Abstract
Background
Iatrogenic nerve injuries can result from direct surgical trauma, mechanical stress on a nerve due to faulty positioning during anesthesia, the injection of neurotoxic substances into a nerve, and other mechanisms. Treating physicians should know the risk factors and the procedure to be followed when an iatrogenic nerve injury arises.
Methods
This review is based on pertinent articles retrieved by a selective search in PubMed and on the authors’ own data from the years 1990—2012.
Results
In large-scale studies, 25% of sciatic nerve lesions that required treatment were iatrogenic, as were 60% of femoral nerve lesions and 94% of accessory nerve lesions. Osteosyntheses, osteotomies, arthrodeses, lymph node biopsies in the posterior triangle of the neck, carpal tunnel operations, and procedures on the wrist and knee were common settings for iatrogenic nerve injury. 340 patients underwent surgery for iatrogenic nerve injuries over a 23-year period in the District Hospital of Günzburg (Neurosurgical Department of the University of Ulm). In a study published by the authors in 2001, 17.4% of the traumatic nerve lesions treated were iatrogenic. 94% of iatrogenic nerve injuries occurred during surgical procedures.
Conclusion
A thorough knowledge of the anatomy of the vulnerable nerves and of variants in their course can lessen the risk of iatrogenic nerve injury. When such injuries arise, early diagnosis and planning of further management are the main determinants of outcome. If adequate nerve regeneration does not occur, surgical revision should optimally be performed 3 to 4 months after the injury, and 6 months afterward at the latest. On the other hand, if postoperative high-resolution ultrasound reveals either complete transection of the nerve or a neuroma in continuity, surgery should be performed without any further delay. If the surgeon becomes aware of a nerve transection during the initial procedure, then either immediate end-to-end suturing or early secondary management after three weeks is indicated.
Traumatic nerve injuries are relatively uncommon and affect primarily younger patients. Iatrogenic nerve damage is even less common. Possible causes include (1, 2):
Direct damage during surgery
Pressure or traction because of positioning during anesthesia
Injection of neurotoxic substances
Compression of a hematoma secondary to drawing blood or through anticoagulation
Tourniquet
Dressings, casts or orthotic devices
Radiation.
Iatrogenic nerve injuries in one series accounted for 17.4% of all traumatic nerve injuries (3). In this study the nerve lesion was associated with a surgical procedure in 94% of patients. Others have also documented that post-operative nerve lesions are most common (4– 7).
Large series indicate that 25.2% of sciatic nerve injuries are secondary to medical intervention (8). Similarly, 60% of femoral nerve lesions (9, 10) and 94% of accessory nerve lesions (11) are iatrogenic.
In a retrospective study from Topuz et al., 29 of 73 patients with post-operative nerve injuries had sciatic nerve damage secondary to an intragluteal injection (12).
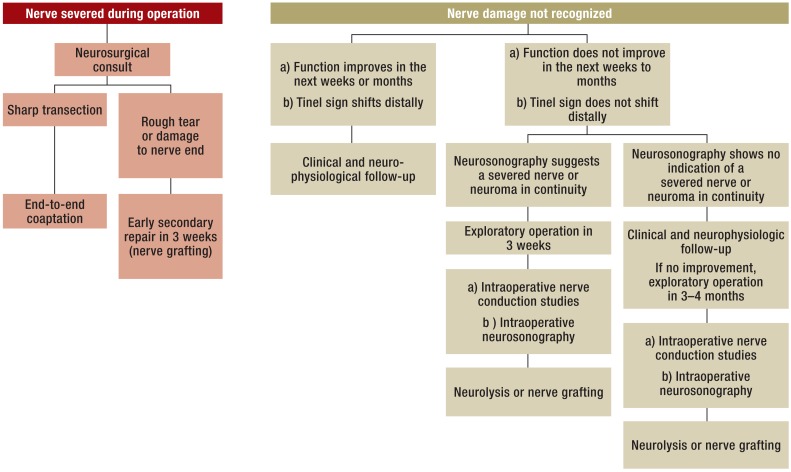
Nerve injuries with neurological deficits have a variable course. If the motor and sensory changes do not resolve spontaneously, operative measures are necessary (Figure 1). In a study by Khan and Birch, 291 of 612 patients with iatrogenic nerve injuries required surgery (13). Carofino et al. reported that 15 of 26 patients with iatrogenic nerve damage following shoulder surgery failed to improve spontaneously and required surgery (14). In view of the results of the above mentioned studies, we will concentrate primarily on the group requiring post-traumatic procedures on peripheral nerves.
