Abstract
Notch signaling plays a critical role in controlling proliferation and differentiation of pulmonary arterial smooth muscle cells (PASMC). Upregulated Notch ligands and Notch3 receptors in PASMC have been reported to promote the development of pulmonary vascular remodeling in patients with pulmonary arterial hypertension (PAH) and in animals with experimental pulmonary hypertension. Activation of Notch receptors by their ligands leads to the cleavage of the Notch intracellular domain (NICD) to the cytosol by γ-secretase; NICD then translocates into the nucleus to regulate gene transcription. In this study, we examined whether short-term activation of Notch functionally regulates store-operated Ca2+ entry (SOCE) in human PASMC. Treatment of PASMC with the active fragment of human Jagged-1 protein (Jag-1) for 15–60 min significantly increased the amplitude of SOCE induced by passive deletion of Ca2+ from the intracellular stores, the sarcoplasmic reticulum (SR). The Jag-1-induced enhancement of SOCE was time dependent: the amplitude was maximized at 30 min of treatment with Jag-1, which was closely correlated with the time course of Jag-1-mediated increase in NICD protein level. The scrambled peptide of Jag-1 active fragment had no effect on SOCE. Inhibition of γ-secretase by N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT) significantly attenuated the Jag-1-induced augmentation of SOCE. In addition to the short-term effect, prolonged treatment of PASMC with Jag-1 for 48 h also markedly enhanced the amplitude of SOCE. These data demonstrate that short-term activation of Notch signaling enhances SOCE in PASMC; the NICD-mediated functional interaction with store-operated Ca2+ channels (SOC) may be involved in the Jag-1-mediated enhancement of SOCE in human PASMC.
Keywords: Jagged, Notch intracellular domain, Notch receptor, pulmonary artery, smooth muscle, store-operated calcium entry
notch signaling is involved in vascular development, cell proliferation and differentiation, angiogenesis, and vascular remodeling (10, 14, 31, 47). In mammals, there are four Notch receptors, Notch1–4, and five Notch ligands, Jagged (Jag-1/2) and Delta-like (Dll1, 3, 4) proteins (14, 17). Upon ligand binding, Notch receptors undergo several proteolytic processes that lead to the release of the Notch intracellular domain (NICD) to the cytosol by γ-secretase. NICD then translocates into the nucleus and functions as a transcriptional regulator associated with the transcription factor, recombination signal binding protein for immunoglobulin-κ J region (RBPjκ). The NICD/RBPjκ complex, in turn, activates transcription of the Notch target genes, such as the transcriptional repressors HES (hairy and enhancer of split) and HEY (HES-related transcriptional factor) (10, 14, 17, 31, 47). Additionally, Notch mediates the transcriptional repression of cyclin-dependent kinase inhibitors p27 and p57 via Hes1 (29, 33, 37).
In pulmonary artery and other types of blood vessels, Notch signaling contributes to controlling proliferation and differentiation of endothelial cells and smooth muscle cells (14, 44). Both Notch receptors and ligands are identified in pulmonary arteries and arterioles (3, 15, 25, 26, 28, 45, 46). Upregulated Notch3 receptors (and ligands) in pulmonary arterial smooth muscle cells (PASMC) have been demonstrated to promote the development of pulmonary vascular remodeling in animals with experimental pulmonary hypertension and patients with idiopathic pulmonary arterial hypertension (IPAH), whereas inhibition of Notch signaling using the γ-secretase inhibitor, DAPT, attenuates the development and reverses the progression of experimental pulmonary hypertension in animals (23). These studies imply that upregulated Notch receptors and ligands and activated Notch signaling are involved in the development of sustained pulmonary vasoconstriction and excessive pulmonary vascular remodeling in animals and patients with pulmonary arterial hypertension.
An increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) in PASMC is a major trigger for pulmonary vasoconstriction and an important stimulus for PASMC proliferation and migration that contribute to causing pulmonary arterial remodeling. In PASMC, [Ca2+]cyt is increased by Ca2+ release or mobilization from the intracellular stores [e.g., sarcoplasmic reticulum (SR)], Ca2+ influx through plasmalemmal Ca2+-permeable channels, and inward Ca2+ transportation by plasmalemmal Na+/Ca2+ exchangers when intracellular Na+ concentration is raised (20, 30, 54). When PASMC are stimulated by vasoconstrictors and growth factors, activation of membrane receptors, such as G protein-coupled receptors (GPCR) or tyrosine kinase receptors (TKR), increases the synthesis of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 activates IP3 receptors on the SR membrane, induces Ca2+ release from the SR to the cytosol, and increases [Ca2+]cyt. The IP3-mediated active depletion of Ca2+ from the SR also mediates store-operated Ca2+ entry (SOCE), previously referred to as capacitative Ca2+ entry, through store-operated Ca2+ channels (SOC) in the plasma membrane. In addition, passive depletion of Ca2+ from the SR by inhibition of Ca2+-Mg2+-ATPase in the SR (SERCA) using cyclopiazonic acid (CPA) or thapsigargin can also cause SOCE in PASMC.
SOCE is an important mechanism involved in maintaining a sustained elevation of [Ca2+]cyt and refilling Ca2+ into the depleted SR (7, 48, 51). Our previous studies showed that SOCE was involved in PASMC proliferation; inhibition of SOCE significantly attenuated growth factor-mediated PASMC proliferation (9, 41, 43, 53). In this study, we aimed at examining whether Jag-1-mediated Notch activation functionally regulates SOCE in human PASMC. The results from this study clearly show that both short-term (15–30 min) and long-term (48 h) activation of Notch by Jag-1 enhances SOCE, which is closely associated with the level of NICD in PASMC.
METHODS AND MATERIALS
Cell culture.
Human PASMC (passage 5 to 10) from normal subjects were purchased from Lonza (Walkersville, MD). Cells were cultured in Medium 199 (Invitrogen/GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Invitrogen/GIBCO), 100 U/ml penicillin plus 100 μg/ml streptomycin (Invitrogen/GIBCO), 50 μg/ml d-valine (Sigma-Aldrich, St. Louis, MO), and 20 μg/ml smooth muscle cell growth supplement (BD Biosciences, Franklin Lakes, NJ) at 37°C.
[Ca2+]cyt measurement.
