-to-474 nm fluorescence ratio was then expressed as a percent maximum ratio.
Statistics.
Values are means ± SD; n is the number of experiments. Effects of multiple treatments were compared using ANOVAs followed by Holm-Sidak-corrected t-tests. P < 0.05 was considered significant. Statistical analysis was calculated using SigmaPlot (version 11, Systat Software, San Jose, CA). Single comparisons of treatment groups were done by t-tests.
RESULTS
C12 triggers loss of barrier function in Calu-3 airway epithelial monolayers; ZVAD, Tg, and Ca2+-free solution + BAPTA-AM prevent this effect.
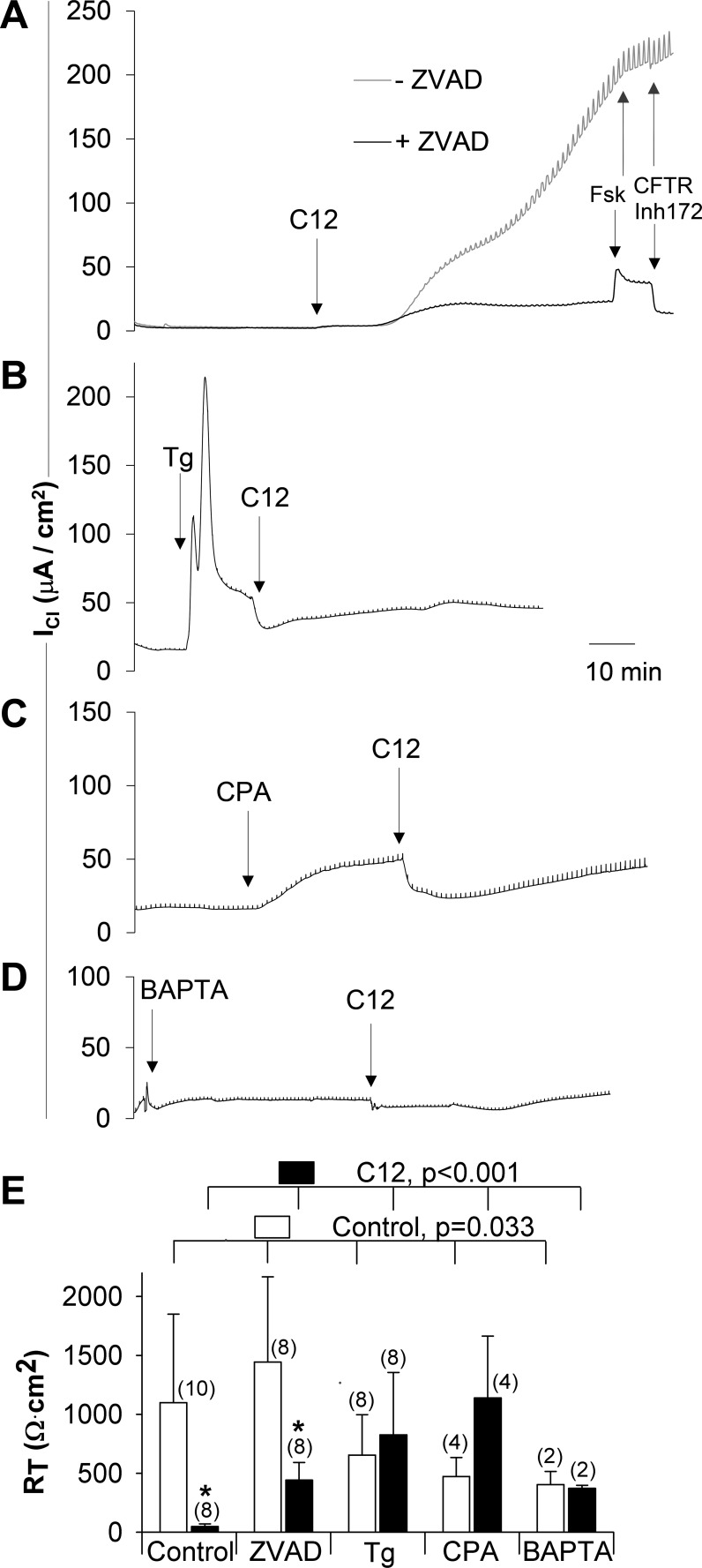
We tested whether previously observed apoptosis-like effects of C12 on Calu-3 cells (27) include loss of barrier function. Transepithelial parameters (ICl and RT) were measured during exposure of cells to C12 or C12 + the caspase blocker ZVAD-fmk (ZVAD). Results from typical experiments are shown in Fig. 1A. Addition of 100 μM C12 to the apical surface of Calu-3 cell monolayers caused an increase in ICl up to a relatively constant plateau of ∼50 μA/cm2 with relatively small decreases in RT (shown by the size of current amplitudes during VT pulses) over the course of 20–30 min. Addition of DMSO at volumes equivalent (0.2%) to those used for C12 addition did not alter electrophysiological properties of Calu-3 cells (data not shown). This first plateau in response to C12 was followed by a second phase of large increases in ICl and decreases in RT (shown by the large increases in ICl pulses required to clamp VT to +1 mV) over the following 45 min. Under the Cl− gradient conditions, this increase in ICl is likely a reflection of the opening of a paracellular ion shunt for Cl−, as the subsequent addition of the CFTR blocker CFTRinh 172 (18) showed small or no effects. After 1 h of treatment with C12, RT of Calu-3 monolayers decreased to very low levels, consistent with loss of barrier function (from 1,100 ± 751 to 48 ± 22 Ω·cm2; Fig. 1E). As also shown in Fig. 1A, pretreatment with ZVAD (50 μM) for 30 min prevented the large second-phase activation of ICl. The initial small increase in ICl and decrease in RT were not affected. As summarized in Fig. 1E, C12 caused a decrease in RT from 1,444 ± 723 to 442 ± 150 Ω·cm2 in ZVAD-treated Calu-3 monolayers; RT was significantly higher in the presence of ZVAD + C12 than C12 alone (Fig. 1E).
Fig. 1.
N-(3-oxododecanoyl)-homoserine lactone (C12) disrupts transepithelial resistance (RT) of Calu-3 cell monolayers; carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (ZVAD), thapsigargin (Tg), cyclopiazonic acid (CPA), and Ca2+-free solution + BAPTA-AM prevent C12's actions. Calu-3 cells grown on Snapwell inserts were mounted in Ussing chambers for measurement of transepithelial Cl currents (ICl) and RT. A: a monolayer was pretreated with C12 (100 μM, apical) and another with ZVAD (50 μM), and C12, forskolin (Fsk, 10 μM), and CFTRinh 172 (10 μM) were added (arrows). B: a monolayer was pretreated with Tg (1 μM) for 15 min and then with C12 (100 μM, apical). C: a monolayer was treated with CPA (10 μM) for 20 min and then with C12. D: a monolayer was treated with Ca2+-free solution + 10 μM BAPTA-AM for 30 min and then with C12. E: summary of RT measurements in Calu-3 cells. Open bars: control, ZVAD, Tg, CPA, and Ca2+-free solution + BAPTA-AM; filled bars: control + C12, ZVAD + C12, Tg + C12, CPA + C12, and Ca2+-free solution + BAPTA-AM + C12. Values are means ± SD for number of experiments shown in parentheses above bars. Statistical comparisons by ANOVA: P < 0.03, control vs. ZVAD, Tg, CPA, and BAPTA-AM; P < 0.001, C12 vs. ZVAD + C12, Tg + C12, CPA + C12, and Ca-free solution + BAPTA-AM + C12. *P < 0.001, control vs. C12 and ZVAD vs. C12 + ZVAD (by t-test).
