Abstract
Background
In adults, MI results in a brisk inflammatory response, myocardium loss and scar formation. We have recently reported the first mammalian large animal model of cardiac regeneration following MI in fetal sheep. We hypothesize that the fetus ability to regenerate functional myocardium following MI is due to differential gene expression regulating the response to MI in the fetus compared to the adult.
Methods
MI was created in adult (n=4) or early gestation fetal (n=4) sheep. Tissue harvested after 3 or 30 days, RNA extracted for microarray, followed by PCA and global gene expression analysis for the gene ontology (GO) terms: “response to wounding”, “inflammatory response”, “extracellular matrix”, “cell cycle”, “cell migration”, “cell proliferation” and “apoptosis”.
Results
PCA demonstrated that the global gene expression pattern in adult infarcts was distinctly different from uninfarcted region at 3 days and remained different 30 days post-MI. In contrast, gene expression in the fetal infarct was different from the uninfarcted region at 3 days, but by 30 days it returned to a baseline expression pattern similar to the uninfarcted region. 3 days post-MI there was an increase in the expression of genes related to all GO terms in fetal and adult infarcts, but this increase was much more pronounced in adults. By 30 days, the fetal gene expression returned to baseline, whereas in the adult remained significantly elevated.
Conclusions
These data demonstrate that the global gene expression pattern is dramatically different in the fetal regenerative response to MI compared to the adult response and may partly be responsible for the regeneration.
Keywords: Myocardial Infarction, Fetal Regeneration, Inflammation, Extracellular Matrix, Gene Expression
Introduction
Cardiovascular diseases are the leading cause of death in the United States and worldwide. Every year, more than one million Americans experience myocardial infarction (MI) and over five million suffer from heart failure [1]. Although reperfusion and drug therapies greatly contributed to improving the pathophysiology of MI, adverse left ventricular remodeling after MI remains the most common cause of heart failure[2–4]. Over the last decade, more interest had been directed to find ways and means to stimulate the regeneration of the infarcted heart. Stem cells constitute a promising strategy to promote myocardial repair and regeneration. However there are some obstacles that this therapy needs to overcome before it can applied to patients on a broader scale.
Following MI, a series of events take place starting with an early phase of inflammation characterized by the infiltration of the infarcted area by inflammatory cells [5, 6]. A remodeling stage follows as matrix metalloproteinases and collagenases degrade the extracellular matrix which eventually results in scar formation, ventricular wall thinning, and a decline in cardiac function [7]. We have recently reported the first mammalian large animal model of cardiac regeneration following in utero MI in fetal sheep [8]. Using this model, we showed that the fetal response to myocardial infarction is dramatically different from the adult and is characterized by minimal inflammation, lack of fibrosis, restoration of cellularity, increased myocardial proliferation, and restoration of cardiac function [8]. In fact, assessment of fetal and adult hearts following MI demonstrated a decline in adult cardiac function whereas in the fetus, restoration of cardiac function was observed in both ejection fraction and lack of akinetic myocardium. In dramatic contrast to the adult response to infarction, the fetal ejection fraction (EF) slightly improved by 3 days, and by 1 month after infarction, the EF had returned to pre-infarction levels. One week following MI, CD45 staining showed high levels of inflammatory cells in the infarct area which increased even more after 4 weeks whereas in the fetus, the inflammatory response was minimal [8]. In addition, at 4 weeks there was no obvious apoptosis in the fetal infarcts whereas the adult infarct showed increased and persistent apoptosis. Moreover, we showed that the regeneration of the myocardium in the fetus is due partly to the proliferation of differentiated cardiomyocytes and the recruitment of cardiac progenitor cells to the infarct region at 3 days and 4 weeks after MI [9].
In the current study, we aim at understanding the mechanisms of fetal regenerative response to cardiac injury and to identify the factors that regulate the response to wounding, inflammation, and extracellular matrix remodeling. We believe that the expression of specific sets of genes plays an important role in the regulation and control of each of these phases. We hypothesized that the fetal response to MI would be associated with a differential gene expression profile which may be responsible for the regenerative healing and restoration of myocardial function in the fetal infarct.
