Abstract
Human senescence has been delayed by a decade. This finding, documented in 1994 and bolstered since, is a fundamental discovery about the biology of human ageing, and one with profound implications for individuals, society and the economy. Remarkably, the rate of deterioration with age seems to be constant across individuals and over time: it seems that death is being delayed because people are reaching old age in better health. Research by demographers, epidemiologists and other biomedical researchers suggests that further progress is likely to be made in advancing the frontier of survival — and healthy survival — to even greater ages.
The finding that mortality at advanced ages can be postponed, and indeed is being postponed, resolved a millennia-old debate about whether survival could be extended among the elderly1–13. The evidence published since 1994 is compelling14–27. Unless radical breakthroughs are achieved, perhaps as a result of research on other species, humans will continue to suffer senescence — but the process is not intractable. Mortality has been postponed considerably, as a result not of revolutionary advances in slowing the process of ageing but of ongoing progress in improving health. The debility that often characterizes the last years of life also seems to have been delayed, although the evidence is mixed, in part because deterioration is difficult to define and measure28–36. The future is uncertain, but it seems plausible that very long lives may be the probable destiny of younger people alive today. If progress in reducing mortality continues at the same pace as it has over the past two centuries23, which is a matter of debate37, then in countries with high life expectancies most children born since the year 2000 will celebrate their 100th birthday — in the twenty-second century28. Longer lifespans will alter the way individuals want to allocate time during their lives and will require radical revision of employment, retirement, health, education and other policies9,38,39.
Although Oliver Wendell Holmes advised people who wanted to live long to “advertise for a couple of parents both belonging to long-lived families”40, genetic factors have a modest role in determining how long individuals live41–45. The progress being made in lengthening lifespans and postponing senescence is entirely due to medical and public-health efforts, rising standards of living, better education, healthier nutrition and more salubrious lifestyles23,46. Future progress in improving health among the elderly, however, probably will be fuelled in part by interventions developed on the basis of deeper understanding — in humans and in nonhuman species — of genetics45,47–51 and of the root causes of ageing6,52–62.
After summarizing the evidence that mortality is being postponed, in this Review I examine the less conclusive evidence that debility is also being postponed. The process of deterioration with age is not being slowed over time: it is being delayed. I discuss this and then turn to the causes of the postponement. In the final sections, I consider probable future developments in human ageing and in biodemographic research on human ageing.
The postponement of mortality
The hypothesis that mortality at higher ages is being postponed was proposed two decades ago on the basis of suggestive data about progress in reducing death rates and about such special populations as Mormon high priests9,10,12. The first conclusive finding that death is being delayed stemmed from an analysis of Swedish mortality data14. Mortality is by far the most readily and reliably measured index of health. Most countries have compiled serviceable vital statistics for decades. Sweden pioneered the systematic collection of data on deaths and has had a good system of national reporting since the 1750s and an almost impeccable one since 1861.
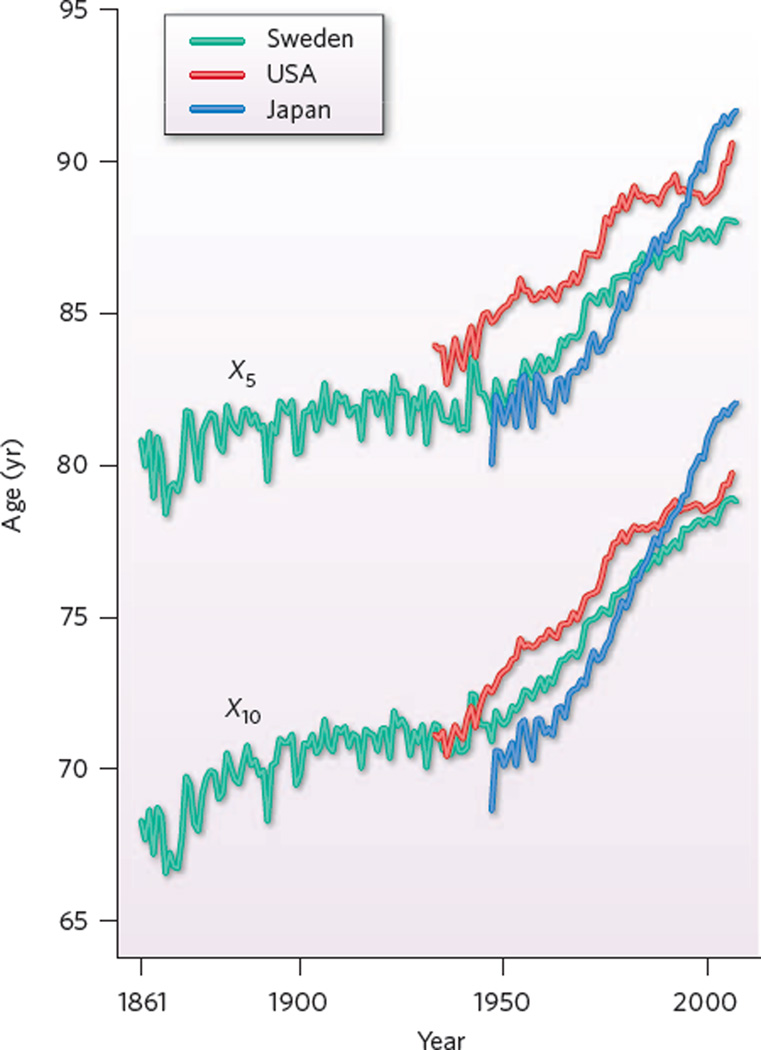
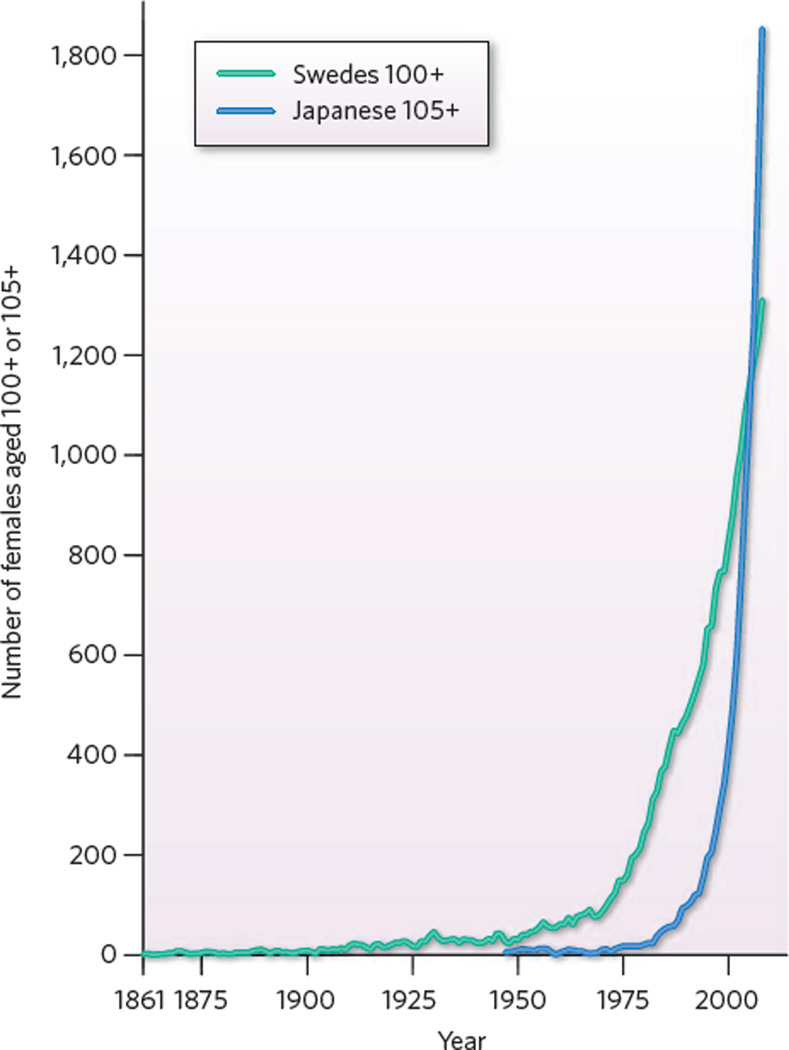
These data permitted study of the age at which remaining life expectancy fell to five years (Fig. 1). The discovery that “the process of aging has been … delayed” was supported by analyses of the decline over time in death rates at advanced ages14. Two subsequent studies15,16, also published in 1994, confirmed that mortality is being postponed in other countries. Research since then shows that postponement is continuing17–27. A remarkable finding is that the delay of death is fuelling a large, rapid increase in the numbers of centenarians63(Fig. 2).
