Abstract
Objective:
The significant weight loss observed with combination naltrexone-sustained release (SR) 32 mg and bupropion SR 360 mg (NB32) therapy is thought to be due, in part, to bupropion stimulation of hypothalamic pro-opiomelanocortin (POMC) neurons, and naltrexone blockade of opioid receptor-mediated POMC autoinhibition, but the neurobiological mechanisms are not fully understood. We assessed changes in brain reactivity to food cues before and after NB32 treatment.
Methods:
Forty women (31.1±8.1 years; body mass index: 32.5±3.9) received 4 weeks of NB32 or placebo, and were instructed to maintain their dietary and exercise habits. Functional magnetic resonance imaging responses (analyzed using SPM2 and clusters (>100 pixels)) to a 5-min food video (preparation of the subject's favorite food) and a 5-min neutral video (manipulation of neutral objects) under conditions of mild food deprivation (∼14 h) were assessed before and after treatment.
Results:
The food cues video induced positive brain activation in visual and prefrontal cortices, insula and subcortical brain regions. The group-by-treatment interaction on regional brain activation was significant and showed that whereas NB32 attenuated the activation in the hypothalamus in response to food cues (P<0.01), it enhanced activation in regions involved in inhibitory control (anterior cingulate), internal awareness (superior frontal, insula, superior parietal) and memory (hippocampal) regions (whole-brain analysis; P<0.05).
Conclusions:
Blunting the hypothalamic reactivity to food cues while enhancing the activation of regions involved with self-control and internal awareness by NB32 might underlie its therapeutic benefits in obesity.
Keywords: fMRI, naltrexone, bupropion
Introduction
Although a number of signals that regulate food intake originate from internal sources that monitor the metabolic state of the body (that is, leptin, insulin, ghrelin and peptide YY), variables other than nutritional needs also profoundly influence food intake. These include pleasurable sensory responses from food, emotional variables and environmental factors.1 Disruption in the sensitivity of the brain to these non-nutritional-related variables could result in excessive eating and obesity. Of particular relevance are the rewarding and conditioned responses triggered by palatable foods. Functional magnetic resonance imaging (fMRI) studies using blood oxygen level-dependent (BOLD) method showed that obese subjects activated brain regions related to motivation (dorsal striatum), salience attribution (orbitofrontal cortex) and taste information processing (insula) while viewing pictures of high-caloric food, which act as powerful food cues that generate food craving.2, 3 Imaging studies have also reported that obese subjects show greater disinhibition, which is associated with decreased activation in the anterior cingulate gyrus when exposed to food cues.4
Combination naltrexone-sustained release (SR) 32 mg and bupropion SR 360 mg (NB32) is a fixed dose drug combination under investigation as a treatment for obesity.5 Bupropion is approved for marketing in the United States for depression and smoking cessation. Functionally, bupropion is thought to increase the level of dopamine (DA) activity at specific brain regions, which appears to lead to a reduction in appetite and increase in energy expenditure. Bupropion is used in the treatment of depression not only for its clinical efficacy but also because of its side effect profile, which includes modest weight loss.6 Bupropion is also used as a treatment for smoking cessation, and ongoing trials are evaluating its utility to treat other types of drug addictions.7 Naltrexone is approved in the United States for the treatment of opioid addiction and for the treatment of alcoholism. Naltrexone works by blocking opioid receptors in the brain and inhibits the reinforcing aspects of addictive substances, reducing their perceived reward.8 Naltrexone might also decrease reward sensitivity to natural reinforcers as shown by reports of reduced reward to sweet-tasting foods in opioid addicts treated with naltrexone.9 Moreover, the combination of naltrexone and bupropion has been demonstrated to result in greater weight loss compared with either agent alone.5 The neurobiological mechanisms underlying the weight loss effects of NB32 are not fully understood but are thought to relate to effects on the reward system as well as bupropion stimulation of hypothalamic pro-opiomelanocortin (POMC) neurons, and naltrexone blockade of opioid receptor-mediated POMC auto-inhibition, with downstream effects to reduce food intake and increase energy expenditure. It may involve hypothalamic and brain stem mechanisms in which synergistic effects with the drug combination on food consumption have been reported.10 The present study assessed changes in brain reactivity to food cues before and after NB32 treatment using fMRI. We hypothesized that NB32 would decrease the reactivity of brain regions involved with reward while decreasing the deactivation of regions involved with regulatory control, relative to placebo.