Next, experiments were performed to test whether Tg could prevent C12-induced loss of barrier function similar to that caused by ZVAD. Results from a typical experiment are shown in Fig. 1B. As already shown in previous reports (5), Tg (1 μM) caused a rapid increase in ICl followed by a secondary plateau, changes that likely resulted from rapid release of Ca2+ from the ER into the cytosol followed by sustained elevation of Cacyto (5) and activation of Cl− and K+ channels (4), as well as a modest reduction of RT, consistent with previously published experiments (21). In the presence of Tg, C12 caused a small, immediate decrease in ICl (compared with an increase in control cells), with only small changes in ICl and RT during the ensuing 45 min. In the presence of Tg, C12 resulted in a modest reduction of RT to 826 ± 529 Ω·cm2 (Fig. 1E). These results were consistent with the idea that C12's effect to reduce barrier function was prevented by Tg. Similar effects were observed in cells treated with CPA (10 μM), another blocker of SERCA pumps (19): CPA caused a rapid increase in ICl, and further addition of C12 reduced ICl, but the characteristic large increases in ICl and decreases in RT were prevented (Fig. 1C).
We tested whether increases in Cacyto were involved in loss of barrier function in response to C12 and, indirectly, Tg's protective effects by treating cells with Ca2+-free solution + BAPTA-AM to prevent/reduce increases in Cacyto. Calu-3 cells in Ussing chambers were incubated with Ca2+-free solution on the apical surface and Ca2+-containing solution on the basolateral surface to ensure tight junction stability (31). Monolayers were then treated with BAPTA-AM (10 μM) for 30 min before C12 was added to the apical side. As shown in Fig. 1, D and E, addition of Ca2+-free solution + BAPTA-AM to the apical side resulted in low ICl and somewhat decreased (compared with controls) RT (404 ± 112 Ω·cm2), but further addition of C12 did not significantly change ICl and RT (372 ± 26 Ω·cm2).
A summary of the effects of C12 on RT and partial prevention of these effects by pretreatment with ZVAD, Tg, CPA, or BAPTA-AM is shown in Fig. 1E. The large C12-induced reduction of RT (from 1,100 ± 751 to 48 ± 22 Ω·cm2) was significantly reduced by pretreatment with ZVAD, Tg, or CPA (Fig. 1E). On average, the C12 effect on RT was reduced by 37% by ZVAD, by 74% by Tg, and by 103% by CPA. ZVAD, Tg, CPA, and BAPTA-AM resulted in modest effects on RT by themselves; however, no single treatment had significant effects compared with control. Subsequent treatments with C12 showed a significant reduction of RT after ZVAD, but not after pretreatment with Tg, CPA, or BAPTA-AM.
C12 causes disassembly of tight junctions of airway epithelia; ZVAD and Tg prevent this effect.
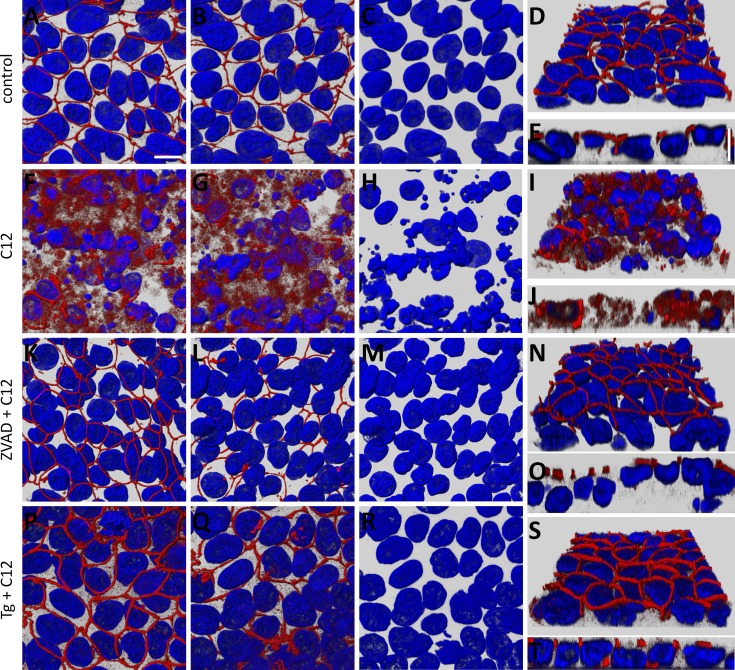
For morphological observations of tight junctions (ZO-1), we fixed and stained Calu-3 cells that had been used for electrophysiological experiments reported in Fig. 1. In control cells, ZO-1 showed characteristic beltlike labeling around the top-most perimeters of cells. ZO-1 was consistently localized apically (Fig. 2, A and D), but not basolaterally (Fig. 2, B and E). Also, nuclei of control cells were large and regularly shaped (Fig. 2C). In contrast, C12-treated cells showed diffuse, disorganized ZO-1 labeling, mostly in the cytosol, with few distinct apical rings (Fig. 2, F and I). Peripheral ZO-1 label was present along the lateral, as opposed to the apical, regions of cells and was diffusely distributed to the basal side of the epithelium (Fig. 2, G and J). Shrinkage of nuclei was often observed in these cells, and many nuclei appeared to have fragmented (Fig. 2H).
Fig. 2.
C12 causes reorganization/disassembly of tight junctions [zonula occludens 1 (ZO-1)] in Calu-3 epithelial cells, and ZVAD and Tg prevent this response. Calu-3 cell monolayers grown on Snapwell inserts and mounted in Ussing chambers for measurement of transepithelial parameters were fixed and labeled with an anti-ZO-1 antibody (red) and stained for nuclei (Hoechst 33342, blue). A–E: untreated control epithelium. x-y images of ZO-1 and nuclei were viewed from the apical side (A) and basolateral side (B), and nuclei alone were viewed from the basolateral side (C). Angled-view projection of ZO-1 and nuclei from the basal surface of a confocal image stack is shown in D; selected orthogonal cut (x-z) view with apical side pointing upward is shown in E. ZO-1 label was prominent at the perimeters of the cells, and there was no apparent ZO-1 staining on the basolateral side. Nuclei were oblong or oval. F–J: C12-treated monolayer; image projections as described in A–E. ZO-1 strands appeared to be disassembled, with distribution throughout the cell and appearance on lateral and basal sides of some cells. Nuclei generally were shrunken, and many appeared to have fragmented. K–O: ZVAD + C12-treated culture from recording in Fig. 1A; image projections as described in A–D. ZO-1 appeared normal, with apical strands surrounding large, oval nuclei. P–T: Tg + C12-treated culture from recording in Fig. 1B; image projections as described in A–E. ZO-1 appeared normal with apical strands; nuclei were large and oval. Scale bar, 10 μm. Each image is typical of images from 3 experiments.
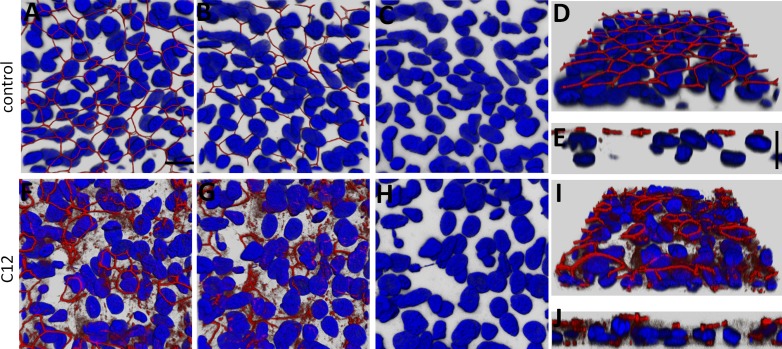
C12 had similar, although less pronounced, effects on ZO-1 distribution and nuclear morphology in primary cultures of human airway epithelial cells. In control conditions, ZO-1 was consistently localized apically (Fig. 3, A and D), but not basolaterally (Fig. 3, B and E). Nuclei of control cells were large and regularly shaped (Fig. 3C). In C12-treated cells, ZO-1 appeared to have been displaced from the apical to the basal surface and lateral regions of the cells or was disassembled into the cytosol or lost altogether in large patches of cells (Fig. 3, F, G, I, and J). Shrinkage and fragmentation of some nuclei were observed (Fig. 3H). These experiments showed that C12 caused disassembly and relocalization of ZO-1 in tight junctions and also nuclear condensation and fragmentation in Calu-3 cell monolayers. Similar, but less pronounced, effects of C12 were observed in primary airway epithelial cell monolayers.
