Abstract
Currently, filtration surgery has been considered as the most effective therapy for glaucoma; however, the scar formation in the surgical area may often lead to failure to the procedure. An implanted drug delivery system may provide localized and sustained release of a drug over an extended period. Poly (ethylene glycol)-poly (ε-caprolactone)-poly (ethylene glycol) (PEG-PCL-PEG, PECE) hydrogel has been successfully synthesized and determined as thermosensitive and biocompatible. In order to overcome the limitations of common local ophthalmic medications, we investigated the function of a self-assembled PECE hydrogel as an intracameral injection-implanted drug carrier to inhibit the formation of postoperative scarring. Following bevacizumal-loaded hydrogel intracameral injection into rabbit eyes, the status of the bleb and filtration fistula formed following the filtering surgery were examined through pathologic evaluation. Due to the sustained release of bevacizumab from the hydrogel, neovascularization and scar formation were inhibited; moreover, there were no corneal abnormalities and other ocular tissue damage found in the rabbits. This suggests that the PECE hydrogel may be considered as the novel biomaterial with potential as a sustained release system in glaucoma filtering surgery. Further studies require in shedding the light on the subject.
Key Words: PEG-PCL-PEG, PECE Hydrogel, Ophthalmic Delivery System, Glaucoma Filtration Surgery, Filtration Fistula
INTRODUCTION
Glaucoma is one of the major causes of irreversible blindness in the world and is usually associated with increased intraocular pressure (IOP) (1,2). Glaucoma filtration surgery is performed by creating a fistula between the subconjunctival space and the anterior chamber to allow the drainage of aqueous humor into the filtering bleb, resulting in lowering the IOP (3). Modulation of wound healing is critical to ensure successful surgery. Unfortunately, glaucoma filtration surgery fails in 30–50% of patients because of fibroblast proliferation and collagen deposition at the site of the filtration bleb, preventing further drainage of aqueous humor (4). There are many ophthalmic drug delivery systems that can be used following filtration surgery. Conventional delivery systems such as eye drops, ointments, or suspensions are the most preferred routes of administration to the anterior segment of the eye. The bioavailability of topical instillation of drugs is, however, usually low since the absorption of eye drops is severely limited by the relatively impermeable cornea. Additionally, multiple subconjunctival and intracameral injections of antiscarring drugs following glaucoma filtering surgery are often required to maintain a high drug concentration (5). Moreover, not only are the frequent injections inconvenient and uncomfortable for the patient, but these agents can also cause cell apoptosis and death. This injection process is usually associated with several undesirable complications such as corneal epithelial defects, cataracts, endophthalmitis and other ocular diseases (6). Frequent instillation of concentrated solutions is required to achieve the desired therapeutic effects. There are few satisfactory treatments due to the lack of an ideal drug delivery system. We may need a novel and promising ophthalmic drug delivery system for these diseases. As it is a new biomaterial, a biodegradable poly (PEG-PCL-PEG, PECE) triblock copolymer was successfully synthesized, resulting in a flowing solution at low temperatures that turned into a nonflowing gel at body temperature (1). The PECE hydrogel applied through intracameral injection could be a novel vector for an ophthalmic drug delivery system and gene therapy. Bevacizumab (Avastin), a synthetic monoclonal antibody used against vascular endothelial growth factor (anti-VEGF), was reported to limit scar formation following glaucoma filtration surgery and to effectively decrease patients’ IOP (7,8). Several reports have described the successful, off-label intravitreal use of bevacizumab for treatment of neovascularization associated with proliferative diabetic retinopathy, age-related macular degeneration, and neovascular glaucoma (9,10). Furthermore, bevacizumab may be a beneficial agent for limiting scar tissue formation and thereby improving success following filtration surgery (11).
Is the hydrogel an ideal drug delivery system?
