Abstract
Oxygen and oxidative stress have become relevant components in clarifying the mechanism that weakens bacterial cells in parallel to the mode of action of bactericidal antibiotics. Given the importance of oxidative stress in the overall defense mechanism of bacteria and their apparent role in the antimicrobial mode of action, it is important to understand how bacteria respond to this stress at a metabolic level. The aim of this study was to determine the impact of oxygen on the metabolism of the facultative anaerobe Enterococcus faecalis using continuous culture, metabolomics, and 13C enrichment of metabolic intermediates. When E. faecalis was rapidly transitioned from anaerobic to aerobic growth, cellular metabolism was directed toward intracellular glutathione production and glycolysis was upregulated 2-fold, which increased the supply of critical metabolite precursors (e.g., glycine and glutamate) for sulfur metabolism and glutathione biosynthesis as well as reducing power for cellular respiration in the presence of hemin. The ultimate metabolic response of E. faecalis to an aerobic environment was the upregulation of fatty acid metabolism and benzoate degradation, which was linked to important changes in the bacterial membrane composition as evidenced by changes in membrane fatty acid composition and the reduction of membrane-associated demethylmenaquinone. These key metabolic pathways associated with the response of E. faecalis to oxygen may represent potential new targets to increase the susceptibility of this bacterium to bactericidal drugs.
INTRODUCTION
Enterococcus faecalis is a Gram-positive facultative anaerobe that naturally inhabits the human gastrointestinal tract. This organism belongs to the lactic acid bacteria (LAB) group, which are identified by a low G+C content and are able to grow in a broad range of temperatures (1). The LAB clade members have been extensively used in the food industry as probiotics, due to their ability to produce bacteriocins; as flavor enhancers; and in the process of cheese ripening (2–4). However, E. faecalis features prominently as an opportunistic pathogen in hospital-acquired infections. It is responsible for a wide variety of infections (e.g., surgical site, urinary tract, visceral, and bloodstream infections and endocarditis) (1, 5–8). In addition, it exhibits a number of characteristics that make it an ideal agent for nosocomial infections: resistance to high temperatures, harsh chemicals, and drying (1) and the ability to survive on various hospital surfaces (1, 9).
E. faecalis strains exhibit intrinsic and acquired resistance to many antibiotics (1, 8), with an increasing occurrence of multidrug-resistant strains (e.g., vancomycin-resistant enterococci [VRE]). VRE pose a serious health threat as vancomycin is considered a last-resort antibiotic. E. faecalis is also involved in the transmission of resistance genes to other medically relevant bacterial species such as Staphylococcus aureus (10). More recently, attention within the investigation of antibiotic-resistant microbes has focused on the link between oxidative stress and bactericidal activity of antimicrobials (11–13). However, the link between the induction of reactive oxygen species (ROS) and antibiotics has been recently debated by different groups who contradict the theory behind the effect of the direct relationship between ROS and antibiotics (14–16). Nevertheless, the fact that E. faecalis is able to detoxify ROS and to produce ROS gives this organism a competitive advantage in terms of its ability to survive and to be more virulent toward the host (11). The arsenal of E. faecalis enzymes related to oxidative stress response has been described previously (17–20), demonstrating that E. faecalis provides an interesting framework for the analysis of the innate and acquired resistance to antibiotics as well as the adaptations to survive in an aerobic environment and hence resist oxidative stress.
Although E. faecalis does not present typical oxygen-dependent metabolic pathways, it is able to respire aerobically when grown in the presence of hemin (21). Proteomic studies have shown that E. faecalis produces approximately 200 different stress proteins when growing under different chemical and physical stresses (20). Many of these proteins are enzymes induced in response to oxygen, such as catalase, NADH peroxidase, NADH oxidase, superoxide dismutase, and glutathione (GSH) reductase (22). Understanding how E. faecalis responds to oxidative stress may provide vital clues into the survival of this organism in the host environment. This work may also point to new drug targets that can weaken the bacterial cells while simultaneously potentiating the action of existing antibiotics.
