ABSTRACT
Pseudomonas aeruginosa airway infections are a major cause of mortality and morbidity of cystic fibrosis (CF) patients. In order to persist, P. aeruginosa depends on acquiring iron from its host, and multiple different iron acquisition systems may be active during infection. This includes the pyoverdine siderophore and the Pseudomonas heme utilization (phu) system. While the regulation and mechanisms of several iron-scavenging systems are well described, it is not clear whether such systems are targets for selection during adaptation of P. aeruginosa to the host environment. Here we investigated the within-host evolution of the transmissible P. aeruginosa DK2 lineage. We found positive selection for promoter mutations leading to increased expression of the phu system. By mimicking conditions of the CF airways in vitro, we experimentally demonstrate that increased expression of phuR confers a growth advantage in the presence of hemoglobin, thus suggesting that P. aeruginosa evolves toward iron acquisition from hemoglobin. To rule out that this adaptive trait is specific to the DK2 lineage, we inspected the genomes of additional P. aeruginosa lineages isolated from CF airways and found similar adaptive evolution in two distinct lineages (DK1 and PA clone C). Furthermore, in all three lineages, phuR promoter mutations coincided with the loss of pyoverdine production, suggesting that within-host adaptation toward heme utilization is triggered by the loss of pyoverdine production. Targeting heme utilization might therefore be a promising strategy for the treatment of P. aeruginosa infections in CF patients.
IMPORTANCE
Most bacterial pathogens depend on scavenging iron within their hosts, which makes the battle for iron between pathogens and hosts a hallmark of infection. Accordingly, the ability of the opportunistic pathogen Pseudomonas aeruginosa to cause chronic infections in cystic fibrosis (CF) patients also depends on iron-scavenging systems. While the regulation and mechanisms of several such iron-scavenging systems have been well described, not much is known about how the within-host selection pressures act on the pathogens’ ability to acquire iron. Here, we investigated the within-host evolution of P. aeruginosa, and we found evidence that P. aeruginosa during long-term infections evolves toward iron acquisition from hemoglobin. This adaptive strategy might be due to a selective loss of other iron-scavenging mechanisms and/or an increase in the availability of hemoglobin at the site of infection. This information is relevant to the design of novel CF therapeutics and the development of models of chronic CF infections.
INTRODUCTION
Iron is an essential component for virtually all forms of life. This includes bacterial pathogens that depend on acquiring iron from their hosts in order to replicate and cause disease (1). A general defensive mechanism of the host is therefore to withhold iron from invading bacteria to prevent their growth, but this defense is countered by bacterial pathogens since they possess specific systems to scavenge iron from their hosts. While the regulation and mechanisms of several of such iron-scavenging systems are well described (1), not much is known about how the within-host selection pressures act on the pathogens’ ability to acquire iron. This is especially relevant in relation to long-term chronic infections in which invading bacteria acquire adaptive mutations in response to the selective pressures encountered in the host.
The opportunistic pathogen Pseudomonas aeruginosa is a common environmental inhabitant which is capable of causing long-term chronic infections in the airways of patients with cystic fibrosis (CF), and P. aeruginosa infections are directly associated with the morbidity and mortality of CF patients. Chronic infections in CF patients provide an opportunity for long-term monitoring of the battle between the infecting bacteria and the host (2–6) and thus offer an opportunity for observing evolutionary adaptation of P. aeruginosa to the human host environment.
Most iron in the human body is bound in hemoglobin, which is an oxygen transport protein in red blood cells (1). If not bound by essential proteins, such as hemoglobin, iron is withheld and stored by binding to proteins like transferrin, lactoferrin, and ferritin. P. aeruginosa is known to scavenge iron from the human host by both siderophore-based systems and heme acquisition systems (7).
Siderophores are low-molecular-weight molecules secreted by bacteria. The strong association of iron to siderophores enables them to remove iron from the human iron storage proteins, whereupon the siderophore-iron complex can be taken up by cognate receptors at the bacterial surface. The major siderophores secreted by P. aeruginosa are pyoverdine and pyochelin (7), and iron-loaded pyoverdine and pyochelin are taken up by the outer membrane receptors FpvA and FptA, respectively (8–10).
Alternatively, iron contained in the heme group of hemoglobin can be taken up by either of two heme uptake systems in P. aeruginosa. The two systems are the Pseudomonas heme utilization (phu) system and the heme assimilation system (has) (11). The two systems are different in the sense that the phu system is dependent on the direct uptake of heme by the outer membrane receptor PhuR, whereas the has system encodes a secreted hemophore, HasA, that returns heme to an outer membrane receptor, HasR.
Furthermore, P. aeruginosa can take up ferrous iron through the feo system (12) or ferric citrate through the fec system (13).
It is not clear in which way the different iron uptake systems in P. aeruginosa play a role for survival in the lungs of CF patients. Detection of pyoverdine in the sputa of some CF patients has led to the suggestion that pyoverdine plays a key role in the infection process (14, 15). On the other hand, measurements of the transcription levels of iron uptake systems in sputum samples have suggested that multiple systems are active and that siderophore-mediated uptake may not be the dominant iron acquisition mechanism in all patients (16, 17).
In an effort to understand the genetic adaptation of P. aeruginosa to the CF airways, we recently mapped all mutational changes in the P. aeruginosa DK2 lineage as it spread among 21 Danish CF patients by interpatient transmission (2). The study showed that the selective forces driving the evolution of P. aeruginosa in the CF airways could be inferred from convergent evolution of DK2 sublineages evolving in parallel in separate hosts. Here we further analyzed the genomic data, and we provide evidence that within-host evolution of P. aeruginosa is characterized by adaptation toward iron acquisition from hemoglobin.
RESULTS AND DISCUSSION
Parallel evolution of mutations in the promoter regions of the phu system.