Materials and methods
Subjects
Before study initiation, participants provided written informed consent approved by the Stony Brook University's Committee on Research Involving Human Subjects. Subjects were screened carefully with a detailed medical history, physical and neurological examination and urine toxicology for psychotropic drugs to ensure they were healthy at the time of the study and that they were not abusing drugs. Subjects (n=46) were included in the study if they were women, right-handed, 18–45 years old, healthy, able to understand and give informed consent and had 27⩽body mass index (BMI)⩽40 kg m−2. Female subjects were used to minimize variability, because the vast majority of individuals who use pharmacotherapy for obesity are women. Exclusion criteria included obesity of known endocrine or genetic origin; history or presence of hepatic, renal, cardiovascular or gastrointestinal diseases; type 1 or type 2 diabetes mellitus requiring pharmacotherapy; serious psychiatric illness; bulimia or anorexia nervosa; history of alcohol or drug abuse or dependence (including nicotine); positive urine pregnancy test; head trauma with loss of consciousness >5 min; and any medical condition that may alter cerebral function or contraindications for magnetic resonance imaging (MRI). Subjects were asked to have their last meal completed by 1900 hours the evening before the day of the imaging visits and were scanned between 15 and 17 h after their last meal. Subjects were informed that blood sugar levels would be checked during the study to help ensure that they refrained from eating.
Study design
Subjects had two imaging visits, one at baseline and one after 4 weeks of treatment with NB32 or placebo. After the baseline visit, half of the participants were randomly assigned to the NB32 group and the other half were assigned to the placebo group. During the imaging visits, subjects underwent fMRI scans with the food video (FV) paradigm under fasting conditions. Study medication was withheld on the days of the scans until after completion of fMRI scans. The subjects were instructed to maintain their usual eating and exercise habits throughout the study to minimize the impact of changes in nutritional status or body weight on the brain activity.
NB32 and placebo administration
The naltrexone/bupropion combination evaluated in this study consisted of daily doses of NB32, combined in a trilayer tablet (each containing naltrexone SR 8 mg, bupropion SR 90 mg and an inert layer between). Study drugs were escalated to full dose over 3 weeks as follows: one tablet in the morning for 7 days→ one tablet in the morning and one tablet in the evening for 7 days→ two tablets in morning and one tablet in evening for following 7 days→ two tablets twice a day thereafter. Active and placebo tablets were blue, round and identical in appearance.
Food video paradigm
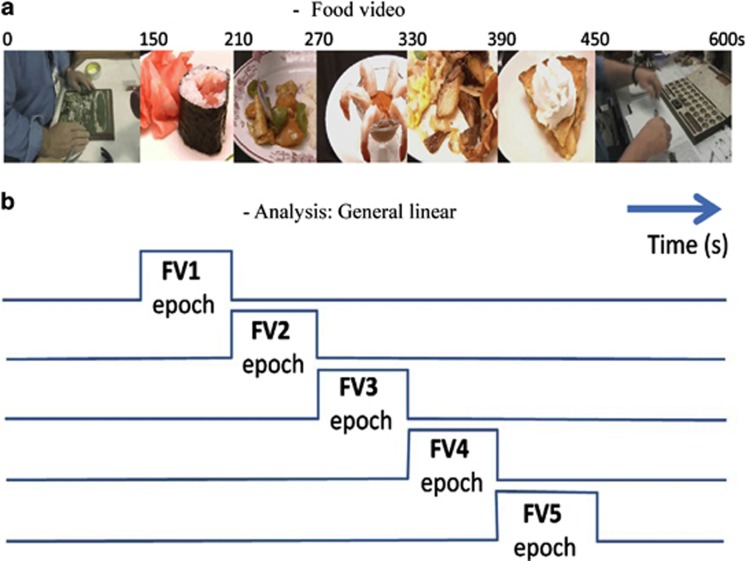
The subjects underwent an fMRI session at the baseline visit and at the week 4 visit. The day before the baseline visit, subjects rated their food preferences on 47 available food items in a pictographic ‘a la carte menu', which corresponded to 47 different FV fragments that were rated from 0 (less preferred) to 10 (most preferred). These food rating (‘liking') scores were used to select the 10 most preferred food items and their corresponding FV fragments for each subject. Each of these 1-min-long high-resolution FV fragments show close views of serving and consumption of the food items that were recorded indoors and saved in audio video interleave format by professional video personnel at the Brookhaven National Laboratory. The preferred FV fragments were contrasted against two different 2.5-min ‘non-food' control video fragments, which include routine administrative/technical work as control items, which were recorded indoors with the same resolution and format by the video personnel. For this purpose, 10-min-long movies with blocked design were created taking into account the subject's preferences. Each movie was composed by a control video epoch (beginning), the five FV fragments (random order) and a control video (end) epoch (Figure 1a). These different movies were presented to the subjects on MRI-compatible goggles connected to a personal computer during fasting condition. Image acquisition was performed continuously during the 10-min duration of the movie. The display software was written in Visual Basic and C languages and synchronized precisely with the MRI acquisition using a trigger pulse.