Figure 1. The postponement of mortality.
Historical trends in X5 and X10, the ages at which remaining life expectancies are, respectively, five and ten years, for females in Sweden (1861–2008), the USA (1933–2006) and Japan (1947–2008). For Swedish women, since 1950 senescence as measured by X10 has been postponed by about eight years. For Japanese women, since 1950 X10 has risen by about 12 years. Note that for all three countries, the curve for X5 follows the same general trajectory as the curve for X10 but at a roughly constant gap of about a decade of age. This indicates that senescence, as captured by these two measures, is being postponed rather than lengthened. Both indicators show that progress in postponing senescence was slow for women in the USA between 1980 and 2000. The prospects are that more rapid progress can be expected in the future77,83: the rapid rise in X5 and X10 in recent years for US women may be a harbinger of this. (Data extended and updated from a graph in ref. 14 using information from the Human Mortality Database (http://www.mortality.org), from Statistics Sweden for Sweden 2008 and from the Japanese Ministry of Health for Japan 2008.)
Figure 2. The emergence of the extremely old.
The numbers of females aged 100+ in Sweden from 1861 to 2008 and aged 105+ in Japan from 1947 to 2007. Very old people were rare until roughly half a century ago. Since then, the number of Swedish centenarians has risen rapidly, and since 1975 the number of Japanese women 105 or older has climbed almost vertically. (Data from the Kannisto–Thatcher Database on Old Age Mortality (http://www.demogr.mpg.de) supplemented with data from Statistics Sweden and the Japanese Ministry of Health.)
The evidence that mortality is being postponed surprised demographers and actuaries. Most of these specialists, whose business it is to forecast mortality trends, believed that human life expectancy was close to an upper limit11,23. They based this view on both data and theory. They analysed extensive data on mortality trends, albeit not for mortality after age 85. Although they could see that death rates were falling, they felt that death rates could not fall much further. This pessimism reflected their inability to imagine the advances that might be made to delay death23, reinforced by their understanding of the theory of mortality.
In particular, they accepted the widely held view1,8,13 that there is only one cause of death at an advanced age, namely old age, and that nothing can be done about it. This notion, which can be traced back to Aristotle’s distinction between premature and senescent death2, led to the conclusion that every species has a characteristic maximum lifespan. Other scholars, from Galen3 in the late second century ad and Cornaro5 in the 1500s to today’s scholars64,65, argued that dietary restriction could extend lifespans, and numerous other conjectures were proposed about the secrets of longevity1,4,66. Evolutionary biologists believed that senescence — deterioration with age — was inevitable for multicellular species6,54–62 but that genetic and other interventions might slow the process54–59. Demographers and actuaries concluded, correctly, that such interventions in humans were unlikely in the short term. Furthermore, many students of human ageing believed that if lives were saved at lower ages, then frail individuals would survive to higher ages, making it more and more difficult with increasing age to reduce mortality and morbidity7.
In addition to the demonstration that mortality at advanced ages is being postponed, other research helped overturn the belief that life expectancy was approaching its limit. The notions of intractable senescent death and of species-specific maximum lifespans were refuted in two back-to-back articles49,67 in Science in 1992. Research on Danish twins born since 1870 found no evidence for an innate maximum lifespan shared by identical twins. Only about 25% of the variation in adult lifespans could be attributed to genetic variation among individuals41,42. This percentage seems to increase slightly with age, but even among the elderly, genetic variation still seems to have only a modest impact44. Its impact might, however, be more significant at the oldest ages43. As discussed in the next section, the progress made in saving lives seems to have also improved health, even in advanced age, but this is still uncertain and is an important topic for research.
The quest to uncover major longevity genes in humans has had little success45. Two variants of the apolipoprotein E gene (APOE) have been shown in multiple studies to be risk factors that lower or raise, respectively, the chances of death at higher ages by a factor of roughly 1.1 or 1.2 relative to the baseline risk faced by people with the common variant47,48. Although studies have found many genes that reputedly affect lifespans, none has an effect as big as the modest effect of APOE, and few have been replicated in multiple studies. All functioning genes in all species contribute directly or indirectly to fertility, survival or both; evolutionary theory and a few empirical studies suggest that variants that substantially increase longevity are probably rare under natural conditions because they reduce reproduction55,57–59,62,68. In the nematode Caenorhabditis elegans, hundreds of genes have been artificially altered to lengthen lifespans50, some with very large effects. The discovery of the first of these genes, age-1, was a seminal advance that revolutionized our understanding of the genetics of ageing51. In humans, it seems likely that polymorphisms at hundreds and perhaps thousands of genetic loci each have a small role in increasing or decreasing the risk of death and debility in advanced age.
The evolutionary theory of ageing has been interpreted as implying that senescence is inevitable for all multicellular species6. A major contribution of researchers in the nascent field of biodemography has been to show that the theory should be expanded to permit greater variation in patterns of ageing, including so-called inverse senescence — the decline of mortality and the improvement of health over all or most of adult life60–62. Research in the laboratory and in the field confirms that, for some species and some periods of adult life, mortality can decline with age and that changes in diet and other environmental factors, as well as genetic changes, can greatly alter age trajectories of survival49−51,54,55,60,64.
Progress in delaying debility
In comparison with death, health is difficult to measure and is often unreliably reported. Estimates of population health are usually based on data from surveys hindered by low participation, especially among the sick. Demographers and epidemiologists have begun to compile serviceable information about the postponement of senescence as captured by various indices of health28–36, but the picture is much less clear and more mixed, especially with regard to data on individuals over age 85 and on cognitive performance, than the cogent perspective provided by mortality statistics.