In this communication, we analyzed the metabolic response of an E. faecalis strain during the rapid transition from anaerobic to aerobic conditions using continuous culture, metabolomics, and 13C enrichment of metabolic intermediates. These data revealed that E. faecalis responds to oxygen by upregulating sulfur, glutathione, and glycolytic metabolism. These alterations in metabolism lead to changes in cell membrane composition by altering fatty acid metabolism and decreasing demethylmenaquinone (DMK) levels.
MATERIALS AND METHODS
Chemicals.
Methanol, chloroform, sodium bicarbonate, and sodium hydroxide were obtained from Merck (Darmstadt, Germany). The internal standard 2,3,3,3-d4-alanine, the derivatization reagent methylchloroformate (MCF), pyridine, d-glucose, glycerol, and vitamin K2 for menaquinone analysis were purchased from Sigma-Aldrich (St. Louis, MO). Anhydrous sodium sulfate was obtained from Fluka (Steinheim, Germany). All chemicals were of analytical grade.
Bacterial strain and culture conditions.
The bacterial strain used throughout all experiments was Enterococcus faecalis 5A-13 (23). This bacterium was maintained on TPA agar plates, which contained peptone (20 g liter−1), NaCl (5 g liter−1), glucose (2 g liter−1), Na2PO4 (2.5 g liter−1), agarose (15 g liter−1), and vancomycin hydrochloride (40 mg liter−1), incubated at 37°C. Preinocula were prepared by transferring a single colony of E. faecalis growing on TPA plates to TP broth (as described above without agar), followed by overnight aerobic incubation at 37°C and 200 rpm. Subsequently, 50 ml of culture broth was harvested by centrifugation (3,000 rpm, 20 min), washed once with 0.9% (wt/vol) NaCl solution, and resuspended into batch-phase culture medium to be used as the inoculum for the bioreactors. A BioFlo 3000 system from New Brunswick Scientific (Enfield, CT) was used throughout all experiments with a working volume of 1.5 liters. Each fermentation started as a batch-phase culture inoculated from the preinoculum culture growing at exponential growth phase. The batch-phase medium contained (NH4)2SO4 (5 g liter−1); KH2PO4 (6 g liter−1); MgSO4 (0.5 g liter−1); vancomycin hydrochloride (40 mg liter−1); hemin chloride (1 mg liter−1); peptone (2 g liter−1); glucose (10 g liter−1); trace metal solution (1 ml/liter) comprised of FeSO4 · 7H2O (3 g liter−1), ZnSO4 · 7H2O (4.5 g liter−1), CaCl2 · 6H2O (4.5 g liter−1), MnCl2 · 4H2O (1 g liter−1), CoCl2 · 6H2O (0.3 g liter−1), CuSO4 · 5H2O (0.3 g liter−1), Na2MoO4 · 2H2O (0.4 g liter−1), H3BO3 (1 g liter−1), KI (0.1 g liter−1), and Na2EDTA (15 g liter−1); and vitamin solution (2 ml liter−1), which contained riboflavin (0.42 g liter−1), calcium pantothenate (5.4 g liter−1), nicotinic acid (6.1 g liter−1), pyridoxal ethyl acetal HCl (1.4 g liter−1), d-biotin (0.06 g liter−1), folic acid (0.042 g liter−1), d-thiamine (1 g liter−1), and myoinositol (12.5 g liter−1). The working volume of the bioreactors was 1.5 liters. Temperature, pH, and oxygen saturation were held constant at 37°C, 7.0, and 0% (under nitrogen atmosphere), respectively. When the cells were grown in the presence of oxygen, the chemostat was set to 20% oxygen. The batch phase was carried out until carbon source (glucose) was exhausted. This was measured using dinitrosalicylic acid (DNS) reagent (24). Once the glucose level was depleted, the bioreactor was switched to continuous culture. Continuous culture allows the researcher to control environmental factors such as pH, nutrient concentration, and metabolic end products, and analysis of the effects of the external factors (such as induction of stress conditions) can be measured and analyzed. This system was set up using the same medium described for the batch phase except with a glucose concentration of 5 g liter−1. The dilution rate was set at 0.06 h−1 (low dilution rate that resembles growth in the host). Continuous culture was left on average for ∼2 residence times (time required for the entire volume of the bioreactor to be replaced twice), and then 13C6-labeled glucose was fed to the cells at 5% (wt/wt) of the total glucose. After a further residence time, samples were then taken for metabolite and 13C-distribution analyses. Oxygen was then introduced into the bioreactor until 20% oxygen saturation was reached. During the addition of oxygen, samples were taken for intracellular metabolite analysis at 3-min intervals over a 15-min period. The bioreactor was then left for a further three residence times (with 5% [wt/wt] of 13C6-labeled glucose being fed to the cells during the final residence time). Samples were harvested under aerobic steady state for metabolite and 13C-distribution analyses. Prior to sampling, the optical density (OD) was monitored to ensure that the cells were in a “steady state” (constant OD values over three residence times). This procedure was repeated three times (biological replicates, n = 3 chemostats). A diagram depicting the experimental procedure is available as Fig. S1 in the supplemental material.