Figure 1.
fMRI paradigm. (a) Example of the timeline of the food-video stimulation. Each 600 seconds video contained 150 seconds control video (CV) fragments at its beginning and end, as well as 5-60 second food video (FV) fragments showing serving and consumption of the subject's favorite food items. (b) The general lineal model implemented in SPM2 was based on a castle design with 5 regressors modeling the FV epochs, which contrasted the FV fragments against the CV fragments.
MRI data acquisition
Subjects underwent MRI in a 4-Tesla whole-body Varian (Palo Alto, CA, USA)/Siemens (Erlangen, Germany) MRI scanner. A T2*-weighted single-shot gradient-echo planar imaging pulse sequence (echo time/repetition time (TE/TR)=20/1600 ms, 4-mm slice thickness, 1-mm gap, 35 coronal slices, 64 × 64 matrix size, 3.125 × 3.125 mm2 in-plane resolution, 90° flip angle, 375 time points, 200.00 kHz bandwidth) with ramp-sampling and whole-brain coverage was used to collect functional images with BOLD contrast. Padding was used to minimize motion. Subject's motion was monitored immediately after each fMRI run using a k-space motion detection algorithm11 written in Interactive Data Language (ITT Visual Information Solutions, Boulder, CO, USA). Earplugs (−28 dB sound pressure level attenuation; Aearo Ear TaperFit 2; Aearo Company, Indianapolis, IN, USA), headphones (−30 dB sound pressure level attenuation; Commander XG MRI Audio System, Resonance Technology Inc., Northridge, CA, USA) and a ‘quiet' acquisition approach were used to minimize the interference effect of scanner noise during fMRI.12 Anatomical images were collected using a T1-weighted three-dimensional modified driven equilibrium Fourier transform pulse sequence13 (TE/TR=7/15 ms, 0.94 × 0.94 × 1.00 mm3 spatial resolution, axial orientation, 256 readout and 192 × 96 phase-encoding steps, 16 min scan time) and a modified T2-weighted hyperecho sequence14 (TE/TR=0.042/10 s, echo train length=16, 256 × 256 matrix size, 30 coronal slices, 0.86 × 0.86 mm2 in-plane resolution, 5 mm thickness, no gap, 2 min scan time), and were reviewed by a neurologist to rule out gross morphological abnormalities of the brain.
fMRI analysis
Image reconstruction was performed using an iterative phase correction method in Interactive Data Language that minimizes signal loss artifacts in echo planar imaging.15 The first four imaging time points were discarded to avoid nonequilibrium effects in the fMRI signal. The statistical parametric mapping package SPM2 (Wellcome Trust Centre for Neuroimaging, London, UK) was used for subsequent analyses. A 4th degree B-spline function without weighting and without warping was used for image realignment (head motion was <2-mm translations and 2° rotations for all scans for all fMRI runs). Spatial normalization to the stereotactic space of the Montreal Neurological Institute was performed using a 12-parameter affine transformation with medium regularization, 16-nonlinear iterations and voxel size of 3 × 3 × 3 mm3 and the standard SPM2 echo planar imaging template. Spatial smoothing was carried out using an 8-mm full-width-half-maximum Gaussian kernel. BOLD–fMRI responses during the FV stimulation paradigm were estimated using a general linear model16 and a castle design matrix with five different FV regressors (Figure 1b), corresponding to the five FV fragments, convolved with low-pass (hemodynamic response function) and high-pass (cutoff frequency: 1/1200 Hz) filters. Thus, five contrast maps reflecting the % BOLD–fMRI signal change from baseline were obtained in fasting conditions and five in satiated conditions for each subject.
Statistical analyses
The BOLD–fMRI signals were included in a one-way analysis of variance (ANCOVA) model in SPM2 with two covariates: a zero-mean regressor reflecting the age of the participants and a zero-mean regressor reflecting the subjects' BMI. Brain activation clusters were corrected for multiple comparisons using the continuous random field calculation implemented in SPM2. The statistical significance for group analyses of the brain activation was based on a family-wise error (FWE) threshold Pcorr<0.05, corrected for multiple comparisons at the voxel level, and a minimum cluster size of 20 voxels. The ANCOVA model included the terms for treatment and the appropriate baseline measurement. Type 3 sum of squares for the least square mean was used for the statistical comparison with 95% confidence interval also reported.