It is known that P. aeruginosa undergoes genetic adaptation to CF patients during long-term chronic infections, and several studies have sequenced the genomes of P. aeruginosa isolates sampled longitudinally from the airways of CF patients to map the mutations that accumulate during infection (2–6). In one such study, we mapped all the mutations that had occurred in the P. aeruginosa DK2 lineage during 36 years of infection (2). Whole-genome analysis of 55 DK2 isolates enabled a fine-grained reconstruction of the evolutionary relationship of the DK2 lineage, and the study identified several genes to be targeted by mutation to optimize pathogen fitness within the host environment (pathoadaptation). Nonetheless, only intragenic mutations (i.e., mutations within genes) were examined to identify such pathoadaptive patterns of mutation. Here, we therefore reanalyzed the data with respect to intergenic regions, since selection might also act on such sequences due to their role in regulation and transcription of neighboring genes.
The 6,402,658-bp genome of the P. aeruginosa DK2 strain contains 4,883 intergenic regions with an average size of 146 bp, and the intergenic regions constitute a total of 714,368 bp. Marvig et al. (2) found 1,365 intergenic mutations, meaning that one would expect an average-length intergenic region to be hit by 0.3 mutations (or 0.0019 mutation/bp). Searching for recurrent patterns of mutation of the same genetic loci makes it possible to identify positive selection for mutations affecting genes important for host adaptation (2, 18, 19). We therefore focused on the intergenic regions with the highest densities of mutations and interestingly found the 180-bp intergenic region containing the promoters of the phu system to be the most frequently mutated, with a total of 13 mutations (0.072 mutation/bp) (Fig. 1). This number of mutations is 38-fold higher than what would be expected by chance and represents a significant increase in mutation density [P(X ≥ 13) ~ pois(X; 0.342) = 2.22e-16, where P(X ≥ 13) is the probability of observing ≥13 mutations given a Poisson distribution with a mean of 0.342 mutations (0.0019 mutation/bp ∗ 180 bp)].
FIG 1 .
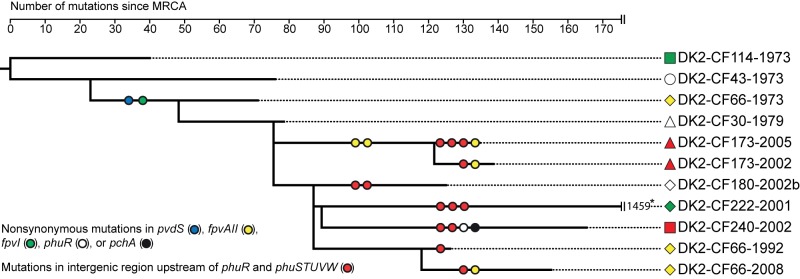
Maximum-parsimony phylogenetic tree showing the genetic relationship of the 11 DK2 clones included in this study. The phylogenetic tree is a subset of a phylogenetic tree from the work of Marvig et al. (2), who recently reported the genome sequences of 55 DK2 isolates. The shown tree depicts the genetic relationship of the 11 DK2 isolates included in this study, and it represents a total of 1,827 mutations (1,486 SNPs and 311 insertion/deletions) identified from whole-genome sequencing. Lengths of branches are proportional to the numbers of mutations except in the case of the truncated branch leading to isolate DK2-CF222-2001. For this hypermutator isolate, the large number of mutations is indicated at the end of the truncated branch. We searched the genomes for nonsynonymous mutations within genes encoding components of the pyoverdine, pyochelin, phu, has, feo, and fec iron acquisition systems (7, 11–13), and circles on the evolutionary branches denote that the specified gene is mutated in the branch. Due to the large number of mutations in the branch leading to the hypermutable isolate DK2-CF222-2001, only phuR and phuSTUVW intergenic mutations are specified. *, in addition to the three phuR and phuSTUVW intergenic mutations, this branch also contains nonsynonymous mutations in pvdS, pvdL, fpvI, the FpvAII gene, fpvR, phuR, fptA, pchH, pchG, pchF, pchE, and pchD (2).
All of the 13 mutations are located within a narrow region from position −91 to −21 relative to the start codon of phuR, and eight of the mutations are within the annotated promoter regions of the phu system (Fig. 2). Furthermore, two positions (positions −35 and −57) were subject to convergent evolution, since they were independently mutated in parallel evolving DK2 sublineages.
FIG 2 .
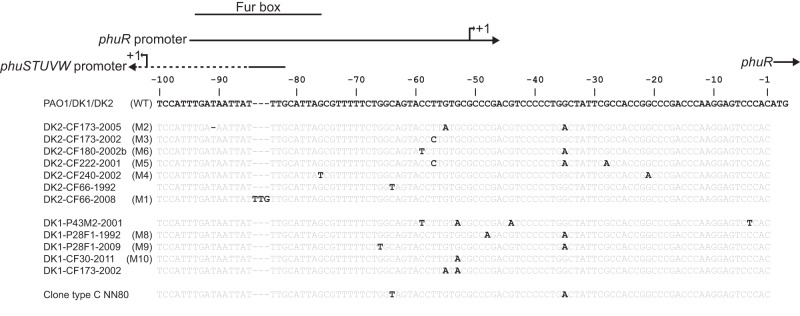
Overview of the intergenic region upstream of phuR. The alignment shows homologue sequences from different isolates with genetic variants highlighted in bold. Wild-type sequences of P. aeruginosa strains PAO1, DK1, DK2, and C are shown at the top of the alignment. Abbreviations of sequence alleles from different isolates are indicated in parentheses (WT and M1 to M10). Positions of promoters and a Fur box are indicated with black lines above the alignment (the phuSTUVW promoter is only partially shown). Positions are relative to the start codon of phuR.