Material and Methods
Animals
All experiments were approved by the IACUC of Nemours Children Hospital - Orlando and the University of Pennsylvania and performed in compliance with NIH Publication No. 85-23, revised 1996, and the European Convention on Animal Care.
MI in adult and fetal sheep
Fetal (65 to 76 days gestation) or adult Dorset sheep were used for all studies. Quantitative echocardiography was performed before infarction, immediately after infarction, and at the time of euthanasia. Animals were sedated with ketamine (11 mg/kg intramuscularly), intubated, and anesthetized with inhaled isoflurane. Cefazolin (1 g intravenously) was given before incision and oxytetracycline (0.06 mg/ kg intramuscularly) before extubation for antibiotic prophylaxis.
MI in adult sheep was generated as mentioned previously [10, 11]. For the fetal model, a laparotomy and hysterotomy was performed to expose the fetus. A left thoracotomy was performed, and the pericardium was opened. The left anterior descending coronary artery and appropriate diagonal branches were suture-ligated to produce an infarct involving 20% of the left ventricular mass. The chest and skin incisions were closed. The amniotic fluid was replaced with sterile normal saline plus 2 million units of penicillin-G added for antimicrobial prophylaxis. The uterus and abdominal incisions were closed before emergence from anesthesia. Analgesia was provided with buprenorphine (0.005 mg/kg intramuscularly) before extubation and flunixin meglumine (2.5 mg/kg intramuscularly) 4h postoperatively. Animals were euthanized at 3 or 30 days after infarction, hearts were excised and RNA was isolated the infarct regions of fetal and adult hearts (n=4 for adult and n=4 for fetal). The remote regions were isolated as controls.
RNA isolation and Ovine Specific Gene Microarray
A 5 mm section of the infarct area or remote region was harvested 3 and 30 days after MI. Total RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA). Total RNA was reverse transcribed to cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and then labeled with the incorporation of fluorescent dCTP. The mixed probes were then hybridized to the oligonucleotide ovine micorarrays (Agilent Technologies, Foster City, CA). The background-subtracted signal intensities were imported into R statistical environment (www.r-project.org) for statistical analysis, after which lowess-normalization of all 15,008 probes was performed across samples. Background-subtracted signal intensities were obtained from (Agilent, Inc.), and imported into R statistical environment (www.r-project.org). Lowess normalization of all 15,008 probes was performed across samples. Normalized intensities were further log2-transformed for statistical analyses. Principle component analysis (PCA) using all probes indicated that the global gene expression patterns were distinguishable between fetal and adult hearts.
Since more than 80% of the probes were mapped to sheep genes not functionally annotated, we mapped microarray probes to human genes through UniGene database (www.ncbi.nlim.nih.gov/unigene), which clusters genes across species based on the similarity of protein sequences. There were totally 9,103 probes mapped to 7,384 unique human Entrez gene IDs.
The difference of group means of each probe was used to represent the differential gene expression between fetal and adult hearts. We then selected several gene groups of interest: “response to wounding” (GO:0009611; 29 genes), “inflammatory response” (GO:0006954; 162 genes), “extracellular matrix” (GO:0031012; 20 genes), “cell cycle” (GO:0007049; 254 genes), “cell migration” (GO:0016477; 33 genes), “cell proliferation” (GO:0008283; 161 genes), and “apoptosis” (GO:0006915; 235 genes) from Gene Ontology database (www.geneontology.org), and evaluated whether these genes were differentially expressed as a whole.
Real-time quantitative PCR
Heart samples from the remote zone or the infarct were homogenized in TRIzol (Life Technologies, Invitrogen), and total cellular RNA was isolated and purified following the manufacturer’s instructions. For mRNA analysis, mRNA was converted into cDNA using the SuperScript First-Strand Synthesis System (Invitrogen). Real-time quantitative PCR was performed with the CFX96 real-time PCR thermal cycler (Bio-Rad, Hercules, CA) to amplify samples in triplicate. Relative gene product amounts were reported for each gene compared with 18S ribosomal RNA. Results were reported as means ± SEM.
Statistical analysis
T-test was used to analyze the data. All data were expressed as the mean ± SD and a P value < 0.05 was considered to be significant. Table 1 summarizes the t-test p values for each GO term.
Table 1.