Functional ROI analyses
Brain activation clusters were further evaluated with region-of-interest (ROI) analyses to identify potential outliers and to report average values in a volume comparable to the image smoothness (for example, resolution elements or ‘resels')17 rather than single-voxel peak values. The volume of the resels was estimated using the random field calculation in SPM2 as a near cubic volume with Cartesian full-width-half-maximum=12.7 × 12.4 × 13.5 mm3. Thus, 9-mm isotropic masks containing 27 imaging voxels (0.73 ml) were defined at the centers of relevant activation clusters to extract the average %BOLD signal from individual contrast maps. These masks were created and centered at the precise coordinates listed in Table 2; the coordinates of the ROI masks were kept fixed across subjects and conditions. In addition to these functional ROIs, we analyzed the BOLD signals in the hypothalamus with anatomical 9-mm cubic ROI centered at Montreal Neurological Institute coordinates (x, y, z)=(0, −3, −6) mm to test for effects of the drug combination on reactivity to food cues in the hypothalamus. The average and s.d. values of BOLD signals within these ROIs were computed for each subject and fMRI run using a custom program written in Interactive Data Language.
Results
Forty-six non-smoking and right-handed women were recruited for the study. Three subjects withdrew from each treatment group (NB32: all because of adverse events (vomiting, headache and depression); placebo: one adverse event (migraine), one lost to follow-up and one failure to comply with protocol). Data from the 40 subjects who adhered to the treatment protocol and completed the fMRI studies are reported here. At baseline, the NB32 group and the placebo group were similar in age and BMI (Table 1). Body weight was not changed in the NB32 group or in the placebo group after 1 month of treatment (Table 1).
Table 1. Baseline characteristics of study subjects and weight over time.
|
Baseline characteristics | ||||
|---|---|---|---|---|
|
NB32 |
Placebo |
|||
| Mean±s.d. | Range | Mean±s.d. | Range | |
| Age (years) | 30.9±7.8 | 20–44 | 31.4±8.5 | 19–45 |
| BMI (kg m−2) | 33.0±4.6 | 27.7–40.4 | 32.0±3.2 | 27.3–37.6 |
|
Weight over time | ||||
| |
NB32 |
Placebo |
Placebo-corrected difference |
P-value |
| Weight | ||||
| Baseline (kg) | 87.9±17.0 | 90.3±14.2 | NA | NA |
| % Change at week 4 | −0.99±0.44 | −0.43±0.44 | −0.56 | 0.38 |
Abbreviations: BMI, body mass index; NA, not applicable; NB32, naltrexone-sustained release (SR) 32 mg and bupropion SR 360 mg.
Baseline values are mean±s.d. Changes are least square mean±s.e.
N=20 for both NB32 and placebo groups.
Brain activation
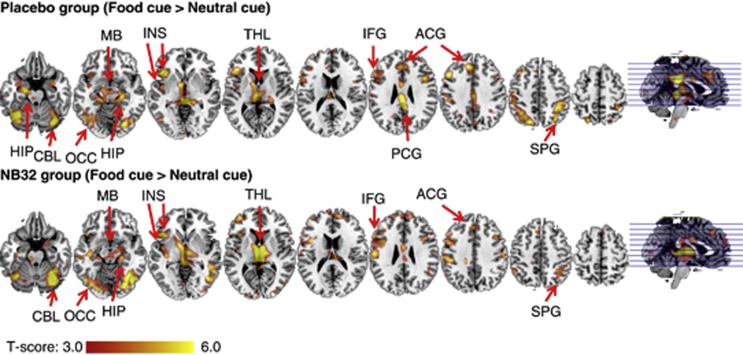
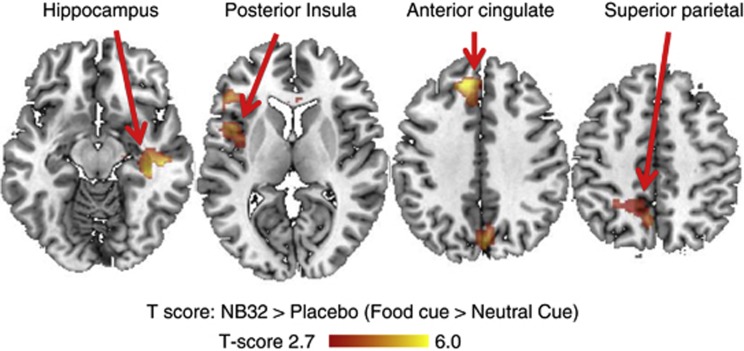
The FV paradigm caused positive BOLD–fMRI responses (from control video epochs to FV epochs) in visual and prefrontal cortices, insula and subcortical brain regions (cerebellum, thalamus and hippocampus) separately for the placebo and NB32 groups and for baseline and treatment (PFWE<0.05, ANCOVA; Figure 2 and Figure 3). At baseline, the NB32 group did not show significant activation in hippocampus, superior parietal cortex and posterior insula, whereas the placebo group showed activation in these regions (PFWE<0.05). However, brain activation differences between the placebo and NB32 groups at baseline were not statistically significant (Table 2). The NB32 group had higher activation in anterior, middle and posterior cingulum, superior frontal and middle temporal cortices, superior parietal cortex and posterior insula after 4-week treatment than that at baseline (Table 2). Conversely, the placebo group had lower activation in anterior, middle and posterior cingulum, superior frontal and superior parietal cortices, hippocampus and parahippocampus after 4-week treatment than that at baseline (Table 2). There was a group-by-treatment interaction on brain activation in the superior frontal (Brodmann area: BA32), dorsal anterior cingulate (BA32), posterior insula, superior parietal (BA5) and hippocampal regions. These group-by-medication interaction effects were statistically significant (PFWE<0.05; Table 2).