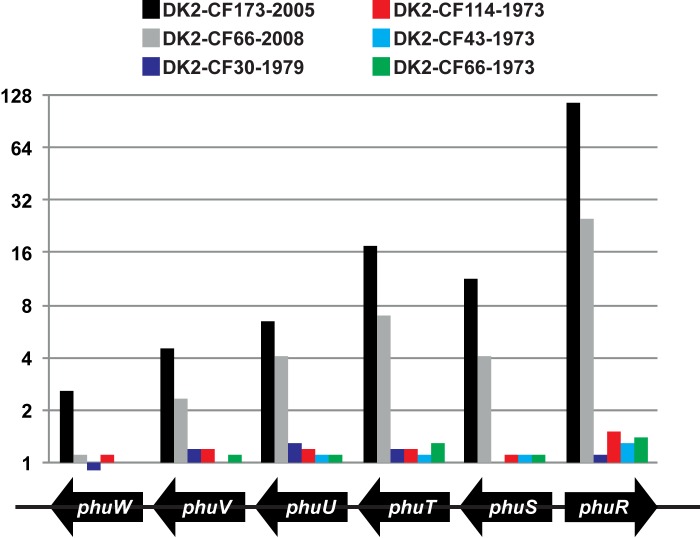
Correlation between promoter mutations and phu transcription in isolates DK2-CF173-2005 and DK2-CF66-2008.
Using Affymetrix GeneChips, we have previously measured the full transcriptomes of six of the 11 DK2 isolates listed in Fig. 1 (4), including four early DK2 isolates without phu promoter mutations and two isolates, DK2-CF173-2005 and DK2-CF66-2008, with phu promoter mutations. We hypothesized that the mutations, due to their location immediately upstream of phuR and phuSTUVW, could cause an effect on the transcription of the phu system. Accordingly, we found the transcription of the phuRSTUVW genes to be upregulated in both of the mutated isolates (DK2-CF173-2005 and DK2-CF66-2008) relative to that for their ancestors and a laboratory reference strain PAO1 (Fig. 3). Most highly upregulated was phuR, showing 116- and 25-fold upregulation, respectively, but also, the genes of the phuSTUVW operon were on average upregulated 8- and 4-fold, respectively.
FIG 3 .
Relative transcriptional levels of genes encoding the phu system. The transcriptomes of six of the DK2 isolates included in this study have previously been measured at exponential growth phase in LB medium (4). The expression of the phu genes is shown for each of the six clinical isolates relative to that for laboratory reference strain PAO1. Values are averages for three replicates, and the values are normalized relative to the transcription of the respective gene in strain PAO1.
The phu system is negatively regulated by the ferric uptake regulator (Fur) (11). As an alternative hypothesis, we therefore speculated that the increased transcription of the phu system in DK2-CF173-2005 and DK2-CF66-2008 might be due to a decreased level or activity of the Fur protein. Nonetheless, no mutations or changes in transcription of the fur gene were found (Table 1) (2).
TABLE 1 .
Relative transcriptional levels of fur and genes encoding the feo iron acquisition pathwaya
| Gene | Relative transcription in strain: |
||||||
|---|---|---|---|---|---|---|---|
| PAO1 | DK2-CF114-1973 | DK2-CF43-1973 | DK2-CF66-1973 | DK2-CF30-1979 | DK2-CF173-2005 | DK2-CF66-2008 | |
| feoA | 1 | 2.9 | 1.6 | 16.7 | 21.2 | 21.6 | 28.1 |
| feoB | 1 | 2 | 1.6 | 5.1 | 6 | 6.8 | 13.4 |
| feoC | 1 | 1.3 | 1.5 | 2.3 | 2.8 | 2.4 | 4.4 |
| fur | 1 | 1.1 | 1.5 | 1.4 | 0.9 | 1.1 | 1 |
The transcriptomes of six DK2 isolates included in this study have previously been measured at exponential growth phage in LB medium (4). We searched the transcriptomes for genes encoding components of the pyoverdine, pyochelin, phu, has, feo, and fec iron acquisition systems (7, 11–13), and the table lists the transcription profiles of those systems in which at least one gene showed differential expression (>3-fold change) in the post-1973 isolates relative to that in the 1973 isolates or strain PAO1. Also, the transcription of the fur gene is shown. Values are averages for three replicates, and the values are normalized relative to the transcription of the respective gene in reference strain PAO1.
Furthermore, in order to determine if iron acquisition systems in general were subject to evolutionary changes in transcription, we searched the transcriptomes for other iron acquisition systems to be differentially transcribed. This search revealed that the feo operon, encoding a ferrous iron uptake system (12), was upregulated in DK2-CF66-1973 and the four isolates sampled after 1973 (Table 1), indicating that several iron acquisition systems might play a role in adaptation of P. aeruginosa to the human host airways.
Effect of intergenic mutations on activities of phu system promoters.
To further investigate the effect of the phu promoter mutations on the activity of the phuR promoter, we cloned the phuR promoter region from six of the mutated DK2 clones in front of a luciferase reporter (luxCDABE) and chromosomally integrated the transcriptional fusion into P. aeruginosa PAO1 at the attB site by use of the mini-CTX2-derived plasmid pHK-CTX-lux. The transcriptional fusions enabled us to compare phuR::lux expression from the mutated promoter regions (M1 to M6) (Fig. 2) relative to the expression from a construct with a wild type promoter region (WT) (Fig. 2). A construct without an inserted promoter region was used to correct for background expression from lux gene cassette integration.
Measurements of phuR::lux expression at exponential growth (optical density at 600 nm [OD600] = 0.15) in Luria-Bertani (LB) medium revealed that all six mutant alleles (M1 to M6) caused a significant increase in promoter activity, with changes in expression from 5- to 112-fold (Table 2). The largest increases in expressions (93- and 112-fold) were observed for the alleles M1 and M2, originating with clones DK2-CF66-2008 and DK2-CF173-2005, respectively. The M1 and M2 alleles contain a 3-bp insertion and a 1-bp deletion, respectively, in the repressor-binding site (Fur box) of the Fur regulator, known to control the expression of the phuR promoter (11). Since Fur mediates strong repression of phuR under iron-rich conditions (11), we find it likely that the indels in the M1- and M2-derived phuR promoters alleviate Fur repression (if there is any repression from Fur).