Number of Genes, Average of (Adult-Fetal), and t-test p-values at 3 and 30 days post MI
| Average of (Adult – Fetus) | p value of t test | ||||
|---|---|---|---|---|---|
| GO Term | Number of Genes | 3 Days | 30 Days | 3 Days | 30 Days |
| Response to wounding (GO:0009611) | 29 | 0.803 | 0.721 | 1.3 x 10−2 | 1.3 x 10−2 |
| Inflammatory response (GO:0006954) | 162 | 0.343 | 1.022 | 1.1 x 10−2 | 7.8 x 10−10 |
| Extracellular matrix (GO:0031012) | 20 | 0.180 | 0.158 | 6.3 x 10−1 | 7 x 10−1 |
| Cell cycle (GO:0007049) | 254 | 0.600 | 0.034 | 1.3 x 10−8 | 5.5 x 10−1 |
| Cell proliferation (GO:0008283) | 161 | 0.411 | 0.284 | 7.5 x 10−5 | 1.9 x 10−3 |
| Cell migration (GO:0016477) | 33 | 0.689 | 0.884 | 2.8 x 10−2 | 3.2 x 10−3 |
| Apoptosis (GO:0006915) | 235 | 0.235 | 0.215 | 5.7 x 10−3 | 7.1 x 10−3 |
Results
Differential gene expression in infarct areas from fetal and adults hearts
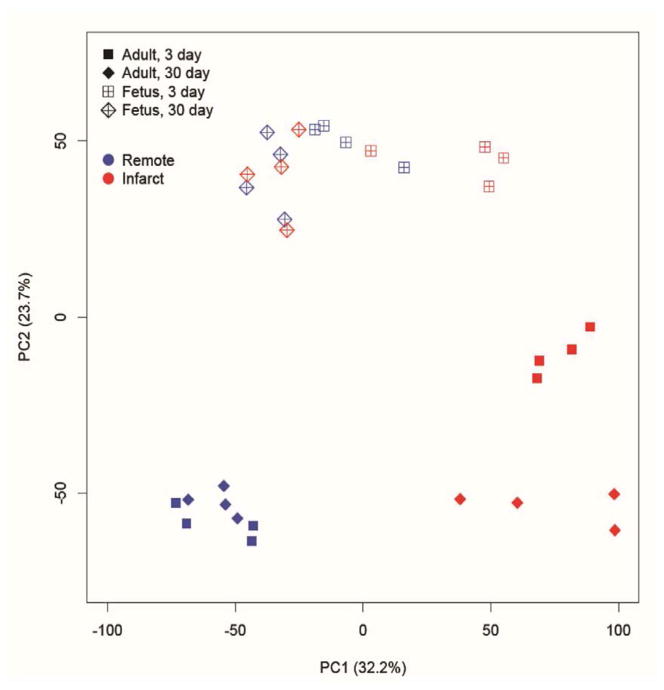
In this novel model, we have generated a unique data set of differentially expressed genes at baseline and following MI in both fetal and adult sheep using the newly available ovine specific gene expression microarray. PCA analysis demonstrated that the fetal and adult gene expression patterns in the remote region were different; however, neither remote region varied significantly between 3 and 30 days after infarction (Figure 1). The infarct region in the adult at 3 and 30 days clustered differently and remained distinct from the remote regions at both time points. In contrast, the fetal infarct clustering pattern was distinct from the fetal remote region at 3 days but returned to a similar expression pattern as the remote or uninfarcted fetal myocardium at 30 days after infarction.
Figure 1.
PCA analysis of microarray data. Open markers correspond to fetal heart and closed markers correspond to adult heart. Box markers correspond to 3 days after infarction and diamond markers correspond to 30 days after infarction. Blue indicates remote and red indicates infarct. PCA two-dimensional scatter plot represent the differential gene expression patterns of infarct and remote zone of adult and fetal hearts at 3 and 30 days post-MI. Axis: X=PC1: PCA Component 1 (32.2% variance); Y=PC2: PCA Component 2 (23.7% variance).