Primary cultured PASMC were incubated in HEPES-buffered solution containing 4 μM fura-2 acetoxymethyl ester (fura-2/AM; Invitrogen/Molecular Probes, Eugene, OR) for 60 min at room temperature (25°C). The cells loaded with fura-2/AM were then placed in a recording chamber on the stage of an inverted fluorescent microscope (Eclipse Ti-E; Nikon, Tokyo, Japan) and superfused with the HEPES-buffered solution for 30 min to wash out extracellular residual fura-2/AM and allow sufficient time for intracellular esterase to convert fura-2/AM to fura-2. The fluorescence intensity emitted at 520 nm in cells excited by illumination at 340 nm and 380 nm was measured by a fluorescent objective lens (S Plan Fluor ×20/0.45 ELWD; Nikon) and an EM-CCD camera (Evolve; Photometrics, Tucson, AZ), and NIS Elements 3.2 software (Nikon). [Ca2+]cyt within a region of interest (5 × 5 μm) in a cell was measured as the ratio of fluorescence intensity (F340/F380) and recorded every 2 s. The HEPES-buffered solution contained (in mM) 137 NaCl, 5.9 KCl, 2.2 CaCl2, 1.2 MgCl2, 14 glucose, and 10 HEPES (pH was adjusted to 7.4 with 10 N NaOH). The Ca2+-free solution was prepared by replacing 2.2 mM CaCl2 with equimolar MgCl2 and adding 1 mM EGTA to chelate residual Ca2+. [Ca2+]cyt measurement was carried out at room temperature (24°C) (50).
Western blot analysis.
Solubilized protein of human PASMC was loaded on an 8% acrylamide gel, transferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA), and immunoblotted with anti-NICD (Notch1) antibody (07–1232, 1:1,000; Millipore). Signals were detected using a Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific/Pierce Biotechnology, Rockford, IL). The protein level was normalized with respect to β-actin (sc-81178; 1:500; Santa Cruz Biotechnology, Santa Cruz, CA), and then the band density was quantified with ImageJ software (National Institutes of Health, Bethesda, MD).
Mouse cell isolation.
Freshly dissociated PASMC were prepared from intrapulmonary arteries of mice according to a modified method previously reported (16). In brief, the lungs were dissected from male mice (7 to 8 wk old) and the intrapulmonary arteries (3rd- to 5th-order of intralobar branches) were isolated in Tyrode solution. The arteries were then incubated for 20 min in Ca2+-free Tyrode solution containing 2 mg/ml collagenase (Worthington Biochemical, Lakewood, NJ) and 1 mg/ml bovine serum albumin (BSA; Sigma-Aldrich) at 37°C. The arteries were then washed in Ca2+-free Tyrode solution and were further digested for 9 min with 0.5 mg/ml elastase (Sigma-Aldrich) and 1 mg/ml BSA at 37°C to obtain single cells. Tyrode solution had an ionic composition (in mM) of 143 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.33 NaH2PO4, 5.5 glucose, and 16.6 HEPES (pH was adjusted to 7.4 with 2 N NaOH). The Ca2+-free Tyrode solution was prepared by replacing 1.8 mM CaCl2 with equimolar MgCl2. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago.
Drugs and chemicals.
All pharmacological reagents were obtained from Sigma-Aldrich except N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT, available as γ-secretase inhibitor IX from Calbiochem/EMD Chemicals, San Diego, CA). CPA and DAPT were dissolved in dimethyl sulfoxide at a concentration of 10 mM as stock solutions. 4-Aminopyridine (4-AP) and tetraethylammonium (TEA) were directly dissolved in distilled water at a concentration of 0.5 and 1 M, respectively, as stock solutions. The active fragment of human Jag-1 protein (aa 188–204; AnaSpec, Fremont, CA) and the scrambled peptide with a random sequence of the amino acids that are the same as the active fragment (scJag-1; AnaSpec) were dissolved in distilled water at a concentration of 5 mM as stock solutions. Aliquots of stock solutions were then diluted into different solutions to make the final concentration on the same day when the experiments were performed.
Statistical analysis.
Pooled data are shown as means ± SE. The statistical significance between two groups was determined by Student's t-test. The statistical significance among groups was determined by Scheffé's test after one-way analysis of variance (ANOVA). Significant difference is expressed in the figures as P < 0.05 or P < 0.01.
RESULTS
Short-term treatment with Jag-1 enhances SOCE in human PASMC.
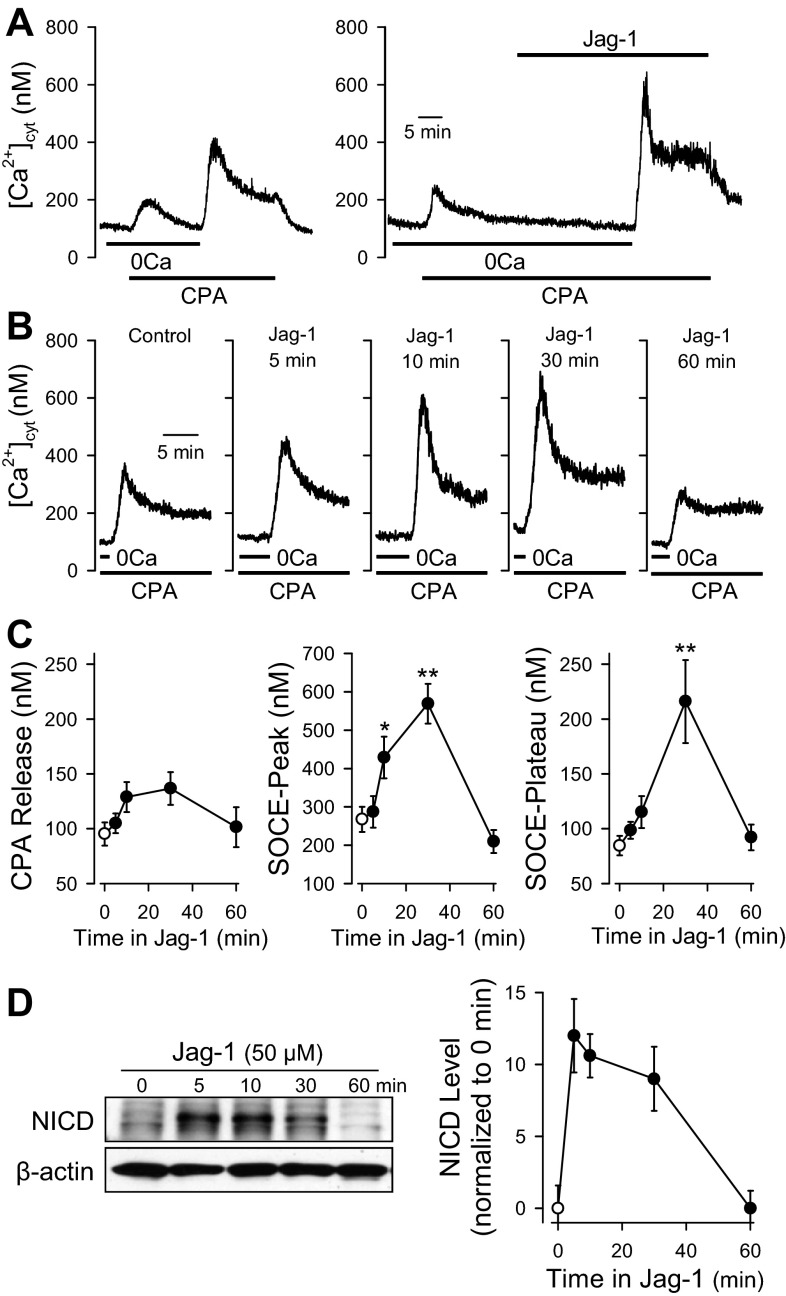