The prevalence of diseases and morbid disorders among the elderly has tended to increase over time28. Part of the rise can be attributed to earlier diagnosis of, for example, type 2 diabetes, hypertension and some cancers. The prevalence of heart disease and arthritis seems to have increased, and individuals are more often reported to have multiple disorders.
Disability is usually measured in terms of self-reported limitations concerning activities of daily living, such as dressing, bathing, shopping and so on. There is increasing evidence that the prevalence of disability may be decreasing28,29,31–34, reflecting better treatment and a postponement of senescence, but some studies find an increase in the prevalence of disability33,35,36.
Health expectancies measure the number of years of healthy life a person can expect to have given current health conditions28. The number of years spent in self-perceived good health has been increasing in most countries studied28, whereas trends in the number of years spent with disability have evolved differently depending on the severity of the disability: a decrease for the most severe levels and an increase for the less severe levels, but with substantial differences among countries28.
There is little available evidence on improvements in morbidity, functioning and health expectancy after age 85, but it seems that, at least in the United States and at least for disability, substantial improvements were made in the 1990s28,29,34. In Denmark, information on centenarians born in 1895–1896 can be compared with similar information on centenarians born in 1905. The later cohort of centenarians was 50% larger than the earlier cohort, mainly because the chance of survival between the ages of 80 and 100 had substantially improved32. Nonetheless, physical disability and cognitive impairments in the two cohorts were comparable, with women in the more recent cohort having slightly better health31.
The prevalence of a disease or condition depends on the net balance between death and incidence, with death delayed by more effective treatment and with incidence increased not only by the greater frequency of the condition but also by improved screening. Three states of health can be distinguished: a person can be healthy, unhealthy or dead. To the extent that the unhealthy state is better than death, greater prevalence of morbidity among the elderly may be a positive development. The unhealthy state can be broken down into two states: having a chronic illness but being able to function, and being disabled. Although it does not reduce morbidity, effective rehabilitation reduces disability. Suffering from erratic heart rhythms but controlling them with a pacemaker, or suffering a stroke but getting physical therapy, increases the prevalence of morbidity but is preferable to being incapacitated by illness, or death. Research on which countries are doing best in minimizing disability — and how — could greatly benefit the elderly.
In the absence of more evidence, indirect assessment is informative. Most kinds of morbidity and disability, including heart disease and dementia, lead to higher death rates. Hence, if poor health after age 85 were not being postponed, it would be difficult to reduce mortality after this age. In the short term and depending on the health index studied, the net result of the twin processes of saving lives and improving health can lead to either increases or decreases in the prevalence of morbidity and disability. This dynamic may explain why health in the elderly sometimes improves when progress in reducing mortality is slow (for example in the United States29,34 (Fig. 1) and Denmark31,32 in the 1980s and 1990s) and worsens when progress is more rapid (for example in Sweden35 and especially Japan (Fig. 1) in recent decades36). In the long term, however, better health at an advanced age might be a precondition for continued improvements in survival. This is a plausible hypothesis, but conclusive evidence is lacking.
Almost all studies of ageing in populations of non-human species focus on mortality as the index of senescence. More laboratory and field studies of age-related patterns of morbidity and disability in other species are needed.
With rising age, women make up an increasing share of the population. In Sweden in 2008, almost 52% of births were boys and, despite higher male death rates, men outnumbered women up to age 60. There were three women for every two men by age 80 and six women for every man among centenarians. In terms of various indices of health and disability, however, older men generally do better than coeval women. This is the health–survival paradox: men seem to be healthier than women, but they die younger69. Social and biological factors interact to determine the prevalence of frail females and dead males, but the relative importance of specific mechanisms is not well understood69–71. Males tend to believe their health is better than it actually is and do not seek medical care as frequently as females: they have fewer appointments with general practitioners but require emergency treatment more often69. Females seem to be better able to survive with poor health72. Males tend to engage in reckless behaviour. This tendency may be partly genetic in origin, having its basis in the different reproductive opportunities males face in comparison with females70,71.
Delay not deceleration
Senescence results from a cumulative imbalance between damage and repair57,62. Progress in reducing damage (by means of public-health efforts to enhance living conditions and to prevent disease, for example) and progress in increasing repair (by medical interventions, for example) are the two fundamental causes of health improvement. It might be thought that such progress would slow the rate of deterioration such that the debilitation that used to occur between ages 70 and 80 would occur between ages 70 and 85 and the debilitation that used to occur between ages 80 and 90 would occur between ages 85 and 100. Remarkably, this does not seem to be the case. Indeed, the pace at which death rates increase with age accelerated somewhat over much of the twentieth century and has been roughly constant in recent decades17–19,24–27. The evidence suggests that deterioration, instead of being stretched out, is being postponed: levels of mortality and other indices of health that used to prevail at age 70 now prevail at age 80, and levels that used to prevail at age 80 now prevail at age 90. The various strands of demographic and epidemiological evidence reviewed above, and some intriguing animal studies64, have been reinforced by new evidence from a study of supercentenarians (that is, people 110 years old and older)73.
Most reported cases of a person being a centenarian — and to an even greater extent a supercentenarian — are erroneous73,74. To verify reputed high ages, correct birth records have to be found. A meticulous research endeavour has yielded a remarkable finding: between the validated ages of 110 and 114, the annual probability of death is constant at a level of 50% per year73. The sparse observations of survival after age 114 are not inconsistent with the hypothesis that mortality stays at this level at all ages after 110. As explained in Box 1, this result implies that at least at advanced ages, human individuals deteriorate at the same rate.
Causes of past and future postponement
Prosperity and medicine seem to be the two main factors contributing to the postponement of senescence34,46. Prosperity matters because older people are healthier when they live in insulated housing, wear appropriate clothing, eat appetizing food and enjoy their days. It matters also because health care can be expensive, especially for innovative treatments, and because more resources can be devoted to biomedical research in richer economies. Furthermore, prosperity is important because citizens of wealthy countries tend to be well educated, and the well educated tend to live healthier and longer lives. Medical treatments, surgical interventions and public-health efforts to prevent or mitigate illness and disability are particularly important for people weakened by senescence. It is not known whether prosperity or medicine is the more important factor, in part because the two interact with each other. Prosperity allows better treatment, further education and more research; healthier populations are more productive and prosperous. Two countries at the same level of income per capita can have different healthy life expectancies, and some countries with modest standards of living do as well as much richer countries75. Understanding why is a research priority.
Many additional factors interact to determine why some people live longer or shorter healthy lives than average59,76. Despite intensive research, the impact of family structure, social networks, obesity and other factors is not well understood77. For instance, opinions differ about the relative importance, in determining health in old age, of early-life conditions versus conditions later in life. On the one hand, some kinds of event in utero and in childhood have significant ramifications for health in old age78, including season of birth79,80. For instance, babies born in November in Europe around 1900 lived several months longer after age 50 than babies born in May79. Evidence, however, is mixed about the lingering consequences of suffering through a famine81, and being a twin in utero does not seem to have any long-term effect82. Furthermore, studies of twins report that less than 10% of the variation in adult lifespan can be attributed to childhood environments shared by the twins41,42. On the other hand, it is known that cigarette smoking during adult years has very serious effects on health even decades later, and patterns of cigarette smoking seem to be the most important key to explaining why progress in reducing adult death rates is faster in some countries than in others77,83. It is also clear that events later in life, such as medical advances or economic growth, are the prime determinants of progress in reducing death rates among the elderly16,17,26,77,84: a striking example is that after the unification of Germany two decades ago, the rates of death of the old and very old in the former East Germany rapidly declined and approached those of the former West Germany85.