Differential gene expression of factors involved in “the response to wounding”
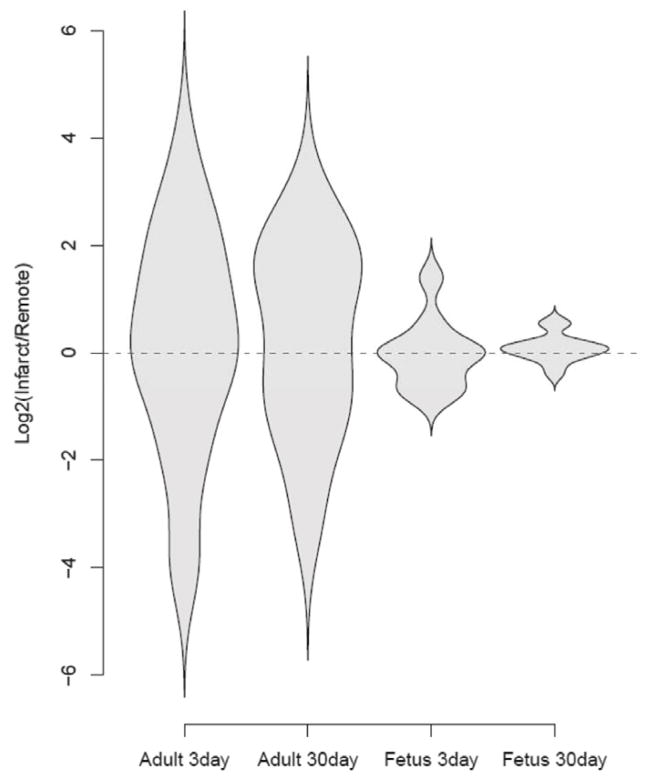
We analyzed this microarray data set of differentially expressed genes with regard to the gene ontology term “Response to Wounding” (GO:0009611). Figure 2 shows the violin plot of this analysis. There was a statistically significant difference in gene expression of factors involved in the response to wounding between the fetus and adult in response to MI. In the adult, the expression of these genes was significantly upregulated in the infarct area compared to the remote zone (uninfarcted myocardium) at 3 and 30 days following MI. Gene expression in the fetal hearts however, was much lower at 3 and 30 days compared to the adult heart. The fetal response in the infarct area was higher than in the remote zone at 3 days. After 30 days, this response resolved and came back to the basal level as in the remote zone. Our data demonstrate broad differences in gene expression, in particular to the response to wounding between the fetal regenerative and the adult reparative response to MI between the remote and infarct areas.
Figure 2.
Violin plots for the genes related to the GO term “response to wounding”. The y-axis represents the log2 of the ratio of the infarct to remote region average gene expression. The violin shapes represent the distribution of the log2 ratios in each group. The adult infarcts demonstrated increased expression of “response to wounding” genes at day 3 and persistence of the expression at 30 days, whereas the fetal infarct gene expression of “response to wounding” genes returned to baseline by 30 days. (29 genes; p<0.005, student’s t test).
Differential gene expression of factors involved in “the inflammatory response”
We previously showed that following MI, adult hearts have high and persistent inflammation compared to fetal hearts. So we analyzed this microarray data set with regard to the gene ontology term “Inflammatory Response” (GO:0006954). Figure 3A shows the violin plot representation of a statistically significant difference in gene expression of factors involved in “Inflammatory Response” between the fetus and the adult in response to MI (p<0.001). As we have seen with the “response to wounding”, our data from the adult hearts show higher levels of genes involved in inflammation in the infarct area compared to the remote zone at 3 and 30 days. The fetal response however was much lower than the adult and the inflammation was resolved as shown by similar expression levels of “inflammatory response” genes between the infarct and the remote area at 30 days following MI. Figure 3B shows the real time PCR analysis of the expression of IL-6 and IL8 in fetal and adult hearts. Both IL-6 and IL-8 are highly expressed in the adult infarct, 3 and 30 days following MI. However in the fetus, 3 days after MI IL-6 gene expression is slightly increased and completely disappeared after 30 days.
Figure 3.
(A) Violin plots for the genes related to the GO term “inflammatory response”. The y-axis represents the log2 of the ratio of the infarct to remote region average gene expression. The violin shapes represent the distribution of the log2 ratios in each group. The adult infarcts demonstrated increased expression of “inflammatory response” genes at day 3 and persistence of the expression at 30 days, whereas the fetal infarct gene expression of “response to wounding” genes returned to baseline by 30 days. (162 genes; p<0.005, student’s t test). (B) Real-time quantitative RT-PCR analysis of mRNA for IL-6 and IL-8 in fetal and adult hearts at 3 and 30 days after MI.