It is obvious that the inhibition of scar tissue formation is limited while the bevacizumab is directly administrated through intracameral injection and subsequently rapidly eliminated from the body. Therefore, a suitable container allowing sustained release of bevacizumab is needed. In clinical application, ocular anterior segment implanted biomaterials should ideally meet the following qualities: (a) the implanted materials should be injectable and the process of implantation simple, moreover, they should have certain viscoelasticity and keep flowing in the ocular anterior segment; (b) the implanted materials should be non-immunogenic to not cause inflammation and foreign body adverse reactions; (c) the implanted materials should completely degrade over a certain time and the materials from the degradation process can be an effectively controlled drug release into the anterior chamber to cure ocular diseases.
To date, various drug delivery devices such as biodegradable amphiphilic block copolymers have been synthesized and applied as drug delivery systems (12-14). For local sustainable release of therapeutic agents, hydrogels are the preferred choice due to their good biodegradability, biocompatibility, huge drug capacity and response to the physical/chemical stimulus (15,16). Hydrogels have been studied extensively in various applications such as medical devices and drug delivery system (17,18). The gelation of hydrogel in the body may lower the rate of diffusion of the entrapped drug following being loaded with therapeutic agents, which enhances the drug retention and bioavailability (19). Furthermore, injectable hydrogels would be in a soluble form prior administration in the body, however once administered, undergo gelation in situ to form a gel. The porous structure of hydrogels makes them perfect carriers for therapeutic macromolecules, such as protein, RNA or DNA. Hydrogel can also be used as a nonviral gene delivery vehicle. It is reported that supercoiled pDNA entrapped in hydrogel expressed maximally at one day and lasted for three days (20,21). For the macrostructure of bevacizumab, the hydrogel systems are suitable carriers for its sustainable delivery. Additionally, because of its thermoresponsive characteristic, the hydrogel can be injected in a liquid form to the juxtascleral region or the vitreous cavity via a small-gauge needle. This system is designed to optimize the antiangiogenic effects and minimize the potential ectopic effects of a large bolus delivery (22). Thermosensitive hydrogels therefore have great potential in the topical delivery of bevacizumab.
Recently, a series of block copolymers consisting of PEG and PCL or PLA, which can form micelles by self-assembling, or thermosensitive hydrogels have been widely reported (23,24). Based on the PEG as the hydrophilic segment, and PCL or PLA as hydrophobic segment, different kinds of hydrogels can be formed that include PCEC hydrogel, PECE hydrogel, PLA-PEG-PLA hydrogel and PEG-PLA hydrogel. Among these, PECE hydrogel contains the basic components of PEG and PCL, which are biocompatible and have been used in several Food and Drug Administration-approved products. For instance, the in vivo gel formation and degradation behavior were noted by subcutaneously injecting aqueous PECE solution into mice. The PECE hydrogel was also an effective, safe, and convenient agent for preventing post-surgical intra-abdominal adhesions, and was proved to be safe in BALB/c mice in vivo by acute toxicity testing (25,26). Consequently, the PECE hydrogel is believed to be promising for in situ gel-forming controlled drug delivery systems that are injectable flowing solutions at low temperature but that turn into gel at body temperature in vivo (27). The good sustained release properties of hydrogels have been confirmed in vitro. The sol–gel–sol transition temperature range could be varied, and the prepared PECE hydrogels have proved to be thermosensitive, biocompatible, and bioabsorbable (28). With further consideration of the loading and releasing properties of PECE hydrogel to small molecules, such as HK, curcumin, DOX, cisplatin and so forth, it is rational to predict that this hydrogel will exhibit excellent performance in the topical delivery of bevacizumab. We have hypothesized that it could be delivered in a liquid form, which is much more convenient and also temperature-sensitive, then it could form a gel in situ and release drugs continuously after intraocular injection or instillation. Furthermore, ocular tissues could absorb the PECE hydrogel completely without any permanent damage. PECE hydrogel may be an implanted drug delivery system for the inhibition of postoperative scarring formation.