A person has little chance of surviving to very old age if he or she smokes cigarettes, gets little exercise and is grossly obese, but even a person who strives to behave in a healthy manner has a probability of only a few per cent of living to age 100 under current health conditions. A century ago, the chance of becoming a centenarian was two orders of magnitude lower (Fig. 2). By contrast, half of the children alive today in countries with high life expectancies may celebrate their 100th birthday28. Personal behaviour is crucial in achieving a relatively long life compared with one’s contemporaries, but the general level of longevity in a population is determined by prosperity and medicine.
Further postponement of senescence in the future depends on progress in improving the health not only of older people but also of younger people, such that they reach old age in better condition. Much can be done on the basis of existing knowledge37. Public-health efforts to make home and outdoor environments safer and to reduce self-hazardous behaviour (cigarette smoking, excess alcohol consumption, obesity, lack of exercise and so on) will be important, as will efforts to provide high-quality health care to everyone. Even in egalitarian societies, lifespans vary considerably86: mortality patterns in 2008 in Sweden imply that most people will live past age 84 but that one in six will die before age 70.
To achieve life expectancies of 100 years or more, new knowledge will be needed37. Research advances suggest that cardiovascular diseases and malignant neoplasms will be prevented and treated much more effectively in the future. Two scourges of senescence are cognitive impairment, often due to Alzheimer’s disease, and sensory deprivation (Shakespeare’s “sans teeth, sans eyes, sans taste, sans everything”). Progress in overcoming these scourges would greatly enhance old age. Genetics research, both in humans45 and in non-human species50, will contribute to a deeper understanding of the mechanisms that cause senescence and may allow individualized medical treatment based on knowledge of individuals’ genomes. Research on dietary restriction64,65 and other non-genetic interventions might also lead to strategies for delaying human ageing. The nascent field of regenerative medicine shows great promise, and in the coming decades it might be possible to rejuvenate organs and tissues. Nanotechnologies also might have a role in postponing senescence.
Forecasts and consequences of longer lives
Demographers generally forecast future levels of mortality by extrapolating past trends23,87,88, occasionally making adjustments for likely changes in trends83. The future may bring disasters (for example epidemics, wars, economic crises or climate change) or breakthroughs (perhaps in taming senescence), but the past has also been characterized by disasters and breakthroughs. Over the past 170 years, in the countries with the highest life expectancies, these events neither decelerated nor accelerated the long-term increase in life expectancy, which proceeded at a pace of 2.5 years per decade, or six hours per day23.
In the early 1860s in Sweden, on average about three people each year (mainly women) celebrated their 100th birthday, whereas in 2007 more than 750 did so, a 250-fold increase. (Figure 2 shows the sharp increase in the number of Swedish female centenarians.) In the year 2107, 50,000 or 60,000 people, more than half the cohort born in 2007, might become centenarians, a further ~75-fold increase, benefiting children who are alive today28.
If it became possible to slow the rate at which death rates rise with age, then senescence would be substantially decelerated. Consider, for instance, remaining life expectancy at age 50. For contemporary Swedish females, halving the annual probability of death after age 50 would increase the expected age at death from 84 to 90. Halving the rate of ageing after age 50, however, would increase the expected age at death to 118. This prospect has encouraged some experts on ageing to advocate a “change in strategy”52 to augment research on diseases with a “systematic attack on ageing itself ”52. Although reductions in deaths from any specific disease will not radically increase life expectancy, advances against many diseases synergistically reinforce each other, especially because co-morbidity is so prevalent among the elderly. Disease-specific progress has propelled, and is likely to continue to propel23,37, increases in remaining lifespan in old age upwards by several months per year. Calls for a new strategy to “slow ageing by seven years”53 should acknowledge that mortality has already been postponed for even longer (Fig. 1) and that continued progress at the same pace as over the past few decades would delay death by another seven years in three or four decades.
If a young person knew that he or she would probably survive past age 100 and live 90 or 95 of those years with good cognitive and physical abilities, they would probably prefer to spend their life differently from how most people do today9,38,39. In many countries, especially countries with long life expectancies, people work hard at the ages when they could have children and spend time with them and then retire at ages when their children are no longer in need of daily care. Devoting two decades or more to education, the next three or four decades to trying to combine work and family, and then enduring leisure for a probable four decades might seem less desirable than spreading and mixing education, work, child rearing and leisure across more years of life.
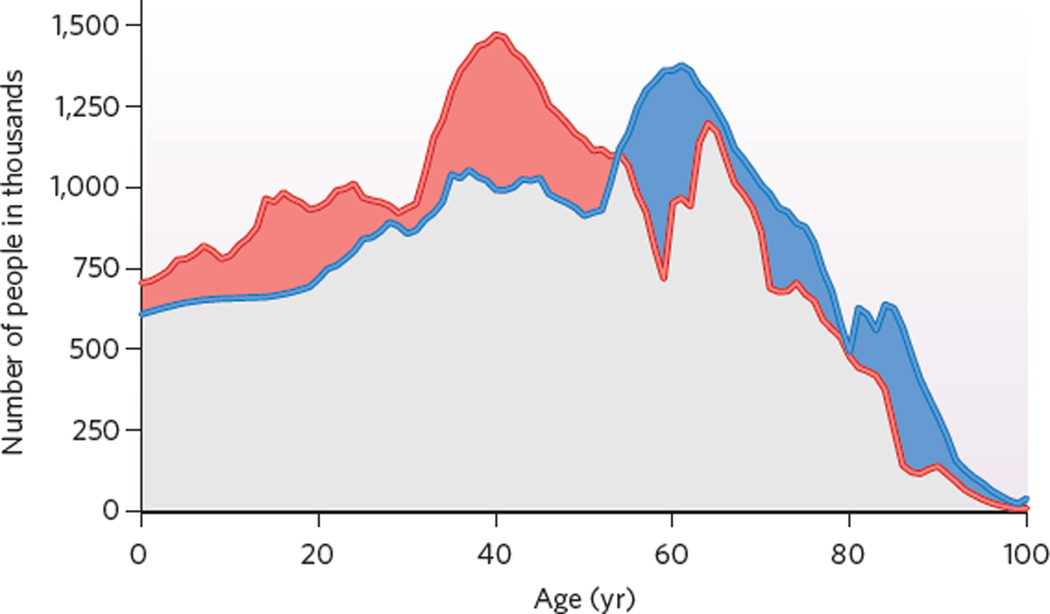
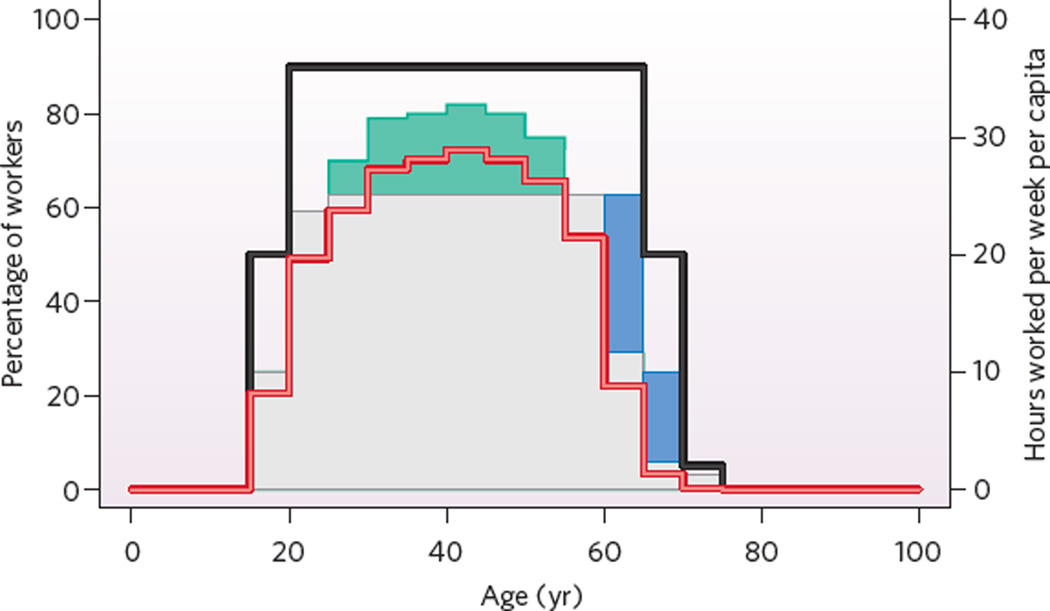
Figure 3 displays the population distribution of Germany in 2005 and the likely distribution in 2025. Figure 4 shows how many people work at various ages and how much they work. The patterns in Figs 3 and 4, which roughly reflect the situation in other rich countries, imply the surprising result that Germans work 16.3 hours per week per capita. If work effort remains at 2005 levels but the population distribution shifts to the 2025 pattern, then work per week per capita will fall to 14.9 hours. To maintain the output of the German economy, additional work effort has to be supplied, and policies seek to do so by increasing employment among people in their late 50s and early 60s. These policies are opposed, however, because people who have worked hard most of their lives resent having to work even longer.
Figure 3. The population of Germany by age.