Differential gene expression of factors that regulate ‘extracellular matrix remodeling”
We recently showed that following MI, adult hearts show extensive remodeling of the extracellular matrix, scar formation and wall thinning at 30 days following MI. We also demonstrated complete myocardial regeneration 30 days following MI. In the current study, we analyzed the microarray data for differentially expressed genes with regard to the gene ontology term “extracellular matrix” (GO:0031012). Figure 4 shows the violin plot of this analysis with a statistically significant difference in gene expression of factors involved in “extracellular matrix remodeling” between the fetus and adult in response to MI (p<0.001). The adult hearts showed high expression of extracellular matrix genes 3 and 30 days after MI. In the fetus on the other hand, we saw much less extracellular matrix remodeling and after 30 days, the expression of genes related to “extracellular matrix remodeling” came back to normal levels as shown by the violin plots for the infarct and border zones.
Figure 4.
Violin plots for the genes related to the GO term “extracellular matrix”. The y-axis represents the log2 of the ratio of the infarct to remote region average gene expression. The violin shapes represent the distribution of the log2 ratios in each group. The adult infarcts demonstrated increased expression of “extracellular matrix” genes at day 3 and persistence of the expression at 30 days, whereas the fetal infarct gene expression of “response to wounding” genes returned to baseline by 30 days. (20 genes; p<0.005, student’s t test).
Differential gene expression of factors that regulate “cell cycle, migration and proliferation”
In Figure 5 we show the violin plot representation of the expression of genes related to “cell cycle” (GO:0007049), “cell migration” (GO:0046477) and “cell proliferation” (GO:0008283). There was a statistically significant difference in the gene expression for all 3 GO terms between the fetus and adult in response to MI (p<0.001). At day 3 in the adult, our results demonstrate high expression of genes related to “cell cycle, proliferation and migration” in the infarct area. At 30 days however, the expression of the “cell cycle and proliferation” genes in the infarct was lower than in the remote zone, probably due to the formation of an acellular scar as we showed previously [9]. Interestingly, cell migration was much higher in the infarct area at 30 days which could be due to the high influx of inflammatory cells into the infarct. In the fetus on the other hand, gene expression of “cell division, proliferation and migration” at 3 days, was similar between the infarct and the remote zone but much lower than the adult. At 30 days, all these responses went back to baseline which could be explained by the complete regeneration of the myocardium at that point.
Figure 5.
Violin plots for the genes related to the GO term “cell cycle (254 genes), proliferation (161 genes) and migration (33 genes)”. The y-axis represents the log2 of the ratio of the infarct to remote region average gene expression. The violin shapes represent the distribution of the log2 ratios in each group. The adult infarcts demonstrated increased expression of “cell cycle, proliferation and migration” genes at day 3 and persistence of the expression at 30 days, whereas the fetal infarct gene expression of “response to wounding” genes returned to baseline by 30 days. (p<0.005, student’s t test).
Differential gene expression of factors responsible of “apoptosis”
We analyzed the microarray data for the gene ontology term “Apoptosis” (GO:0006915). As shown in figure 6, infarct samples from adult hearts show significantly higher expression of genes associated with apoptosis compared to samples from the remote zone. At day 3, fetal infarcts demonstrated higher expression of “apoptosis genes” compared to the remote zone but their levels went back to baseline after 30 days.
Figure 6.
Violin plots for the genes related to the GO term “apoptosis”. The y-axis represents the log2 of the ratio of the infarct to remote region average gene expression. The violin shapes represent the distribution of the log2 ratios in each group. The adult infarcts demonstrated increased expression of “apoptosis” genes at day 3 and persistence of the expression at 30 days, whereas the fetal infarct gene expression of “response to wounding” genes returned to baseline by 30 days. (235 genes; p<0.005, student’s t test).
