Abstract
RATIONALE
Stress can reinstate previous cocaine seeking long after drug is no longer present. However, little is known regarding the effect of chronic drug exposure and subsequent drug abstinence on responsivity to stress.
OBJECTIVE
To determine the effect of acute (24-hour) and prolonged (14-day) drug-free periods in cocaine-experienced mice on behavioral, endocrine and molecular outputs following stress exposure.
METHODS
Mice were administered a cocaine binge (15 mg/kg) for two weeks. Following a 24-hour or 14-day drug-free period, stress responsivity, along with levels of anxiety, were measured using the forced swim test and elevated zero maze. Alterations in levels of plasma corticosterone, corticotrophin releasing factor (CRF) mRNA, brain-derived neurotrophic factor (BDNF) mRNA, and histone acetylation at their respective promoters were examined following stress exposure.
RESULTS
At both acute and prolonged abstinence time points, behavioral measures were essentially unaltered, however, cocaine-experienced mice exhibited an augmented corticosterone response to forced swim stress compared to saline-treated mice. Stress exposure increased BDNF mRNA levels in the ventral tegmental area (VTA) and nucleus accumbens (NAc) only in cocaine-experienced mice following a prolonged, but not acute, drug-free period. Increased BDNF mRNA in the NAc was associated with an increase in acetylated histone 3 (AcH3) at the BDNF I promoter. CRF mRNA levels were increased in the amydala (AMYG); however, this was not associated with alterations in histone acetylation at the promoter.
CONCLUSION
These results demonstrate that drug history and prolonged abstinence can alter the endocrine and molecular responses to stress, which may facilitate the reinstatement of drug seeking behaviors.
Keywords: chronic binge cocaine, cocaine withdrawal, FST, VTA, NAc, AMYG, BDNF, CRF, corticosterone, chromatin immunoprecipitation
Introduction
Exposure to stress following cocaine abstinence can precipitate relapse in humans (Hall et al. 1991; McMahon 2001) and facilitate reinstatement in animal models (Ahmed and Koob 1997; Erb et al. 1996; Kreibich and Blendy 2004; Shalev et al. 2000). While the effect of stress on cocaine addiction has been well documented, little is known about how prolonged drug abstinence affects stress reactivity. Human addicts report an increase in anxiety and depression-like symptoms immediately following cocaine abstinence that decreases with time (Coffey et al. 2000; Satel et al. 1991; Weddington et al. 1990). In rodent models, similar increases in anxiety (Paine et al. 2002; Perrine et al. 2007; Rudoy and Van Bockstaele 2007; Sarnyai et al. 1995) and pro-depressive phenotypes (Perrine et al. 2007) have been observed during acute cocaine abstinence. The effects of prolonged cocaine abstinence on these phenotypes, however, have yet to be examined.
Alterations in the hypothalamic-pituitary-adrenal (HPA) axis, the primary endocrine stress pathway, have been observed in human addicts following prolonged cocaine abstinence. Although baseline cortisol levels are normal, former cocaine addicts demonstrate augmented cortisol levels upon exposure to a stressor (Kreek and Koob 1998; Schluger et al. 2001). Recently, corticosterone was found to be increased in cocaine-experienced rats exposed to restraint stress compared to their non-stress counterparts following a 24-hour drug-free period (Mantsch et al. 2007). While these findings correlate with the hyperactive HPA axis observed in former cocaine addicts, it is unclear whether this HPA dysfunction is recapitulated in animal models following long-term cocaine abstinence. As stress exposure can facilitate relapse long after drug use is discontinued, it is relevant to determine if this hyperactivity is sustained following prolonged drug-free periods.
The persistence of drug craving and relapse behavior suggests that exposure to drugs of abuse results in long-term adaptations in the brain. These adaptations likely involve alterations in gene expression. Both stress exposure and cocaine administration can activate expression of several genes, including brain-derived neurotrophic factor (BDNF) and corticotrophin releasing factor (CRF). Increases in BDNF in the ventral tegmental area (VTA) and nucleus accumbens (NAc) during cocaine abstinence have been correlated with increased drug craving (Grimm et al. 2003; Lu et al. 2004). In addition, local injections of BDNF into the VTA can potentiate cocaine seeking following prolonged cocaine abstinence (Lu et al. 2004) and local BDNF injections in the NAc during self-administration can facilitate stress-induced reinstatement (Graham et al. 2007). CRF mRNA and protein levels are also altered following acute and chronic cocaine administration, as well as during short-term cocaine abstinence (Mantsch et al. 2007; Sarnyai et al. 1993; Zhou et al. 1996). CRF is important in stress-induced reinstatement, specifically the CRF projection from the amygdala (AMYG) to the bed nucleus of the stria terminalis (BNST) (Erb et al. 2001; Erb and Stewart 1999). Blockade of CRF receptors in the BNST, but not the AMYG, blocks footshock-induced reinstatement of cocaine (Erb et al. 2001; Lu et al. 2001). These studies indicate that both BDNF and CRF are involved in stress-induced cocaine reinstatement, however changes in these genes over the course of cocaine abstinence may also alter stress reactivity thus increasing susceptibility to drug relapse.
The long-lasting effects of cocaine on gene expression may be perpetuated by alterations in brain chromatin remodeling. Chromatin consists of DNA wrapped tightly around histone proteins in repeated units (nucleosomes) throughout the chromosome structure. The central core of the nucleosome contains heterotetramers of histones H3 and H4, along with heterodimers of histones H2A and H2B. Biochemical modifications of these histones play an important role in determining chromatin structure and access to gene transcription (Felsenfeld and Groudine 2003; Marmorstein 2001). Specifically, increases in histone acetylation result in changes in chromatin structure that have been shown to accompany induction of gene transcription (Kingston and Narlikar 1999; Sudarsanam and Winston 2000). A limited number of studies have examined alterations in chromatin structure following exposure to drugs of abuse (Black et al. 2006; Freeman et al. 2007; Kumar et al. 2005; Pandey et al. 2008; Schroeder et al. 2008). However, to date no studies have evaluated the effects of stress on chromatin remodeling following chronic drug exposure.