TABLE 2 .
Activities of the phuR and phuS promoters originating with different clinical isolates of P. aeruginosaa
| Strain | Promoter | Origin of promoter | Allele | Mean luminescence (± SD) | Fold change | P value |
|---|---|---|---|---|---|---|
| PAO1 | phuR | PAO1 | WT | 365 (±1,018) | 1 | |
| PAO1 | phuR | DK2-CF66-2008 | M1 | 34,111 (±3,379) | 93 | 0.00021 |
| PAO1 | phuR | DK2-CF173-2005 | M2 | 40,726 (±3,422) | 112 | 0.00004 |
| PAO1 | phuR | DK2-CF173-2002 | M3 | 1,879 (±3,422) | 5 | 0.16 |
| PAO1 | phuR | DK2-CF240-2002 | M4 | 7,584 (±496) | 21 | 0.00038 |
| PAO1 | phuR | DK2-CF222-2001 | M5 | 8,968 (±610) | 25 | 0.00023 |
| PAO1 | phuR | DK2-CF180-2002 | M6 | 6,723 (±701) | 18 | 0.00088 |
| PAO1 | phuR | DK1-P28F1-1992 | M8 | 13,329 (±1,482) | 37 | 0.00024 |
| PAO1 | phuR | DK1-P28F1-2009 | M9 | 12,205 (±603) | 33 | 0.00007 |
| PAO1 | phuR | DK1-CF30-2011 | M10 | 9,563 (±1,586) | 26 | 0.0011 |
| PAO1 | phuS | PAO1 | WT | 7,444 (±1,777) | 1 | |
| PAO1 | phuS | DK2-CF173-2005 | M2 | 12,030 (±3,191) | 1.6 | 0.01 |
Luminescence production from laboratory reference strain PAO1 (37) with phuR::lux reporter fusions was measured at exponential growth (OD600 = 0.15) in Luria-Bertani (LB) medium and normalized for differences in cell density. Mean luminescence production and standard deviations (SD) were calculated for three biological replicates. Statistical analysis concerning the difference between two means was done using a Student t test, and the P values denote the probability of the mutated alleles having expression equal to that of the wild type (WT).
Using the same cloning strategy, we tested a phuS::lux reporter fusion to compare the expression from the mutated promoter region of DK2-CF173-2005 to the expression from a construct with a wild-type promoter region. Similar to the results for the phuR promoter, we observed that the mutations also resulted in a significant (P = 0.01) increase in phuS promoter activity (Table 2), albeit the mutations had a larger effect on the activity of the phuR promoter.
phuR promoter mutations confer a growth advantage in the presence of hemoglobin.
The increased expression from the mutated phu promoters suggested that there has been positive selection in the CF airways toward iron acquisition from hemoglobin. To test this hypothesis, we replaced the wild-type phu promoters of isolate DK2-CF30-1979 with the mutated phu promoters of isolate DK2-CF173-2005 by allelic replacement and tested whether the constructed mutant strain, DK2-CF30-1979-M2, had a growth advantage relative to the isogenic wild-type strain, DK2-CF30-1979. We chose to test the consequence of the phu promoter mutations in the genetic background of isolate DK2-CF30-1979 because this isolate is an immediate ancestor of isolate DK2-CF173-2005 (4). For the growth experiment, we used FeCl3-free ABTGC minimal medium (which contains glucose and Casamino Acids), supplemented with hemoglobin and apotransferrin.
Confirming our hypothesis, we found that the allelic replacement mutant DK2-CF30-1979-M2 grew significantly faster than its isogenic wild-type counterpart when hemoglobin was present as the sole iron source (Table 3), while no difference was observed for rich medium and medium supplemented with Fe3+ as the sole iron source. We suggest that the growth advantage of the mutant is facilitated by an enhanced uptake of iron derived from hemoglobin.
TABLE 3 .
Growth rates of strains DK2-CF30-1979 and DK2-CF30-1979-M2 at exponential growth phase in different mediaa
| Growth medium | Doubling time (h) |
P value | |
|---|---|---|---|
| DK2-CF30-1979 | DK2-CF30-1979-M2 | ||
| LB | 1.27 ± 0.05 | 1.35 ± 0.07 | 0.16 |
| ABTGC + 10 µM Fe3+ | 2.74 ± 0.02 | 2.69 ± 0.03 | 0.23 |
| ABTGC + 10 µM Fe3+ + 100 µg/ml apo-TF | 3.08 ± 0.10 | 3.07 ± 0.04 | 0.91 |
| ABTGC + 2.5 µM Hb + 100 µg/ml apo-TF | 2.76 ± 0.24 | 2.13 ± 0.09 | 0.01 |
The abbreviations Hb and apo-TF are used for hemoglobin and apotransferrin, respectively. Note that the ABTGC minimal medium standard recipe was modified so that no iron source other than the one stated in the table was added to the growth medium. Mean doubling times were calculated from three biological replicates. Statistical analysis concerning difference between two means was done using a Student t test, and the P values denote the probability of the two strains having equal means.
Adaptation toward heme utilization is a general adaptive mechanism.
Our results demonstrate parallel adaptation of the DK2 lineage toward hemoglobin utilization in five different CF patients. This indicates that similar selective conditions for heme utilization exist across different patients. Next, we speculated on whether the acquisition of phu promoter mutations is an adaptive mechanism specific to the DK2 lineage or if phuR promoter mutations constitute a general adaptive genetic mechanism of P. aeruginosa toward heme utilization in the CF airways. To further investigate the generality, we compared our findings to other lineages of P. aeruginosa isolated from CF infections.