Figure 2.
The effect of NB32 and placebo on brain activity during food cue stimulation. Significant activated clusters during FV stimulation. SPG, superior parietal gyrus; ACG, anterior cingulate gyrus; PCG, posterior cingulate gyrus; IFG, inferior frontal gyrus; THL, thalamus; INS, insula; MB, midbrain; OCC, occipital cortex; HIP, hippocampus; CBL, cerebellum.
Figure 3.
The greater effect of NB32 as compared with placebo in response to visual food cues.
Table 2. Spatial coordinates of significant activated clusters during food video stimulation in the MNI stereotactic space (P<0.05; t-tests). Average t-score values in isotropic cubic regions of interest (27 voxels; 0.73 cc) centered at the (x, y, z) coordinates.
| Region |
Coordinates |
t-score |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
mm |
PL |
NB |
PL>NB |
NB>PL |
NB>PL |
||||||||
| BA | x | y | z | BL | Tx | Tx>BL | BL | Tx | Tx>BL | BL | Tx | (Tx>BL) | |
| Superior frontal | 32 | −12 | 39 | 39 | NS | NS | −3.3 | NS | 3.7 | NS | NS | 3.8 | 4.2 |
| Anterior cingulate | 32 | −9 | 30 | 33 | NS | NS | −4.7 | NS | 3.8 | NS | NS | 4.1 | 4.8 |
| Hippocampus | 20 | 36 | −27 | −12 | 4.4 | NS | NS | NS | 4.7 | 4.4 | NS | 3.4 | 4.0 |
| Hippocampus | 20 | 33 | −6 | −27 | NS | NS | −6.0 | NS | NS | NS | NS | NS | 3.4 |
| Superior parietal | 5 | 18 | −45 | 66 | 3.2 | −3.6 | NS | NS | NS | 3.2 | NS | 3.8 | 4.4 |
| Posterior insula | 48 | 42 | −30 | 18 | 3.6 | NS | NS | NS | NS | 3.6 | NS | 3.1 | 4.0 |
Abbreviations: BA, Brodmann area; BL, baseline (food cue>neutral cue); MNI, Montreal Neurological Institute; NB, NB32 group; NB>PL(Tx>BL), group × treatment interaction; NS, not significant; PL, placebo group; Tx, after 4-week treatment (food cue>neutral cue). Tx>BL, treatment interaction.
N=20 for both the NB32 and placebo groups.
The BOLD–fMRI responses in the anatomical ROI in the hypothalamus did not show significant group differences at baseline. Hypothalamic activation after 4-week treatment was lower than that at baseline for the NB32 group (P<0.002) but there were no significant differences in hypothalamic activation in the placebo group (P>0.4). The group-by-medication interaction effect in the hypothalamus was also significant (P<0.01).
Discussion
Contrary to our initial hypothesis, participants treated with NB32 had enhanced activation in superior parietal (BA5), posterior insular, superior frontal (BA32), dorsal anterior cingulate (BA32), hippocampal regions and decreased activation in hypothalamus while viewing FVs as compared with placebo-treated participants under fasted conditions. We did not observe changes in brain regions such as nucleus accumbens and amygdala, which have been reported to become activated during imaging studies in response to food cues. It is possible that the food cue paradigm used in this study was not sufficient to induce activation in these areas.