To examine the behavioral, endocrine and molecular alterations in drug-experienced mice following stress exposure, we exposed mice to a two-week chronic cocaine binge administration paradigm followed by an acute (24-hour) and prolonged (14-day) abstinence period. This cocaine administration paradigm was chosen as it closely mimics human cocaine use in several parameters, including repeated administration of cocaine and circadian rhythm (Unterwald et al. 1994; Zhou et al. 1996). Stress responsivity and anxiety were measured using the forced swim test and elevated zero maze, respectively.
Materials and Methods
Animals
Mice, 129SvEv:C57BL/6 F1 hybrid mouse strain (2-4 months; 20-40 g; mixed sexes), were group housed and maintained on a 12 h light/dark cycle with food and water available ad libitum in accordance with the University of Pennsylvania Animal Care and Use committee. All experimental testing was conducted between the hours of 9 AM and 1 PM.
Drug administration
Cocaine (15 mg/kg, i.p.; NIDA Drug Supply, Research Triangle Park, NC) or saline was administered in the home cage at 9 AM, 10 AM and 11 AM for 14 days. Mice were monitored for health, with weight measured daily. Statistical analysis examining weight in saline- and cocaine-treated mice was carried out using a repeated measures ANOVA with Fisher’s post-hoc tests. Following 14 days of chronic drug administration, animals remained in their home cage drug-free for a period of either 24 hours or 14 days.
Behavioral Experiments
Mice were exposed to the forced swim stress or the elevated zero maze and locomotor chambers at two time points during cocaine abstinence; 24 hours and 14 days. All cohorts of mice (n=9-12 per group) were counterbalanced for sex, age and drug treatment. Separate cohorts were used to measure behavior in the forced swim test, elevated zero maze and locomotor activity at each withdrawal time point. Plasma corticosterone levels, as well as mRNA levels of CRF and BDNF, were measured in the non-stress and forced swim stress-exposed cohorts. A separate cohort of mice was used for the chromatin immunoprecipitation experiment (Table 1). All behavioral testing was conducted between the hours of 9:00 AM and 1:00 PM. For all studies, mice were acclimated to the behavioral testing facility for one hour prior to testing.
Table 1.
Number of mice in each cohort with a description of behavioral, endocrine and molecular endpoints
| Experimental endpoints for each cohort |
||||
|---|---|---|---|---|
| Length of drug abstinence period |
Forced swim, corticosterone levels, mRNA analysis |
Elevated plus maze |
Locomotor activity |
Forced swim, chromatin immunoprecipitation |
| 24-hour | 9-12/group | 9-12/group | 9-12/group | 3-6/group |
| 14-day | 9-12/group | 9-12/group | 9-12/group | 3-6/group |
Note: All mice received either a cocaine binge or saline treatment as described in the materials and methods.
Forced swim stress
Mice were placed into plastic cylinders (23 cm tall × 14 cm diameter) containing water (22-24°C) filled to a depth of 15 cm for a 6-minute swim. Non-stress controls were kept in their home cage for the duration of the behavioral test. The Viewpoint Tracking System (Champagne au Mont d’Or, France) was used to video-record and register time spent immobile. Statistical analysis was carried out using an unpaired student’s t-test.
Elevated zero maze
The zero maze (Stoelting, Wood Dale, IL), consisting of two open areas (wall height, 1.3 cm) and two closed areas (wall height, 30.5 cm), was elevated 61 cm from the ground. Each mouse was placed in a closed area and allowed to explore the maze for a period of 300 seconds. The Viewpoint Tracking System was used to video-record and register the time spent in the open areas. In addition, ethologically relevant parameters, including stretch attend posture (SAP), head dips and rears, were measured by a trained, blind observer. Statistical analysis was completed for all behavioral parameters using an unpaired student’s t-test.
Locomotor activity
Locomotor activity was measured using a photobeam frame (MedAssociates, St. Albans, VT). Mice were placed individually into the testing cages and beam break data were recorded by MedAssociates software. The behavioral test lasted 30 minutes, with ambulations measured in 5 min intervals. Statistical analysis was completed using repeated-measures ANOVA.
Corticosterone measurement
Trunk blood was collected 30 minutes after exposure to swim stress. Plasma corticosterone levels were measured by radioimmunoassay using a commercially available kit (ICN Biomedicals, Cleveland, OH). Data were analyzed using a two-way ANOVA with subsequent Bonferroni/Dunn post-hoc tests.
Quantitative real-time polymerase chain reaction (QPCR)
All mice were sacrificed 30 minutes after exposure to swim stress following either 24 hours or 14 days of cocaine abstinence. RNA isolation and cDNA synthesis. Mice were sacrificed by cervical dislocation and brains rapidly removed and dissected on ice. Brains were first sliced using a mouse brain matrix into 1 mm slices. Specific regions were identified and macro-dissected using their approximate mouse stereotaxic coordinates (AMYG, bregma −1.2 mm; BNST, bregma +0.26mm; HIPPO, bregma −1.70; NAc, bregma +1.10 mm; VTA, bregma −3.64 mm) (Franklin and Paxinos, 2001). Hypothalamus was removed from the ventral side of the brain prior to placement in the mouse brain matrix. RNA was isolated from brain tissue using TRIzol/chloroform in conjunction with a RNeasy Mini kit (Qiagen). cDNA was synthesized using Oligo dT primer (Operon) and Superscript II reverse transcriptase (Invitrogen). All QPCR reactions were run using the Stratagene MX3000 and the MXPro QPCR software. SYBR-green QPCR. Reactions were assembled using Applied Biosystems 2X SYBR-Green master mix along with 300 nM primers (final concentration). Cycling parameters were 95°C for 10 minutes followed by 40 cycles of 95°C (30 s) and 60°C (1 min), ending with a melting curve analysis to control for the amplification of a single gene product. All reactions were performed in triplicate and the median cycle threshold was used for analysis. mRNA levels of target genes were normalized to the housekeeping gene, TATA Binding Protein (TBP). Primer sequences are available upon request. Taqman QPCR. Multiplex reactions were assembled using Taqman Universal PCR Master Mix (Applied Biosystems) and appropriate primers.