In addition to the DK2 lineage, our previous investigations have revealed another distinct clone type, known as the DK1 clone type, which has also spread among Danish CF patients (21). We sequenced and analyzed the phuR promoter region of five DK1 isolates sampled in the years 1992 to 2011 in addition to an ancestral DK1 isolate from 1973. Whereas the sequence of the phuR promoter of the ancestral 1973 isolate (DK1-P33F0-1973) was identical to the wild-type sequence of strains PAO1 and DK2, all five evolved DK1 isolates had accumulated 1 to 4 single nucleotide polymorphisms (SNPs) in the promoter region, and three of the DK1 SNPs were identical to SNPs found in the evolved DK2 isolates (Fig. 2). We tested the activities of three of the mutated promoters from the DK1 isolates (M8 to M10) and found that all three mutated promoters resulted in increased levels of transcription, similar to what has been observed for mutated DK2 alleles (Table 2). Our results provide strong evidence for convergent adaptive evolution of different lineages of P. aeruginosa toward iron acquisition from hemoglobin.
To rule out that the adaptive trait was specific for P. aeruginosa CF infections at the Copenhagen CF Center, we analyzed the available public data for the genomic evolution of the P. aeruginosa C lineage, which was isolated from a patient attending the CF clinic at Hannover Medical School, Germany (6). Interestingly, the C lineage, which has colonized this patient for a period of more than 20 years, also accumulated two SNPs in the phuR promoter region (Fig. 2). Remarkably, the two SNPs are identical to SNPs found in the DK1 and DK2 lineages, and this observation suggests that these mutations were also positively selected for in the host environment.
The research team at Hannover Medical School also investigated the microevolution of a PA14 lineage as it infected a patient over 14 years. Nonetheless, the PA14 lineage did not accumulate SNPs in any iron acquisition systems. Likewise, a lineage investigated by Smith et al. (5) over an infection course of 90 months also did not reveal any mutations in iron acquisition systems, except for a nonsynonymous mutation in pvdS (which correlated with the loss of pyoverdine production) and an intergenic SNP upstream of fptA (5). We therefore conclude that despite an apparent selection for phu promoter mutations in three independent P. aeruginosa lineages, not all lineages accumulate phu promoter mutations during CF infections.
Selection against pyoverdine secretion might lead to a shift in iron source.
The siderophore pyoverdine has previously been found in sputum of CF patients, and thus pyoverdine-mediated uptake of iron has been considered important for the survival of P. aeruginosa in the CF airways (14). Nonetheless, we observed that all three lineages (DK1, DK2, and C) had accumulated nonsynonymous mutations in the alternative sigma factor PvdS, which is required for pyoverdine synthesis (Fig. 1 and Fig. 4). Accordingly, the evolved C clone NN80 was observed to have lost its ability to produce pyoverdine, in contrast to its predecessors (C clones NN2 and NN11) (6).
FIG 4 .
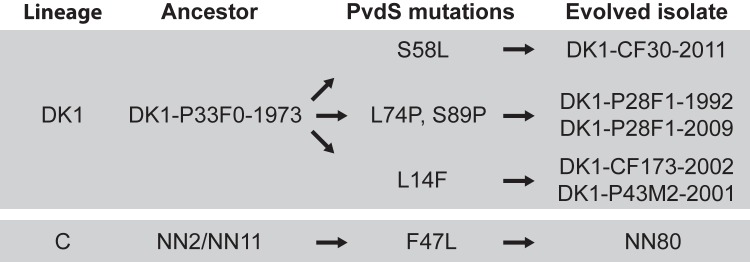
Overview of pvdS mutations in the DK1 and C lineages. Mutations that have accumulated in evolved isolates relative to sequences of their ancestor are shown. The pvdS mutation found in the DK2 lineage is shown in Fig. 1.
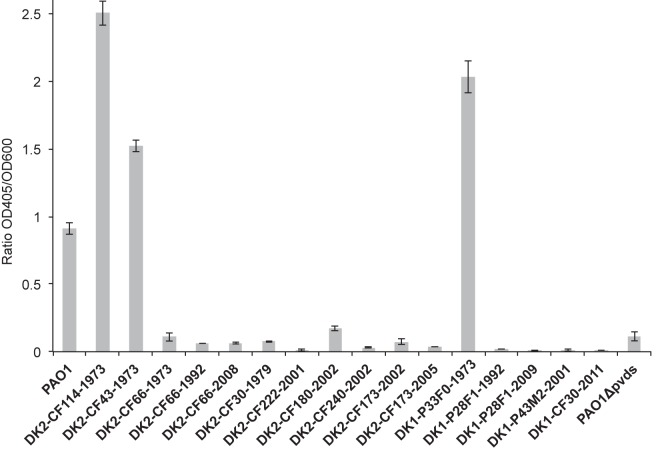
This led us to examine the production of pyoverdine in the DK1 and DK2 isolates, and we observed a negative correlation between pyoverdine production and mutations in PvdS (Fig. 5). Accordingly, only the ancestral DK1 and DK2 isolates carrying wild-type alleles of pvdS were able to produce pyoverdine, whereas all isolates carrying mutated alleles of pvdS were unable to produce pyoverdine (DK1-CF173F-2002 was not tested).
FIG 5 .
Pyoverdine production in isolates of P. aeruginosa. The presence of pyoverdine secreted into the supernatant of bacterial cultures grown in pyoverdine-inducing medium was quantified by measurement of the absorbance at OD405 and normalized against the cell density (OD600). The means and standard deviations calculated from three biological replicates are shown in the bar plot.
Siderophores are generally regarded as highly immunogenic (22), and selection against pyoverdine production might have driven the accumulation of pvdS mutations, leading to a loss of pyoverdine production in the evolved isolates. At the same time, we observed a positive selection for phuR promoter mutations in the CF airways, leading to a bacterial growth advantage when acquiring iron from hemoglobin. We therefore propose a model in which the CF airways impose selective pressure on the invading bacteria, forcing them to adapt toward a shift to hemoglobin as an alternative iron source. This is of particular interest because inflammation may cause microbleeds, which lead to the presence of hemoglobin at the delicate CF lung epithelia in the presence of both host and bacterial proteases (23). Also, hemoglobin is reported to be expressed by alveolar epithelial cells (24).