Figure 1.
Algorithmic approach to iatrogenic peripheral nerve damage (modified from Antoniadis G, Pedro M, König R: Iatrogene Nervenläsionen-chirurgische Therapieoptionen. Neurologisch: Fachmagazin für Neurologie 2/13, 24-26)
We will address the causes, diagnostic approaches and treatment strategies for iatrogenic nerve damage. Our goal is to increase the reader’s level of suspicion for such lesions so that they can be treated in a more timely fashion than is generally the case today.
Causes of iatrogenic nerve injuries
During surgery nerves can be cut ( Figure 2), crushed, tied off, penetrated and twisted by screws, or even traumatized during the removal of osteosynthetic devices. In addition, they can be stretched by retractors, cut with an electric knife, or thermally damaged by hardening bone cement or during coagulation.
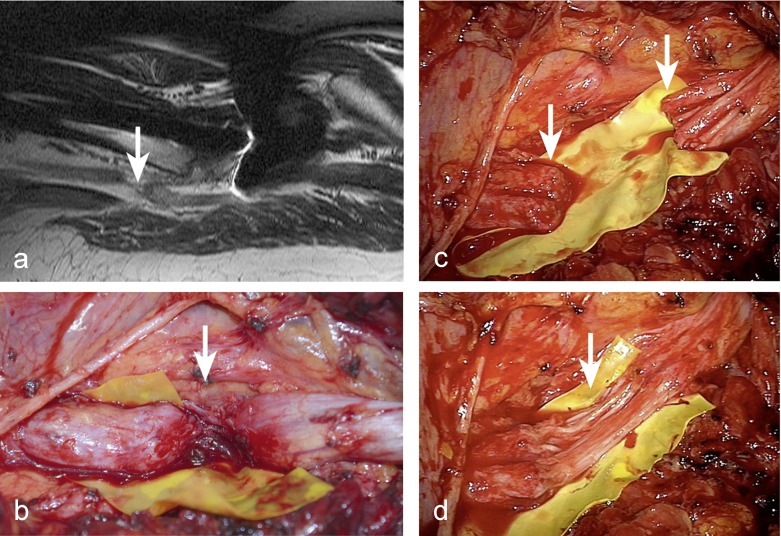
Figure 2.
The sciatic nerve was severed during total hip replacement for hip dysplasia 3 months previously. The nerve injury was not recognized and the patient was treated conservatively for several months.
a) After MRI examination revealed that the nerve was completely separated, the patient was referred to our clinic.
b) The operation revealed a completely severed sciatic nerve in the upper third of the thigh.
c) After the neuroma was resected,
d) nerve grafting using 12 sural nerve grafts from both calves was performed
Nerves can also be cut because they are not clearly exposed in the operative field or not recognized as a nerve but mistaken for a tendon (15– 18) or vessel. Finally, they can be removed along with a nerve sheath tumor or lymph node. The latter is the most common cause for damage to the accessory nerve during neck dissections involving the posterior triangle (Figure 3).
Figure 3.