METHODS
The PECE hydrogel has great biocompatibility, biodegradability, and sustained release properties in the eye and it is hoped it will be a creative and promising ophthalmic drug delivery system in the future. So we speculate that PECE hydrogel through intracameral injection is as a novel potential, in situ, sustained ophthalmic drug delivery system that can be applied to prevent scarring formation after trabeculectomy. However, the toxicity of PECE hydrogel was concentration-dependent according to our pilot study with different concentrations (5 wt %, 10 wt %, 15 wt % and 20 wt %). Endothelia of rabbits were treated with 5 wt %, 10 wt% and 15 wt% PECE hydrogel seemed regular. However, 5 wt% and 10 wt% PECE hydrogel were not conducive to sustained release, because they were degraded more early than 15 wt% PECE hydrogel. In addition, 20 wt% PECE hydrogel could result in endothelial injury more severely than 15 wt% PECE hydrogel. We eventually found that the slight toxicity of 15 wt% PECE hydrogel is reasonable.
PECE triblock copolymer was dissolved in a balanced salt solution (BSS) at a designated temperature and at the concentration of 15 wt% to form PECE hydrogels; these PECE hydrogels were then kept at 4° C before being used (28). In the self-assembly process hydrogel can be directly loaded with appropriate drugs. We loaded bevacizumab into the hydrogel, building a bevacizumab controlled-release system, and applied this kind of controlled-release system following filtration surgery through intracameral injections into rabbit eyes. Bleb survival and characteristics were evaluated over a 28-day period. The rabbits were sacrificed with an overdose of pentobarbital on the 28th day. Histology of the surgical eyes was performed to evaluate and grade the amount of scarring and fibrosis in each group.
RESULTS
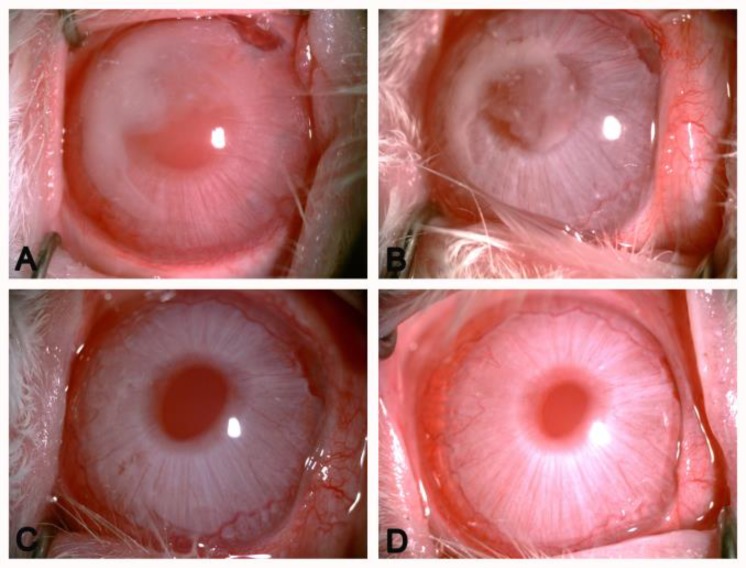
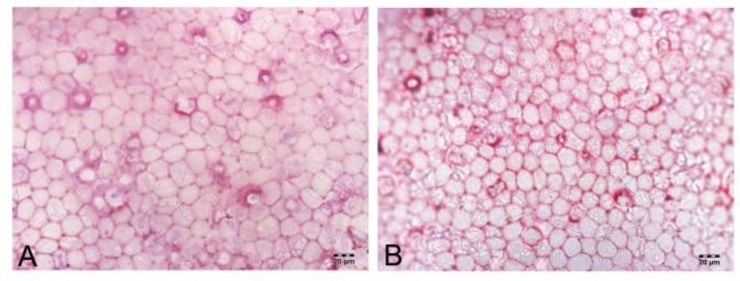
The rabbits’ eyes were examined carefully to detect the toxic effect of PECE hydrogel on ocular tissues and to evaluate the biocompatibility of novel hydrogel following injection into the rabbits’ anterior chambers. In the whole experimental process, the rabbits’ anterior chambers that were injected PECE hydrogels did not show any corneal abnormalities, cataract, inflammatory signs, or other ocular disorders by slit-lamp biomicroscopy and indirect ophthalmoscopy. Bevacizumab could be administered slowly, smoothly and efficiently by release from a hydrogel controlled-release system. In the anterior chamber, PECE hydrogel on the corneal endothelia burst into fragments on contact with the aqueous humor because of hydrolysis. Hydrogel was absorbed completely within 3 weeks (Figure 1). These phenomena indicated