In the absence of extracellular Ca2+ (0Ca), extracellular application of the SERCA inhibitor CPA (10 μM) induced a transient increase in [Ca2+]cyt due to Ca2+ leakage from the SR in human PASMC (n = 24; Fig. 1A, left). Restoration of extracellular Ca2+ to 2.2 mM caused another rise of [Ca2+]cyt via SOCE (Fig. 1A, left; n = 24). The SOCE-mediated increase in [Ca2+]cyt was composed of an initial transient phase (termed as SOCE-peak) followed by a relatively sustained (or plateau) phase (termed as SOCE-plateau) (Fig. 1A, left; n = 24).
Fig. 1.
Short-term treatment with Jagged-1 (Jag-1) enhances store-operated Ca2+ entry (SOCE) in human pulmonary arterial smooth muscle cells (PASMC). SOCE was induced by passive depletion of Ca2+ from the sarcoplasmic reticulum (SR) with 10 μM cyclopiazonic acid (CPA) in human PASMC. The active fragment (aa 188–204) of human Jag-1 protein was used for stimulation of Notch receptors in human PASMC. A: representative records of cytosolic free Ca2+ concentration ([Ca2+]cyt) showing CPA-induced increases in [Ca2+]cyt due to Ca2+ release and SOCE in control PASMC (left) and PASMC treated with Jag-1 (50 μM). 0Ca, absence of extracellular Ca2+. B: representative records of changes in [Ca2+]cyt due to SOCE in PASMC treated with 50 μM Jag-1 for 0 (Control), 5, 10, 30, and 60 min, respectively. C: summarized data (means ± SE) showing the effects of Jag-1 on CPA-induced increases in [Ca2+]cyt due to Ca2+ release (left) and SOCE (middle and right) in human PASMC treated with 50 μM Jag-1 for 0 (Control), 5, 10, 30, and 60 min, respectively. Note that the pretreatment with Jag-1 for 30 min dramatically enhanced the amplitude of SOCE. Summarized data were obtained from 17 to 27 cells. *P < 0.05 or **P < 0.01 vs. 0 min. D: Western blot analysis of Notch intracellular domain (NICD) in PASMC treated with Jag-1 (50 μM) for 0, 5, 10, 30, or 60 min (left) using a specific antibody (1:1,000) against the NICD of Notch1. β-Actin (1:500) was used as a control to ensure equal loading. Summarized data (means ± SE, n = 3 experiments) show NICD levels normalized to the level in control PASMC (Jag-1, 0 min).
An active fragment (aa 188–204) of human Jag-1 protein was used to activate Notch receptors in human PASMC. As shown in Fig. 1A (right), treatment with Jag-1 after extracellular application of CPA had no effect on the resting [Ca2+]cyt in PASMC superfused with Ca2+-free solution, but significantly enhanced the increases in [Ca2+]cyt due to SOCE. The Jag-1-mediated enhancement of SOCE was time dependent. Pretreatment of the cells with 50 μM Jag-1 for 5 min negligibly affected the amplitude of SOCE (Fig. 1, B and C; n = 20); pretreatment with Jag-1 for 10 min, however, significantly enhanced the transient component of SOCE (SOCE-peak, n = 27, P < 0.05) and appeared to have a trend to augment the plateau component of SOCE (SOCE-plateau, n = 27, P > 0.05 by Scheffé's test whereas P < 0.05 by Student t-test) (Fig. 1, B and C, middle and right). When the cells were pretreated with Jag-1 for 30 min, both transient (SOCE-peak, n = 17, P < 0.01) and plateau (SOCE-plateau, n = 17, P < 0.01) components of SOCE were significantly increased (Fig. 1, B and C, middle and right). Unexpectedly, the amplitude of SOCE in PASMC pretreated with Jag-1 for 60 min was not significantly changed in comparison to control cells (Fig. 1, B and C, middle and right). Pretreatment of the cells with Jag-1, however, had negligible effect on the amplitude of increases in [Ca2+]cyt due to CPA-induced Ca2+ leakage (Fig. 1, B and C, left; P > 0.05 among all the data points shown in Fig. 1C, left). These data indicate that short-term activation of Notch receptors in human PASMC with Jag-1 significantly enhances SOCE induced by the passive depletion of Ca2+ in the SR.
To examine whether the Jag-1-mediated enhancement of SOCE is associated with an increase in NICD, we used Western blot analysis and a specific antibody against the Notch-1 intracellular domain (NICD) and examined the time course of Jag-1-mediated effects on NICD levels in PASMC. Similar to the time course of Jag-1-mediated enhancement of SOCE, short-term (5–60 min) treatment of human PASMC with 50 μM Jag-1 also significantly increased protein level of NICD (Fig. 1D). The Jag-1-mediated increase in NICD started at 5 min and was maintained at the high level for ∼30 min (Fig. 1D). The time course curve shows that the Jag-1-mediated increase in NICD (which peaks at 5 min) precedes the Jag-1-mediated enhancement of both transient and plateau phases of SOCE (which peaks at 30 min) (Fig. 1, D, right, and C, middle and right). These data imply that increased NICD and/or release of NICD from Notch receptors by γ-secretase-mediated cleavage contributes to the enhancement of SOCE in PASMC.