The number of people in 2005 (red line) and 2025 (projected, blue line) by single year of age in Germany. Note that the projected population distribution in 2025 is similar to the distribution in 2005 but shifted 20 years to the right. The differences between the two distributions are mainly due to mortality; international immigration and emigration are relatively unimportant in Germany. The area coloured in red represents the loss of people younger than age 54 from 2005 to 2025; the area coloured in blue represents the gain of people older than 54. (Data from the Human Mortality Database (http://www.mortality.org) and the Coordinated Population Projection for Germany, German Statistical Office.)
Figure 4. Employment in Germany by age.
Proportion of people by age working in Germany in 2005 (red line) and hypothetically in 2025 (black line), and hours worked per week per worker in 2005 (grey–green area) and hypothetically in 2025 (grey–blue area). Under the hypothetical scenario, at most ages a greater proportion of people would work, as shown by the difference between the black and red lines. At ages under 55, the employed in 2025 would work fewer hours per week on average than those in 2005: they would not contribute the work denoted by the green area. At ages between 60 and 70, the employed in 2025 would work more hours per week on average than those in 2005: they would contribute the additional work effort denoted by the blue area. In the hypothetical scenario, people aged from 20 to 65 work an average of about 25 hours per week: the 90% who work contribute about 28 hours of effort on average. If one-tenth of the population between ages 20 and 65 does not work at all, then another one-tenth could work 40 hours per week, four-tenths could work 30 hours per week and the remaining four-tenths could work 20 hours per week to maintain the work output at 2005 levels. (Calculations based on data from the EU Labour Force Survey.)
A sensible alternative is to compensate younger people for working when they are older by allowing them to work fewer hours per week over the whole course of their lives. The total output of the German economy can be maintained if citizens work more years but correspondingly fewer hours per week. One option is depicted in Fig. 4. The twentieth century was a century of the redistribution of wealth; the twenty-first century will probably be a century of the redistribution of work39.
Perspectives
Before the discovery that senescence could be postponed, geriatric medicine was viewed as a laudable but rather futile effort to palliate the misery of those in the process of dying. Today, however, geriatrics is becoming a more attractive and increasingly important speciality. Similarly, research on ageing, once an observational backwater, is now an engaging, proactive science, with considerable funding from the US National Institute on Aging but much less support in other countries.
The demography of ageing has developed into one of the most exciting fields of demography89, with research on the biodemography of ageing56,89–91 and recently on the evolutionary biodemography of ageing60–62,91–93 emerging as innovative initiatives. The discipline of demography is based on a mathematical foundation, benefits from extensive data, is of interest to policy-makers and the public, and is linked with both the social sciences and the biological sciences, and it has had, and will continue to have, a major role in the deepening understanding of ageing. Dobzhansky asserted: “nothing in biology can be understood except in the light of evolution”94. It can similarly be asserted that nothing in evolution can be understood except in the light of demography (and vice versa), because evolution is driven by (and drives) the dynamics of fertility, mortality and migration of non-human species and, to a considerable extent, of humans as well. These dynamics are the core of demography95,96.
As the average length of life has increased, the variance in age at death has decreased86: the countries with the highest life expectancies are the countries with the smallest disparities in lifespan. Most people in richer countries and an increasing proportion of those in developing countries can look forward to long, mostly healthy lives. This is arguably the most significant achievement of modern civilization.
Although many policy-makers realize that the world’s population is ageing, the pace of this change and its social, economic and health-care consequences have not been adequately recognized. Perhaps because the discovery did not arise from laboratory experiments or clinical trials, most biomedical researchers do not fully appreciate — and some seem to be unaware of53 — the postponement of mortality. That senescence can be postponed and is being postponed should, however, guide and encourage gerontological research, geriatric practice and policy reform.
The millennia-long debate about whether survival could be extended among the elderly has had a remarkable resolution. At advanced age, death can be delayed not because the rate of increase of mortality with age is being slowed but because people are reaching very old age in better health. Slowing the pace of deterioration has proved intractable, at least until now. But another aspect of ageing, the level of health among older people, can be changed. Taken together, these findings are so perplexing that they can be dubbed the ‘longevity riddle’: why do the evolutionary forces that shaped human ageing provide a licence to alter the level of health but not the rate of debilitation? A related question is why humans differ substantially in their level of health at any age but not in their rate of ageing (Box 1). Research is under way to discover whether and how the rate of ageing can be slowed37,52,53. At least as significant could be research on why and how senescence has been postponed and how it could be further delayed37. Priorities include research on nona genarians, centenarians and supercentenarians, on male versus female health and survival, and on how improvements in health and reductions in mortality in the young and the middle aged influence health and mortality in old age. Research on the hypothesis advanced in this article — that the rate at which the chance of death increases with age for humans may be a basic biological constant, very similar and perhaps invariant across individuals and over time — would fundamentally contribute to our understanding of how and why we age.