water gel injected in rabbit eyes with good biocompatibility. We also tested the high IOP of eyes that had been injected with bevacizumab-loaded hydrogel and found that the anterior chamber injected bevacizumab-loaded hydrogel maintained relatively lower IOP, compared with the Bevacizumab group and BSS group following trabeculectomy (Table 1). We speculated that following a bevacizumab-loaded hydrogel injection into the anterior chamber of rabbits, the main degradation products, as a single amino acid, had non-toxic side effects on the trabecular meshwork cells in rabbit eyes. In addition, there was a period of time for hydrogel degradation, so PECE hydrogel released bevacizumab constantly to encourage antiscar formation and control IOP in rabbit eyes. We will evaluate this hypothesis in the future since this study has been performed as a pilot report. Finally, the pathological tissue slices of the injected eyes, including the cornea, lens, iris, ciliary body and retina and found there were no tissue anomalies. The structure of each layer of the above tissues showed no infiltration by inflammatory cells or obvious irritations to rabbit eyes (data not shown). Dual staining of corneal endothelium with Typan blue and Alizarin red was also performed. The rabbits’ endothelial cells were nearly a regular hexagon shape and intact following the bevacizumab-loaded hydrogel intracameral injection (Figure 2). Repeated measures ANOVA was analyzed (F=10.135, P=0.012), suggesting that the difference between the three groups was statistically significant. Then we found there was no significant difference between BSS group and Bevacizumab group, while a significant decrease in IOP was found in the Bevacizumab-loaded hydrogel group compared with the BSS group or Bevacizumab group (P <0.01 and P <0.05, respectively) (n=3 rabbit in each group).
Figure 1.
In vivo gel formation of PECE hydrogel in the rabbit anterior chamber. PECE was absorbed completely within 3 weeks. A: 1d after injection. B: 7d after injection. C: 14d after injection D: 21d after injection (× 40 magnification).
Table 1.
Preoprative and postoperative intraocular pressures following the filtration surgery (mmHg).
| Group | Preoperative IOP |
Postoperative IOP
|
|||||
|---|---|---|---|---|---|---|---|
| 1(d) | 3(d) | 7(d) | 14(d) | 21(d) | 28(d) | ||
| BSS Group | 16.997±0.335 | 14.773±0.836 | 15.000±0.000 | 14.773±0.510 | 14.887±0.196 | 15.443±0.510 | 15.220±0.191 |
| Bevacizumab Group | 17.220±0.381 | 15.443±0.510 | 15.443±0.510 | 14.997±0.335 | 14.110±0.191 | 13.553±0.387 | 13.777±0.387 |
| Bevacizumab-loaded Hydrogel Group | 16.667±0.557 | 15.553±0.508 | 14.887±0.196 | 14.220±0.191 | 13.443±0.510 | 13.110±0.840 | 12.997±0.335 |
Figure 2.