Sampling and extraction procedure for intracellular metabolite analysis.
The sampling, quenching, and intracellular metabolite extraction were based on our previously published protocol (25). In summary, 50 ml of culture broth, in triplicate, was harvested and quenched from the bioreactors by rapidly mixing the sampled broth with cold glycerol-saline solution (3:2) followed by centrifugation at −20°C. The cell pellets were resuspended in cold glycerol-saline washing solution (1:1) followed by centrifugation at −20°C. Intracellular metabolites were extracted from the cell pellets after addition of cold methanol-water solution (1:1) at −20°C and the internal standard (2,3,3,3-d4-alanine), followed by three freeze-thaw cycles. Samples were then centrifuged (−20°C), and the supernatant was collected. The remaining cell pellet was then resuspended in cold pure methanol (−20°C) for a second round of extraction. The mixture was again centrifuged (−20°C), and the supernatant was collected and pooled with the previous one. The cell pellet was then resuspended in bidistilled water and centrifuged, and supernatant was collected. Twenty milliliters of cold bidistilled water (4°C) was added to the metabolite extracts, frozen, and subsequently freeze-dried using a VirTis freeze-dryer from SP Scientific (Newtown Square, PA). The remaining cell debris was used for analysis of 13C-labeled distribution in the biomass-derived amino acids.
Samples were taken in triplicate for steady-state analysis (technical replicates). During the transition state from the anaerobic to the aerobic environment, one 50-ml sample was collected every 3 min for a total of 12 min, i.e., five samples per chemostat (biological replicates, n = 3 chemostats). We have included a diagram in the experimental design which can be seen in Fig. S1 in the supplemental material.
Extracellular metabolite analysis.
Ten milliliters of culture was taken from the bioreactor and centrifuged. The supernatant was collected and membrane filtered (0.22-μm pore size). The filtrate was then separated into three aliquots (3 ml), and internal standard (2,3,3,3-d4-alanine) was added to each of them before they were frozen and subsequently freeze-dried on a VirTis freeze-dryer (SP Scientific).
Hydrolysis of protein biomass for amino acid composition analysis.
The procedure for hydrolysis of protein biomass for amino acid composition analysis has been adapted from a protocol of Christensen and Nielsen (26). Following extraction of intracellular metabolites, the remaining cell debris was resuspended in 100% methanol (1 ml) and evaporated to dryness. The cell debris was then hydrolyzed using 6 M HCl (1 ml), incubated at 100°C for 24 h, and then evaporated to dryness using a SpeedVac system from Thermo Scientific (Waltham, MA) coupled to a cold trap.
Chemical derivatization of metabolites and GC-MS analysis.
Intracellular and extracellular metabolites and amino acids derived from biomass hydrolysates were made volatile for gas chromatography-mass spectrometry (GC-MS) analysis by resuspending the dry samples in 200 μl of 1 M NaOH and derivatized using a methylchloroformate (MCF) method according to our standard laboratory procedure described previously (25). The derivatized samples were analyzed by GC-MS according to the parameters established previously (27) using a gas chromatograph (GC7890) from Agilent Technologies (Santa Clara, CA) coupled to a quadrupole mass spectrometer (MSD5975; Agilent Technologies, Santa Clara, CA). The GC-capillary column was a Zebron ZB-1701 column (Phenomenex, Torrance, CA) with dimensions of 30 m by 250 μm (inside diameter [i.d.]) by 0.15 μm, a 290°C injection temperature, and an ion source at 70 eV and 230°C, and the quadrupole temperature was set to 200°C. The carrier gas was helium with a flow of 1 ml/min.