Dose dependence of Jag-1-mediated SOCE enhancement in human PASMC.
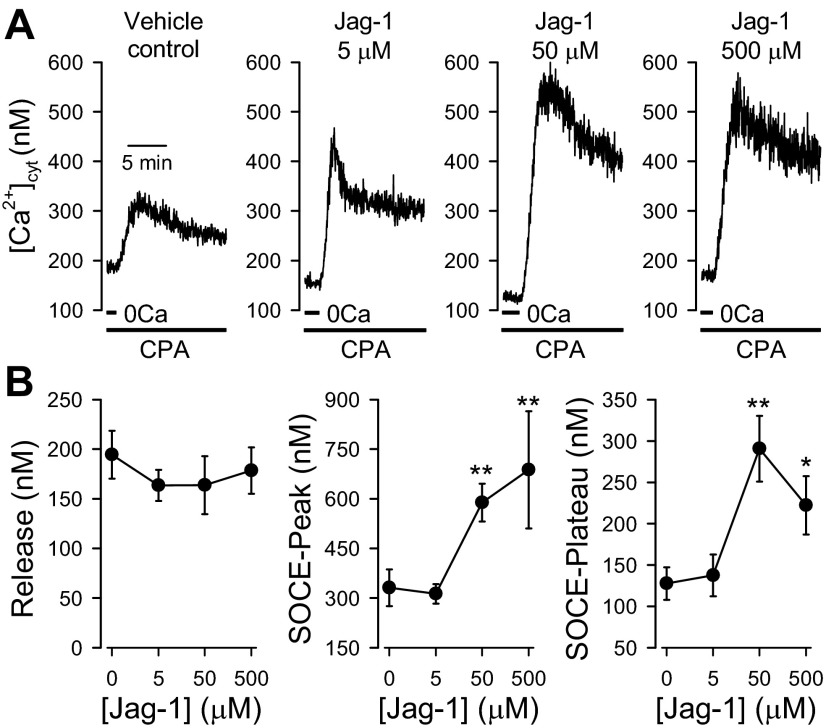
On the basis of the results shown in Fig. 1, the optimal time for Jag-1 to exert the maximal augmenting effect on SOCE was ∼30 min. To examine the concentration dependence of Jag-1-mediated enhancement of SOCE, we compared the amplitudes of SOCE in human PASMC pretreated with different doses of Jag-1 for 30 min and in cells pretreated with vehicle. Pretreatment with 5 μM Jag-1 for 30 min did not significantly affect either the transient or the plateau component of SOCE (Fig. 2A, left, and B; n = 20) compared with that in cells treated with vehicle (water) (n = 20). Pretreatment with 50 μM (n = 17; P < 0.01) and 500 μM (n = 14, P < 0.05) Jag-1 for 30 min, however, significantly enhanced both components of SOCE (Fig. 2). Consistent with the results shown in Fig. 1C, pretreatment of the cells with different concentrations of Jag-1 for 30 min had no effect on the amplitude of the increase in [Ca2+]cyt due to CPA-induced Ca2+ leakage (P > 0.05; Fig. 2B, left). These data indicate that the Jag-1-mediated enhancement of SOCE is dose dependent and that the EC50 values are approximately 37 μM and 27 μM, respectively, for the transient (or peak) and plateau phases of SOCE.
Fig. 2.
Dose dependence of Jag-1-mediated enhancement of SOCE in human PASMC. SOCE was induced by passive depletion of Ca2+ from the SR with 10 μM CPA in human PASMC. The active fragment (aa 188–204) of human Jag-1 protein was used for activation of Notch receptors. A: representative records showing the increases in [Ca2+]cyt due to SOCE in control PASMC (Vehicle control) and PASMC treated with 5, 50, or 500 μM Jag-1 (for 30 min). B: summarized data (means ± SE, n = 17–20 cells) showing the amplitude of the increase in [Ca2+]cyt due to Ca2+ release or leakage (left) and the amplitude of the peak (middle) and plateau (right) increases in [Ca2+]cyt in PASMC treated with 5, 50, or 500 μM Jag-1 (for 30 min). *P < 0.05, **P < 0.01 vs. control PASMC (0 μM) treated with vehicle (1% distilled water).
Inhibition of γ-secretase attenuates Jag-1-induced enhancement of SOCE in human PASMC.
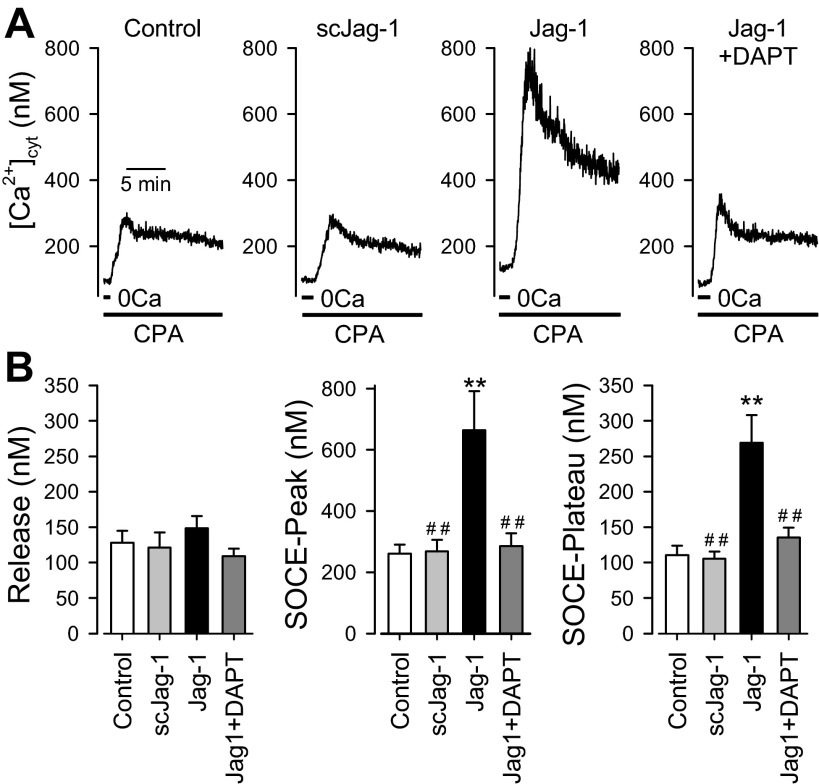
In this study, we used the active fragment (aa 188–204) of human Jag-1 protein (Jag-1) to activate Notch receptors and the peptide with a scrambled sequence of the same amino acids (scJag-1) as the active fragment of Jag-1 as a negative control. In contrast to Jag-1, pretreatment of human PASMC with 50 μM scJag-1 for 30 min had no effect on the transient and plateau components of SOCE (n = 20) in comparison to the cells treated with vehicle used to dissolve scJag-1 (n = 20) (Fig. 3A, left, and B, middle and right). Furthermore, there was no significant difference of the amplitude of increase in [Ca2+]cyt due to CPA-induced Ca2+ leakage in cells treated with vehicle, Jag-1, or scJag-1 (P > 0.05; Fig. 3B, left). These data clearly demonstrate that the inactive peptide, scJag-1, had no effect on SOCE and that the augmenting effect of Jag-1 on SOCE is apparently due to Jag-1-mediated activation of Notch receptors in PASMC.