Fig. 3.
C12 causes reorganization/degradation of tight junctions in primary cultures of airway epithelia. Well-differentiated primary airway epithelial cell cultures grown on filters at an air-liquid interface were fixed, labeled with an anti-ZO-1 antibody (red), and stained for nuclei (Hoechst 33342, blue). A–E: untreated control epithelium. x-y images of ZO-1 and nuclei were viewed from the apical side (A) and basolateral side (B), and nuclei alone were viewed from the basal side (C). Angled-view projection of ZO-1 and nuclei from the apical surface of a confocal image stack are shown in D; selected orthogonal cut (x-z) view with apical side pointing upward is shown in E. ZO-1 label was prominent at the perimeters of the cells. Nuclei were oval or oblong. F–J: monolayer treated for 3 h with 100 μM C12; image projections as described in A–E. ZO-1 strands appeared to be disassembled in large patches of cells, with distribution throughout the cell and appearance on lateral and basal sides of some cells. Nuclei appeared misshapen in many cells. Scale bar, 10 μm. Each image is typical of images from 2 experiments.
Calu-3 cells treated with ZVAD prior to C12 exhibited ZO-1 staining (Fig. 2, K–O) similar to controls (Fig. 2, A–E). In cells treated with ZVAD + C12, most nuclei appeared normal, although shrinkage was observed in small number of nuclei (Fig. 2M). Calu-3 cells treated with Tg prior to C12 exhibited labeling of ZO-1 and staining of nuclei (Fig. 2, P–T) very similar to controls, although more ZO-1 label appeared to have migrated toward the basolateral surface than in controls (compare Fig. 2, S and T, with Fig. 2, D and E).
Since ZVAD largely prevented the damaging effects of C12 on barrier function (Fig. 1) and structure of tight junctions and nuclei of airway epithelia (Fig. 2), it seems likely that C12 caused degradation of barrier function and tight junctions as part of the apoptosis program in airway epithelia. A corollary of these results was that Tg, like ZVAD, was blocking C12-triggered apoptosis in Calu-3 cells.
C12-activation of caspase 3/7 is prevented by Tg and Ca2+-free solution + BAPTA-AM.
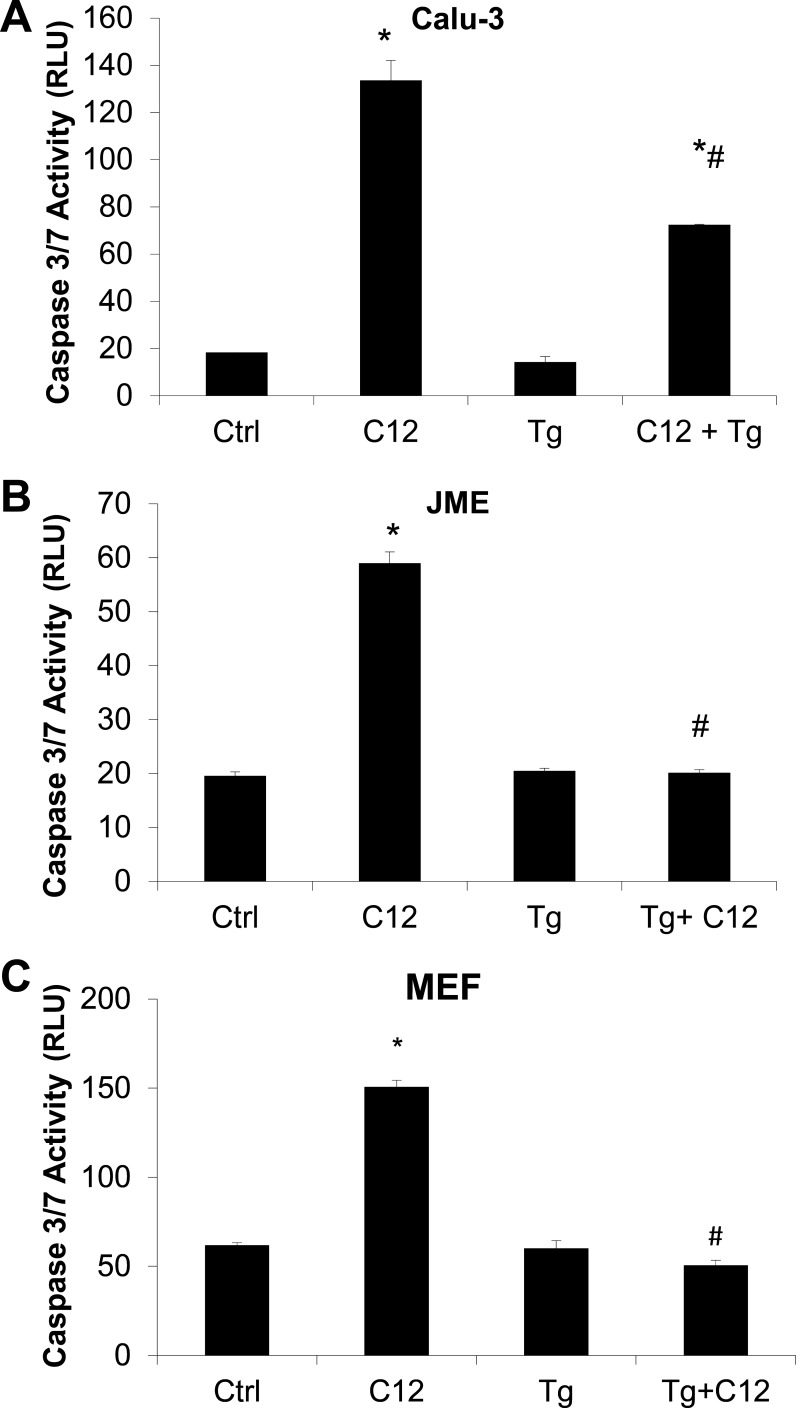
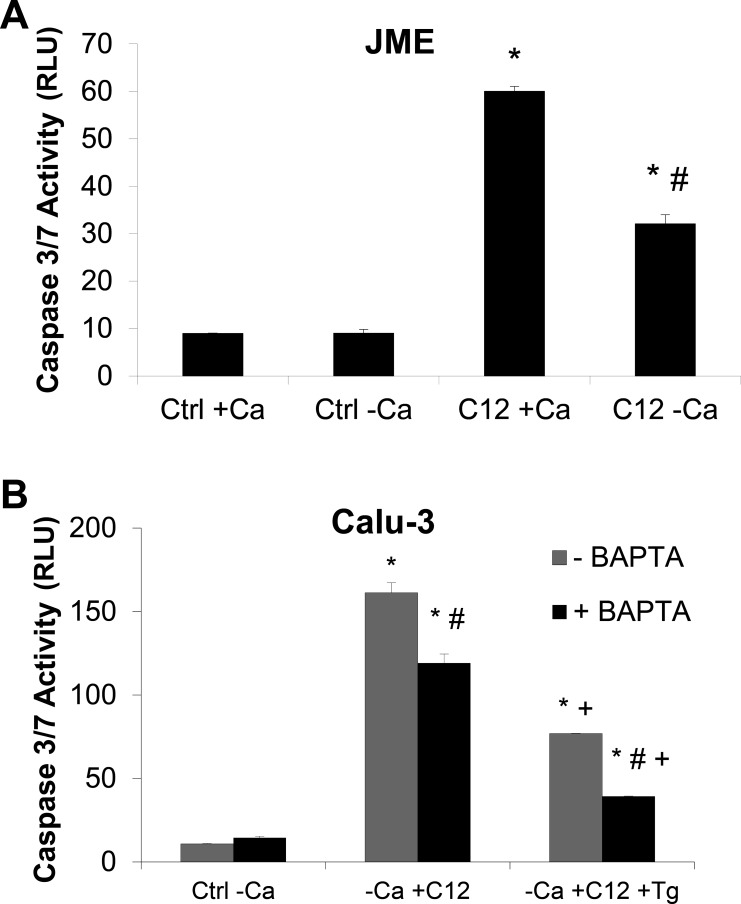
The role of Tg in prevention of C12-triggered apoptosis was investigated further by testing the effect of C12 in the presence and absence of Tg on caspase 3/7 activity. Calu-3 cells grown on plastic six-well plates were exposed simultaneously to C12 and Tg for 2 h, and caspase 3/7 activity was evaluated. As shown in Fig. 4A, C12 increased caspase 3/7 activity in Calu-3 cells, and these effects were reduced by Tg. Addition of DMSO at volumes equivalent (0.1%) to C12 volumes did not alter caspase 3/7 activity of Calu-3 cells compared with Ringer solution alone (n = 6, data not shown). Tg alone also did not activate caspase 3/7 activity over the 2-h duration of these experiments. Tg elicited even more pronounced preventive effects on C12-triggered caspase activity in the nasal cell line JME (Fig. 4B). The preventive effect of Tg was also observed in MEFs (Fig. 4C), showing that Tg prevented C12-induced apoptosis in fibroblasts, as well as in airway epithelia. These data showed that, in human airway epithelia and MEFs over a 2-h period, C12 triggered caspase 3/7, while Tg did not, and Tg reduced C12-induced activation of caspase 3/7.