Comment
Cardiovascular diseases remain the leading cause of mortality in the United States and worldwide. Every year, more than one million American is affected by MI and despite the currently available therapies, the maximum life expectancy after MI is five years [1, 3, 12]. Stem and progenitor cells have shown promising results in promoting myocardial regeneration; however these therapies are not ready yet to be translated to patients. Unfortunately these were short term improvements and the transplanted cells were unable to engraft in the myocardium and many died after transplantation. One proposed explanation is that the cells are being introduced to an already hostile environment flooded with inflammatory cells, pro-inflammatory cytokines, apoptotic bodies and mediators, and high levels of reactive oxygen species. This environment would negatively affect the regeneration of the myocardium by limiting the viability of the existent cardiomyocytes and also inhibiting the migration, proliferation and differentiation of stem and progenitor cells.
In this current study we demonstrate that post MI, fetal and adult hearts present a distinct gene expression profile and marked differences in their response to MI. We show that following MI, fetal hearts present decreased expression of genes involved in the regulation of inflammation, extracellular matrix remodeling, apoptosis, cell cycle, cell migration and proliferation and response to wounding compared to adult hearts. We believe that these differences play a crucial role in promoting cardiac regeneration that we see in the fetal hearts.
Our data show that in the adult hearts, the expression of genes known to play an important role in the “response to wounding” was significantly upregulated as early as 3 days following MI and remained high even 30 days after injury. In the fetus on the other hand, the response to injury was not as intense and went back to baseline at 30 days. This distinctive trend in the response to injury between fetal and adult hearts correlates with the echocardiographic data which demonstrated the healing and complete regeneration of the fetal heart whereas in the adult, the infarct continued to increase in size and heart function decreased continuously.
From our research in fetal dermal and tendon wound healing, we previously showed that adult dermis or tendon wounds are associated with sustained inflammation and heal with scar formation. Similar wounds in the fetus showed minimal inflammation and healed regeneratively without scar formation. These fetal wounds are characterized by a decreased expression of genes associated with inflammation [13, 14]. The fetal regenerative healing takes place with a diminished inflammatory response and fibrosis has been associated to inflammation in different organs including the heart [15–18]. In adults, MI induces a dramatic inflammatory response leading to scar formation, ventricular remodeling and decreased cardiac function [5, 7, 19]. We previously showed that there is an association between decreased inflammation and fetal regenerative cardiac healing [8]. In this current study, we show that the global expression of genes closely involved in the regulation of inflammation, apoptosis and extracellular matrix remodeling in adult hearts is different from fetal hearts following MI. In fact, the reparative healing process in adult hearts correlated with a high and robust expression of inflammatory genes. The fetal heart on the other hand, demonstrated minimal inflammatory response and extracellular remodeling allowing a regenerative healing to take place.
Injecting stem cells or cardiac progenitor cells into the heart has shown very limited ability to induce cardiac regeneration after MI due to low number of cells that survive and the inability of most of the injected cells to differentiate into cardiomyocytes. Laflamme et al. used a pro-survival cocktail that inhibited different mechanisms of cell death and improved success levels of ES cell-derived myocardial graft formation in infarcted rat hearts [20]. These findings support the notion that promoting cardiac progenitor/stem cells proliferation and migration may be a reasonable therapeutic strategy. Our data show that the expression of genes associated with cell cycle and proliferation is significantly higher in adult hearts compared to fetal hearts. This could explain the increased proliferation of cardiac fibroblasts in the adult hearts. Prior studies on fetal hearts showed that the majority of proliferating cells in the fetus are differentiated cardiomyocytes instead of local or circulating progenitor cells [21]. Our data show that in the adult hearts, the signals important for cell migration are dramatically upregulated at day 3 and 30 after MI, but there was no regeneration which could be due to insufficient amount of stem cells in the adult heart to migrate and repair the infarct. In the fetus on the other hand, we see a significant decrease in the expression of genes involved in cell migration especially at 30 days post-infarct. This could be explained by the fact that there was a complete regeneration of the infarct at that timepoint, confirmed by echocardiographic data.
In conclusion, this study provides basis for further research into therapies to minimize apoptosis, avoid fibrosis, promote myocardial regeneration, and maintain cardiac function. Here we show that there are important differences in the expression of genes associated with the response to injury, inflammatory response, extracellular matrix remodeling, cell proliferation and migration, and apoptosis between the fetal and adult hearts. Understanding the mechanisms behind this differential response could be used to identify and target factors that are crucial to promote a regenerative response and may allow the development of potential treatment strategies to promote cardiac regeneration in the adults.