Chromatin Immunoprecipitation (ChIP)
A separate cohort of mice exposed to chronic cocaine binge and subsequent 14-day abstinence were sacrificed 30 minutes following exposure to an acute forced swim stress. Macrodissected brain tissue was minced in cold PBS and suspended in 1% formaldehyde/PBS for 10 min at RT. Cross-linking was quenched by the addition of glycine to a final concentration of 0.125 M, after which the tissue was sedimented by centrifugation. Pellets were resuspended in ChIP whole lysis buffer (10 mM Tris-Hcl, pH 8.0, 10 mM NaCl, 3 mM MgCl2, 1% NP-40, 1% SDS plus protease and phosphatase inhibitors) and dounce homogenized. Lysate was sonicated (BioRuptor, Diagenode SA, Sparta, NJ) for 5 minutes at 200 W. Insoluble debris was removed by centrifugation at 13,000g for 10 min at 4°C, supernatant was collected and flash frozen in liquid nitrogen. Cross-linking was reversed by the addition of NaCl to a final concentration of 192mM, overnight incubation at 65°C and purification using a Mineulute PCR purification kit (Quigen). For immunoprecipitations, chromatin was precleared by incubation for 1h at 4°C with 125ul of protein G-agarose in a total volume of 1 ml ChIP dilution buffer (20 mM Tris-HCl, pH 8.1, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 2 mM EDTA). After this preclearing, the supernatant was evenly divided and incubated overnight with 10 μg of AcH3 antibody (Upstate) or control IgG. Immunoprecipitation was performed as described (Rubins et al. 2005).
Promoter enrichment was quantified using Q-PCR and calculated by comparing the difference in abundance of the control DNA sequences (28S rRNA loci) to the promoter sequence of interest (BDNF or CRF) in genomic DNA (input) to the immunoprecipitated DNA. A promoter enrichment of >1 indicates that AcH3 is present at the promoter region tested, while a value of <1 indicates that no AcH3 is present. Data were analyzed using a two-way ANOVA with Bonferroni/Dunn post-hoc. Primer sequences are available upon request.
Results
Weight change
Over the course of the 14-day chronic binge, mice given cocaine showed significant weight loss compared to their saline counterparts (significant by drug, F(1,38)=26.722, p<0.0001; significant by time, F(13,38)=5.215,p<0.0001; significant drug × time interaction, F(1,13)=5.999, p<0.0001, repeated measures ANOVA) (data not shown). This decrease in body mass was statistically significant by the third day (p<0.001, Fisher’s post-hoc test) and remained significantly decreased throughout the remainder of the chronic binge paradigm. No health problems were noted during the drug administration protocol.
Behavioral responsivity to swim stress is not altered during cocaine abtinence
Mice were exposed to a 6-minute swim stress 24 hours or 14 days following the last injection of cocaine. As shown in Figure 1a (24-hour) and 1b (14-day), no alterations in time spent immobile were observed following either 24-hour or 14-day forced abstinence from chronic binge cocaine.
Figure 1.
Behavioral outputs from forced swim test, elevated zero maze and locomotor activity either 24 hours (a, b, c and d) or 14 days (e, f, g and h) following exposure to chronic binge cocaine. Duration of immobility in the forced swim test is shown following either 24-hour (a) and 14-day (b) abstinence periods. Behavioral parameters in the elevated plus maze, including time spent in the open arms (b and f) and ethologically relevant behaviors (c and g), were also examined following both short (24-hour; b and c) and long (14-day; f and g) cocaine abstinence periods. Ambulations, a measure of locomotor activity, are shown in figure 1d (24-hour) and figure 1h (14-day). All data is expressed as mean ± SEM. *p<0.01, unpaired student’s t-test.
Alterations in elevated zero maze performance are evident 14 days following chronic cocaine binge
To determine whether chronic cocaine binge alters anxiety levels, mice were placed in the elevated zero maze following either a 24-hour or 14-day drug-free period. At the 24-hour time point, no difference in time spent in open arms was observed in cocaine-treated mice compared to their saline-treated counterparts (Figure 1c). However, following a 14-day abstinence period (Figure 1d), mice exposed to chronic binge cocaine spent significantly more time in the open arms (p<0.01, unpaired student’s t-test), which can indicate a decrease in anxiety, an increase in exploratory behavior or a combination of the two. In addition, ethologically relevant behaviors were measured during the anxiety paradigm (Figure 1e). Stretch attend posture (SAP), a measure of risk-assessment, was significantly decreased in mice previously exposed to cocaine (p<0.01, unpaired student’s t-test). Furthermore, these same cocaine-treated mice exhibited significantly more head dips than their saline counterparts (p<0.01, unpaired student’s t-test). The significant increase in head dips would support either a decrease in anxiety or an increase in exploratory behavior.
Alterations observed in elevated zero maze are not dependent on an increase in locomotor activity
Locomotor activity was examined in both saline- and cocaine-treated mice at 24 hours and 14 days following the cocaine binge. At both time points examined, no effect of cocaine exposure was observed, although there was a significant effect of time (24 hours, F(5,21)=93.845, p<0.0001, repeated measures ANOVA: 14 days, F(5,22)=83.981, p<0.0001, repeated measures ANOVA) (Figure 1g and 1h).
HPA axis activation is augmented in cocaine-experienced mice
To determine if the hypothalamic-adrenal-pituitary (HPA) axis was altered by chronic binge cocaine, we examined corticosterone levels following exposure to the swim stress (Figure 2). Following a 24-hour abstinence period, exposure to swim caused a significant increase in corticosterone in both saline- and cocaine-treated mice (F(1,25)=153.0, p<0.0001, Two-way ANOVA) (Figure 2a). Additionally, this stress-induced augmentation was greater in cocaine-experienced mice following prolonged drug abstinence (p<0.001, compared to saline-swim stress, Bonferroni/Dunn post-hoc) (Figure 2b). Two-way ANOVA demonstrated an overall affect of stress (F(1,36)=130.6, p<0.0001) and drug (F(1,36)=6.399, p<0.02), as well as a significant stress × drug interaction (F(1,36)=7.673, p<0.01). These results clearly show that exposure to a chronic binge regimen of cocaine administration alters the HPA axis response to stress long after the drug is no longer present.
Figure 2.
Corticosterone levels following exposure to a swim stress at 24-hour (a) and 14-day (b) withdrawal from chronic binge cocaine. All data is expressed as mean ± SEM. *p<0.01, and **p<0.001, Bonferroni/Dunn post-hoc.