The NB32-treated subjects, when compared with subjects who received placebo, showed increased activation in the superior frontal gyrus (BA32) and dorsal anterior cingulate (BA32) when exposed to the food cues. The superior frontal gyrus is associated with self-awareness, which is involved in introspection and linked to sensory perception. Introspection is a very high-level cognitive task that may include increases in attentional, memory and cognitive demands. When the brain diverts its resources to do challenging tasks, regions related to self-awareness are not engaged during sensory perception and can be suppressed.18 In this study, the decreased activation or no activation to the food cues in the superior frontal gyrus during baseline condition and after the placebo treatment would be consistent with the suppression of introspection. As the treatment with naltrexone reduces sensory perception of food cues,19 the enhanced reactivity of the superior frontal gyrus in NB32-treated group would be suggestive of an enhancement in self-awareness.
The anterior cingulate regulates both cognitive (dorsal cingulate) and emotional processing (ventral cingulate). The dorsal cingulate is associated with response conflict, decision-making and self-control. Using positron emission tomography to measure regional brain glucose metabolism in healthy controls (BMI range, 19–37 kg m−2) during baseline (no stimulation), we showed a negative correlation between BMI and metabolic activity in the prefrontal cortex and cingulate gyrus (BA 32).20 Moreover, baseline metabolism in the prefrontal cortex and cingulate gyrus was positively associated with performance on tests of memory and executive function. These findings suggest that excessive weight might impair the activity of the prefrontal cortex and cingulate. In obese subjects, decreased metabolism in the prefrontal cortex and cingulate, which was associated with low levels of striatal DA D2 receptors,21 might contribute to excessive food intake by interfering with self-regulation, thus favoring impulsive and compulsive food intake. Thus, the enhanced reactivity of the dorsal cingulate in subjects that received NB32 treatment during exposure to food cues would be consistent with an enhancement in self-control. Indeed, the therapeutic effects of bupropion in smoking cessation are believed to be partly through enhancement of the anterior cingulate activation with concomitant improvement in the ability to resist craving.22 These reports mirror the results of the current study in which elevated baseline dorsal anterior cingulate activity was found in NB32-treated subjects. As bupropion increases DA and norepinephrine,23 and both norepinephrine cells originating in the locus ceruleus and DA cells originating in the ventral tegmental area innervate the ventral anterior cingulate directly,24 the enhanced activation of the cingulate could reflect increases in catecholamine signaling.
Subjects treated with NB32 also showed an enhanced response in the hippocampus, which is a brain region connected to the hypothalamus and insula. Preclinical studies show that hippocampal damage can result in hyperphagia.25 The hippocampus modulates DA release in the ventral striatum, which is a mechanism by which the saliency of a stimulus is modulated.26 It also regulates activity in prefrontal regions involved with inhibitory control.27 Imaging studies have shown that obese and previously obese individuals have decreased responses in posterior hippocampus when tasting a liquid meal when compared with lean subjects. Persistence of an abnormal hippocampal response in the previously obese was associated with their susceptibility to relapse.28 In two independent fMRI studies one of which evaluated activation responses to an implantable gastric stimulator developed for the treatment of obesity and the other measured the activation responses to balloon gastric distention, we showed activation of the hippocampus presumably from downstream stimulation of the vagus nerve and the solitary nucleus.29, 30 In these studies, we found the activation in the hippocampus was associated with a sensation of fullness. Thus, enhanced reactivity of the hippocampus by NB32 could also help interfere with excessive food intake.
Subjects treated with NB32 also showed enhanced brain activity in the insula, in addition to the enhanced response in the superior frontal and anterior cingulate regions. These regions appear to have a critical role in the initiation, maintenance, and adjustment of attentional control31 and in interoception.18, 32 Interoception refers to the monitoring of internal bodily states to maintain or procure homeostasis, possibly by rousing the organism through affective, motivational and attentional mechanisms. The insula is organized into multiple regions along a posterior-to-anterior gradient.33 The posterior insula has been related to primary interoceptive operations, which is connected with primary and secondary somatosensory cortices and receives inputs from the hypothalamus, the amygdala and the limbic system.34 Somatic and visceral sensory processing, taste perception and food cues activate the posterior insula.33 The signals are then sent to the anterior insula, which is associated with affective and cognitive processes. Imaging studies showed hunger is associated with an increase in regional cerebral blood flow in the mid-posterior insular cortex, which attenuates with satiation.35 Similarly, using fMRI we showed that distention of the stomach (using an inflatable balloon to mimic the gastric distension that occurs with food intake) activated the posterior insula, which most likely reflected its role in the awareness of body states.29 Interestingly, obese individuals showed decreased cerebral blood flow35 and decreased functional connectivity strength36 in the posterior insula when they were hungry, suggesting impairment in interoceptive awareness. Thus, overeating in obese subjects could reflect in part an impaired awareness of internal hunger/satiety states secondary to impaired function of the posterior insula. Naltrexone, which reduces food intake in animal models of obesity and binge eating,37 has been shown to modulate insular and dorsal striatal activity during food-related cues.19 Thus, the reported loss of body weight with treatment with NB325, 10 could also relate to the greater reactivity of the posterior insula and a concomitant enhanced awareness and attention to interoceptive signals that reflect satiety (that is, fullness).