Acknowledgments
The research was supported by NIH Diabetes Pathfinder Grant 7DP2 DK-083085-01 (KWL) and HL063954 (RCG). R. Gorman and J. Gorman were supported by individual Established Investigator Awards from the American Heart Association, Dallas, TX.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Bolognese L, Ducci K, Angioli P, Falsini G, Liistro F, Baldassarre S, et al. Elevations in troponin I after percutaneous coronary interventions are associated with abnormal tissue-level perfusion in high-risk patients with non-ST-segment-elevation acute coronary syndromes. Circulation. 2004;110:1592–7. doi: 10.1161/01.CIR.0000142856.56565.56. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–9. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 4.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. The New England journal of medicine. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 5.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–64. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 7.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. Journal of molecular and cellular cardiology. 1995;27:1281–92. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 8.Herdrich BJ, Danzer E, Davey MG, Allukian M, Englefield V, Gorman JH, 3rd, et al. Regenerative healing following foetal myocardial infarction. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2010;38:691–8. doi: 10.1016/j.ejcts.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allukian M, 3rd, Xu J, Morris M, Caskey R, Dorsett-Martin W, Plappert T, et al. Mammalian cardiac regeneration after fetal myocardial infarction requires cardiac progenitor cell recruitment. The Annals of thoracic surgery. 2013;96:163–70. doi: 10.1016/j.athoracsur.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson BM, Gorman JH, Moainie SL, Guy TS, Narula N, Narula J, et al. Extension of borderzone myocardium in postinfarction dilated cardiomyopathy. Journal of the American College of Cardiology. 2002;40:1160–7. doi: 10.1016/s0735-1097(02)02121-6. discussion 8–71. [DOI] [PubMed] [Google Scholar]
- 11.Jackson BM, Gorman JH, 3rd, Salgo IS, Moainie SL, Plappert T, St John-Sutton M, et al. Border zone geometry increases wall stress after myocardial infarction: contrast echocardiographic assessment. American journal of physiology Heart and circulatory physiology. 2003;284:H475–9. doi: 10.1152/ajpheart.00360.2002. [DOI] [PubMed] [Google Scholar]
- 12.Kaul P, Armstrong PW, Chang WC, Naylor CD, Granger CB, Lee KL, et al. Long-term mortality of patients with acute myocardial infarction in the United States and Canada: comparison of patients enrolled in Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO)-I. Circulation. 2004;110:1754–60. doi: 10.1161/01.CIR.0000142671.06167.91. [DOI] [PubMed] [Google Scholar]
- 13.Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000;12:671–6. doi: 10.1006/cyto.1999.0598. [DOI] [PubMed] [Google Scholar]
- 14.Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. The Journal of surgical research. 1998;77:80–4. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- 15.Elias JA, Freundlich B, Kern JA, Rosenbloom J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990;97:1439–45. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- 16.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. The Journal of experimental medicine. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Antonini JM, Rojanasakul Y, Castranova V, Scabilloni JF, Mercer RR. Potential role of apoptotic macrophages in pulmonary inflammation and fibrosis. Journal of cellular physiology. 2003;194:215–24. doi: 10.1002/jcp.10220. [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Yang XP, Liu YH, Xu J, Cingolani O, Rhaleb NE, et al. Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension. 2004;43:229–36. doi: 10.1161/01.HYP.0000107777.91185.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Hiasa Y, Ohara Y, Miyazaki S, Ogura R, Miyajima H, et al. Relation between neutrophil counts on admission, microvascular injury, and left ventricular functional recovery in patients with an anterior wall first acute myocardial infarction treated with primary coronary angioplasty. The American journal of cardiology. 2007;100:35–40. doi: 10.1016/j.amjcard.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 20.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature biotechnology. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 21.Drenckhahn JD, Schwarz QP, Gray S, Laskowski A, Kiriazis H, Ming Z, et al. Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Developmental cell. 2008;15:521–33. doi: 10.1016/j.devcel.2008.09.005. [DOI] [PubMed] [Google Scholar]