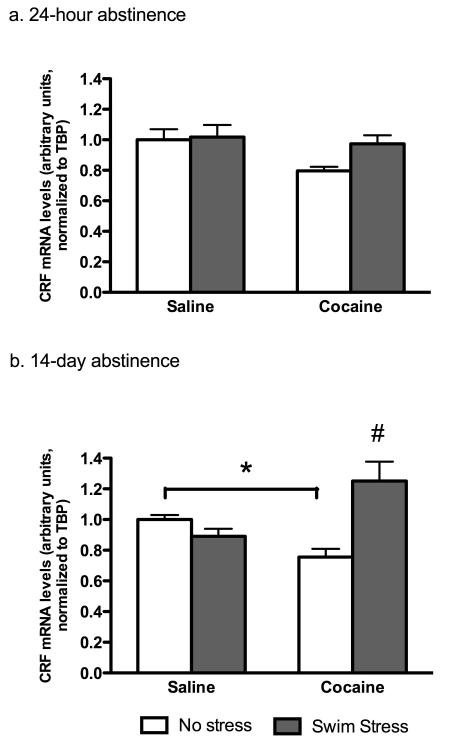
CRF mRNA expression is stimulated in the AMYG after stress exposure following long-term withdrawal from cocaine
CRF-containing cell bodies in the AMYG are important in facilitating stress-induced reinstatement to drug seeking (Erb et al. 2001); however, it is not known how swim stress exposure during acute and prolonged drug-free periods affects CRF gene expression in this brain area. Previous studies have demonstrated increases in CRF mRNA in several brain areas, including the AMYG, within 30 minutes following exposure to a stressor (Imaki et al. 1996; Stout et al. 2002; Yamano et al. 2004). Following a drug-free period of 14 days, only cocaine-experienced mice demonstrated a significant increase in AMYG CRF after exposure to swim stress (Figure 3b) (p<0.02 as compared to all other groups, Bonferroni/Dunn post-hoc). Statistical analysis revealed a significance by stress (F(1,25)=7.857, p<0.01, two-way ANOVA), in addition to a significant interaction between drug administration and stress exposure (F(1,25)=18.58, p<0.001, two-way ANOVA). Additionally, CRF mRNA was decreased in mice that received chronic cocaine binge (cocaine-no stress group as compared to their saline-no stress counterparts) (p<0.03, Bonferroni/Dunn post-hoc). This effect is likely due to prolonged drug abstinence from chronic binge cocaine rather than the drug exposure as no differences in CRF mRNA levels were found in either saline or cocaine-treated mice at the 24-hour forced abstinence time point regardless of their stress exposure (Figure 3a). Of interest, there is a general effect of stress to increase CRF mRNA in the NAc at 24 hours and in the hypothalamus and VTA at 14 days following cocaine abstinence in both cocaine and saline treated mice (Table 2).
Figure 3.
Alterations in CRF mRNA in AMYG following short (24-hour, a) and long-term (14-day, b) cocaine abstinence. All data is expressed as mean ± SEM. *p<0.03, and #p<0.01 compared to all other groups, Bonferroni/Dunn post-hoc.
Table 2.
Gene expression changes following stress expoure during acute and prolonged cocaine abstinence
| 24 hours | ||||||
|---|---|---|---|---|---|---|
|
Genes of
Interest |
Brain Regions | |||||
| AMYG | BNST | HYPO | NAc | VTA | HIPPO | |
| BDNF | No change | No change | N/A | Effect of drug (p=0.025) |
Effect of drug (p=0.0113) |
Interaction (p=0.0476) |
| CRF | No change | No change | No change | Effect of stress (p=0.0287) |
No change | N/A |
| 14 days | ||||||
|---|---|---|---|---|---|---|
|
Genes of
Interest |
Brain Regions | |||||
| AMYG | BNST | HYPO | NAc | VTA | HIPPO | |
| BDNF | Effect of drug (p=0.0351) |
N/A | N/A | Effect of stress (p=0.0492); Interaction (p=0.0437) |
Effect of drug (p=0.0103); Interaction (p=0.0007) |
Effect of stress (p=0.0006) |
| CRF | Effect of stress (p=0.0096); Interaction (p=0.0002) |
No change | Effect of stress (p=0.0043) |
No change | Effect of stress (p=0.0048) |
N/A |
Note: N/A; Insufficient RNA for analysis
BDNF mRNA is increased in the VTA and NAc after stress exposure following long-term abstinence from cocaine
BDNF is increased in the VTA and the NAc following chronic cocaine exposure (Grimm et al. 2003) and this increase partially mediates drug craving during prolonged cocaine abstinence (Lu et al. 2004). In addition, BDNF mRNA and protein levels are decreased following chronic stress (Murakami et al. 2005; Takeda et al. 2006; Vaidya et al. 1999). However, the effect of stress on BDNF levels during cocaine abstinence is not known. Therefore, BDNF mRNA levels were examined in the VTA and NAc after exposure to an acute stressor following either a 24-hour or 14-day drug-free period in both saline- and cocaine-treated mice. As shown in Figure 4, following a 24-hour forced abstinence period, there was a small but significant decrease in BDNF mRNA levels in cocaine-experienced mice regardless of stress exposure in both the VTA (a) (significant by drug, F(1,27)=6.864, p=0.01, two-way ANOVA) and NAc (c) (significant by drug, F(1,16)=6.576, p=0.02, two-way ANOVA). However, following a drug-free period of 14 days, exposure to an acute swim stress significantly increased BDNF mRNA levels in the VTA only in cocaine-experienced mice (significant by drug, F(1,27)=7.613, p=0.01, twoway ANOVA; significant drug × stress interaction, F(1,27)=14.65, p<0.001, two-way ANOVA; p<0.01 as compared to all other groups, Bonferroni/Dunn post-hoc) (Figure 4b). In the NAc, BDNF mRNA levels were increased after stress exposure in cocaine-treated mice compared to their no-stress counterparts following a 14-day, but not 24-hour, forced abstinence period (Figure 4d) (significant by stress, F(1,24)=4.292, p<0.05, two-way ANOVA; significant drug × stress interaction, F(1,24)=4.535, p<0.05, two-way ANOVA; p<0.01 as compared to cocaine-no stress, Bonferroni/Dunn post-hoc). In addition, BDNF mRNA levels in the NAc were significantly decreased in the non-stress cocaine-treated mice as compared to the non-stress saline-treated mice (p<0.05, Bonferroni/Dunn post-hoc). There is a general effect of cocaine on BDNF mRNA in the amygdala (trend toward a decrease) regardless of stress exposure and a general effect of stress on BDNF mRNA in the hippocampus (trend toward and increase) regardless of drug exposure at 14 days of cocaine abstinence (Table 2).