Finally, subjects treated with NB32 also showed an enhanced response in the superior parietal cortex (BA 5), which is a brain region involved with somatosensory processing,38 spatial attention and conscious pain perception.39 The activation of BA5 after NB32 suggests increased attention to the processing of the food cue experiment.
The enhanced regions observed in the current study (anterior cingulate, frontal cortex and hippocampus) overlap with regions of relatively decreased response to a Simon spatial incompatibility task in individuals with bulimia nervosa.40 Women with bulimia nervosa have less self-regulatory control and respond more impulsively and make more errors to the task than healthy subjects. These tasks usually need greater engagement from frontostriatal regions. The enhanced activation of these regions after NB32 could reflect a functional enhancement of self-regulatory control processes in response to food cues.
Activation in the hypothalamus to food cues during fasting condition has been previously been demonstrated in fMRI studies.41, 42, 43 The hypothalamus activation was attenuated when the lean subjects were overfed,43 but not in overweight and obese individuals,41 nor in obese subjects who have lost weight.42 A separate fMRI study demonstrated a delayed hypothalamic response after glucose ingestion in obese subjects.44 Taken together, these studies suggest that obese subjects may have sluggish or absent homeostatic responses to satiety in the hypothalamus. In a post hoc analysis, we found that after NB32 treatment, subjects displayed attenuated activation in the hypothalamus in response to food cues. This overall attenuation of the hypothalamic response to food cues presumably occurs in juxtaposition to the enhanced activation of a subset of hypothalamic neurons (the POMC neurons), and reveals the complexity of the hypothalamus as an integrator of hunger and satiety signals. Interestingly, overall attenuated hypothalamic activity to food cues has also been observed following treatment with sibutramine, a predominantly serotonin and norepinephrine reuptake inhibitor thought to operate through a distinct mechanism of action compared with naltrexone/bupropion.41
It is noteworthy that little weight loss was observed in the NB32 subjects in this study. The study was designed to be of short duration and subjects were instructed to maintain their typical diet and exercise to minimize weight loss and the associated metabolic changes that could influence regional brain activity and confound the direct pharmacological effects of NB32 on brain. Thus, the changes in BOLD signal reported are more likely to be related to NB32 treatment than to any potential compensatory effects of weight loss.
Limitations of this study are: (1) when the subjects were retested 1 month after the treatment (placebo or drug), they showed decreased activation in response to the FV stimulation paradigm (Table 2). Decreased response to repeated stimulation paradigms (that is, semantic memory, working memory and visual attention) have previously been reported in other fMRI studies. These studies suggest that practice/habituation effects can reduce brain activation for visual tasks that involve attention and memory. (2) Only female subjects were enrolled in this study. Although this is consistent with the population of most obesity pharmacotherapeutic studies, caution should be used in extending these findings to men. (3) We did not observe significant correlations between subjective appetitive responses and brain activation responses to food cues, which would have facilitated the interpretation of our findings.
Conclusion
In a fasted condition and compared with the placebo group, the NB32 group following exposure to food cues had decreased activation in the hypothalamus and enhanced activation in the dorsal anterior cingulate, superior frontal, posterior insula, hippocampal and superior parietal regions, which are brain regions involved in inhibitory control, internal awareness, memory/conditioning and somatosensory processing. These findings suggest that, in addition to hypothalamic mechanisms, NB32-induced weight loss may also be because of changes in cortical reactivity to food cues, particularly brain regions implicated in interoception, memory and self-control.
Acknowledgments
We thank Karen Apelskog-Torres for study protocol preparation, Millard Jayne for subject recruitment, Barbara Hubbard and Pauline Carter for patient care and Orexigen Therapeutics for scientific review of the manuscript. The functional MR study was carried out at Brookhaven National Laboratory with support from Orexigen Therapeutics Inc. (GJW) and in part from Intramural Research Program of the National Institute on Alcoholism and Alcohol Abuse, Z01AA000550 (NDV, FT, MJ).