Acetate quantification.
Acetate levels in the spent culture medium were assayed using the commercial Enzymatic BioAnalysis/Food Analysis UV method from R-Biopharm AG (Darmstadt, Germany), according to the manufacturer's instructions.
Demethylmenaquinone extraction and quantification.
Approximately 10 ml of culture broth (OD at 600 nm [OD600] of ∼1.0) was freeze-dried, and the residue was extracted with 25 ml of chloroform-methanol (2:1, vol/vol) for 15 min under vigorous stirring over a magnetic stirrer. The mixture was then centrifuged for 15 min at 4°C (4,000 rpm), and the supernatant was collected and dried under a stream of N2 gas. The lipid fraction was resuspended into 2 ml of hexane and passed through a solid-phase extraction (SPE) column (Strata SI-1 silica, 55 μm, 70 Å; Phenomenex) in order to isolate the menaquinone fraction. The analytes were eluted from the cartridges using a mixture of hexane-diethyl ether (96:4, vol/vol). The eluents were collected and dried under an N2 stream and later resuspended in 20 μl of acetone prior to high-pressure liquid chromatography (HPLC) analysis. The separation and quantification of menaquinones were performed using a System Gold Complete HPLC system coupled to a Gemini-NX C18 column (250 by 4.6 mm, 5 μm) from Phenomenex and UV detection at 245 nm according to methodology proposed by Suvarna et al. (28). The sample was eluted under isocratic conditions with methanol-diethyl ether solution (3:1, vol/vol) as mobile phase at 0.7 ml/min for 20 min. Vitamin K2 (menaquinone-4) from Sigma was used as a standard to build up the calibration curve.
Fatty acid composition of cell envelopes.
Approximately 5 mg of freeze-dried bacterial biomass was used for determining the profile of membrane-associated fatty acids. Dried biomass samples were transferred to a GC-MS amber vial for saponification with an excess amount of base (KOH in methanol) and butylated hydroxytoluene (BHT) solution. BHT is a powerful antioxidant that preserves unsaturated and polyunsaturated fatty acids from oxidation. The mixture was homogenized by 1 h of incubation at 55°C. Then, the hydrolysate was transferred into silanized glass tubes and derivatized by MCF as previously described (25). After the derivatization step, the samples were analyzed by GC-MS as described above.
Metabolite identification and data analysis.
The metabolite derivatives detected by GC-MS were identified using the Automated Mass Spectral Deconvolution and Identification System (AMDIS) software. This software is very useful for deconvolution and identification of chromatographic peaks. To normalize the data, the intensity of each metabolite peak was divided by the intensity of the standard peak (2,3,3,3-d4-alanine) and also by the amount of biomass in each sample. A comparative metabolite profile was determined using Pathway Activity Profiling (PAPi) analysis (29), and the statistical analysis was performed using an R package also developed in-house (30). For general pattern identification and to consider the reproducibility of the methods and sample replicates, the software GeneSpring (Agilent Gene Spring MS Proteomics & Metabolomics Analysis 1.1.1) was used based on raw GC-MS data rather than analysis of only identified metabolites. In order to determine the statistical significance of our findings, analysis of variance (ANOVA) and the Student t test were used.
13C-labeling distribution analysis.
13C-labeling patterns in the amino acids derived from the cell pellet hydrolysate were determined by analyzing the ratio of 13C to 12C in the major mass fragments produced by MS fragmentation of the respective MCF derivatives. The ratios were compared to those found in natural metabolites (from cells grown in [12C]glucose). Evidence for labeling was determined by an increase in the relative level of 13C labeling in the metabolites from samples grown in [13C]glucose. The labeling patterns of the amino acids were used to infer changes in the metabolic flux throughout the central carbon metabolism. The labeling pattern analysis was also extended to include an examination of the patterns found within some key intracellular metabolites.