Figure 4.
BDNF mRNA levels in the VTA and NAc following both 24-hour (a,c) and 14-day (b,d) forced abstinence from chronic binge cocaine. All data is expressed as mean ± SEM. #Significant by drug, p<0.05, two-way ANOVA; *p<0.01, Bonferroni/Dunn post-hoc.
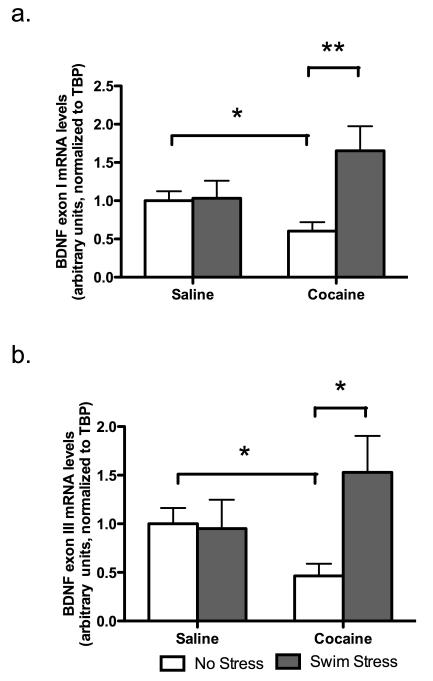
Both BDNF exon I and BDNF exon III mRNA levels are increased in the NAc after stress exposure following long-term abstinence from cocaine
The BDNF gene consists of five exons, four of which encode unique untranslated 5′exons with individual promoters (Exons I-VI) and a common 3′ coding exon (Exon V)(Timmusk et al. 1993). The existence of multiple BDNF promoters is thought to confer tissue- and stimuli-specific regulation of this gene in the brain (Dias et al. 2003; Nair et al. 2007). Therefore, levels of BDNF exon I and III mRNA were measured to determine whether they are differentially contributing to total levels of BDNF. BDNF exon I mRNA levels were increased in the NAc of cocaine-experienced animals following an acute swim stress at the 14-day time point (significant by stress, F(1,24)=5.383, p<0.03; significant drug × stress interaction, F(1,24)=4.771, p<0.05; p<0.01 compared to non-stress cocaine group, Bonferroni/Dunn post-hoc) (Figure 5a). Similar results were observed with BDNF exon III (significant drug × stress interaction, F(1,23)=4.285, p<0.05; p<0.05 compared to non-stress cocaine group, Bonferroni/Dunn post-hoc) (Figure 5b). Furthermore, both BDNF exon I and BDNF exon III mRNA were decreased in the non-stressed cocaine-experienced mice compared to non-stressed saline-treated mice (p<0.05, Bonferroni/Dunn post-hoc). These data suggest that the total BDNF mRNA alterations observed previously in the NAc might be driven by the alterations in both BDNF exon I and exon III mRNA levels.
Figure 5.
Alterations in BDNF exon I (a) and III (b) mRNA levels in the NAc following stress exposure after long-term (14-day) forced abstinence from cocaine. All data is expressed as mean ± SEM. *p<0.05, **p<0.01, Bonferroni/Dunn post-hoc.
Acetylation at Histone 3 is increased at BDNF promoter I following 14 days of abstinence and exposure to swim stress in the NAc of cocaine-treated mice
As BDNF and CRF gene expression were increased only in cocaine-experienced mice exposed to an acute swim stress following a 14-day drug-free period, changes in chromatin structure during this drug abstinence period may explain this selective gene activation. Chromatin immunoprecipitation, a technique that measures specific DNA-protein associations, and subsequenc quantification by real-time PCR demonstrated and increase binding by acetylated H3 (AcH3) at the BDNF I promoter in the NAc of cocaine-experienced mice exposed to an acute swim stress (Figure 6c) (significant by drug, F(1,17)=18.35, p<0.001; significant drug × stress interaction, F(1,17)=6.862, p<0.02; significant from corresponding no-stress group, p<.01, Bonferroni/Dunn post-hoc). Interestingly, no AcH3 binding was observed in any other group at the BDNF I promoter in the NAc, suggesting that increased H3 acetylation occurs following stress exposure only in cocaine-treated mice. No significant difference was observed in AcH3 binding at the BDNF III promoter in the NAc (Figure 6d). It is important to note, however, that there is more AcH3 binding in this promoter region, regardless of drug treatment or stress exposure, than at the BDNF I promoter, suggesting an transcriptionally active state of the gene. No alterations in AcH3 levels were observed in either the BDNF I (Figure 6a) or BDNF III (Figure 6b) promoter region in the VTA at the 14-day abstinence time point. No significant changes in acetylated H3 were observed at the CRF promoter region in the AMYG following long-term drug abstinence and subsequent exposure to swim stress (data not shown).
Figure 6.
AcH3 binding at the BDNF I (a,c) and III (b,d) promoter in the NAc and VTA following long-term forced abstinence (14-day) from chronic binge cocaine and subsequent stress exposure. All data is expressed as mean ± SEM. *p<0.01, Bonferroni/Dunn post-hoc.
Discussion
Exposure to stressful stimuli in an animal with prior drug experience can elicit cocaine craving and mediate reinstatement of cocaine-seeking behavior (Ahmed and Koob 1997; Erb et al. 1996). This suggests that prior drug experience alters an animal’s response to a stressor, particularly following prolonged cocaine abstinence. While much attention has been focused on periods of drug taking and drug relapse, the neurobiological changes that occur during the intermediate drug-free state have been largely unexplored. Understanding these changes may help explain why humans still relapse years, even decades, after cessation of drug use. Animal models of relapse demonstrate a progressive enhancement of drug craving that increases over an extended period of drug abstinence (Grimm et al. 2001). This incubation of craving phenomenon may help explain why susceptibility to relapse in humans lasts indefinitely. Thus investigation of the behavioral, endocrine and molecular changes that occur during this protracted drug-free period are imperative to determining mechanisms underlying drug relapse.