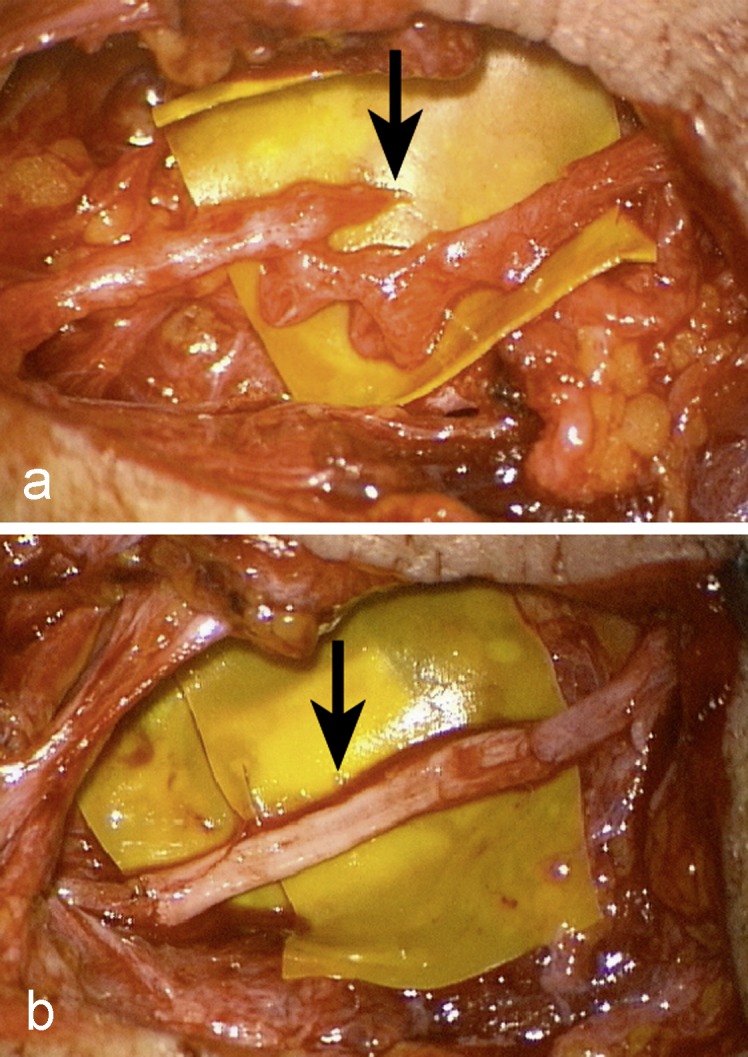
The accessory nerve was damaged during a lymph node resection in the posterior triangle of the neck.
a) The trapezius muscle remained paralyzed; surgical exploration revealed a completely severed nerve.
b) An autologous sural nerve graft was used to bridge the defect.
Nerve sheath tumors are rarely recognized clinically. They are in almost all cases benign (schwannoma, neurofibroma) and with appropriate surgical technique, they can be removed without causing a functionally relevant neurological deficit (19). The results are less satisfactory when the tumor is not recognized as such. Knight et al. reported a series of 234 benign solitary schwannomas removed surgically. In 36 cases, the previous operative procedure requiring secondary surgery had been been performed at another hospital. In 28 cases there was a neurological deficit and/or neuropathic pain following the initial procedure (20). In another series of 88 benign peripheral nerve sheath tumors, the correct diagnosis was made preoperatively in only 8% (7/88), even though all had a positive Tinel sign at the site of the tumor (21). In 31 cases, the preoperative diagnosis was “unspecified lump”; in 16, “unspecified tumor”; and in 13, “ganglion”. However, many of the patients had been treated prior to the widespread availability of magnetic resonance imaging (MRI) and neurosonographic studies.
A crucial aspect, to which every surgeon must be alert, is the highly variable location of peripheral nerves. A nerve often fails to take the course depicted in anatomic atlases. If one is alert to the possibility of variation in location, the intraoperative risk of a nerve injury can be reduced.
In addition to extensive anatomic knowledge, a precise and clean surgical approach is essential. When planning the skin incision, attention should be paid to the underlying cutaneous nerves. If during the course of surgery nerves are exposed and make further progress difficult, they should be extensively neurolyzed in order to mobilize them away from the operative field.
High-risk procedures
Surgical procedures that often lead to nerve damage include (6):
Osteosynthesis and osteotomy
Arthrodesis
Lymph node biopsy in posterior triangle of neck
Carpal tunnel syndrome surgery
Varicose vein surgery
Excision of Baker cyst
Inguinal herniorrhaphy.
In our series of 340 patients with iatrogenic nerve lesions on whom we operated between 1990 and December 2012, we identified the following causes: 45%, major procedures (trauma, abdominal surgery, orthopaedic procedures); 27%, minor procedures; 15%, neurosurgical procedures; and 4%, non-surgical causes. 9% of cases could not be assigned to a category (Table 1).