Other iron acquisition systems might be affected by mutations.
Several iron acquisition systems and mutations other than the ones that we have investigated in detail here might play a role in survival of P. aeruginosa in the lungs of CF patients. Accordingly, we also found nonsynonymous mutations in the FpvAII gene and the genes fpvI, fpvR, phuR, pchA, pchDEFGH, and fptA when searching for mutations in genes of the pyoverdine, pyochelin, phu, has, feo, and fec iron acquisition systems (Fig. 1). We anticipate that the identification of such mutations can facilitate further investigations of the adaptation of P. aeruginosa to human host airways. For example, it remains to be elucidated whether the mutations in the pch and fptA genes affect the function the pyochelin iron uptake system in the DK2 lineage and if isolates with mutations in the pyoverdine system are unable to cheat on other pyoverdine producers.
Conclusions and implications.
Our results provide evidence that the selective conditions by which evolution is directed in the CF airways can result in acquisition of phu promoter mutations in P. aeruginosa during chronic CF infections and that such mutations provide a growth advantage in relation to acquisition of iron from hemoglobin. This adaptive trait may be directly selected for due to an abundance of heme-bound iron in the CF lung. Furthermore, we also observed that phu promoter mutations coincided with the loss of pyoverdine production, suggesting that selection for increased heme utilization may be secondary to the loss of the pyoverdine iron uptake system. Therefore, targeting heme utilization might be a promising strategy for the treatment of CF infections.
CF patients commonly experience iron deficiency, and P. aeruginosa possibly contributes to iron deficiency by depletion of the host iron storage and by causing inflammation (25, 26). In this regard, expanding our knowledge of adaptation of P. aeruginosa to the CF lung may help to lessen the impact of P. aeruginosa infection and improve the condition of patients.
MATERIALS AND METHODS
Bacterial strains and media.
Isolates of the P. aeruginosa DK1 and DK2 clone types were sampled from Danish CF patients attending the Copenhagen Cystic Fibrosis Clinic. Isolation and identification of P. aeruginosa from sputum were done as previously described (27). The isolates are named according to their clone type, the patient from whom they were isolated, and their isolation year (e.g., isolate DK2-CF30-1979). Luria-Bertani (LB) broth was used for routine preparations of bacterial cultures. ABTGC minimal medium was composed of 2 g/liter (NH4)2SO4, 6 g/liter Na2HPO4, 3 g/liter KH2PO4, 3 g/liter NaCl, 1 mM MgCl2, 0.1 mM CaCl2, 0.01 mM FeCl3, 2.5 mg/liter thiamine supplemented with 1% glucose, and 0.5% Casamino Acids. For the growth rate experiments (Table 3), no FeCl3 was added to ABTGC minimal medium unless otherwise stated. Human hemoglobin (Sigma-Aldrich) and human apotransferrin (Sigma-Aldrich) were added to concentrations of 2.5 µM and 100 µg/ml, respectively. Pyoverdine-inducing medium was composed of ABTGC minimal medium with 50 µM iron chelator 2,2′-dipyridyl (DIPY). Escherichia coli strain CC118(λpir) was used for maintenance of recombinant plasmids (28) in medium supplemented with 8 µg/ml of tetracycline. Allelic replacement constructs were transferred to P. aeruginosa by triparental mating using the helper strain E. coli HB101/pRK600 (29). For marker selection in P. aeruginosa, 50 µg/ml of tetracycline was used. Genetic techniques were performed using standard methods, and Sanger sequencing was used for verification of genetic construct and allelic replacement mutants.
Sequencing of phuR promoter region and pvdS gene in DK1 isolates.
Sequencing of DK1 isolates was performed as described earlier (4). Accordingly, genomic DNA was purified from P. aeruginosa isolates using a Wizard Genomic DNA purification kit (Promega, Madison, WI) and sequenced on Illumina’s GAIIx or Hiseq2000 platform. Reads were mapped against the reference genome sequence using the software program Novoalign (Novocraft Technologies, Selangor, Malaysia) (30), and pileups of read alignments were produced by the software program SAMtools, release 0.1.7 (31).
Construction of reporter fusions and luminescence measurements.
The lux gene cassette (luxCDABE) was subcloned from the plasmid pUC18-mini-Tn7T-Gm-lux (32) fragment into mini-CTX2 (33) using the restriction sites XhoI and PstI to produce pHK-CTX2-lux, used for the transcriptional fusion experiments. For phuR::lux reporter fusions, a 220-bp fragment containing the intergenic region upstream of phuR was amplified from genomic DNA using Phusion polymerase (Thermo Scientific) with the primers PhuR_F-PstI (5′ GAGACTGCAGAGGCTGGGAGTGCTGCTCAT 3′) and PhuR_R-XhoI (5′ ACATCTCGAGAAGGGCGGGGAGAGCGGCAT 3′) and ligated with T4 DNA ligase into pHK-CTX2-lux after double digestion of the PCR fragment and vector with the restriction enzymes XhoI and PstI. For phuS::lux reporter fusions, a 220-bp fragment containing the intergenic region upstream of phuS was amplified with the primers PhuS_F-XhoI (5′ ACATCTCGAGAGGCTGGGAGTGCTGCTCAT 3′) and PhuS_R-PstI (5′ GAGACTGCAGAAGGGCGGGGAGAGCGGCAT 3′) and ligated into pHK-CTX2-lux after double digestion of the PCR fragment and vector with the restriction enzymes XhoI and PstI. The resulting plasmids were introduced into P. aeruginosa strain PAO1 by transformation as previously described (32).
Allelic replacement of phuR promoter region in DK2-CF30-1979.