Human addicts frequently cite anxiety and depression-like symptoms during abstinence periods (Coffey et al. 2000; Satel et al. 1991; Weddington et al. 1990). Therefore, to examine the behavioral effects of prior drug experience followed by a period of abstinence, alterations in anxiety as well as in stress-responsivity were measured. Previous studies in rats have reported increased anxiety (Paine et al. 2002; Perrine et al. 2007; Rudoy and Van Bockstaele 2007; Sarnyai et al. 1995) while others have shown no change in these behaviors (Basso et al. 1999; Hoplight et al. 2005). The present study reports on these behaviors following both acute and prolonged cocaine abstinence in mice. No alterations in anxiety (elevated zero maze) were observed 24 hours following cessation of cocaine treatment. Studies have shown marked differences in anxiety behavior across rodent strains and in different laboratories, as well as in manipulations prior to testing (Crabbe et al. 1999; Hogg 1996; Wahlsten et al. 2006), indicating that anxiety measures can be highly sensitive to a wide range of factors. Furthermore, behavioral paradigms may vary in their sensitivity to abstinence-induced anxiety, as a previous study found that anxiety associated with acute abstinence from cocaine was evident in the defensive burying task but not the elevated plus maze (Basso et al. 1999).
In the present study cocaine-treated mice that experienced prolonged forced abstinence spent considerably more time in the open arms of the maze than drug-naïve mice; a behavior often associated with a decrease in anxiety. However, this was accompanied by an increase in head dips, indicating an increase in exploratory behavior (Rodgers and Cole 1994; Rodgers and Johnson 1995; Shepherd et al. 1994). This type of increased activity in open arm of the elevated plus maze and light compartment of the light-dark box has been observed in rats withdrawn from cocaine self-administration (Mantsch et al. 2008; Mantsch et al. 2007). Thus, the more active behaviors observed in cocaine-experienced animals during abstinence could be reflective of a generalized increase in novelty exploration. Cocaine-experienced mice also demonstrated a decrease in stretch attend posture, indicating a decrease in risk assessment (Shepherd et al. 1994). Indeed, human cocaine addicts exhibit increased novelty seeking and impulsivity (Adams et al. 2003; Monterosso et al. 2001; Sher et al. 2000); however, it is unclear whether these traits are indicative of a pre-existing condition or are a consequence of chronic drug administration. Similar findings are observed in animal models, as rats that exhibit increased locomotor activity upon exposure to a novel environment acquire psychostimulant self-administration more rapidly and at lower doses than low-novelty responders (Mantsch et al. 2001; Piazza et al. 1989). In addition, in a delay-of-reward task, chronic cocaine increases impulsivity in rats (Paine et al. 2003; Richards et al. 1999). Paradigms tailored specifically to address these behaviors associated with novelty seeking and impulsivity should be examined following cocaine exposure and prolonged abstinence.
Stress responsivity can be evaluated in paradigms such as the forced swim test. In this paradigm, an increase in immobility is interpreted as depressive-like behavior (Cryan et al. 2002). While a previous study in rats demonstrated a depressive-like phenotype in this paradigm during acute cocaine withdrawal (Perrine et al. 2007), no behavioral differences in the forced swim test were observed in this study between the saline- and cocaine-treated mice following acute and prolonged cocaine abstinence.
In the present studies chronic binge cocaine and subsequent abstinence augmented corticosterone release upon exposure to an acute stressor. These data are consistent with past findings demonstrating that HPA hyperactivity occurs following restraint stress during acute cocaine withdrawal (Mantsch et al. 2007), and we further demonstrated that this hyperactivation was still apparent following forced abstinence of 14 days. Thus, alterations observed in the HPA axis during acute abstinence persist. Human studies have previously demonstrated a hyperactive HPA axis following a stressor during extended periods of abstinence (Kreek and Koob 1998; Schluger et al. 2001), suggesting that alterations in the HPA axis may be contributing to drug craving. Future studies examining the significance of this cocaine-induced HPA hyperactivity will enable us to determine whether this long-term alteration plays a role in stress-induced craving.
Activation of CRF in the AMYG following stress exposure may play a role in mediating stress-induced drug craving. Previous studies in rats have demonstrated increases in AMYG CRF mRNA levels following acute but not chronic cocaine administration (Rivier and Lee 1994; Zhou et al. 2003), with similar changes observed in protein levels at these time points (Sarnyai et al. 1993; Zhou et al. 1996). CRF protein levels are increased in the AMYG of cocaine-experienced rats following a 6-week abstinence period (Zorrilla et al. 2001). In addition, stress-induced reinstatement is dependent on activation of extra-hypothalamic CRF brain circuits, specifically the CRF projection from the AMYG to the bed nucleus of the stria terminalis (BNST) (Erb et al. 2001; Erb and Stewart 1999). Our data demonstrate a general effect of stress induced increase in CRF mRNA in NAc, hypothalamus and VTA, irrespective of saline or cocaine exposure. However, CRF gene expression is increased in the AMYG following exposure to stressful stimuli only in cocaine-experienced mice. Of interest, CRF mRNA is reduced in cocaine-experienced mice not exposed to stress, which may be related to the decreased anxiety seen behaviorally at this time point, however more experiments are needed to confirm this association. CRF mRNA levels were not altered in response to either drug treatment or stress exposure at the 24-hour time point in the AMYG, suggesting that long-term cocaine abstinence is necessary for the molecular alterations that facilitate stress-induced activation in this particular brain region.
BDNF is also important in facilitating drug seeking following prolonged cocaine abstinence (Graham et al. 2007), and its actions in mesolimbic brain regions may contribute to the development of drug craving (Grimm et al. 2003; Lu et al. 2004). BDNF protein levels localized in the mesolimbic dopamine pathway have been shown to increase in a time-dependent manner during abstinence periods and that this increase is positively correlated with the animal’s response to drug-associated cues. Subsequent studies demonstrated that local injection of BNDF into the VTA or NAc shell potentiated reinstatement of drug seeking (Graham et al. 2007; Lu et al. 2004). In the present study, BDNF mRNA levels were decreased in the VTA and NAc of cocaine-treated mice during acute abstinence (24 hour), regardless of stress exposure. This BDNF decrease was subtle but sustained in the NAc of non-stressed cocaine-experienced mice 14 days following cessation of cocaine administration. Our data demonstrate that stress-induced increases in mesolimbic BDNF are elicited only following prolonged cocaine abstinence. Specifically, only cocaine-experienced mice demonstrated a significant increase in BDNF mRNA levels in the VTA and NAc when exposed to an acute swim stress 14 days following cessation of drug treatment. Thus, prior drug experience followed by prolonged abstinence enables the stress-induced activation of BDNF gene transcription.