Fig. 4.
Effects of C12, Tg, and C12 + Tg on caspase 3/7 activity in airway epithelial cells and fibroblasts. Calu-3 cells (A), JME cells (B), and mouse embryonic fibroblasts (MEFs; C) were grown in plastic wells, exposed to 50 μM C12, 1 μM Tg, or C12 + Tg for 2 h, and then processed for caspase 3/7 activity. C12, but not Tg, increased caspase 3/7 activity [expressed as relative light units (RLU)]. Cells treated with C12 + Tg exhibited small or no increases in caspase 3/7 activity [i.e., compared with control (Ctrl)]. Values are means ± SD (n = 3 experiments). *P < 0.05 vs. Ctrl; #P < 0.05 vs. C12.
We tested whether increases in Cacyto were responsible for C12's proapoptotic effects or Tg's protective effects against C12-induced apoptosis in JME and Calu-3 cells. As shown for JME cells, C12 caused less activation of caspase 3/7 in cells incubated in Ca2+-free solution than in cells incubated in solutions containing 1 mM Ca2+ for 2 h (Fig. 5A). As shown for Calu-3 cells incubated in Ca2+-free solution, addition of BAPTA-AM reduced C12's proapoptotic activation of caspase 3/7 and C12-triggered caspase 3/7 activity was reduced even further by treatment with BAPTA-AM and Tg (Fig. 5B). The experiments testing C12 activation of caspase 3/7 (Figs. 4 and 5) were consistent with RT measurements (Fig. 1), in that C12's activation of caspase 3/7 in airway epithelial cells was largely prevented by release of Ca2+ from the ER or by buffering of Cacyto.
Fig. 5.
Ca2+-free solutions, BAPTA-AM, and Tg reduce C12-induced caspase 3/7 in JME (A) and Calu-3 (B) cells. JME and Calu-3 cells were grown in plastic wells, exposed to Ca2+-containing (+Ca) or Ca2+-free (−Ca) solution or Ca2+-free solution containing BAPTA-AM (10 μM) for 30 min, treated with 50 μM C12 for 2 h, and then processed for caspase 3/7 activity. Values are means ± SD (n = 2–3 experiments). *P < 0.05 vs. Ctrl. +P < 0.05 vs. no Tg. #P < 0.05 vs. no BAPTA-AM.
C12-triggered release of ER-roGFP is prevented by Tg.
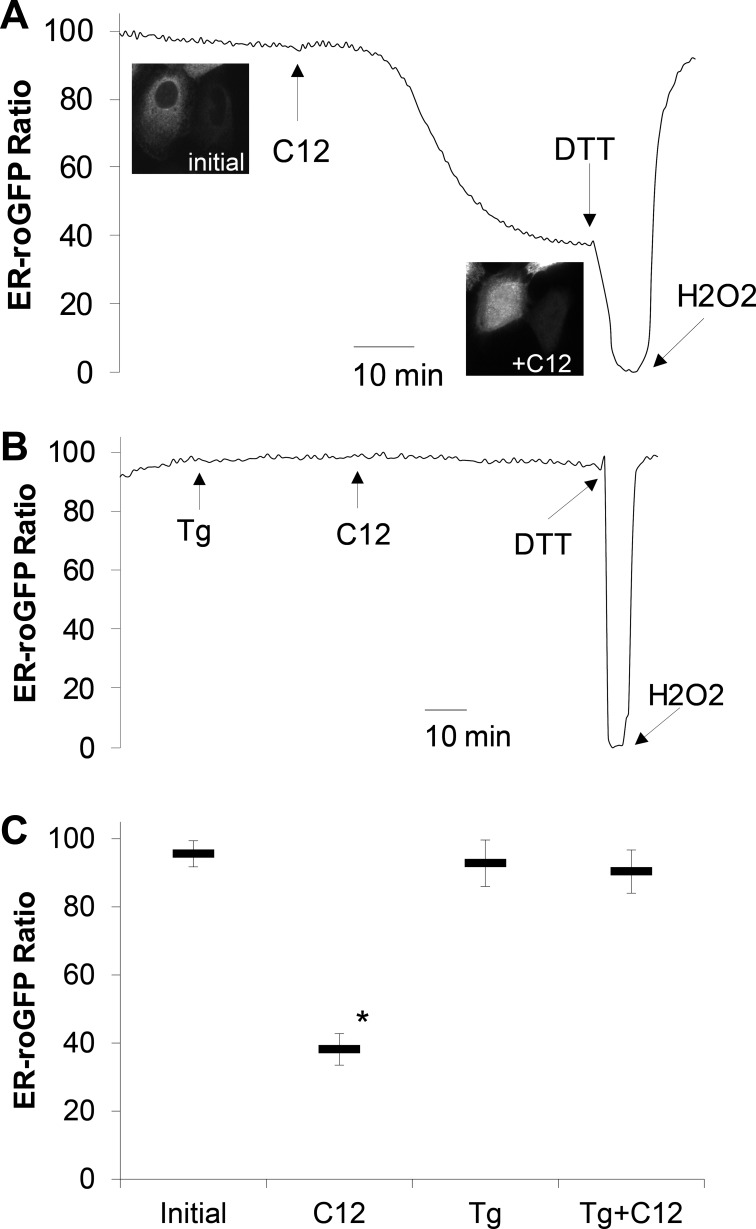
A previous study showed that C12 activation of apoptosis in JME cells was accompanied by release of ER-roGFP into the cytosol and nucleus (27). We tested whether this response was also prevented by Tg in JME cells, which are much easier to transfect than Calu-3 cells. As in previous studies (27), control JME cells had a highly oxidized redox potential, as measured with ER-roGFP (Fig. 6A), consistent with its typical, reticular, ER-like appearance throughout the cytosolic region and exclusion from the nucleus (Fig. 6, inset: Initial). Similar to previous results (27), C12 reduced the redox potential measured by ER-roGFP (Fig. 6A), concomitant with ER-roGFP leakage from the ER into the cytosol and nucleus (Fig. 6A, inset: C12). As proposed previously (27), a likely explanation for the C12-induced change (oxidized → reduced) in redox potential measured by ER-roGFP is that the oxidized ER contents leaked into the reduced cytosol, resulting in reduction of the ER-roGFP. Tg did not affect redox potential of ER-roGFP (Fig. 6B), but in the presence of Tg, C12 did not reduce ER-roGFP, indicating that ER-roGFP remained localized in the ER. A summary of quantitative ER-roGFP experiments is shown in Fig. 6C. Under control conditions, ER-roGFP was nearly maximally oxidized, and C12 caused a marked reduction of ER-roGFP. ER-roGFP exhibited similarly reduced redox potentials in control cells and cells treated with Tg or Tg followed by C12. Thus Tg prevented C12-induced release of ER-roGFP from its typically oxidized ER location into the more reduced cytosol.