RESULTS AND DISCUSSION
When E. faecalis was grown in anaerobic glucose-limited continuous culture at a dilution rate of 0.06 h−1, steady-state conditions were achieved after 15 h (three residential times). Steady-state cells were rapidly transitioned to aerobic growth conditions by introducing air into the bioreactor until 20% oxygen saturation was reached. The optical density remained constant even after oxygen challenge. Anaerobic glucose metabolism produced lactate and acetate as the main end products. In response to oxygen, the level of acetate decreased 2-fold, with a concomitant increase in lactate and fatty acid levels. Metabolomic analysis of the cells before, during, and after the transition between anaerobic and aerobic conditions demonstrated that there were a number of major metabolic changes in response to oxygen. The complete metabolite profiles of the intracellular, extracellular, and transition states are available as Data Set S1 in the supplemental material.
Upregulation of sulfur and glutathione metabolism and increased flux through glycolysis in response to oxygen.
E. faecalis exposed to oxygen rapidly consumed glutamate (Fig. 1). A significant increase in intracellular pyroglutamate levels and extracellular glutathione levels indicated that glutathione biosynthesis was upregulated in response to oxygen (Fig. 2 and 3). Sixteen amino acids showed a significant increase in extracellular levels aerobically, with the exception of serine and cysteine (Fig. 3). Serine was transported from the medium at a greater rate under aerobic growth, which indicates a higher demand for this metabolite. Serine is the precursor of glycine, which is also a precursor for glutathione. Serine can also be converted into cysteine through the activity of the enzymes serine O-acetylacetylase (cysE) and cysteine synthase (cysK) coupled with the release of acetate (31). Similarly, depleted levels of cysteine also indicated a higher demand for this amino acid intracellularly in response to oxygen. Cysteine itself has strong antioxidant activity (32) and is a key precursor for glutathione and a key metabolite in the synthesis of other sulfur-containing compounds, including thiamine, coenzyme A (CoA), and biotin, which have all been implicated in the oxidative stress response in other organisms such as Escherichia coli and Bacillus subtilis (33, 34).
FIG 1.
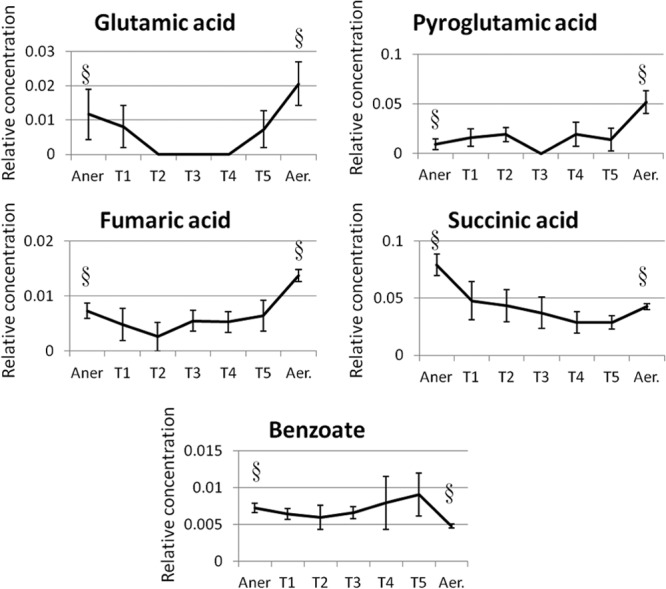
Changes in metabolite levels over time during transition from anaerobic to aerobic steady states (Aner, anaerobic steady state; T1, T2, T3, T4, and T5, transition states 1, 2, 3, 4, and 5, respectively; Aer, aerobic steady state). The relative metabolite levels have been normalized by the level of internal standard (2,3,3,3-d4-alanine) and biomass concentration. The error bars show the standard error between three biological replicates. The section signs (§) indicate the steady-state levels of the different metabolites before and after the transition state.
FIG 2.