Mechanisms underlying susceptibility to gene activation may be due to chromatin remodeling during this prolonged drug-free period. Modification of histone proteins, specifically increases in acetylation, are associated with an open chromatin structure, allowing for transcription to occur more rapidly. Indeed, previous studies have demonstrated chromatin remodeling at the BDNF promoter 30 minutes following the last cocaine injection in a chronic cocaine administration paradigm (Kumar et al. 2005), indicating that alterations in histone structure play a role in cocaine–induced expression of this gene.
A number of studies have demonstrated that changes in chromatin structure accompany induction of gene transcription in response to cocaine exposure. In the present study, we wanted to determine if promoter remodeling and subsequent gene transcription occurs as a result of a combination of stress and drug effects. Therefore we examined AcH3 binding at BDNF promoters in the NAc and VTA and the CRF promoter in the AMYG as these were the regions where stress and drugs appear to interact most robustly with respect to changes in gene expression. While no significant changes in AcH3 binding were observed at the CRF promoter or the BDNF promoters (I and III) in the VTA, a significant increase was observed in the NAc of stressed cocaine-experienced mice following prolonged abstinence. This correlates with the increase in total BDNF mRNA expression. Furthermore, no AcH3 binding was observed at the BDNF promoter I in the NAc of saline-treated animals, regardless of stress exposure suggesting a closed chromatin structure. Interestingly, in the NAc, mRNA levels of both exon I and exon III closely mirrored the total BDNF mRNA expression, indicating that both of these transcripts contribute to the alterations in total BDNF mRNA observed following prolonged cocaine abstinence. Although the BNDF III promoter did not show any alterations in AcH3 in the NAc, its overall levels of AcH3 were greater than levels at the BDNF I promoter. Thus, stress-induced gene activation could have occurred at this promoter without additional modifications to histone structure. Total BDNF mRNA was also increased in the VTA following stress exposure during prolonged abstinence only in cocaine-experienced mice. While neither stress exposure nor drug treatment altered the levels of AcH3 at these two promoters, levels of AcH3 binding were greater at the BDNF I and III promoter in the VTA than in the NAc. Therefore, chromatin remodeling might have been necessary in the NAc, but not in the VTA, to allow the activation of BDNF gene expression following stress exposure. Future studies examining specific transcription factor binding to the BDNF promoters need to be completed to fully understand the complex regulation of this important gene.
This series of studies examined behavioral, endocrine and molecular endpoints following chronic binge cocaine administration and either acute or prolonged abstinence. As stress and anxiety are both observed following cocaine abstinence, it is important to examine the endocrine and molecular alterations observed in the context of these behaviors. Alterations in the endocrine and molecular circuitry were observed 30 minutes following the swim stress and would not have affected the behavior in this paradigm. However, as a single stress exposure may not lead to cocaine relapse in human addicts, but rather repeated exposure to various stressors, examination of behavioral alterations following repeated exposure to a swim stress during the cocaine abstinence period may more closely mimic human stress-induced relapse. Exposure to a single forced swim stress increased corticosterone, as well as CRF and BDNF mRNA only in cocaine-experienced mice following prolonged abstinence, therefore repeated exposure to this swim stress may repeatedly active these circuits, possibly resulting in alterations in stress responsivity in the forced swim test. While no behavioral alterations were observed in the swim test, changes in behavior were observed in the elevated zero maze, a paradigm that examines anxiety in addition to exploratory behavior. An increase in time spent in open arms, indicating a decrease in anxiety, was observed only in cocaine-experienced mice following prolonged abstinence. Of interest, at the same timepoint, a decrease in CRF mRNA was seen in the AMYG. Previous studies have demonstrated a correlation between increased AMYG CRF mRNA and increases in anxiety in the elevated plus maze (Shepard et al. 2000). Therefore, the decrease in AMYG CRF mRNA observed in non-stressed cocaine-treated mice following prolonged cocaine abstinence may be associated with the decrease in anxiety observed in the elevated plus maze. Future studies examining the role of AMYG CRF mRNA levels following prolonged cocaine abstinence and anxiety levels need to be completed to further understand this relationship.
Anxiety and depression are important features of cocaine abstinence in humans and may facilitate drug relapse; therefore, modeling these behaviors in animals is imperative to investigating relapse susceptibility. The use of a variety of behavioral paradigms designed to measure stress-reactivity, anxiety behavior and novelty seeking, such as learned helplessness and open field, may help tease apart the effects of cocaine abstinence on stress-mediated behaviors and anxiety. Our findings demonstrate that stress-induced changes in HPA axis function and gene expression are altered following cocaine exposure. We have shown that the HPA axis is hyperactive in response to a stressor following both acute and long-term cocaine abstinence. This persistent hyperactivity may cause further alterations in brain homeostasis such as changes in mRNA and protein levels, neurotransmitter levels, and synaptic plasticity. The observed increases in BDNF and CRF gene expression following stress only occur in cocaine-experienced animals that have undergone prolonged abstinence. This suggests that these gene changes, which appear to be in part mediated by chromatin remodeling, may contribute to the mechanisms that underlie drug craving and relapse. As both BDNF and CRF have been shown to be involved in cocaine reinstatement in animal models, further examination of the way in which cocaine abstinence alters stress-induced regulation of these genes is vital to understanding how stress enhances susceptibility to relapse.
Acknowledgements
The authors would like to acknowledge Tamar L. Gur, Cedric Mombereau and Steve Mague for their excellent technical assistance.