Table 1. Type and frequency of surguical procedures that led to iatrogenic nerve damage* (1990-2012)*.
| Type of operation | Number of patients | Procedure (patients) |
|---|---|---|
|
Major procedures (trauma, abdominal surgery, orthopedic surgery) (45%) |
152 |
|
|
Minor procedures (27%) |
93 |
|
| Neurosurgical procedures (15%) | 50 | |
| Non-surgical measures (4%) | 13 | |
| Miscellaneous (9%) | 32 | No attributable cause |
| Total | 340 |
*over a period of 23 years in the Neurosurgical Department of the University of Ulm/District Hospital of Günzburg. Some of these results have already been published (3, 29)
High-risk sites
Sites especially likely to be affected include the carpal tunnel and wrist, as well as the posterior triangle of the neck and the knee, especially the popliteal fossa (3). In these areas, the nerves tend to be superficial, close together or in close proximity to the surgical target, such as a lymph node or Baker cyst. It is well-known that the ulnar nerve at the elbow and the common peroneal nerve at the head of the fibula are especially susceptible to pressure injuries secondary to false positioning or casts (3, 6, 22).
Frequently affected nerves
Between 1990 and 2012 we performed surgery on 340 patients with iatrogenic nerve injuries at the District Hospital of Günzburg (Neurosurgical Department of the University of Ulm). This is the largest series of such injuries to be published. Patients were referred from throughout Germany. Over the years the number of procedures increased steadily, from 10 in 2000 to 27 in 2007 (Table 1).
Among the 340 patients, the median nerve was most commonly affected with 17% (58/340); it was followed by the accessory nerve (54/340, 16%); radial nerve (44/340, 13%); common peroneal nerve (43/340, 13%); ulnar nerve (29/340, 8.5%); and femoral nerve (17/340, 5%) (Table 2). 41 of the 58 injuries to the median nerve occurred during carpal tunnel operations. Injuries were less common during endoscopic procedures (17/41) than following open decompression (24/41). Nerve injuries during the standard procedure are more likely when one tries to mimic the endoscopic approach with a mini-incision and fails to adequately visualize the flexor retinaculum (Figure 4)
Table 2. The most commonly affected nerves among 340 iatrogenic nerve injuries treated surgically between 1990 and 2012*.
| Nerve | Number | Percentage (95% confidence interval) |
|---|---|---|
| Median | 58 | 17% (13%; 21%) |
| Accessory | 54 | 16% (12%; 20%) |
| Radial | 44 | 13% (9%; 17%) |
| Common peroneal | 43 | 13% (9%; 17%) |
| Ulnar | 29 | 8,5% (5.5%; 11%) |
| Femoral | 17 | 5% (3%; 7%) |
*The sural nerve was most commonly used for nerve grafting
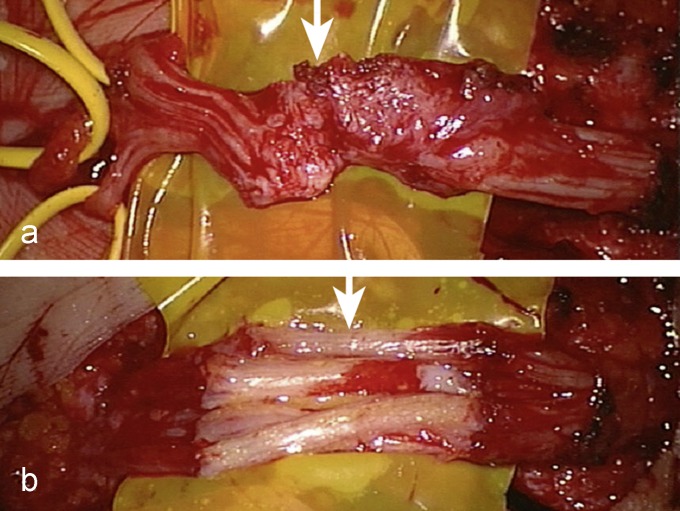
Figure 4.