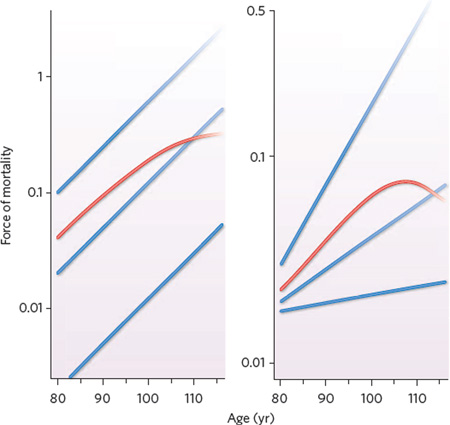
Box 1 | Dynamics of heterogeneous populations.
The two graphs shown here illustrate how the age pattern of mortality for a heterogeneous population (red) can be qualitatively different from the age patterns for the subpopulations that comprise it (blue).
The graph on the left depicts a population that consists of subpopulations with different levels of age-specific mortality but with the same constant rate of ageing (that is, pace of increase in mortality with age). The rate of ageing determines the slopes of the blue trajectories: the trajectories are straight lines because the vertical axis is on a logarithmic scale. The dying out of the subpopulations is indicated by the fading of the blue colour. The subpopulation with the highest level of mortality dies out first, leaving a population that consists of the second subpopulation and, increasingly, the third subpopulation. If there is a continuous range of subpopulations, with the three shown in blue representing illustrative cases, then the force of mortality for the population as a whole — the surviving population — can follow a trajectory similar to the one shown in red. Mortality in the population as a whole can level off at a constant value that reflects a balance between the increasing mortality of each subpopulation and the changing mix of subpopulations97–99.
The graph on the right is analogous, but the subpopulations differ in their rate of ageing; there are also differences in the initial level of mortality, at age 80. Regardless of the magnitude of the differences in the initial level of mortality, populations with subpopulations that age at different rates typically show an eventual decline in the force of mortality for the population as a whole, as indicated by the red curve98.
Mortality for humans seems to level off after age 110 (ref. 73), consistent with the graph on the left. Exceptionally long-lived people seem to reach advanced ages not because they senesce more gradually than others (as depicted by the lowest blue curve in the right-hand graph) but because they reach old age in a better state of health (as depicted in the lowest blue curve in the left-hand graph). Genetic defects that speed the process of ageing in people who suffer from segmental progeria have been found. But no mutation, at least in humans, has been discovered that decelerates the process, and it may be that no such mutation is prevalent in human populations. Hence, I propose the hypothesis that except for individuals with accelerated-ageing disorders, all older humans have a similar, and perhaps essentially the same, rate of increase in mortality with age. I believe that this should be tested.
This hypothesis is consistent with the finding that the process of senescence is being delayed rather than being decelerated. Longevity is increasing because the set of blue curves in the left-hand graph are shifting down or, equivalently, shifting to the right. There has not been a decline in the slope of the blue curves, such as is depicted in the right-hand graph.
Reference 100 provides an accessible introduction to the dynamics of heterogeneous populations.
Acknowledgements
I thank Z. Zhang, A. Baudisch, K. Christensen, G. Doblhammer, J. Haaga, B. Jeune, S. Leek, R. Rau, S. Scherneck, R. Suzman and N. Vaupel. My research is supported in part by the Max Planck Society and by the US National Institute on Aging (NIA P01-08761).
Footnotes
The author declares no competing financial interests.
References
- 1. Jeune B. Living longer—but better? Aging Clin. Exp. Res. 2002;14:72–92. doi: 10.1007/BF03324421. This engaging account, with 238 references, summarizes ideas and theories about longevity from Genesis and the Sumerian legend of Gilgamesh to the present.
- 2.Barnes J, editor. The Complete Works of Aristotle: The Revised Oxford Translation. Princeton Univ. Press; 1984. Aristotle; pp. 740–744. [Google Scholar]
- 3.Hygiene: De Sanitate Tuenda. Ch. 2. Vol. 1. Thomas; 1951. Galen. [Google Scholar]
- 4.Bacon R. In: The Code of Health and Longevity. Sinclair J, editor. Constable: 1806. [Google Scholar]
- 5.Cornaro L. The Art of Living Long. Springer; 2005. [Google Scholar]
- 6.Hamilton WD. The moulding of senescence by natural selection. J. Theor. Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- 7.Gruenberg EM. The failures of success. Milbank Q. 1977;55:3–24. [PubMed] [Google Scholar]
- 8.Fries JF. Aging, natural death, and the compression of morbidity. N. Engl. J. Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 9.Vaupel JW, Gowan AE. Passage to Methuselah: some demographic consequences of continued progress against mortality. Am. J. Public Health. 1986;76:430–433. doi: 10.2105/ajph.76.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guralnik JM, Yanagishita M, Schneider EL. Projecting the older population of the United States: lessons from the past and prospects for the future. Milbank Q. 1988;66:283–308. [PubMed] [Google Scholar]
- 11.Olshansky SJ, Carnes BA, Cassel C. In search of Methuselah: estimating the upper limits to human longevity. Science. 1990;250:634–640. doi: 10.1126/science.2237414. [DOI] [PubMed] [Google Scholar]
- 12.Manton KG, Stallard E, Tolley HD. Limits to human life expectancy: evidence, prospects, and implications. Popul. Dev. Rev. 1991;17:603–637. [Google Scholar]
- 13.Hayflick L. Biological aging is no longer an unsolved problem. Ann. NY Acad. Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- 14. Vaupel JW, Lundström H. In: Studies in the Economics of Aging. Wise DA, editor. Univ. Chicago Press; 1994. pp. 79–104. The discovery of the postponement of mortality, as demonstrated by four complementary analyses of a then-new Swedish data set, was first reported in this book chapter.
- 15.Kannisto V, Lauritsen J, Thatcher AR, Vaupel JW. Reductions in mortality at advanced ages: several decades of evidence from 27 countries. Popul. Dev. Rev. 1994;20:793–810. [Google Scholar]
- 16.Kannisto V. Development of Oldest-Old Mortality, 1950–1990: Evidence from 28 Developed Countries. Odense Univ. Press; 1996. [Google Scholar]
- 17.Kannisto V. The Advancing Frontier of Survival: Life Tables for Old Age. Odense Univ. Press; 1996. [Google Scholar]
- 18.Vaupel JW. The remarkable improvements in survival at older ages. Phil. Trans. R. Soc. Lond. B. 1997;352:1799–1804. doi: 10.1098/rstb.1997.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaupel JW, et al. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- 20.Tuljakurpar S, Li N, Boe C. A universal pattern of mortality decline in the G7 countries. Nature. 2000;405:789–792. doi: 10.1038/35015561. [DOI] [PubMed] [Google Scholar]
- 21.Wilmoth JR, Deegan LJ, Lundström H, Horiuchi S. Increase of maximum life-span in Sweden, 1861–1999. Science. 2000;289:2366–2368. doi: 10.1126/science.289.5488.2366. [DOI] [PubMed] [Google Scholar]
- 22.Kannisto V. Mode and dispersion of the length of life. Population. 2001;13:159–171. [Google Scholar]
- 23. Oeppen J, Vaupel JW. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. This article shows that life expectancy has increased by more than two years per decade since 1840 in the countries with highest life expectancies and that there is no imminent limit to further increases.