Combined staining of rabbit endothelia with Typan blue and alizarin red after PECE injection into anterior chamber. Staining of intercellular borders with alizarin red showed the mosaic pattern of normal cells and low proportion of dead cells. A: control eye without treatment B: PECE-bevacizumab injection eye (×400 magnification)
DISSCUSSION
To assess whether these PECE hydrogel applications through intracameral injection can be a sustained and secure ophthalmic drug delivery system in clinical practice, further studies on toxicity evaluation could be conducted. More evidence on the ocular drug delivery nanocarriers with antiscarring drugs following glaucoma filtration surgery will support our hypothesis. Hydrophilic agents can easily mix with hydrogel and form sustained release medications. Intracameral injection of hydrogel at relatively low concentration has little effect on cell viability, IOP and histopathology of ocular tissues in toxicity evaluation (28). This is especially useful for antiscarring treatment after glaucoma filtration surgery in clinical practice. Although, there are many anatomical differences between rabbit and human eyes (29), the overall pattern of wound healing is similar to scars found in humans (30). As a result, intracameral injection of drug-loaded hydrogel could avoid the need for frequent subconjunctival injections and decrease the toxic ocular side effects caused by intraoperative topical applications. This approach should be further investigated.
CONCLUSION
The results indicated that PECE hydrogel is of good biodegradability and biocompatibility when injected into the anterior chamber of rabbits. The drug-loaded hydrogels provide a great opportunity to increase the therapeutic efficacy of glaucoma filtration surgery. This technique not only avoids frequent subconjunctival or intracameral injection of drugs given thereby significantly reducing the side effects of drugs on the surrounding normal tissue, but can also control the filtration postoperative IOP and inhibit filtration postoperative scarring and neovascularization. These findings indicate its potential application in ophthalmology as an implantable drug delivery system for the treatment to ocular anterior segment diseases in the future.
DISCLOSURE
The authors report no conflicts of interest in this work. This work was supported by the National Nature Science Foundation of China (No. 30901658) and International Science & Technology Cooperation Program of China (No. 2013DFG52300). Dr. Ribo Peng and Dr. Gang Qin contributed equally to this work and are to be regarded as equivalent authors.
References
- 1.Gong CY, Wu QJ, Dong PW, Shi S, Fu SZ, Guo G, Hu HZ, Zhao X, Wei YQ, Qian ZY. Acute toxicity evaluation of biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PEG-PCL-PEG hydrogel. J Biomed Mater Res B Appl Biomater. 2009 Oct;91(1):26–36. doi: 10.1002/jbm.b.31370. PMID: 19365823. [DOI] [PubMed] [Google Scholar]
- 2.Volotinen M, Mäenpää J, Kautiainen H, Tolonen A, Uusitalo J, Ropo A, Vapaatalo H, Aine E. Ophthalmic timolol in a hydrogel vehicle leads to minor inter-individual variation in timolol concentration in aqueous humor. Eur J Pharm Sci. 2009 Feb;36(2-3):292–6. doi: 10.1016/j.ejps.2008.10.004. PMID: 19013521. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006 Mar;90(3):262–7. doi: 10.1136/bjo.2005.081224. PMID: 16488940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Van Bergen T, Van de Veire S, Van de Vel I, Moreau H, Dewerchin M, Maudgal PC, Zeyen T, Spileers W, Moons L, Stalmans I. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009 Nov;50(11):5217–25. doi: 10.1167/iovs.08-2662. PMID: 19474408. [DOI] [PubMed] [Google Scholar]