The median nerve was damaged during an endoscopic carpal tunnel operation eight months previously in another hospital. A severed nerve was identified during exploratory surgery. a) Both nerve stumps were connected by a bridge of scar tissue. b) After resection of the neuroma, the median nerve was repaired with sural nerve grafts.
The surgical repair involved nerve reconstruction (end-to-end coaptation or nerve grafting) in 51% of cases; in 42% neurolysis was performed (external neurolysis, epineurectomy, or internal neurolysis); in 7% a neuroma was excised.
Clinical features
The diagnosis of an iatrogenic nerve injury is straightforward. If a previously asymptomatic patient develops a neurological deficit following a medical intervention, generally an operation, then this deficit is usually a result of the intervention. Although the nerve damage is usually recognized immediately, there are some nerves to which injuries are not apparent unless they are specifically sought. This is especially true for the accessory nerve which can, for example, be cut during a lymph node biopsy in the posterior triangle, usually performed under local anesthesia. The patient may complain of a sharp pain “like an electrical shock” at the moment the nerve is transected. The accompanying shoulder pain because of loss of function of the trapezius muscle is often misinterpreted as post-operative wound pain or arthritis. Such patients have immediate post-operative problems with the simultaneous abduction and rotation of the arm at the shoulder, the motion employed, for example, in combing one’s hair.
If function is lost in an entire nerve or part thereof, and the deficit fits with the area where surgery was performed, then one must assume that the medical intervention had caused the problem. The following questions must be immediately clarified:
What type of injury has occurred (transection, pressure, stretching)?
Where is the site of the lesion?
What can be done to ameliorate the problem?
The most important step in obtaining a precise diagnosis and then formulating a strategy is an accurate history coupled by an immediate detailed neurological examination by a neurologist or neurosurgeon with experience in traumatic nerve lesions. An electrophysiological evaluation is required to precisely identify the level of the lesion and assess the potential for regeneration. In the hands of an experienced specialist, modern imaging procedures such as MRI and neurosonography can deliver important information for planning therapy.
The most important prognostic factor which can be influenced by the physician is the timing of the corrective operation. It should be performed 3–4 months after the injury—at the latest after 6 months. When nerves are completely severed, after a few weeks progressive loss of neuronal cells occurs, negatively influencing the potential for regeneration following successful nerve reconstruction (23, 24). After 6 months the likelihood of surgery restoring or at least improving nerve function is significantly reduced (25).
In reality, then timing is usually less than ideal. In our previously cited study from 2001 involving 126 patients, only 43 (35%) were operated on during the first 6 months after the iatrogenic injury; 40 (32%) were treated within a year and 41 (33%) after more than a year. Two-thirds of the patients were seen for the first time after the ideal interval of 6 months (3).
The following factors are usually responsible for the delayed diagnosis and therapy:
The nerve damage is not recognized.
The nerve damage is identified but one waits too long hoping for spontaneous improvement.
The surgeon fails to acknowledge the nerve damage and thus delays appropriate corrective measures.
Therapy
If it is noted during an operation that a nerve has been severed, it should be repaired immediately during the same operation (primary repair) or within 2–3 weeks (early secondary repair) (26). The same is true when the nerve is torn or damaged but not cleanly cut. The same operative approach is used as for any other nerve injury. The repair ideally is done with microsurgical tools and magnifying devices, insuring maximal visualization for the repair.
Once again, this ideal situation with immediate repair is seldom achieved. Usually the cause of the damage is unknown. In our experience, the operative report rarely provides useful information. When the mechanism for the damage is unknown but there is reason to think that the nerve may regenerate itself, we prefer to wait 3 months with monthly neurological examinations. If at this time, the deficit has not changed or only minimally improved, the nerve should be surgically explored in the next month. If the neurosonographic examination after exposure of the nerve identifies a neuroma, one should not delay. The operation should ideally occur within 3 weeks (26).