Abbreviations
- AcH3
acetylated histone 3
- AMYG
amygdala
- BDNF
brain-derived neurotrophic factor
- BNST
bed nucleus of the stria terminalis
- ChIP
chromatin immunoprecipitation
- CRF
corticosterone releasing factor
- HPA axis
hypothalamic-pituitary-adrenal axis
- NAc
nucleus accumbens
- VTA
ventral tegmental area
References
- Adams JB, Heath AJ, Young SE, Hewitt JK, Corley RP, Stallings MC. Relationships between personality and preferred substance and motivations for use among adolescent substance abusers. Am J Drug Alcohol Abuse. 2003;29:691–712. doi: 10.1081/ada-120023465. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine-but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997;132:289–95. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Black YD, Maclaren FR, Naydenov AV, Carlezon WA, Jr., Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci. 2006;26:9656–65. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Dansky BS, Carrigan MH, Brady KT. Acute and protracted cocaine abstinence in an outpatient population: a prospective study of mood, sleep and withdrawal symptoms. Drug Alcohol Depend. 2000;59:277–86. doi: 10.1016/s0376-8716(99)00126-x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–45. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–63. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–5. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128:408–12. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421:448–53. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. Persistent Alterations in Mesolimbic Gene Expression with Abstinence from Cocaine Self-Administration. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–37. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–7. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Effects of commitment to abstinence, positive moods, stress, and coping on relapse to cocaine use. J Consult Clin Psychol. 1991;59:526–32. doi: 10.1037//0022-006x.59.4.526. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Hoplight BJ, Vincow ES, Neumaier JF. The effects of SB 224289 on anxiety and cocaine-related behaviors in a novel object task. Physiol Behav. 2005;84:707–14. doi: 10.1016/j.physbeh.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Imaki T, Naruse M, Harada S, Chikada N, Imaki J, Onodera H, Demura H, Vale W. Corticotropin-releasing factor up-regulates its own receptor mRNA in the paraventricular nucleus of the hypothalamus. Brain Res Mol Brain Res. 1996;38:166–70. doi: 10.1016/0169-328x(96)00011-3. [DOI] [PubMed] [Google Scholar]
- Kingston RE, Narlikar GJ. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 1999;13:2339–52. doi: 10.1101/gad.13.18.2339. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci. 2004;24:6686–92. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–14. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–11. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu D, Ceng X. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol. 2001;415:203–8. doi: 10.1016/s0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157:31–9. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Taves S, Khan T, Katz ES, Sajan T, Tang LC, Cullinan WE, Ziegler DR. Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neurosci Lett. 2007;415:269–73. doi: 10.1016/j.neulet.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein R. Protein modules that manipulate histone tails for chromatin regulation. Nat Rev Mol Cell Biol. 2001;2:422–32. doi: 10.1038/35073047. [DOI] [PubMed] [Google Scholar]
- McMahon RC. Personality, stress, and social support in cocaine relapse prediction. J Subst Abuse Treat. 2001;21:77–87. doi: 10.1016/s0740-5472(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96:1825–37. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–39. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, Duman RS, Vaidya VA. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32:1504–19. doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–47. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Paine TA, Jackman SL, Olmstead MC. Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol. 2002;13:511–23. doi: 10.1097/00008877-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–37. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2007;54:355–64. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 1999;146:432–9. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Rivier C, Lee S. Stimulatory effect of cocaine on ACTH secretion: role of the hypothalamus. Mol Cell Neurosci. 1994;5:189–95. doi: 10.1006/mcne.1994.1021. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC. The elevated plus-maze: pharmacology, methodology and ethology. Wiley; 1994. Wiley. [Google Scholar]
- Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Rubins NE, Friedman JR, Le PP, Zhang L, Brestelli J, Kaestner KH. Transcriptional networks in the liver: hepatocyte nuclear factor 6 function is largely independent of Foxa2. Mol Cell Biol. 2005;25:7069–77. doi: 10.1128/MCB.25.16.7069-7077.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy CA, Van Bockstaele EJ. Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1119–29. doi: 10.1016/j.pnpbp.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Alterations of corticotropin-releasing factor-like immunoreactivity in different brain regions after acute cocaine administration in rats. Brain Res. 1993;616:315–9. doi: 10.1016/0006-8993(93)90224-b. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Satel SL, Price LH, Palumbo JM, McDougle CJ, Krystal JH, Gawin F, Charney DS, Heninger GR, Kleber HD. Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Am J Psychiatry. 1991;148:1712–6. doi: 10.1176/ajp.148.12.1712. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Borg L, Ho A, Kreek MJ. Altered HPA axis responsivity to metyrapone testing in methadone maintained former heroin addicts with ongoing cocaine addiction. Neuropsychopharmacology. 2001;24:568–75. doi: 10.1016/S0893-133X(00)00222-0. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, Akbarian S. Drug-Induced Activation of Dopamine D(1) Receptor Signaling and Inhibition of Class I/II Histone Deacetylase Induce Chromatin Remodeling in Reward Circuitry and Modulate Cocaine-Related Behaviors. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl) 2000;150:337–46. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–95. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000;68:818–29. [PubMed] [Google Scholar]
- Stout SC, Owens MJ, Nemeroff CB. Regulation of corticotropin-releasing factor neuronal systems and hypothalamic-pituitary-adrenal axis activity by stress and chronic antidepressant treatment. J Pharmacol Exp Ther. 2002;300:1085–92. doi: 10.1124/jpet.300.3.1085. [DOI] [PubMed] [Google Scholar]
- Sudarsanam P, Winston F. The Swi/Snf family nucleosome-remodeling complexes and transcriptional control. Trends Genet. 2000;16:345–51. doi: 10.1016/s0168-9525(00)02060-6. [DOI] [PubMed] [Google Scholar]
- Takeda H, Tsuji M, Yamada T, Masuya J, Matsushita K, Tahara M, Iimori M, Matsumiya T. Caffeic acid attenuates the decrease in cortical BDNF mRNA expression induced by exposure to forced swimming stress in mice. Eur J Pharmacol. 2006;534:115–21. doi: 10.1016/j.ejphar.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–89. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor upregulation during ‘binge’ cocaine administration. J Pharmacol Exp Ther. 1994;270:1387–1397. [PubMed] [Google Scholar]
- Vaidya VA, Terwilliger RM, Duman RS. Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 1999;262:1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci U S A. 2006;103:16364–9. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, Dax EM, Herning RI, Michaelson BS. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47:861–8. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Yoshioka M, Toda Y, Oshida Y, Chaki S, Hamamoto K, Morishima I. Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. J Vet Med Sci. 2004;66:1323–7. doi: 10.1292/jvms.66.1323. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Ho A, Kreek MJ. Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic ‘binge’ cocaine. Brain Res Mol Brain Res. 2003;114:73–9. doi: 10.1016/s0169-328x(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther. 1996;279:351–8. [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–81. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]