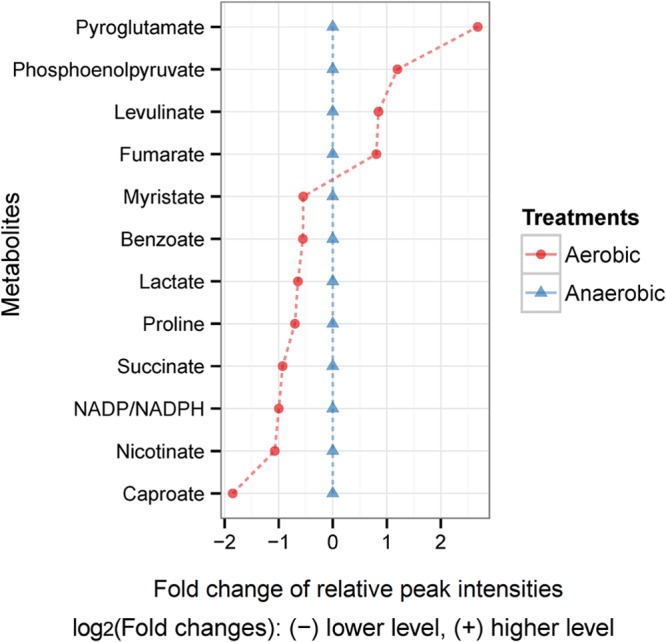
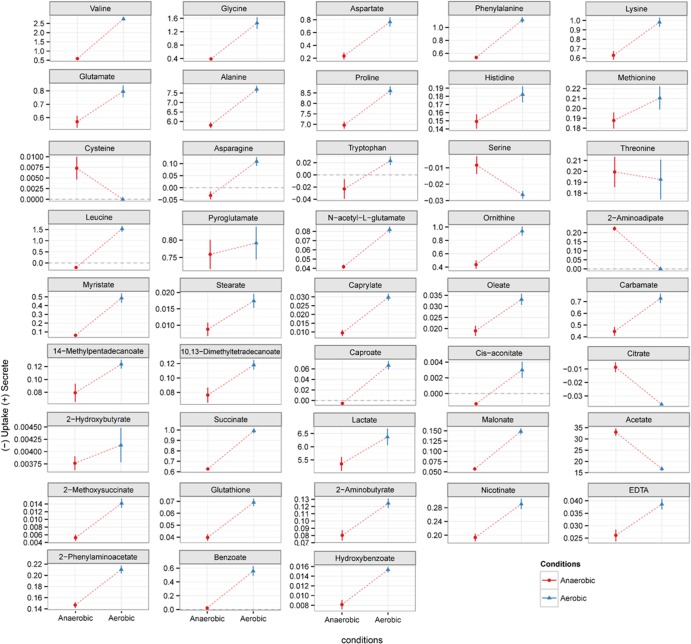
Intracellular metabolite levels of E. faecalis cells detected at significantly different levels (P < 0.05) when comparing anaerobic and aerobic growth conditions. Metabolite levels were normalized by internal standard (2,3,3,3-d4-alanine) and biomass concentration. The relative level of metabolites under the anaerobic condition has been set to 0 and compared to their comparative level under the aerobic condition.
FIG 3.
Extracellular metabolite levels of E. faecalis cultures grown under anaerobic and aerobic conditions. The metabolite levels were normalized by internal standard (2,3,3,3-d4-alanine), subtracted from the metabolite profile present in the noninoculated medium, and normalized by biomass. All presented metabolites were detected at statistically significant levels (P < 0.05).
Increased labeling in glutamate and pyroglutamate under aerobic conditions was another indication of upregulation of pathways that lead to the formation of compounds related to glutathione and sulfur metabolism (Fig. 4). We therefore hypothesize that the major response of E. faecalis cells in response to oxygen is the upregulation of glutathione biosynthesis and sulfur metabolism, particularly because glutathione and other sulfur-containing metabolites play an important role in protecting cells against oxidative stress (35). This is a rapid response to oxygen because this metabolic response could be detected within 12 min during the transition stage between anaerobic and aerobic growth (Fig. 1). Glutathione is a small peptide composed of cysteine, glycine, and glutamate. It exists in two forms within the cell, the reduced form (GSH) and the oxidized form (GSSH). The interconversion between these two forms allows glutathione to donate reducing equivalents (H+/e−) to unstable ROS in order to neutralize oxidative stress agents (36). The increase in glutathione activity under aerobic conditions in E. faecalis has been previously implicated based on proteomics data where the level of glutathione reductase increased in response to oxygen (37). Our metabolome data therefore confirmed this observation.