A 1,296-bp fragment containing the intergenic region upstream of phuR was amplified from genomic DNA of DK2-CF173-2005 using Phusion polymerase (Thermo Scientific) with the primers PhuSi_F-XbaI (5′-ACATTCTAGACGGACGTCGCTGGCCTCG-‘3) and PhuRi_R-SacI (5′-GAGAGAGCTCTCTCGTGGCCCTGGCGGTAG-3′). The PCR fragment was ligated into the vector pNJ1 (34) after digestion with the restriction enzymes XbaI and SacI. The allelic replacement construct was transferred into strain DK2-CF30-1979 by triparental mating, and merodiploid mutants were selected by plating the conjugation mixture on LB agar plates with tetracycline. Colonies were restreaked on selective plates before being streaked on 8% (wt/vol) sucrose-LB plates without NaCl. Sucrose-resistant and tetracycline-sensitive colonies were restreaked on sucrose-LB plates and screened for the presence of mutated alleles by PCR followed by restriction fragment length polymorphism (RFLP) analysis. Positive mutants were finally sequenced by Sanger sequencing at LGC genomics (Germany).
Measurement of growth and luminescence in reporter fusion strains.
Overnight cultures of the reporter fusion strains were diluted 40 times in fresh LB, and aliquots of 100 µl were transferred to a black (clear-bottom) 96-well microtiter plate (Nunc). Three technical replicates were used for each strain, and measurements of growth (OD600) and luminescence were recorded in a Synergy Hybrid H1 reader (Bio-Tek) with 6-min intervals for 10 h and under shaking conditions (200 rpm) at 37°C. Data were analyzed using a custom-made script in the R software environment, version 2.15.2 (35). The experiment was repeated three times to obtain biological replicates.
Growth rate measurements.
Growth rate experiments were carried out in 250 ml baffled shake flasks containing 50 ml of growth medium under shaking (200 rpm) at 37°C. Culture flasks were inoculated to a starting OD600 of 0.005 in 50-ml minimal medium, and measurements of OD600 were started 9 h after the inoculation and recorded every 30 min. In the experiment where the cells were cultivated in LB, the measurements were started after 2 h. The experiment was stopped when the cells reached stationary growth phase, typically after around 23 h of growth in minimal medium. Growth experiments were repeated three times for each strain under each condition to obtain biological replicates.
Pyoverdine quantification assay.
Pyoverdine concentrations were quantified as previously described (36). All strains were grown in pyoverdine inducing medium for up to an OD600 of >1.5. Cultures were moved into 2-ml microcentrifuge tubes and centrifuged at 16,000 × g for 2 min. The supernatants were diluted in 100 mM Tris-HCl buffer (pH 8), and pyoverdine concentrations were quantified by measurement of the absorbance at OD405. Finally, the values of absorbance at OD405 were normalized against the cell densities (OD600) for each strain. The procedure was repeated for three independent biological replicates.
ACKNOWLEDGMENTS
This work was supported by the Danish Council for Independent Research (http://fivu.dk/forskning-og-innovation/rad-og-udvalg/det-frie-forskningsrad), the Lundbeck Foundation (http://www.lundbeckfonden.com), and the Villum Foundation (http://villumfonden.dk/).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Lei Yang for help with initial pyoverdine measurements, Sandra B. Andersen for helpful discussion, Helle K. Johansen for collection of the DK1 and DK2 strains, and Christian Munck and Morten O. A. Sommer for help with luminescence recordings.
Footnotes
Citation Marvig RL, Damkiær S, Khademi SMH, Markussen TM, Molin S, Jelsbak L. 2014. Within-host evolution of pseudomonas aeruginosa reveals adaptation toward iron acquisition from hemoglobin. mBio 5(3):e00966-14. doi:10.1128/mBio.00966-14.
REFERENCES
- 1. Skaar EP. 2010. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 6:e1000949. 10.1371/journal.ppat.1000949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marvig RL, Johansen HK, Molin S, Jelsbak L. 2013. Genome analysis of a transmissible lineage of Pseudomonas aeruginosa reveals pathoadaptive mutations and distinct evolutionary paths of hypermutators. PLoS Genet. 9:e1003741. 10.1371/journal.pgen.1003741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rau MH, Marvig RL, Ehrlich GD, Molin S, Jelsbak L. 2012. Deletion and acquisition of genomic content during early stage adaptation of Pseudomonas aeruginosa to a human host environment. Environ. Microbiol. 14:2200–2211. 10.1111/j.1462-2920.2012.02795.x [DOI] [PubMed] [Google Scholar]
- 4. Yang L, Jelsbak L, Marvig RL, Damkiær S, Workman CT, Rau MH, Workman CT, Hansen SK, Folkesson A, Johansen HK, Ciofu O, Høiby N, Sommer MO, Molin S. 2011. Evolutionary dynamics of bacteria in a human host environment. Proc. Natl. Acad. Sci. U. S. A. 108:7481–7486. 10.1073/pnas.1018249108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 103:8487–8492. 10.1073/pnas.0602138103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cramer N, Klockgether J, Wrasman K, Schmidt M, Davenport CF, Tümmler B. 2011. Microevolution of the major common Pseudomonas aeruginosa clones C and PA14 in cystic fibrosis lungs. Environ. Microbiol. 13:1690–1704. 10.1111/j.1462-2920.2011.02483.x [DOI] [PubMed] [Google Scholar]
- 7. Lamont IL, Konings AF, Reid DW. 2009. Iron acquisition by Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. Biometals 22:53–60. 10.1007/s10534-008-9197-9 [DOI] [PubMed] [Google Scholar]
- 8. Poole K, Neshat S, Krebes K, Heinrichs DE. 1993. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J. Bacteriol. 175:4597–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Chial M, Ghysels B, Beatson SA, Geoffroy V, Meyer JM, Pattery T, Baysse C, Chablain P, Parsons YN, Winstanley C, Cordwell SJ, Cornelis P. 2003. Identification of type II and type III pyoverdine receptors from Pseudomonas aeruginosa. Microbiology 149:821–831. 10.1099/mic.0.26136-0 [DOI] [PubMed] [Google Scholar]