GJW reports having received lecture fees and research funding from Orexigen Therapeutics Inc.; ED was an employee of Orexigen Therapeutics Inc. during the study. The remaining authors declare no conflicts of interest.
References
- Patel KA, Schlundt DG. Impact of moods and social context on eating behavior. Appetite. 2001;36:111–118. doi: 10.1006/appe.2000.0385. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17–24. doi: 10.1586/14737175.7.1.17. [DOI] [PubMed] [Google Scholar]
- Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24:541–550. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Heberlein A, Muschler MA, Bleich S, Frieling H. Opioid modulators for alcohol dependence. Expert Opin Investig Drugs. 2011;20:1073–1086. doi: 10.1517/13543784.2011.592139. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Busch EL, O'Brien CP, Elman I. Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence. Psychopharmacology. 2012;220:559–564. doi: 10.1007/s00213-011-2503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab. 2009;94:4898–4906. doi: 10.1210/jc.2009-1350. [DOI] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. NeuroImage. 2003;20:1411–1418. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. NeuroImage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Hennig J, Scheffler K. Hyperechoes. Magn Reson Med. 2001;46:6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- Caparelli E, Tomasi D. K-space spatial low-pass filters can increase signal loss artifacts in echo-planar imaging. Biomed Signal Process Control. 2008;3:107–114. doi: 10.1016/j.bspc.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Franckowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Worsley K, Evans A, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Beaver J, Makwana A, Searle G, Long C, Nathan PJ, et al. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans Mol Psychiatry 201116826–35.,785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity. 2009;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KF, DeSanty KP, Kast RE. Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother. 2006;6:1249–1265. doi: 10.1586/14737175.6.9.1249. [DOI] [PubMed] [Google Scholar]
- Mundorf ML, Joseph JD, Austin CM, Caron MG, Wightman RM. Catecholamine release and uptake in the mouse prefrontal cortex. J Neurochem. 2001;79:130–142. doi: 10.1046/j.1471-4159.2001.00554.x. [DOI] [PubMed] [Google Scholar]
- Forloni G, Fisone G, Guaitani A, Ladinsky H, Consolo S. Role of the hippocampus in the sex-dependent regulation of eating behavior: studies with kainic acid. Physiol Behav. 1986;38:321–326. doi: 10.1016/0031-9384(86)90101-0. [DOI] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci USA. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taepavarapruk P, Howland JG, Ahn S, Phillips AG. Neural circuits engaged in ventral hippocampal modulation of dopamine function in medial prefrontal cortex and ventral striatum. Brain Struct Funct. 2008;213:183–195. doi: 10.1007/s00429-008-0177-1. [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, et al. Gastric distention activates satiety circuitry in the human brain. NeuroImage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci USA. 2006;103:15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD.Interoceptive cortex in the posterior insula: comment on Garcia-Larrea et al. 2010 Brain 133, 2528 Brain 2011134(Pt 4e166author reply e165. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD, Zhang ET. Retrograde analyses of spinothalamic projections in the macaque monkey: input to posterolateral thalamus. J Comp Neurol. 2006;499:953–964. doi: 10.1002/cne.21155. [DOI] [PubMed] [Google Scholar]
- Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, et al. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring HU, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH. Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behav Pharmacol. 2009;20:537–548. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Grefkes C, Palomero-Gallagher N, Schleicher A, Zilles K. Subdivisions of human parietal area 5 revealed by quantitative receptor autoradiography: a parietal region between motor, somatosensory, and cingulate cortical areas. NeuroImage. 2005;25:975–992. doi: 10.1016/j.neuroimage.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Witting N, Kupers RC, Svensson P, Arendt-Nielsen L, Gjedde A, Jensen TS. Experimental brush-evoked allodynia activates posterior parietal cortex. Neurology. 2001;57:1817–1824. doi: 10.1212/wnl.57.10.1817. [DOI] [PubMed] [Google Scholar]
- Marsh R, Horga G, Wang Z, Wang P, Klahr KW, Berner LA, et al. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry. 2011;168:1210–1220. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Napolitano A, Skeggs A, Miller SR, Delafont B, Cambridge VC, et al. Distinct modulatory effects of satiety and sibutramine on brain responses to food images in humans: a double dissociation across hypothalamus, amygdala, and ventral striatum. J Neurosci. 2010;30:14346–14355. doi: 10.1523/JNEUROSCI.3323-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr. 2007;86:965–971. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes. 1999;48:1801–1806. doi: 10.2337/diabetes.48.9.1801. [DOI] [PubMed] [Google Scholar]