Fig. 3.
Inhibition of Notch signaling by the γ-secretase inhibitor DAPT attenuates Jag-1-mediated SOCE enhancement in human PASMC. SOCE was induced by passive depletion of Ca2+ from the SR with 10 μM CPA in human PASMC. The active fragment (aa 188–204) of human Jag-1 protein was used for activation of Notch receptors. A: representative records showing the increases in [Ca2+]cyt due to SOCE in control PASMC and PASMC treated with scrambled Jag-1 (scJag-1, 50 μM for 30 min), Jag-1 (50 μM for 30 min), and Jag-1 (50 μM) plus DAPT (10 μM, an inhibitor of γ-secretase, for 30 min). B: summarized data (means ± SE, n = 17–20 cells) showing the amplitude of the increase in [Ca2+]cyt due to Ca2+ release or leakage (Release, left) and the amplitude of the peak (middle) and plateau (right) increases in [Ca2+]cyt in control PASMC and PASMC treated with scJag-1, Jag-1, or Jag-1 + DAPT (for 30 min). **P < 0.01 vs. vehicle control (Control, PASMC treated with 1% distilled water); ##P < 0.01 vs. Jag-1.
Upon activation of the Notch receptor by its ligand, such as Jag-1, the γ-secretase-mediated production of NICD is an important step for activation of Notch signaling. In the next set of experiments, we examined whether inhibition of γ-secretase with DAPT attenuates Jag-1-mediated enhancement of SOCE in PASMC. Treatment with 10 μM DAPT alone decreased the amplitude of SOCE compared with vehicle treatment (data not shown). This is most likely due to inhibition of endogenous basal levels of Notch signaling in PASMC. As shown in Fig. 3, treatment of the cells with 10 μM DAPT before and during application of Jag-1 almost abolished the Jag-1-mediated enhancement of the transient and plateau components of SOCE (n = 20; P < 0.01) (Fig. 3A, right, and B, middle and right). DAPT, alone or in combination with Jag-1 treatment, had no effect on the amplitude of the increase in [Ca2+]cyt due to CPA-mediated Ca2+ leakage from the SR (Fig. 3B, left). These data indicate that γ-secretase-mediated cleavage of the Notch intracellular domain or release of NICD to the cytosol is involved in or required for the enhancement of SOCE induced by short-term (5–30 min) treatment with Jag-1.
Prolonged treatment of human PASMC with Jag-1 enhances SOCE.
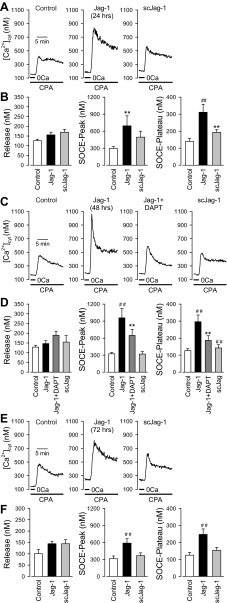
The results shown in Fig. 1 indicate that short-term treatment (5–60 min) of human PASMC with 50 μM Jag-1 caused a transient increase in NICD, which precedes the transient enhancement of SOCE (Fig. 1C, middle and right, with Fig. 1D, right). In the next set of experiments, we examined whether the long-term Jag-1 treatment affected the amplitude of SOCE in human PASMC. In cells treated with 50 μM Jag-1 for 24 h, the amplitude of both transient and plateau components of SOCE was significantly increased (n = 17, P < 0.01) in comparison to the cells treated with vehicle (n = 19) (Fig. 4, A and B, middle and right). In cells treated with Jag-1 for 48 h, the amplitude of both transient and plateau components of SOCE was also significantly increased (n = 30, P < 0.01) in comparison to the cells treated with vehicle (n = 30, P < 0.01) or scJag-1 (n = 20, P < 0.01) (Fig. 4, C and D, middle and right). Inhibition of γ-secretase with DAPT (10 μM, n = 20) significantly attenuated the enhancement of SOCE induced by prolonged (48 h) treatment with Jag-1 (Fig. 4, C and D; P < 0.01 vs. Jag-1 alone). Furthermore, in human PASMC treated with 50 μM Jag-1 for 72 h, the amplitude of both transient and plateau components of SOCE was significantly increased (n = 20) in comparison to the cells treated with vehicle (n = 19) or scJag-1 (n = 20, P < 0.05) (Fig. 4, E and F, middle and right). However, the enhancement of SOCE induced by 72-h treatment with Jag-1 was less than in the enhancement induced by 48-h treatment (compare Fig. 4D, middle and right, with Fig. 4F, middle and right). There was no significant difference on CPA-induced Ca2+ release among cell groups treated with vehicle (control, 1% distilled water), Jag-1, scJag-1, or DAPT (P > 0.05; Fig. 4, B, D and F, left). These data indicate that prolonged (24–72 h) treatment of human PASMC with 50 μM Jag-1 also induces a sustained enhancement of SOCE, which may be due to a different mechanism than short-term treatment with Jag-1 (e.g., a transcriptional upregulation of SOC channels).