A severed nerve should be reconstructed, if possible. Usually this requires nerve grafting. The sural nerve on the lateral calf is usually used as a source. Other cutaneous nerves such as the saphenous nerve and the medial antebrachial cutaneous nerve can also be used (26). If the nerve appears to be intact, then intraoperative nerve conduction studies help assess how functional it is in the area of damage. If conductivity is impaired, then the affected segment of the nerve surrounded by scar tissue—usually thickened and diagnosed as a neuroma in continuity—is excised and replaced by a transplant. In other cases, when conductivity studies are more promising, it suffices to free the nerve up from the surrounding reactive tissues (neurolysis). In recent years intraoperative neurosonography has been employed, facilitating the evaluation of individual nerve fascicles, helping distinguish between a complete neuroma in continuity without any residual fascicles and a partial lesion still containing functioning fascicles (27).
The combination of the functional evaluation (nerve conduction studies) and the morphologic assessment (neurosonography) is very helpful in the surgical management of traumatic injuries in peripheral nerve surgery. The exact approach is documented in the interdisciplinary guidelines of the AWMF “Versorgung peripherer Nervenverletzungen” (26).
A key factor in improving the prognosis is physical therapy, both after the deficit is identified and then post-operatively, until re-innervation of the affected muscles has occurred. Electric stimulation therapy is also worthwhile in our option. In this way, the muscle structures can be better maintained until nerve regeneration has occurred.
Results of therapy
In 2001, we reported our first 126 patients with surgically treated iatrogenic nerve damage. 97 cases with mean follow-up of 18 months (3 months to 7 years 8 months) could be evaluated. This represents a subgroup of the patients shown in Table 1. 45 patients (46%) showed a slight to definite improvement in their motor and sensory changes, as well as pain. 23 patients (24%) had a very good response with remission of their neurological symptoms. In 25 patients (26%) the neurological deficits and pain did not change, while 4 patients (4%) reported worsening pain. Overall, 70% of the patients showed post-operative improvement (3). The results would have almost certainly been better if the cohort had not included nerve lesions with a generally accepted poor outlook (such as common peroneal nerve lesions) and if more than one-third of the patients had been referred within 6 months after their iatrogenic injury.
Lesions of the accessory nerve, radial nerve and tibial nerve have an especially good prognosis. The accessory nerve is an exception in that repairs after 6 months often produce good functional results (28).
Patients with nerve deficits as a result of improper positioning or extended pressure generally do not require surgery. The lesions are almost always incomplete. 90% will heal spontaneously (7, 29). However, this process may take many months.
Conclusion
Iatrogenic nerve injuries are not as uncommon as one might expect. They account for around 20% of the traumatic nerve lesions seen at special centers (as reflected in our data and that of R. Birch [personal communication] in London) and require careful attention. They usually are the result of surgical procedures. The connection between the medical intervention, usually an operation, and the nerve damage is usually obvious.
In our opinion and that of the AWMF guideline authors, it is key that the patients be examined as soon as possible after the injury by someone with experience in traumatic nerve inquires to make the correct diagnosis and identify the level of the defect, so that the appropriate therapy can be planned (26). Frequently the patients encounter therapeutic nihilism.
The treating physician cannot influence the injury, the involved nerve, the site and degree of damage, the age of the patient and other patient-specific factors, but he or she can make a correct diagnosis and insure therapy is performed at the most favorable time. It is not acceptable when patients with iatrogenic nerve injures are simply re-assured or even not informed about their nerve injury. In this way, valuable time is lost, and the hope for improvement of the neurological deficit is reduced or even lost.
The British orthopedic surgeons Birch, Bonney and Wynn Parry (1998) said it best (4, p. 313): “Although some allowance has to be made for the damage itself, little or no allowance should be made for failure to recognise the fact of nerve injury, failure to diagnose depth of affection and nature and extent of injury, and failure to take appropriate action.“ Birch et al. attribute “the principal causes of clinical error and negligence [to]: failure of knowledge, failure to observe and failure to use common sense.” (4, p. 326, emphasis taken from the original)
Key Messages.
Iatrogenic nerve injuries can occur during all surgical procedures. Complete familiarity with the anatomy of the region can markedly reduce the risk of a nerve injury.
If a nerve is severed during an operation, it should be repaired primarily with an end-to-end coaptation or reconstructed shortly thereafter (early secondary repair).