- 24.Cheung SLK, Robine J-M. Increase in common longevity and the compression of mortality: the case of Japan. Popul. Stud. (Camb.) 2007;61:85–97. doi: 10.1080/00324720601103833. [DOI] [PubMed] [Google Scholar]
- 25.Canudas-Romo V. The modal age at death and the shifting mortality hypothesis. Demogr. Res. 2008;19:1179–1204. [Google Scholar]
- 26.Rau R, Soroko E, Jasilionis D, Vaupel JW. Continued reductions in mortality at advanced ages. Popul. Dev. Rev. 2008;34:747–768. [Google Scholar]
- 27.Bongaarts J. Trends in senescent life expectancy. Popul. Stud. (Camb.) 2009;63:203–213. doi: 10.1080/00324720903165456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. This paper concludes, on the basis of a thorough review of research on mortality, morbidity and disability, that both survival and health are improving at higher ages.
- 29.Freedman VA, et al. Resolving inconsistencies in trends in old-age disability: report from a technical working group. Demography. 2004;41:417–441. doi: 10.1353/dem.2004.0022. [DOI] [PubMed] [Google Scholar]
- 30.Christensen K, McGue M, Petersen I, Jeune B, Vaupel JW. Exceptional longevity does not result in excessive levels of disability. Proc. Natl Acad. Sci. USA. 2008;105:13274–13279. doi: 10.1073/pnas.0804931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engberg H, Christensen K, Andersen-Ranberg K, Vaupel JW, Jeune B. Improving activities of daily living in Danish centenarians — but only in women: a comparative study of two birth cohorts born in 1895 and 1905. J. Gerontol. A. 2008;63:1186–1192. doi: 10.1093/gerona/63.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeune B, Brønnum-Hansen H. Trends in health expectancy at age 65 for various health indicators, 1987–2005, Denmark. Eur. J. Ageing. 2008;5:279–285. doi: 10.1007/s10433-008-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagger C, et al. Inequalities in healthy life years in the 25 countries of the European Union in 2005: a cross-national meta-regression analysis. Lancet. 2008;372:2124–2131. doi: 10.1016/S0140-6736(08)61594-9. [DOI] [PubMed] [Google Scholar]
- 34.Cutler DM, Wise DA, editors. Health at Older Ages: The Causes and Consequences of Declining Disability among the Elderly. Univ. Chicago Press; 2008. [Google Scholar]
- 35.Parker MG, Schön P, Lagergren M, Thorslund M. Functional ability in the elderly Swedish population from 1980 to 2005. Eur. J. Ageing. 2008;5:299–309. doi: 10.1007/s10433-008-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robine J-M, Saito Y. In: Frontiers of Japanese Demography. Goldstein JR, editor. Springer; in the press. [Google Scholar]
- 37. Sierra F, Hadley E, Suzman R, Hodes R. Prospects for life span extension. Annu. Rev. Med. 2009;60:457–469. doi: 10.1146/annurev.med.60.061607.220533. This comprehensive overview of an extensive literature provides a balanced account of possible advances in research on health and survival at higher ages and of potential challenges.
- 38.Lee RD, Goldstein JR. In: Life Span: Evolutionary, Ecological, and Demographic Perspectives. Carey JR, Tuljapurkar S, editors. Population Council; 2003. pp. 183–207. [Google Scholar]
- 39.Vaupel JW, Loichinger E. Redistributing work in aging Europe. Science. 2006;312:1911–1913. doi: 10.1126/science.1127487. [DOI] [PubMed] [Google Scholar]
- 40.Holmes OW. Over the Teacups. Vol. 181. Houghton Mifflin: 1890. [Google Scholar]
- 41.McGue M, Vaupel JW, Holm NV, Harvald B. Longevity is moderately heritable in a sample of Danish twins born 1870–1880. J. Gerontol. A. 1993;48:B237–B244. doi: 10.1093/geronj/48.6.b237. [DOI] [PubMed] [Google Scholar]
- 42.Herskind AM, et al. Untangling genetic influences on smoking, body mass index and longevity: a multivariate study of 2464 Danish twins followed for 28 years. Hum. Genet. 1996;98:467–475. doi: 10.1007/s004390050241. [DOI] [PubMed] [Google Scholar]
- 43.Perls TT, et al. Life-long sustained mortality advantage of siblings of centenarians. Proc. Natl Acad. Sci. USA. 2002;99:8442–8447. doi: 10.1073/pnas.122587599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hjelmborg JvB, et al. Genetic influences on human lifespan and longevity. Hum. Genet. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 45.Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nature Rev. Genet. 2006;7:436–448. doi: 10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riley JC. Rising Life Expectancy: A Global History. Cambridge Univ. Press; 2001. [Google Scholar]
- 47.Gerdes LU, Jeune B, Andersen-Ranberg K, Nybo H, Vaupel JW. Estimation of apolipoprotein E genotype-specific relative mortality risks from the distribution of genotypes in centenarians and middle-aged men: apolipoprotein E gene is a ‘frailty gene’, not a ‘longevity gene’. Genet. Epidemiol. 2000;19:202–210. doi: 10.1002/1098-2272(200010)19:3<202::AID-GEPI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 48.Ewbank DC. Differences in the association between apolipoprotein E genotype and mortality across populations. J. Gerontol. A. 2007;62:899–907. doi: 10.1093/gerona/62.8.899. [DOI] [PubMed] [Google Scholar]
- 49.Curtsinger JW, Fukui HH, Townsend DR, Vaupel JW. Demography of genotypes: failure of the limited life-span paradigm in Drosophila melanogaster. Science. 1992;258:461–463. doi: 10.1126/science.1411541. [DOI] [PubMed] [Google Scholar]
- 50.Tissenbaum HA, Johnson TE. In: Molecular Biology of Aging. Guarente L, Partridge L, Wallace DC, editors. Cold Spring Harbor Laboratory Press; 2008. pp. 153–183. [Google Scholar]
- 51.Johnson TE. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249:908–912. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- 52.Butler RN, et al. New model for health promotion and disease prevention for the 21st century. Br. Med. J. 2008;337:149–150. doi: 10.1136/bmj.a399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farrelly C. Has the time come to take on time itself? Br. Med. J. 2008;337:148–149. [Google Scholar]
- 54.Finch CE. Longevity, Senescence and the Genome. Univ. Chicago Press; 1990. [Google Scholar]
- 55.Rose MR. Evolutionary Biology of Aging. Oxford Univ. Press; 1994. [Google Scholar]
- 56.Wachter KW, Finch CE, editors. Between Zeus & the Salmon. US Natl Acad. Press; 1997. [PubMed] [Google Scholar]
- 57.Kirkwood TBL. Time of Our Lives: The Science of Human Aging. Oxford Univ. Press; 1999. [Google Scholar]
- 58.Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- 59.Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120:437–443. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 60.Vaupel JW, Baudisch A, Dölling M, Roach DA, Gampe J. The case for negative senescence. Theor. Popul. Biol. 2004;65:339–351. doi: 10.1016/j.tpb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Baudisch A. Hamilton’s indicators of the force of selection. Proc. Natl Acad. Sci. USA. 2005;102:8263–8268. doi: 10.1073/pnas.0502155102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baudisch A. Inevitable Aging? Contributions to Evolutionary-Demographic Theory. Springer; 2008. This pioneering work uses models based on evolutionary theory and demographic concepts to demonstrate that senescence is not inevitable for all species.