Fig. 4.
Prolonged treatment with Jag-1 enhances SOCE in human PASMC. SOCE was induced by passive depletion of Ca2+ from the SR with 10 μM CPA in human PASMC. The active fragment (aa 188–204) of human Jag-1 protein was used for stimulation of Notch receptors. A: representative records showing the increase in [Ca2+]cyt due to SOCE in control PASMC and PASMC treated with Jag-1 (50 μM for 24 h) and scrambled Jag-1 (scJag-1, 50 μM for 24 h). B: summarized data (means ± SE, n = 17–30 cells) showing the amplitude of the increase in [Ca2+]cyt due to Ca2+ release or leakage (left) and the amplitude of the peak (middle) and plateau (right) increases in [Ca2+]cyt in control PASMC and PASMC treated with Jag-1 or scJag-1 (for 24 h). C: representative records showing the increase in [Ca2+]cyt due to SOCE in control PASMC and PASMC treated with Jag-1 (50 μM for 48 h), Jag-1 + DAPT (50 μM and 10 μM, respectively, for 48 h), and scrambled Jag-1 (scJag-1, 50 μM for 48 h). D: summarized data (means ± SE, n = 17–30 cells) showing the amplitude of the increase in [Ca2+]cyt due to Ca2+ release or leakage (left) and the amplitude of the peak (middle) and plateau (right) increases in [Ca2+]cyt in control PASMC and PASMC with Jag-1, Jag-1 + DAPT, and scJag-1 (for 48 h). E: representative records showing the increase in [Ca2+]cyt due to SOCE in control PASMC and PASMC treated with Jag-1 (50 μM for 72 h) and scrambled Jag-1 (scJag-1, 50 μM for 72 h). F: summarized data (means ± SE, n = 17–30 cells) showing the amplitude of the increase in [Ca2+]cyt due to Ca2+ release or leakage (left) and the amplitude of the peak (middle) and plateau (right) increases in [Ca2+]cyt in control PASMC and PASMC treated with Jag-1 or scJag-1 (for 72 h). **P < 0.01 vs. vehicle control (Control, PASMC treated with 1% distilled water); ##P < 0.01 vs. Jag-1.
DISCUSSION
In this study, we have demonstrated that short-term Jag-1 treatment significantly enhances SOCE in PASMC in a time- and dose-dependent manner. Additionally, long-term treatment with Jag-1 also enhances SOCE in PASMC, and inhibition of γ-secretase with DAPT attenuates the Jag-1-induced enhancement of SOCE. These data suggest that activation of Notch signaling by Jag-1 contributes to the regulation of SOCE in human PASMC. The Notch-mediated enhancement of SOCE may occur via two different mechanisms: 1) direct functional interaction of NICD with the SOC and/or its regulatory proteins (short-term effect), and 2) potential transcriptional upregulation of genes encoding the SOC channel proteins (long-term effect) in human PASMC.
The Notch signaling pathway is an important evolutionarily conserved pathway for the cell fate determination during embryonic development, vascular morphogenesis, angiogenetic process, phenotypic switching, and vascular remodeling following injury (10, 14, 17, 31, 47). The Notch receptors (Notch1, Notch2, Notch3, and Notch4) are single-transmembrane-spanning proteins that receive signals from cell-bound ligands encoded by the Jagged (Jag-1/2) and Delta-like (Dll1, 3, and 4) gene families in mammals (14, 17). After ligand binding, the Notch receptor undergoes several proteolytic events that eventually lead to release of NICD from the plasma membrane by γ-secretase. The NICD translocates to the nucleus, where it forms an active transcriptional complex with RBPjκ to induce transcription of downstream Notch target genes, including HES and HEY. Several lines of evidence suggest that Notch signaling is crucial to vascular smooth muscle cell (SMC) identity and proliferative capacity. Notch signaling is critical in determining lineage fate of vascular SMC in the late developing embryo (38). Additionally, Notch3 is expressed exclusively on vascular SMC from arteries, not veins, in the adult, and expression of the Notch3 gene has been linked to change of vascular SMC into an undifferentiated state (1, 32). Mice with disrupted Notch-related molecules display various abnormalities in vascular formation, such as endothelial cell (EC) proliferation and migration, SMC differentiation, vascular remodeling processes, and arterial-venous identification (5, 6, 8, 14, 18, 19, 49, 52). In PASMC, Notch signaling is a very important pathway in controlling proliferation and differentiation. We have previously shown that the expression level of Notch3 in PASMC is upregulated in patients with pulmonary arterial hypertension (PAH) and that the severity of disease (determined by pulmonary vascular resistance and pulmonary arterial pressure) in patients with PAH and rodents with experimental pulmonary hypertension correlates with the amount of Notch3 receptor protein in the lung (23). Expression of Jag-1 in the pulmonary artery has been well studied due to its association with Alagille syndrome, which is caused by mutation of Jag-1. Patients with Alagille syndrome have major abnormalities in pulmonary arteries resulting from inactivating mutations in Jag-1 (22, 36).
In this study, we showed that the short-term treatment (10–30 min) of human PASMC with Jag-1 markedly enhanced SOCE. These data suggest that the role of NICD in enhanced SOCE may not be due to transcriptional regulation. Although the roles of NICD on the regulation of transcriptional factors in the nucleus are well studied, to our knowledge, there is no report focused on the direct interaction of NICD with functional proteins in the plasma membrane such as ion channels. There are two possible mechanisms underlying the enhancement of SOCE by Jag-1 in human PASMC: Jag-1 itself binds directly with SOC to potentiate the channel activity or Jag-1 interacts with Notch receptors and then released NICD activates SOC channels. We can exclude the former possibility because the enhancing effect of short-term treatment with Jag-1 on SOCE was attenuated by the addition of DAPT, an inhibitor of γ-secretase. It is likely that short-term effects of Jag-1 are due to the functional interaction between NICD and SOC, and not through any transcriptional pathways that generally take hours (not minutes) until functional expression of target proteins. However, this molecular mechanism by which NICD directly interacts with the SOC or its regulatory proteins still remains unclear. Further experiments are necessary to determine the exact mechanism by which NICD interacts with SOC and its regulatory proteins in human PASMC.