Fig. 6.
C12 causes release of endoplasmic reticulum (ER)-targeted redox-sensitive green fluorescent protein (roGFP) into the cytosol, and Tg prevents this response. JME cells grown on cover glasses were transfected with ER-targeted roGFP (ER-roGFP) and then mounted in the imaging microscope. Relative redox potential in the ER (redoxer), along with morphology of ER-roGFP, was recorded in response to C12 (50 μM), Tg (1 μM), and Tg + C12. A: C12 caused, after a delay, redox potential of C12 to decrease (become more reduced) over 40 min. Insets: under control conditions, roGFP was localized exclusively to the ER lumen and absent in the cytosol and nuclear lumen (Initial); after 45 min of C12 treatment, ER-roGFP was distributed smoothly throughout the ER and the cytosol and in the nucleus, likely resulting from leakage of ER-roGFP from the oxidized ER lumen into the reduced cytosol and nucleus (+C12). B: Tg had no effect on redox potential, which remained oxidized, and subsequent addition of C12 also had no effect. Probe was still responsive to changes in redoxer, as shown by DTT-induced reduction and H2O2-induced oxidation of ER-roGFP at the end of the experiment. In cells treated with Tg or Tg + C12, roGFP was localized in a reticular pattern specific for the ER but was excluded from the nucleus, similar to distribution in control cells. C: summary of relative redox potential in the ER (redoxer) in control (Initial), C12, Tg, and Tg + C12. Values are means ± SD (n = 3 experiments). *P < 0.05 vs. Initial.
C12-triggered depolarization of Δψmito is prevented by Tg.
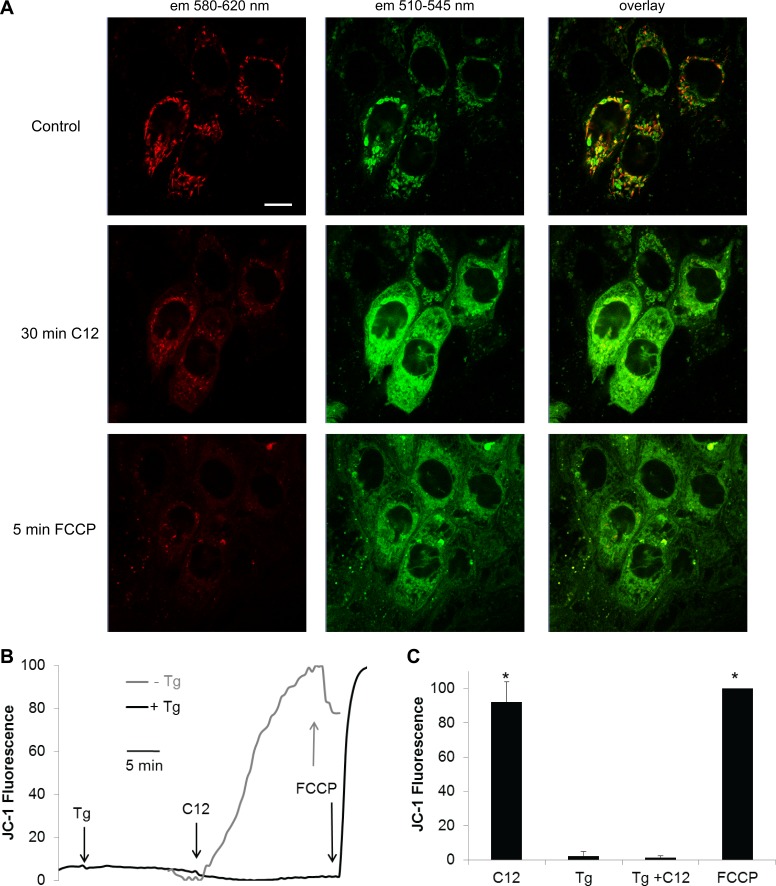
We tested whether C12's characteristic rapid, apoptosis-associated depolarization of Δψmito (27, 28) was prevented by Tg. In control Calu-3 cells, JC-1 was localized to puncta (mitochondria) throughout the cytosol (Fig. 7A, control). These JC-1-stained puncta exhibited red (emission: 580–620 nm) and green (emission: 510–545 nm) fluorescence, perhaps indicating different Δψmito of Calu-3 mitochondria in control conditions. C12 (50 μM) caused JC-1's red fluorescence to decrease and green fluorescence to increase and redistribute to the cytosol, consistent with depolarization of Δψmito (Fig. 7A, 30 min C12). Further treatment with FCCP (10 μM) to elicit maximal depolarization of Δψmito caused a further decrease in red JC-1 fluorescence and, after a short time, also a decrease in JC-1 green fluorescence, consistent with loss of JC-1 from the cells as it was released first into the cytosol and then diffused out of the cell (Fig. 7A, 5 min FCCP).
Fig. 7.
C12 depolarizes mitochondrial membrane potential (Δψmito) in Calu-3 cells, and Tg prevents the depolarization. Calu-3 cells grown on cover glasses were incubated in 10 μM JC-1 for 10 min, washed, and mounted in a confocal (A) or wide-field (B and C) fluorescence microscope. A: confocal images of Calu-3 cells (excitation 488 nm; emission: 580–620 and 510–545 nm) in control conditions and the same cells after 30 min of treatment with 50 μM C12 (30 min C12) and at the end of the experiment following treatment with 10 μM FCCP to induce maximal depolarization (5 min FCCP). Scale bar, 10 μm. B: quantitative measurement of JC-1 green fluorescence (excitation: 490 ± 5 nm; emission: 510–540 nm), normalized to JC-1 fluorescence during controls (0%) and maximal JC-1 fluorescence during FCCP (100%), during treatment with C12 or Tg (1 μM) followed by C12. C: summary of steady-state JC-1 green fluorescence in control cells and during treatment with Tg, C12, or Tg + C12. Values are means ± SD (n = 4–5 experiments). *P < 0.05 vs. control.
In separate experiments performed on JC-1-labeled Calu-3 cells using the wide-field fluorescence microscope, quantitative measurements of JC-1 fluorescence showed that, similar to previous experiments (27) and consistent with confocal images in Fig. 7A, C12 caused a slow increase in JC-1 green fluorescence (excitation: 490 ± 5 nm; emission: 510–540 nm) as mitochondria depolarized slowly during 20–30 min; in the presence of C12, FCCP caused little further increase in fluorescence, and intensities usually decreased as JC-1 was released from the cells (Fig. 7B). These quantitative measurements of JC-1 green fluorescence were also consistent with the confocal images in Fig. 7A. Previous experiments showed that this response did not occur during treatment with the 4-C derivative of C12 dissolved in the same amount of DMSO (0.1%), showing that this apparent depolarization resulted from C12's selective effects on mitochondria (27). As also shown in Fig. 7B, treatment with Tg (1 μM) had little effect on JC-1 green fluorescence, and, in contrast to the control untreated cells, further treatment with C12 caused no or only a small increase in JC-1 green fluorescence; FCCP caused a large increase in JC-1 green fluorescence, showing that the mitochondria were fully polarized in the presence of C12 + Tg and then were fully depolarized by FCCP (Fig. 7B). As summarized in Fig. 7C, C12 caused, on average, ∼90% depolarization of Δψmito in Calu-3 cells over the course of 20–30 min; Tg did not depolarize Δψmito, and C12 had essentially no effect in cells pretreated with Tg. These data showed that C12 slowly, but nearly completely, depolarized Δψmito in airway epithelia and that this effect was blocked by pretreatment with Tg.
Potential role of mitochondrial Ca2+ in Tg's apoptotic effects.