- 5.Lattanzio FA Jr, Sheppard JD Jr, Allen RC, Baynham S, Samuel P, Samudre S. Do injections of 5-fluorouracil after trabeculectomy have toxic effects on the anterior segment? J Ocul Pharmacol Ther. 2005 Jun;21(3):223–35. doi: 10.1089/jop.2005.21.223. PMID: 15969640. [DOI] [PubMed] [Google Scholar]
- 6.Cui LJ, Sun NX, Li XH, Huang J, Yang JG. Subconjunctival sustained release 5-fluorouracil for glaucoma filtration surgery. Acta Pharmacol Sin. 2008 Sep;29(9):1021–8. doi: 10.1111/j.1745-7254.2008.00833.x. PMID: 18718170. [DOI] [PubMed] [Google Scholar]
- 7.Horsley MB, Kahook MY. Anti-VEGF therapy for glaucoma. Curr Opin Ophthalmol. 2010 Mar;21(2):112–7. doi: 10.1097/ICU.0b013e3283360aad. PMID: 20040875. [DOI] [PubMed] [Google Scholar]
- 8.Grewal DS, Jain R, Kumar H, Grewal SP. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy a pilot study. Ophthalmology. 2008 Dec;115(12):2141–2145. doi: 10.1016/j.ophtha.2008.06.009. e2. PMID: 18692246. [DOI] [PubMed] [Google Scholar]
- 9.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006 Mar;113(3):363–372. doi: 10.1016/j.ophtha.2005.11.019. e5. PMID: 16458968. [DOI] [PubMed] [Google Scholar]
- 10.Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (Avastin) injection. Retina. 2006 Mar;26(3):354–6. doi: 10.1097/00006982-200603000-00017. PMID: 16508439. [DOI] [PubMed] [Google Scholar]
- 11.Memarzadeh F, Varma R, Lin LT, Parikh JG, Dustin L, Alcaraz A, Eliott D. Postoperative use of bevacizumab as an antifibrotic agent in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3233–7. doi: 10.1167/iovs.08-2441. PMID: 19182254. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006 Jun 6;315(1-2):12–7. doi: 10.1016/j.ijpharm.2006.01.029. PMID: 16616442. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Griffith M, Li F. Alginate microsphere-collagen composite hydrogel for ocular drug delivery and implantation. J Mater Sci Mater Med. 2008 Nov;19(11):3365–71. doi: 10.1007/s10856-008-3486-2. PMID:18545941. [DOI] [PubMed] [Google Scholar]
- 14.Peng CL, Shieh MJ, Tsai MH, Chang CC, Lai PS. Self-assembled star-shaped chlorin-core poly(epsilon-caprolactone)-poly(ethylene glycol) diblock copolymer micelles for dual chemo-photodynamic therapies. Biomaterials. 2008 Sep;29(26):3599–608. doi: 10.1016/j.biomaterials.2008.05.018. PMID: 18572240. [DOI] [PubMed] [Google Scholar]
- 15.Miyata T, Asami N, Uragami T. A reversibly antigen-responsive hydrogel. Nature. 1999 Jun;399(6738):766–9. doi: 10.1038/21619. PMID: 10391240. [DOI] [PubMed] [Google Scholar]
- 16.Chao GT, Qian ZY, Huang MJ, Kan B, Gu YC, Gong CY, Yang JL, Wang K, Dai M, Li XY, Gou ML, Tu MJ, Wei YQ. Synthesis, characterization, and hydrolytic degradation behavior of a novel biodegradable pH-sensitive hydrogel based on polycaprolactone, methacrylic acid, and poly (ethylene glycol) J Biomed Mater Res A. 2008 Apr;85(1):36–46. doi: 10.1002/jbm.a.31362. PMID: 17688254. [DOI] [PubMed] [Google Scholar]
- 17.Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD, Leroux JC, Atkinson BL, Binette F, Selmani A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000 Nov;21(21):2155–61. doi: 10.1016/s0142-9612(00)00116-2. PMID: 10985488. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Parsons SP, Huizinga JD. Measurement of intracellular chloride ion concentration in ICC in situ and in explant culture. Neurogastroenterol Motil. 2010 Jun;22(6):704–9. doi: 10.1111/j.1365-2982.2010.01501.x. PMID: 20403100. [DOI] [PubMed] [Google Scholar]