Intracellular free Ca2+ plays an important role in the regulation of contraction, proliferation, and migration of PASMC. An increase in [Ca2+]cyt in PASMC is a major trigger for pulmonary vasoconstriction and an important stimulus for PASMC proliferation that leads to pulmonary vascular remodeling under pathological conditions. Among Ca2+ influx pathways, SOCE is essential for maintaining a high level of [Ca2+]cyt and for refilling intracellular Ca2+ stores (i.e., SR) in PASMC. High levels of [Ca2+]cyt and sufficient levels of Ca2+ in the SR are required for proliferation of vascular smooth muscle cells (7, 48, 51). SOCE is enhanced while SOC are upregulated during PASMC proliferation to increase Ca2+ influx and provide sufficient Ca2+ for activation of the intracellular mechanisms responsible for cell proliferation and growth (9, 43, 53). In the present study, we demonstrated that the NICD production by Jag-1 stimulation enhanced both transient and plateau components of SOCE in human PASMC. The data further indicate that the NICD is involved in regulating SOCE in human PASMC. Enhancement of SOCE by Jag-1 is thought to cause enhanced PASMC proliferation, which may be a reason for the abnormal proliferation under pathological conditions.
The Jag-1-induced enhancement of SOCE was time dependent in human PASMC (see Fig. 1): SOCE was enhanced by the pretreatment with Jag-1 for 10 and 30 min, but the exposure to Jag-1 for 60 min did not affect the amplitude of SOCE. The expression analysis of NICD protein indicated that the SOCE response was positively correlated with the NICD level. This result also supported the hypothesis that the NICD functionally coupled the activity of SOC in human PASMC. It has been reported that the expression level of NICD protein is dependent on the time after Jag-1 stimulation in human keratinocytes (34), after the injury in rat tracheal epithelium (27), during differentiation in human bone marrow-derived cells (4), and after hypoxia in human glioblastoma cells (2), which influence the expression of the downstream transcription factors. In addition to a short-term effect of Jag-1, the long-term treatment (24 to 72 h) of Jag-1 caused an enhancement of SOCE in human PASMC. In this case, the enhancement of SOCE by the long-term treatment with Jag-1 was potentially due to the upregulation of SOC. It has been reported that Jag-1 treatment for 4 h increases the mRNA expression of transient receptor potential canonical subfamily 6 (TRPC6) channels in human glioblastoma cells (21). Although DLL1, another ligand of Notch receptor, has been reported to modulate the expression and activity of voltage-dependent Na+ channels, independently of Notch signaling, in human prostate cancer cells (39), our results clearly show that the augmentation of SOCE after long-term treatment with Jag-1 was mediated by NICD in human PASMC based on DAPT experiments and Western blot analysis.
Recently, it has been revealed that phosphoinositol lipids are endogenous modulators of several types of ion channels. Phosphatidylinositol 4,5-bisphosphate (PIP2) is a major phosphoinositide of the plasma membrane and involved in multiple cellular functions in various cell types. PIP2 directly modulates the activity of the G protein-coupled inward rectifier K+ channels, KCNQ channels, epithelial Na+ channels, and TRP channels (12, 35, 42). These reports suggest that phospholipids released into cytosolic component are involved in not only the cellular process mediated by phosphoinositol cascade but the modulation of activity of ion channels through direct binding. Similarly, the direct (short-term effect of Jag-1-mediated activation of Notch signaling) and indirect (long-term effect of Jag-1-mediated activation of Notch signaling) regulation of ion channel activity by NICD may be an important regulatory mechanism of PASMC function and proliferation.
PAH is a fatal and progressive disease characterized pathologically by severe pulmonary vascular remodeling. A central aspect of pulmonary vascular remodeling is adventitial, medial, and intimal hypertrophy caused by excessive proliferation of fibroblasts and myofibroblasts in the adventitia, PASMC in the media, and endothelial cells in the intima. The concentric pulmonary vascular wall remodeling or thickened arterial and arteriole wall narrows the intra-arterial lumen, increases pulmonary vascular resistance, and ultimately causes pulmonary hypertension (11, 30). Pulmonary hypertension affects ∼100,000 individuals and is the cause of death in 20,000 people each year in the United States (13). Although certain conditions, such as hypoxia, fenfluoramine ingestion, collagen vascular disease, portal hypertension, and intracardiac left-to-right shunting, contribute to the induction of this disease (40), the mechanism of how the lung remodels its vascular architecture in PAH remains unknown. Since SOC are upregulated in PASMC isolated from patients with idiopathic pulmonary arterial hypertension (IPAH) and from animals with hypoxia-induced pulmonary hypertension, Ca2+ entry through these upregulated cation channels may play an important pathogenic role in the initiation and progression of pulmonary vascular remodeling under pathological conditions (9, 24, 41). Activity and expression of SOC are involved in sustained pulmonary vasoconstriction and excessive pulmonary vascular remodeling in patients with IPAH and animals with hypoxia-induced pulmonary hypertension.
In summary, the data from this study indicate that Jag-1-mediated activation of Notch signaling enhances SOCE (potentially by NICD) in human PASMC. Our data provide compelling evidence that Notch signaling is potentially involved in the regulation of SOCE in human PASMC. The mechanisms responsible for the augmenting effect of Jag-1 on SOCE in human PASMC may include 1) direct functional interaction of NICD with the SOC channels or/and its regulatory proteins (short-term effect) and 2) potential transcriptional upregulation of genes encoding the SOC proteins (long-term effect). Elucidation of the sequence of events underlying the Notch-mediated enhancement of Ca2+ signaling in PASMC will provide critical insights leading to in-depth understanding of the pathogenic mechanisms of PAH.
GRANTS
This work was supported, in part, by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL-110543, HL-066012, and HL-098053).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.Y., A.Y., K.A.S., and J.X.-J.Y. conception and design of research; H.Y., A.Y., E.A.K., and N.M.P. performed experiments; H.Y., A.Y., E.A.K., N.M.P., K.A.S., A.Z., and J.X.-J.Y. analyzed data; H.Y., A.Y., E.A.K., N.M.P., K.A.S., F.L.P., P.A.T., and J.X.-J.Y. interpreted results of experiments; H.Y., A.Y., E.A.K., N.M.P., K.A.S., A.Z., and J.X.-J.Y. prepared figures; H.Y. and J.X.-J.Y. drafted manuscript; H.Y., A.Y., E.A.K., N.M.P., K.A.S., A.Z., F.L.P., P.A.T., and J.X.-J.Y. edited and revised manuscript; H.Y., A.Y., E.A.K., N.M.P., K.A.S., A.Z., F.L.P., P.A.T., and J.X.-J.Y. approved final version of manuscript.