- 10. Heinrichs DE, Young L, Poole K. 1991. Pyochelin-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. Infect. Immun. 59:3680–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ochsner UA, Johnson Z, Vasil ML. 2000. Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa. Microbiology 146:185–198 [DOI] [PubMed] [Google Scholar]
- 12. Cartron ML, Maddocks S, Gillingham P, Craven CJ, Andrews SC. 2006. Feo—transport of ferrous iron into bacteria. Biometals 19:143–157. 10.1007/s10534-006-0003-2 [DOI] [PubMed] [Google Scholar]
- 13. Vasil ML, Ochsner UA. 1999. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol. 34:399–413. 10.1046/j.1365-2958.1999.01586.x [DOI] [PubMed] [Google Scholar]
- 14. Haas B, Kraut J, Marks J, Zanker SC, Castignetti D. 1991. Siderophore presence in sputa of cystic fibrosis patients. Infect. Immun. 59:3997–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin LW, Reid DW, Sharples KJ, Lamont IL. 2011. Pseudomonas siderophores in the sputum of patients with cystic fibrosis. Biometals 24:1059–1067. 10.1007/s10534-011-9464-z [DOI] [PubMed] [Google Scholar]
- 16. Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, Malfroot A, Cornelis P, Newman DK. 2013. Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. mBio 4(4):e00557-13. 10.1128/mBio.00557-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Konings AF, Martin LW, Sharples KJ, Roddam LF, Latham R, Reid DW, Lamont IL. 2013. Pseudomonas aeruginosa uses multiple pathways to acquire iron during chronic infection in cystic fibrosis lungs. Infect. Immun. 81:2697–2704. 10.1128/IAI.00418-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marvig RL, Søndergaard MS, Damkiær S, Høiby N, Johansen HK, Molin S, Jelsbak L. 2012. Mutations in 23S rRNA confer resistance against azithromycin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56:4519–4521. 10.1128/AAC.00630-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lieberman TD, Michel JB, Aingaran M, Potter-Bynoe G, Roux D, Davis MR, Jr, Skurnik D, Leiby N, LiPuma JJ, Goldberg JB, McAdam AJ, Priebe GP, Kishony R. 2011. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat. Genet. 43:1275–1280. 10.1038/ng.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. Jelsbak L, Johansen HK, Frost AL, Thøgersen R, Thomsen LE, Ciofu O, Yang L, Haagensen JA, Høiby N, Molin S. 2007. Molecular epidemiology and dynamics of Pseudomonas aeruginosa populations in lungs of cystic fibrosis patients. Infect. Immun. 75:2214–2224. 10.1128/IAI.01282-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wandersman C, Delepelaire P. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611–647. 10.1146/annurev.micro.58.030603.123811 [DOI] [PubMed] [Google Scholar]
- 23. Cosgrove S, Chotirmall SH, Greene CM, McElvaney NG. 2011. Pulmonary proteases in the cystic fibrosis lung induce interleukin 8 expression from bronchial epithelial cells via a heme/meprin/epidermal growth factor receptor/Toll-like receptor pathway. J. Biol. Chem. 286:7692–7704. 10.1074/jbc.M110.183863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newton DA, Rao KM, Dluhy RA, Baatz JE. 2006. Hemoglobin is expressed by alveolar epithelial cells. J. Biol. Chem. 281:5668–5676. 10.1074/jbc.M509314200 [DOI] [PubMed] [Google Scholar]
- 25. Pond MN, Morton AM, Conway SP. 1996. Functional iron deficiency in adults with cystic fibrosis. Respir. Med. 90:409–413. 10.1016/S0954-6111(96)90114-6 [DOI] [PubMed] [Google Scholar]
- 26. Reid DW, Withers NJ, Francis L, Wilson JW, Kotsimbos TC. 2002. Iron deficiency in cystic fibrosis: relationship to lung disease severity and chronic Pseudomonas aeruginosa infection. Chest 121:48–54. 10.1378/chest.121.1.48 [DOI] [PubMed] [Google Scholar]
- 27. Høiby N, Frederiksen B. 2000. Microbiology of cystic fibrosis, p 83–107 In Hodson M, Geddes D. (ed), Cystic fibrosis, 2nd ed. Arnold, London, United Kingdom [Google Scholar]
- 28. Herrero M, de Lorenzo V, Timmis KN. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kessler B, de Lorenzo V, Timmis KN. 1992. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol. Gen. Genet. 233:293–301. 10.1007/BF00587591 [DOI] [PubMed] [Google Scholar]
- 30. Krawitz P, Rödelsperger C, Jäger M, Jostins L, Bauer S, Robinson PN. 2010. Microindel detection in short-read sequence data. Bioinformatics 26:722–729. 10.1093/bioinformatics/btq027 [DOI] [PubMed] [Google Scholar]
- 31. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup 2009. The Sequence Alignment/map format and SAMtools. Bioinformatics 25:2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi KH, Schweizer HP. 2006. Mini-Tn7 insertion in bacteria with secondary, non-glmS-linked attTn7 sites: example Proteus mirabilis HI4320. Nat. Protoc. 1:170–178. 10.1038/nprot.2006.26 [DOI] [PubMed] [Google Scholar]
- 33. Hoang TT, Kutchma AJ, Becher A, Schweizer HP. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59–72. 10.1006/plas.1999.1441 [DOI] [PubMed] [Google Scholar]
- 34. Yang L, Hengzhuang W, Wu H, Damkiaer S, Jochumsen N, Song Z, Givskov M, Høiby N, Molin S. 2012. Polysaccharides serve as scaffold of biofilms formed by mucoid Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 65:366–376. 10.1111/j.1574-695X.2012.00936.x [DOI] [PubMed] [Google Scholar]
- 35. Team RDC 2009. R: a language and environment for statistical computing. http://www.R-project.org
- 36. Imperi F, Tiburzi F, Visca P. 2009. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 106:20440–20445. 10.1073/pnas.0908760106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holloway BW, Krishnapillai V, Morgan AF. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73–102 [DOI] [PMC free article] [PubMed] [Google Scholar]