If a neurological deficit is noticed immediately after an operation, the patient should be closely monitored with neurological, neurophysiological and neurosonographic methods. If no improvement occurs after 3 months, the injured nerve should be explored. If neurosonography now shows a complete separation or neuroma in continuity, an operation should be performed immediately.
The two most commonly affected nerves are the accessory nerve after a lymph node biopsy and the median nerve after open or endoscopic carpal tunnel operations.
The most common causes of nerve injuries are surgical repair of fractures and implantation of joint prostheses.
Acknowledgments
Translated from the original German by Walter Burgdorf, MD.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Kretschmer T, Heinen CW, Antoniadis G, Richter HP, Konig RW. Iatrogenic nerve injuries. In: Spinner RJ, Winfree CJ, editors. Neurosurg Clin N Am. Vol. 20. Philadelphia: W.B. Saunders Company; 2004. pp. 73–90. [DOI] [PubMed] [Google Scholar]
- 2.Wilbourn AJ. Iatrogenic nerve injuries. Neurol Clin. 1998;16:55–82. doi: 10.1016/s0733-8619(05)70367-4. [DOI] [PubMed] [Google Scholar]
- 3.Kretschmer T, Antoniadis G, Braun V, Rath SA, Richter HP. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg. 2001;94:905–912. doi: 10.3171/jns.2001.94.6.0905. [DOI] [PubMed] [Google Scholar]
- 4.Birch R, Bonney G, Parry CW. Iatropathic Injury. In: Birch R, Bonney G, Parry W, editors. Surgical Disorders of the Peripheral Nerves. Edinburgh: Churchill Livingstone; 1998. pp. 293–333. [Google Scholar]
- 5.Muller-Vahl H. Iatrogenic lesions of peripheral nerves in surgery. Langenbecks Arch Chir. 1984;364:321–323. doi: 10.1007/BF01823225. [DOI] [PubMed] [Google Scholar]
- 6.Stöhr M. Injektion, Operation, Lagerung, Strahlentherapie. ed 2. Stuttgart: Thieme; 1996. Iatrogene Nervenläsionen. [Google Scholar]
- 7.Tackmann W, Richter H-P, Stöhr M. Berlin: Springer; 1989. Kompressionssyndrome peripherer Nerven. [Google Scholar]
- 8.Kim DH, Murovic JA, Tiel R, Kline DG. Management and outcomes in 353 surgically treated sciatic nerve lesions. J Neurosurg. 2004;101:8–17. doi: 10.3171/jns.2004.101.1.0008. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Kline DG. Surgical outcome for intra- and extrapelvic femoral nerve lesions. J Neurosurg. 1995;83:783–790. doi: 10.3171/jns.1995.83.5.0783. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Murovic JA, Tiel RL, Kline DG. Intrapelvic and thigh-level femoral nerve lesions: management and outcomes in 119 surgically treated cases. J Neurosurg. 2004;100:989–996. doi: 10.3171/jns.2004.100.6.0989. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Cho YJ, Tiel RL, Kline DG. Surgical outcomes of 111 spinal accessory nerve injuries. Neurosurgery. 2003;531:106–112. doi: 10.1227/01.neu.0000089058.82201.3d. discussion 1102-3. [DOI] [PubMed] [Google Scholar]
- 12.Topuz K, Kutlay M, Simsek H, Atabey C, Demircan M, Senol Güney M. Early surgical treatment protocol for sciatic nerve injury due to injection - a retrospective study. Br J Neurosurg. 2011;5:509–515. doi: 10.3109/02688697.2011.566380. [DOI] [PubMed] [Google Scholar]
- 13.Kahn R, Birch R. Iatropathic injuries of peripheral nerves. J Bone Joint Surg Br. 2001;83:1145–1148. doi: 10.1302/0301-620x.83b8.12251. [DOI] [PubMed] [Google Scholar]
- 14.Carofino BC, Brogan DM, Kircher MF, et al. Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg Am. 2013;95:1667–1674. doi: 10.2106/JBJS.L.00238. [DOI] [PubMed] [Google Scholar]