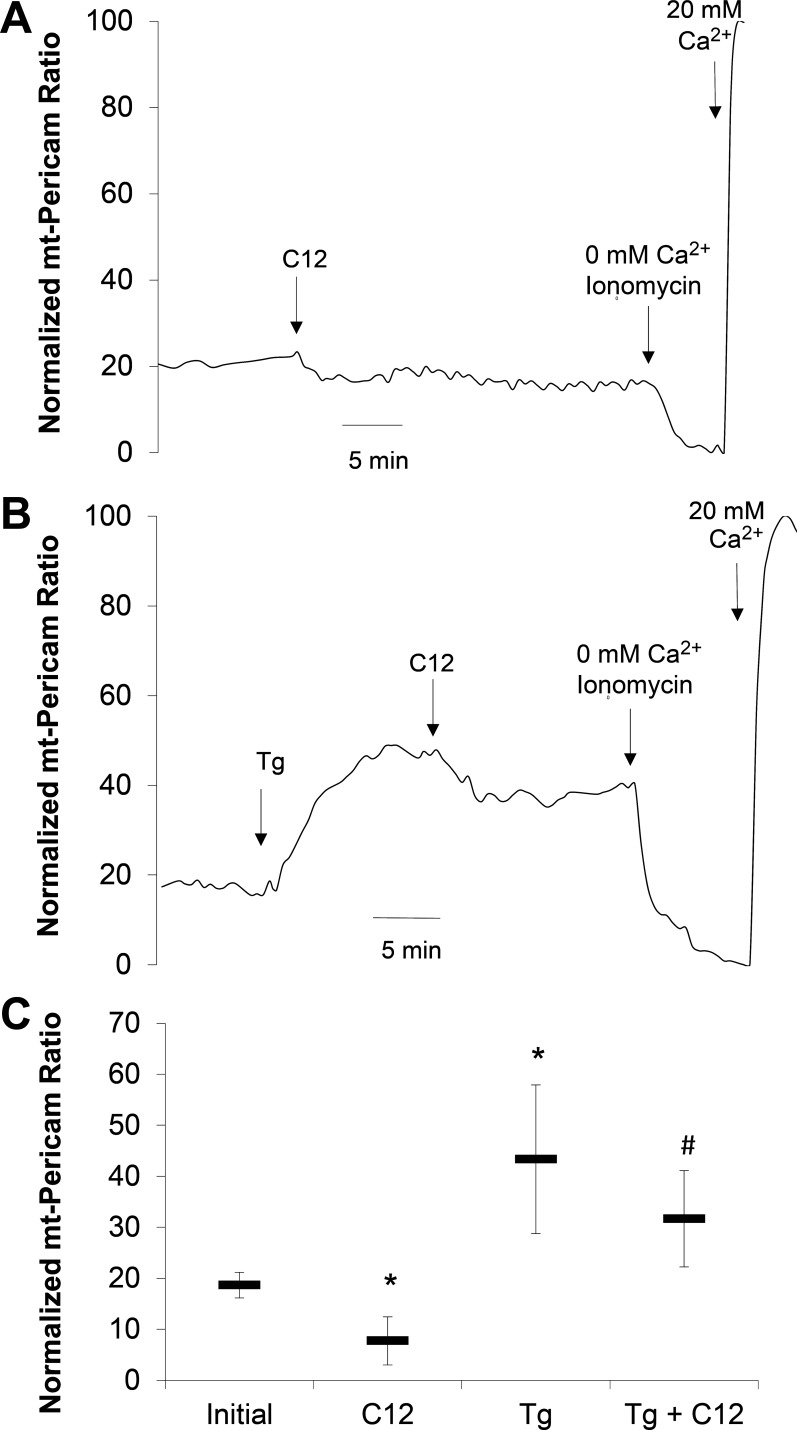
We considered the possibility that the apparently contradictory effects of Tg and BAPTA-AM on proapoptotic effects of C12 were explained by effects on mitochondria. Tg releases Ca2+ from the ER into the cytosol, and it is likely that at least some of this Ca2+ enters mitochondria (23), which has been proposed in some studies to activate mitochondrial metabolism (20) and prevent apoptosis (41). This possibility was tested by measuring Camito in JME cells. Typical results from JME cells transfected with the mitochondria-targeted Ca2+ sensor pericam and treated with C12 and Tg are shown in Fig. 8. Pericam localized to puncta throughout the cells, but not in the nucleus, very similar to localization in JC-1-stained cells (data not shown). C12 (50 μM) caused Camito to decrease (Fig. 8A), even though C12 raises Cacyto in airway epithelial cells (28). In contrast, Tg (1 μM) caused Camito to increase, and subsequent treatment with C12 caused Camito to decrease only slightly and not below baseline (Fig. 8, B and C). Localization of pericam remained unchanged during these treatments (data not shown).
Fig. 8.
C12 decreases and Tg increases mitochondrial Ca2+ concentration (Camito) in JME cells. Pericam-transfected JME cells grown on coverslips were mounted on the imaging microscope, and Camito was measured during treatment with C12 or Tg followed by C12. Cells were permeabilized and treated with low- and high-Ca2+ solutions to permit normalization of pericam signals. mt-Pericam, mitochondrial pericam. A: C12 (50 μM) caused Camito to decrease. B: Tg (1 μM) caused Camito to increase and reach a plateau; subsequent treatment with C12 caused a small decrease below this elevated level. C: summary of steady-state, normalized Camito measured in controls (initial) and during treatment with C12, Tg, or Tg + C12. Values are means ± SD (n = 2–3 experiments). *P < 0.05 vs. control. #P < 0.05 vs. C12.
DISCUSSION
C12-induced degradation of tight junctions results from activation of proapoptotic caspases.
One important conclusion emerging from this study was that C12 caused loss of barrier function and disassembly/breakdown of tight junctions in Calu-3 cell epithelial monolayers. In control Calu-3 monolayers, RT was routinely >500–2,000 Ω·cm2, and the tight junction-associated protein ZO-1 was distributed in a ring around the apical aspects of cells that had large, oval nuclei. In contrast, in cells treated with C12 for 2–3 h, RT decreased nearly to zero, and ZO-1 was found in regions along the lateral or basal aspects of the cells and/or diffused throughout the cytosol. In these C12-treated cells, shrinkage and fragmentation of nuclei characteristic of cells undergoing apoptosis were observed (32). Similar C12-induced loss of barrier function and reorganization of tight junction protein localization have been observed in Caco-2 intestinal epithelia (39). In primary airway epithelial cell monolayers, C12 caused loss of ZO-1 in patches of surface cells that was often accompanied by the presence of ZO-1 in the lateral and basal membranes, which normally do not exhibit ZO-1 staining. Although further work is required to characterize C12's apparent disassembly of ZO-1, the staining patterns indicate that ZO-1 may be redistributed downward along the lateral membrane before being internalized inside Calu-3 cells. The loss of ZO-1 staining in many regions may indicate that ZO-1 was being degraded. The results in primary cells are important, in that these cells show a proapoptotic response of the surface epithelia that might be expected to be exposed to biofilms and the highest concentrations of C12 similar to Calu-3 cell monolayers.
In cells pretreated with the caspase blocker ZVAD, C12 caused RT to decrease from ∼1,444 to ∼442 Ω·cm2, perhaps resulting from C12-induced release of Ca2+ from the ER and activation of K+ and Cl− channels (27). RT was significantly higher in the presence of ZVAD + C12 than in the presence of C12 alone (48 Ω·cm2), and morphologies of ZO-1 and nuclei appeared very similar to normal untreated cells. These data indicate that barrier function and tight junctions of Calu-3 monolayers remained intact in the presence of ZVAD + C12, but not in the presence of C12, and that C12-induced loss of barrier function and disassembly/degradation of tight junctions likely occurred as part of an apoptotic response in airway epithelia that also included shrinkage and fragmentation of nuclei, activation of caspase 3/7, permeabilization of the ER leading to loss of ER-roGFP from the ER into the cytosol, and depolarization of Δψmito. Similar proapoptotic effects of C12 in multiple cell types have been reported (10, 27, 37, 38).