REFERENCES
- 1.Campos AH, Wang W, Pollman MJ, Gibbons GH. Determinants of Notch-3 receptor expression and signaling in vascular smooth muscle cells: implications in cell-cycle regulation. Circ Res 91: 999–1006, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Chigurupati S, Venkataraman R, Barrera D, Naganathan A, Madan M, Paul L, Pattisapu JV, Kyriazis GA, Sugaya K, Bushnev S, Lathia JD, Rich JN, Chan SL. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res 70: 418–427, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Crosnier C, Attie-Bitach T, Encha-Razavi F, Audollent S, Soudy F, Hadchouel M, Meunier-Rotival M, Vekemans M. JAGGED1 gene expression during human embryogenesis elucidates the wide phenotypic spectrum of Alagille syndrome. Hepatology 32: 574–581, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Doi H, Iso T, Shiba Y, Sato H, Yamazaki M, Oyama Y, Akiyama H, Tanaka T, Tomita T, Arai M, Takahashi M, Ikeda U, Kurabayashi M. Notch signaling regulates the differentiation of bone marrow-derived cells into smooth muscle-like cells during arterial lesion formation. Biochem Biophys Res Commun 381: 654–659, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 18: 2730–2735, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18: 2474–2478, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta 1772: 895–906, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA 101: 15949–15954, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol 92: 277–309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54: S10–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Huang CL. Complex roles of PIP2 in the regulation of ion channels and transporters. Am J Physiol Renal Physiol 293: F1761–F1765, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary hypertension surveillance–United States, 1980–2002. MMWR Surveill Summ 54: 1–28, 2005 [PubMed] [Google Scholar]
- 14.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol 23: 543–553, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Jones EA, Clement-Jones M, Wilson DI. JAGGED1 expression in human embryos: correlation with the Alagille syndrome phenotype. J Med Genet 37: 658–662, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko EA, Burg ED, Platoshyn O, Msefya J, Firth AL, Yuan JX. Functional characterization of voltage-gated K+ channels in mouse pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 293: C928–C937, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 18: 2469–2473, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14: 1343–1352, 2000 [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX. New mechanisms of pulmonary arterial hypertension: role of Ca2+ signaling. Am J Physiol Heart Circ Physiol 302: H1546–H1562, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyriazis GA, Belal C, Madan M, Taylor DG, Wang J, Wei Z, Pattisapu JV, Chan SL. Stress-induced switch in Numb isoforms enhances Notch-dependent expression of subtype-specific transient receptor potential channel. J Biol Chem 285: 6811–6825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 16: 243–251, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med 15: 1289–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Loomes KM, Taichman DB, Glover CL, Williams PT, Markowitz JE, Piccoli DA, Baldwin HS, Oakey RJ. Characterization of Notch receptor expression in the developing mammalian heart and liver. Am J Med Genet 112: 181–189, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Loomes KM, Underkoffler LA, Morabito J, Gottlieb S, Piccoli DA, Spinner NB, Baldwin HS, Oakey RJ. The expression of Jagged1 in the developing mammalian heart correlates with cardiovascular disease in Alagille syndrome. Hum Mol Genet 8: 2443–2449, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Ma XB, Jia XS, Liu YL, Wang LL, Sun SL, Song N, Wang EH, Li F. Expression and role of Notch signalling in the regeneration of rat tracheal epithelium. Cell Prolif 42: 15–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development 129: 1075–1082, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Monahan P, Rybak S, Raetzman LT. The notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology 150: 4386–4394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O'Brien C, Walls D, Redmond EM, Cahill PA. Notch and vascular smooth muscle cell phenotype. Circ Res 103: 1370–1382, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Notch-mediated CBF-1/RBP-Jκ-dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell Physiol 289: C1188–C1196, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Murata J, Ohtsuka T, Tokunaga A, Nishiike S, Inohara H, Okano H, Kageyama R. Notch-Hes1 pathway contributes to the cochlear prosensory formation potentially through the transcriptional down-regulation of p27Kip1. J Neurosci Res 87: 3521–3534, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-κB and PPARgamma. Cell Death Differ 9: 842–855, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. EMBO J 27: 2809–2816, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet 16: 235–242, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep 9: 377–383, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev 21: 2511–2524, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Scorey N, Fraser SP, Patel P, Pridgeon C, Dallman MJ, Djamgoz MB. Notch signalling and voltage-gated Na+ channel activity in human prostate cancer cells: independent modulation of in vitro motility. Prostate Cancer Prostatic Dis 9: 399–406, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43: 5S–12S, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Song MY, Makino A, Yuan JX. STIM2 contributes to enhanced store-operated Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Pulm Circ 1: 84–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37: 175–195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 283: L144–L155, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Thistlethwaite PA, Li X, Zhang X. Notch signaling in pulmonary hypertension. Adv Exp Med Biol 661: 279–298, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development 122: 2251–2259, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108: 161–164, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Wang T, Baron M, Trump D. An overview of Notch3 function in vascular smooth muscle cells. Prog Biophys Mol Biol 96: 499–509, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Deng X, Hewavitharana T, Soboloff J, Gill DL. Stim, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle. Clin Exp Pharmacol Physiol 35: 1127–1133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet 8: 723–730, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Yamamura A, Yamamura H, Zeifman A, Yuan JX. Activity of Ca2+-activated Cl− channels contributes to regulating receptor- and store-operated Ca entry in human pulmonary artery smooth muscle cells. Pulm Circ 1: 269–279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang XR, Lin MJ, Sham JS. Physiological functions of transient receptor potential channels in pulmonary arterial smooth muscle cells. Adv Exp Med Biol 661: 109–122, 2010 [DOI] [PubMed] [Google Scholar]
- 52.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435: 98–104, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Zhang S, Yuan JX, Barrett KE, Dong H. Role of Na+/Ca2+ exchange in regulating cytosolic Ca2+ in cultured human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 288: C245–C252, 2005 [DOI] [PubMed] [Google Scholar]