- 63.Vaupel JW, Jeune B. In: Exceptional Longevity: From Prehistory to the Present. Jeune B, Vaupel JW, editors. Odense Univ. Press; 1995. pp. 109–116. [Google Scholar]
- 64.Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- 65.Sohal RS, Weindurch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perls TT, Silver MH, Lauerman JF. Living to 100. Perseus; 1999. [Google Scholar]
- 67.Carey JR, Liedo P, Orozco D, Vaupel JW. Slowing of mortality rates at older ages in large medfly cohorts. Science. 1992;258:457–461. doi: 10.1126/science.1411540. [DOI] [PubMed] [Google Scholar]
- 68.Doblhammer G, Oeppen J. Reproduction and longevity among the British peerage. Proc. R. Soc. Lond. B. 2003;270:1541–1547. doi: 10.1098/rspb.2003.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality; sex differences in health and aging. Aging Clin. Exp. Res. 2008;20:91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Austad S. Why women live longer than men. Gend. Med. 2006;3:79–92. doi: 10.1016/s1550-8579(06)80198-1. [DOI] [PubMed] [Google Scholar]
- 71.Bonduriansky R, Maklakov A, Zajitschek F, Brooks R. Sexual selection, sexual conflict and the evolution of ageing and life span. Funct. Ecol. 2008;22:443–453. [Google Scholar]
- 72.Olsen TS, Dehlendorff C, Andersen KK. The female stroke survival advantage: relation to age. Neuroepidemiology. 2009;32:47–52. doi: 10.1159/000170906. [DOI] [PubMed] [Google Scholar]
- 73.Maier H, Gampe J, Jeune B, Robine J-M, Vaupel JW, editors. Supercentenarians. Springer; in the press. [Google Scholar]
- 74.Jeune B, Vaupel JW, editors. Validation of Exceptional Longevity. Odense Univ. Press; 1999. [Google Scholar]
- 75.Preston SH. The changing relation between mortality and level of economic development. Popul. Stud. (Camb.) 1975;29:231–248. [PubMed] [Google Scholar]
- 76.Rogers RG, Hummer RA, Nam CB. Living and Dying in the USA: Behavioral, Health, and Social Differentials of Adult Mortality. Academic; 2000. [Google Scholar]
- 77.Crimmins EM, Preston SH, editors. Divergent Trends in Life Expectancy. National Research Council; in the press. [Google Scholar]
- 78.Barker DJP, Bergmann RL, Ogra PL, editors. The Window of Opportunity: Pre-Pregnancy to 24 Months of Age. Karger; 2008. [DOI] [PubMed] [Google Scholar]
- 79.Doblhammer G. The Late Life Legacy of Very Early Life. Springer; 2004. [Google Scholar]
- 80.Moore SE, Cole TJ, Poskitt EME, Sonko BJ. Season of birth predicts mortality in rural Gambia. Nature. 1997;388:434–435. doi: 10.1038/41245. [DOI] [PubMed] [Google Scholar]
- 81.Kannisto V, Christensen K, Vaupel JW. No increased mortality in later life for cohorts born during famine. Am. J. Epidemiol. 1997;145:987–994. doi: 10.1093/oxfordjournals.aje.a009067. [DOI] [PubMed] [Google Scholar]
- 82.Christensen K, Vaupel JW, Holm NV, Yashin AI. Mortality among twins after age 6: fetal origins hypothesis versus twin method. Br. Med. J. 1995;310:432–436. doi: 10.1136/bmj.310.6977.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H, Preston SH. Forecasting United States mortality using cohort smoking histories. Proc. Natl Acad. Sci. USA. 2009;106:393–398. doi: 10.1073/pnas.0811809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaupel JW, Wang Z, Andreev KF, Yashin AI. Population Data at a Glance: Shaded Contour Maps of Demographic Surfaces over Age and Time. Odense Univ. Press; 1997. p. 22. 39; 52; 54; 59–63. [Google Scholar]
- 85.Vaupel JW, Carey JR, Christensen K. It’s never too late. Science. 2003;301:1679–1681. doi: 10.1126/science.1090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edwards RD, Tuljapurkar S. Inequality in life spans and a new perspective on mortality convergence across industrialized countries. Popul. Dev. Rev. 2005;31:645–675. [Google Scholar]
- 87.Wilmoth JR. The future of human longevity: a demographer’s perspective. Science. 1998;280:395–397. doi: 10.1126/science.280.5362.395. [DOI] [PubMed] [Google Scholar]
- 88.Alho JM, Spencer BD. Statistical Demography and Forecasting. Springer; 2005. [Google Scholar]
- 89.Haaga JG, et al. What’s next for the demography of aging? Popul. Dev. Rev. 2009;35:323–365. [Google Scholar]
- 90.Carey JR, Vaupel JW. In: Handbook of Population. Poston DL Jr, Micklin M, editors. Springer; 2005. pp. 625–658. [Google Scholar]
- 91.Carey JR, Tuljapurkar S, editors. Life Span: Evolutionary, Ecological, and Demographic Perspectives. Population Council; 2003. [Google Scholar]
- 92.Chu CYC, Chien H-K, Lee RD. Explaining the optimality of U-shaped age-specific mortality. Theor. Popul. Biol. 2008;73:171–180. doi: 10.1016/j.tpb.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steinsaltz DR, Evans SN, Wachter KW. A generalized model of mutation-selection balance with applications to aging. Adv. Appl. Math. 2005;35:16–33. [Google Scholar]
- 94.Dobzhansky T. Nothing in biology makes sense except in the light of evolution. Am. Biol. Teach. 1973;35:125–129. [Google Scholar]
- 95.Preston SH, Heuveline P, Guillot M. Demography: Measuring and Modeling Population Processes. Blackwell; 2000. [Google Scholar]
- 96.Caselli G, Vallin J, Wunsch G. Demography: Analysis and Synthesis. Vols 1–4. Academic; 2006. [Google Scholar]
- 97.Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- 98.Finkelstein M, Esaulova V. Asymptotic behavior of a general class of mixture failure rates. Adv. Appl. Probab. 2006;38:244–262. [Google Scholar]
- 99.Steinsaltz DR, Wachter KW. Understanding mortality rate deceleration and heterogeneity. Math. Popul. Stud. 2006;13:19–37. [Google Scholar]
- 100.Vaupel JW, Yashin AI. Heterogeneity’s ruses. Am. Statist. 1985;39:176–185. [PubMed] [Google Scholar]