- 15.Geldmacher J. Median nerve as free tendon graft. Hand. 1972;4 doi: 10.1016/0072-968X(72)90012-5. [DOI] [PubMed] [Google Scholar]
- 16.McGeorge D, Sturzenegger M, Buchler U. Tibial nerve mistakenly used as a tendon graft. Reports of three cases. J Bone Joint Surg Br. 1992;74:365–366. doi: 10.1302/0301-620X.74B3.1587878. [DOI] [PubMed] [Google Scholar]
- 17.Oppikofer C, Tschopp H. Tibial nerve used as a tendon graft. Helv chir Acta. 1990;57:923–929. [PubMed] [Google Scholar]
- 18.Weber RV, Mackinnon SE. Median nerve mistaken for palmaris longus tendon: Restoration of function with sensory nerve transfers. Hand. 2007;2:1–4. doi: 10.1007/s11552-006-9011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Murovic JA, Tiel RL, Kline DG. Operative outcomes of 546 Louisiana State University Health Sciences Center peripheral nerve tumors. Neurosurg Clin N Am. 2004;15:177–192. doi: 10.1016/j.nec.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007;89:382–387. doi: 10.1302/0301-620X.89B3.18123. [DOI] [PubMed] [Google Scholar]
- 21.Kehoe NJ, Reid RP, Semple JC. Solitary benign peripheral-nerve tumours. Review of 32 years’ experience. J Bone Joint Surg Br. 1995;77:497–500. [PubMed] [Google Scholar]
- 22.Winfree CJ, Kline DG. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63:5–18. doi: 10.1016/j.surneu.2004.03.024. discussion 18. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Novikov LN, Kellerth JO, Wiberg M. Early nerve repair after injury to the postganglionic plexus: an experimental study of sensory and motor neuronal survival in adult rats. Scand J Plast Reconstr Surg Hand Surg. 2003;37:1–9. doi: 10.1080/alp.37.1.1.9. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Novikov LN, Wiberg M, Kellerth JO. Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: a quantitative morphological study. Exp Brain Res. 2001;139:216–223. doi: 10.1007/s002210100769. [DOI] [PubMed] [Google Scholar]
- 25.Antoniadis G, Pedro MT, König R. Traumatische Nerven- und Plexusschäden: Prä- und klinische Versorgungsalgorithmen und Behandlungsoptionen. Traumatische Nerven- und Plexusschäden. Neurochirurgie Scan. 2013;1:127–142. [Google Scholar]
- 26.Deutsche Gesellschaft für Handchirurgie (DGH), Deutsche Gesellschaft für Neurologie (DGN), Deutsche Gesellschaft für Neurochirurgie (DGNC), Deutsche Gesellschaft für Orthopädie und Orthopädische Chirurgie (DGOOC), Deutsche Gesellschaft der Plastischen, Rekonstruktiven und Ästhetischen Chirurgen (DGPRÄC), Deutsche Gesellschaft für Unfallchirurgie (DGU) Leitlinen: Versorgung peripherer Nervenverletzungen. http://www.awmf.org/leitlinien/detail/ll/005-010.html (Stand 30.06.2013)
- 27.Koenig RW, Schmidt TE, Heinen CPG, et al. Intraoperative high-resolution ultrasound: a new technique in the management of peripheral nerve disorders. Clinical article Journal of Neurosurgery. 2011;114:514–521. doi: 10.3171/2010.9.JNS10464. [DOI] [PubMed] [Google Scholar]
- 28.Camp SJ, Birch R. Injuries to the spinal accessory nerve: a lesson to surgeons. J Bone Joint Surg Br. 2011;93:62–67. doi: 10.1302/0301-620X.93B1.24202. [DOI] [PubMed] [Google Scholar]
- 29.Kretschmer T, Antoniadis G, Borm W, Richter HP. Iatrogenic nerve injuries - Part 1: Frequency distribution, new aspects, and timing of microsurgical treatment. Chirurg. 2004;75:1104–1112. doi: 10.1007/s00104-004-0879-8. [DOI] [PubMed] [Google Scholar]
- 30.Birch R. Iatrogenous Injuries. In: Birch R, editor. Surgical disorders of peripheral nerves. London: Springer Verlag; 2011. pp. 483–525. [Google Scholar]