Although C12 caused similar changes in morphology of tight junctions in airway epithelial cells (present work) and in Caco-2 intestinal epithelial cells (39), there was one major difference in these responses: C12's effects appeared to be largely restricted to alteration of intercellular junctions through changes in the phosphorylation status of junctional proteins in Caco-2 intestinal cells, while in the present experiments C12 caused damage to tight junctions as part of a cascade of events related to activation of apoptosis in airway epithelia (and also in fibroblasts). It seems possible that C12 and Tg affect tight junctions in airway and intestinal epithelial cells, with subsequent downstream signaling leading to activation of caspases and apoptotic degradation in airway, but not intestinal, cells.
Tg prevents C12-induced apoptosis.
A second major conclusion from this study was that pretreatment of cells with Tg, the main molecular function of which is thought to be inhibition of the Ca2+-ATPase (SERCA) in the ER (2), prevented proapoptotic effects of C12, including loss of barrier function and tight junction structure, activation of caspase 3/7, release of ER-roGFP from the ER into the cytosol, and depolarization of Δψmito. Another SERCA pump inhibitor, CPA (2), also provided protection against C12-induced decrease of RT, indicating that the effect of Tg on the SERCA pump is a likely explanation for its effects. Since similar blocking effects occurred during treatment with Tg and CPA and also with Ca2+-free solution + BAPTA-AM, the data indicate that C12's apoptosis-related reduction of barrier function in Calu-3 cells was largely prevented by release of Ca2+ from the ER or buffering of Cacyto. Tg and CPA caused modest decreases in RT (compared with controls) on their own, effects that likely resulted from their well-known ability to raise Cacyto and activate ion channels in the plasma membranes. The antiapoptotic effects of Tg were unusual, because Tg is commonly used as a proapoptotic agent, although the proapoptotic effects require many hours (12 h, instead of 2 h) (40). It is thought that Tg-triggered apoptosis in other cells is mediated through Ca2+ release from the ER into the cytosol and uptake into mitochondria, which subsequently depolarize and release cytochrome c to initiate apoptosis (23).
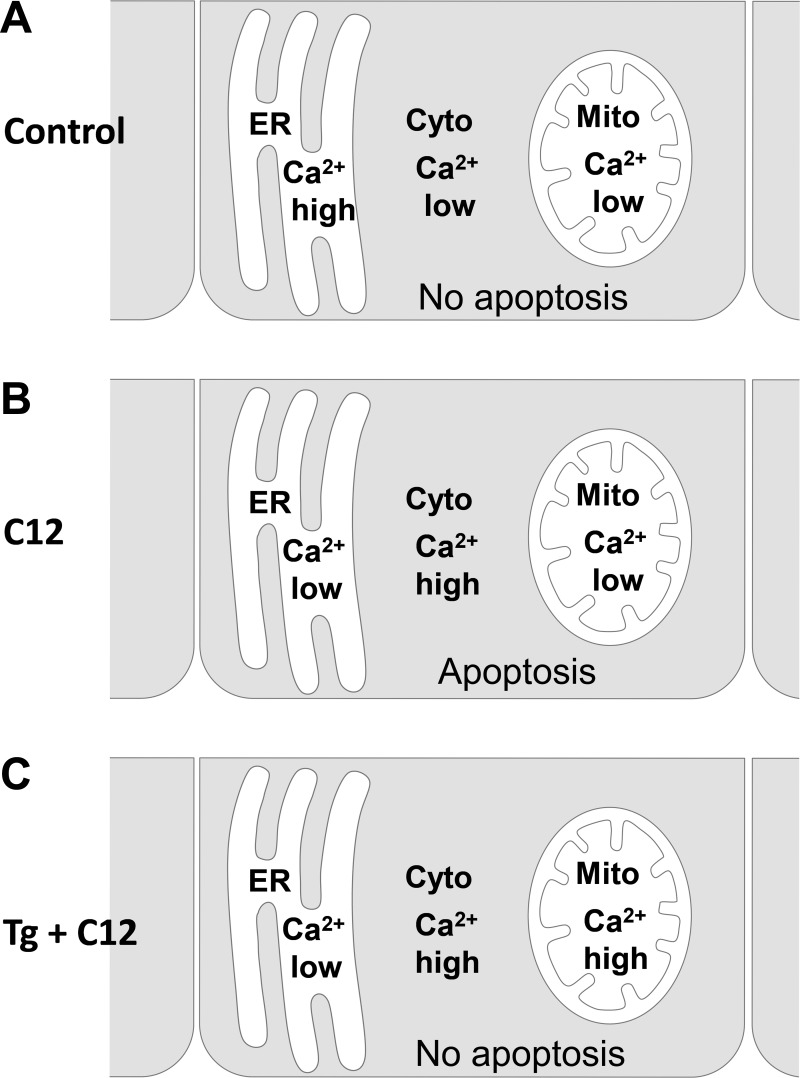
The molecular mechanism(s) by which C12 induces apoptosis and Tg prevents it is not known. A number of correlative data about Cacyto, Camito, and Ca2+ concentration in the ER (Caer) presented here (Fig. 8) and previously (4, 27, 30) may be informative about the mechanism. A summary of our results and speculations about C12 and Tg are presented in Fig. 9. In control conditions (Fig. 9A), Cacyto and Camito are low, while Caer is high. In the presence of C12 (Fig. 9B), Caer and Camito are lower than in control conditions, while Cacyto is increased, and the apoptotic cascade is triggered. In contrast, in the presence of Tg + C12, Caer is low but Camito (Fig. 8) and Cacyto (30) are higher than control, and C12-triggered apoptosis is blocked. Because Ca2+-free solution and/or Ca2+-free solution + BAPTA-AM were inhibitory to C12-induced degradation of barrier function and activation of caspase 3/7, it seems unlikely that C12 activated apoptosis by increasing Cacyto or that the antiapoptotic effects of Tg were mediated through an increase in Cacyto. Also, since Tg and C12 decrease Caer, it seems unlikely that C12's proapoptotic effect and Tg's protective effect resulted from release of Ca2+ from the ER. A more likely possibility is that Camito plays a key role, perhaps by regulating mitochondrial metabolism: C12, which activates apoptosis, lowered Camito and might be expected to inhibit mitochondrial metabolism, while Tg, which prevents C12-induced apoptosis, increased Camito and might be expected to increase mitochondrial metabolism (41).
Fig. 9.
C12 and Tg regulate apoptosis and ER, mitochondrial (mito), and cytosolic (cyto) Ca2+ concentration in airway epithelial cells.
The mechanisms by which C12 activates apoptosis and Tg prevents this effect have not been identified. The data are consistent with the idea that C12 has multiple, yet selective, effects on airway epithelial cells (e.g., C6 and C4 homoserine lactone have no effect on caspase 3/7 of airway epithelia). The inositol 1,4,5-trisphosphate receptor is activated, and Ca2+ is released from the ER; Camito decreases, and mitochondria depolarize and release cytochrome c; proteins and oxidizing contents are released from the ER into the cytosol; nuclei shrink and fragment; and tight junctions degrade, and barrier function is lost (27, 30, present data). Tg pretreatment inhibits most of these proapoptotic effects. Although further experiments are required to determine whether Tg's ability to prevent C12-induced apoptosis is mediated through alteration of mitochondrial metabolism or some other mechanism, this study presents a new pharmacological concept that Tg, known as a proapoptotic chemical, can control C12-induced cell death in airway epithelia.
GRANTS
This work was supported by National Institutes of Health Grants GM-10141 and PN2-EY-018241 and by Cystic Fibrosis Research, Inc. Confocal microscopy was performed at the Microimaging Facility at Children's Hospital Oakland Research Institute, which is supported by the Jordan Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.S., B.R., M.P., Z.F., B.I., H.F., and T.E.M. are responsible for conception and design of the research; C.S., B.R., M.P., S.S., Z.F., B.I., H.F., and T.E.M. performed the experiments; C.S., B.R., M.P., S.S., Z.F., B.I., H.F., and T.E.M. analyzed the data; C.S., B.R., M.P., Z.F., B.I., H.F., and T.E.M. interpreted the results of the experiments; C.S., B.R., and Z.F. prepared the figures; C.S., Z.F., B.I., H.F., and T.E.M. edited and revised the manuscript; C.S., B.R., M.P., S.S., Z.F., B.I., H.F., and T.E.M. approved the final version of the manuscript; T.E.M. drafted the manuscript.