- 19.Chitkara D, Shikanov A, Kumar N, Domb AJ. Biodegradable injectable in situ depot-forming drug delivery systems. Macromol Biosci. 2006 Dec;6(12):977–90. doi: 10.1002/mabi.200600129. PMID: 17128422. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Ning W, Wang J, Choi A, Lee PY, Tyagi P, Huang L. Controlled gene delivery system based on thermosensitive biodegradable hydrogel. Pharm Res. 2003 Jun;20(6):884–8. doi: 10.1023/a:1023887203111. PMID: 12817892. [DOI] [PubMed] [Google Scholar]
- 21.Lee PY, Li Z, Huang L. Thermosensitive hydrogel as a Tgf-beta1 gene delivery vehicle enhances diabetic wound healing. Pharm Res. 2003 Dec;20(12):1995–2000. doi: 10.1023/b:pham.0000008048.58777.da. PMID: 14725365. [DOI] [PubMed] [Google Scholar]
- 22.Kang Derwent JJ, Mieler WF. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans Am Ophthalmol Soc. 2008;106:206–13. discussion 213-4. PMID: 19277236. [PMC free article] [PubMed] [Google Scholar]
- 23.Kissel T, Li Y, Unger F. ABA-triblock copolymers from biodegradable polyester A-blocks and hydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery systems for proteins. Adv Drug Deliv Rev. 2002 Jan;54(1):99–134. doi: 10.1016/s0169-409x(01)00244-7. PMID: 11755708. [DOI] [PubMed] [Google Scholar]
- 24.Liu CB, Gong CY, Huang MJ, Wang JW, Pan YF, Zhang YD, Li GZ, Gou ML, Wang K, Tu MJ, Wei YQ, Qian ZY. Thermoreversible gel-sol behavior of biodegradable PCL-PEG-PCL triblock copolymer in aqueous solutions. J Biomed Mater Res B Appl Biomater. 2008 Jan;84(1):165–75. doi: 10.1002/jbm.b.30858. PMID: 17455282. [DOI] [PubMed] [Google Scholar]
- 25.Yang B, Gong C, Qian Z, Zhao X, Li Z, Qi X, Zhou S, Zhong Q, Luo F, Wei Y. Prevention of post-surgical abdominal adhesions by a novel biodegradable thermosensitive PECE hydrogel. BMC Biotechnol. 2010 Sep;10:65. doi: 10.1186/1472-6750-10-65. PMID: 20825683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang B, Gong C, Zhao X, Zhou S, Li Z, Qi X, Zhong Q, Luo F, Qian Z. Preventing postoperative abdominal adhesions in a rat model with PEG-PCL-PEG hydrogel. Int J Nanomedicine. 2012;7:547–57. doi: 10.2147/IJN.S26141. PMID: 22346350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong C, Shi S, Dong P, Kan B, Gou M, Wang X, Li X, Luo F, Zhao X, Wei Y, Qian Z. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int J Pharm. 2009 Jan;365(1-2):89–99. doi: 10.1016/j.ijpharm.2008.08.027. PMID: 18793709. [DOI] [PubMed] [Google Scholar]
- 28.Yin H, Gong C, Shi S, Liu X, Wei Y, Qian Z. Toxicity evaluation of biodegradable and thermosensitive PEG-PCL-PEG hydrogel as a potential in situ sustained ophthalmic drug delivery system. J Biomed Mater Res B Appl Biomater. 2010 Jan;92(1):129–37. doi: 10.1002/jbm.b.31498. PMID: 19802831. [DOI] [PubMed] [Google Scholar]
- 29.Einmahl S, Behar-Cohen F, D'Hermies F, Rudaz S, Tabatabay C, Renard G, Gurny R. A new poly (ortho ester)-based drug delivery system as an adjunct treatment in filtering surgery. Invest Ophthalmol Vis Sci. 2001 Mar;42(3):695–700. PMID: 11222529. [PubMed] [Google Scholar]
- 30.Skuta GL, Parrish RK 2nd. Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987 Nov-Dec;32(3):149–70. doi: 10.1016/0039-6257(87)90091-9. PMID: 3328315. [DOI] [PubMed] [Google Scholar]