ACKNOWLEDGMENTS
We thank K. E. Magnusson (Linkoping University, Sweden) for informative discussions.
REFERENCES
- 1.Bryan A, Watters C, Koenig L, Youn E, Olmos A, Li G, Williams SC, Rumbaugh KP. Human transcriptome analysis reveals a potential role for active transport in the metabolism of Pseudomonas aeruginosa autoinducers. Microbes Infect 12: 1042–1050, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen SB, Andersen A, Poulsen JC, Treiman M. Derivatives of thapsigargin as probes of its binding site on endoplasmic reticulum Ca2+ ATPase. Stereoselectivity and important functional groups. FEBS Lett 335: 345–348, 1993 [DOI] [PubMed] [Google Scholar]
- 3.DiMango E, Zar HJ, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest 96: 2204–2210, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu Z, Bettega K, Carroll S, Buchholz KR, Machen TE. Role of Ca2+ in responses of airway epithelia to Pseudomonas aeruginosa, flagellin, ATP, and thapsigargin. Am J Physiol Lung Cell Mol Physiol 292: L353–L364, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Haws C, Finkbeiner WE, Widdicombe JH, Wine JJ. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl− conductance. Am J Physiol Lung Cell Mol Physiol 266: L502–L512, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol 28: 491–533, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hybiske K, Fu Z, Schwarzer C, Tseng J, Do J, Huang N, Machen TE. Effects of cystic fibrosis transmembrane conductance regulator and ΔF508CFTR on inflammatory response, ER stress, and Ca2+ of airway epithelia. Am J Physiol Lung Cell Mol Physiol 293: L1250–L1260, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Illek B, Fu Z, Schwarzer C, Banzon T, Jalickee S, Miller SS, Machen TE. Flagellin-stimulated Cl− secretion and innate immune responses in airway epithelia: role for p38. Am J Physiol Lung Cell Mol Physiol 295: L531–L542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irie Y, Parsek MR. Quorum sensing and microbial biofilms. Curr Top Microbiol Immunol 322: 67–84, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Jacobi CA, Schiffner F, Henkel M, Waibel M, Stork B, Daubrawa M, Eberl L, Gregor M, Wesselborg S. Effects of bacterial N-acyl homoserine lactones on human Jurkat T lymphocytes—OdDHL induces apoptosis via the mitochondrial pathway. Int J Med Microbiol 299: 509–519, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Jahoor A, Patel R, Bryan A, Do C, Krier J, Watters C, Wahli W, Li G, Williams SC, Rumbaugh KP. Peroxisome proliferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer. J Bacteriol 190: 4408–4415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S, Chow SC, Nicotera P, Orrenius S. Intracellular Ca2+ signals activate apoptosis in thymocytes: studies using the Ca2+-ATPase inhibitor thapsigargin. Exp Cell Res 212: 84–92, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC, Lehmann M, Meijler MM, Janda KD, Ulevitch RJ. Modulation of gene expression via disruption of NF-κB signaling by a bacterial small molecule. Science 32: 259–263, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Wood MR, Brogan AP, Lehmann M, Mee JM, Iwata K, Pan Q, Fearns C, Knaus UG, Meijler MM, Janda KD, Ulevitch RJ. N-(3-oxo-acyl)homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J Biol Chem 281: 28822–28830, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Leonhard-Marek S, Becker G, Breves G, Schröder B. Chloride, gluconate, sulfate, and short-chain fatty acids affect calcium flux rates across the sheep forestomach epithelium. J Dairy Sci 90: 1516–1526, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Li L, Hooi D, Chhabra SR, Pritchard D, Shaw PE. Bacterial N-acylhomoserine lactone-induced apoptosis in breast carcinoma cells correlated with down-modulation of STAT3. Oncogene 23: 4894–4902, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Li H, Wang L, Ye L, Mao Y, Xie X, Xia C, Chen J, Lu Z, Song J. Influence of Pseudomonas aeruginosa quorum sensing signal molecule N-(3-oxododecanoyl) homoserine lactone on mast cells. Med Microbiol Immunol (Berl) 198: 113–121, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest 10: 1651–1658, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason MJ, Garcia Rodriguez C, Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma-membrane—comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem 266: 20856–20862, 1991 [PubMed] [Google Scholar]
- 20.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391–425, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Moon S, Singh M, Krouse ME, Wine JJ. Calcium-stimulated Cl− secretion in Calu-3 human airway cells requires CFTR. Am J Physiol Lung Cell Mol Physiol 273: L1208–L1219, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA 101: 17404–17409, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 3: 566–578, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Rumbaugh KP. Convergence of hormones and autoinducers at the host/pathogen interface. Anal Bioanal Chem 387: 425–435, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Sachs LA, Finkbeiner WE, Widdicombe JH. Effects of media on differentiation of cultured human tracheal epithelium. In Vitro Cell Dev Biol Anim 39: 56–62, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Schuster M, Greenberg EP. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int J Med Microbiol 296: 73–81, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Schwarzer C, Fu Z, Patanwala M, Hum L, Lopez-Guzman M, Illek B, Kong W, Lynch SV, Machen TE. Pseudomonas aeruginosa biofilm-associated homoserine lactone C12 rapidly activates apoptosis in airway epithelia. Cell Microbiol 14: 698–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzer C, Fu Z, Shuai S, Babbar S, Zhao G, Li C, Machen TE. Pseudomonas aeruginosa homoserine lactone triggers apoptosis and Bak/Bax-independent release of mitochondrial cytochrome cin fibroblasts. Cell Microbiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarzer C, Illek B, Suh JH, Remington SJ, Fischer H, Machen TE. Organelle redox of CF and CFTR-corrected airway epithelia. Free Radic Biol Med 43: 300–316, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzer C, Wong S, Shi J, Matthes E, Illek B, Ianowski JP, Arant RJ, Isacoff E, Vais H, Foskett JK, Maiellaro I, Hofer AM, Machen TE. Pseudomonas aeruginosa homoserine lactone activates store-operated cAMP and cystic fibrosis transmembrane conductance regulator-dependent Cl secretion by human airway epithelia. J Biol Chem 285: 34850–34863, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedar AW, Forte JG. Effects of calcium depletion on the junctional complex between oxyntic cells of gastric glands. J Cell Biol 22: 173–188, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen S. Programmed cell death: concept, mechanism and control. Biol Rev Camb Philos Soc 67: 287–319, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am J Physiol Lung Cell Mol Physiol 266: L493–L501, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Shiner EK, Terentyev D, Bryan A, Sennoune S, Martinez-Zaguilan R, Li G, Gyorke S, Williams SC, Rumbaugh KP. Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signaling. Cell Microbiol 8: 1610, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Smith RS, Fedyk ER, Springer TA, Mukaida N, Iglewski BH, Phipps RP. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-κB and activator protein-2. J Immunol 167: 366–374, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Smith RS, Kelly R, Iglewski BH, Phipps RP. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J Immunol 169: 2636–2642, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun 71: 5785–5793, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valentine CD, Anderson MO, Papa FR, Haggie PM. X-box binding protein 1 (XBP1s) is a critical determinant of Pseudomonas aeruginosa homoserine lactone-mediated apoptosis. PLoS Pathog 9: e1003576, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vikström E, Bui L, Konradsson P, Magnusson KE. Role of calcium signalling and phosphorylations in disruption of the epithelial junctions by Pseudomonas aeruginosa quorum sensing molecule. Eur J Cell Biol 89: 584–597, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Olberding KE, White C, Li C. Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ 18: 38–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat Cell Biol 7: 1021–1028, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]