FIG 4.
Isotope labeling patterns of lactate (A), glutamate (B), pyroglutamate (C), and glycine (D). Light gray bars describe the natural level of 13C expected in the mass ions of each compound. Dark gray and black bars indicate the relative concentration of 13C in each metabolite after culture in medium enriched with 13C-labeled glucose. The asterisks (*) indicate metabolite levels with statistically significant differences between the two growth conditions.
Higher intracellular levels of phosphoenolpyruvate combined with increased extracellular levels of lactate, alanine, and glycine, and increased 13C labeling in lactate, suggested an unexpected upregulation of glycolysis in response to oxygen (Fig. 2 to 4). Lactate and alanine are synthesized from pyruvate, the end product of glycolysis, and the glycine precursor is 3-phosphoglycerate, another glycolytic precursor. Higher levels of these metabolites suggest higher availability of their precursor. Detecting higher 13C labeling in lactate under aerobic growth conditions reinforces our hypothesis of a higher availability of glycolytic products for biosynthesis of these metabolites.
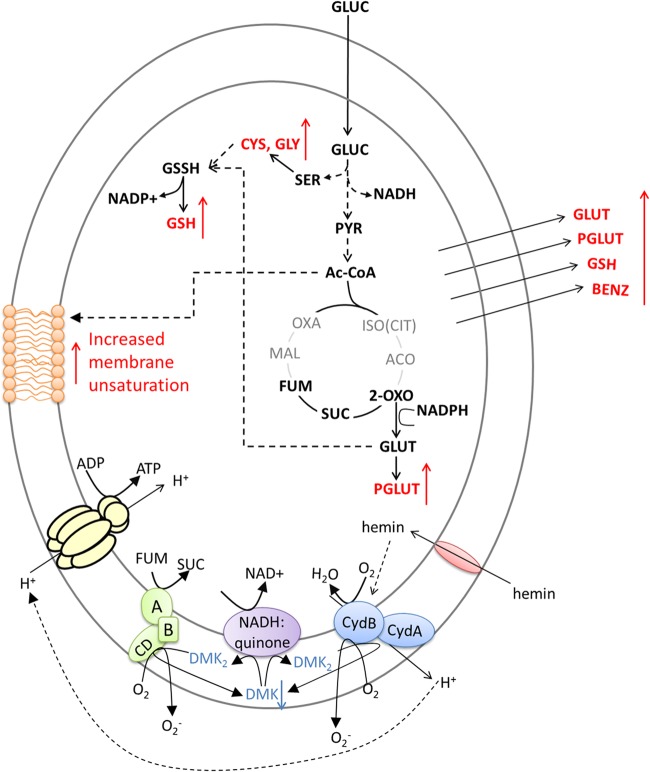
Analysis of E. faecalis metabolism shows that it does not display typical oxygen-dependent pathways such as a tricarboxylic acid (TCA) cycle and oxidative phosphorylation, as it lacks key enzymes such as fumarase and aconitase. However, there is evidence that it is able to respire aerobically using a cytochrome bd terminal oxidase, when grown in the presence of hemin (21). A conceptualized model of an adapted functional electron transport chain (ETC) has been proposed and involves a putative hemin transporter, a cytochrome bd, and a fumarate reductase. DMK is reduced to demethylmenaquinol (DMK2) by accepting the cytosolic equivalents through a putative NADH:quinone oxidase. The previously hemin-activated cytochrome bd and fumarate reductase are the final electron acceptors and reduce succinate to fumarate and O2 to H2O (38). The conceptualized model is shown in Fig. 5. It seems reasonable that an increased glycolytic flux could compensate for the lack of a tricarboxylic acid cycle by overproducing NADH cofactor to be further regenerated in the adapted ETC. Additionally, higher glycolytic flux allows for increased availability of important metabolites involved in key stress defense mechanisms. As mentioned above, a number of glycolytic intermediates and products are important for the synthesis of glutathione (Fig. 5).
FIG 5.
Overall metabolic response of E. faecalis to oxygen. A first-line defense drives the cellular metabolism toward glutathione production. Consequently, an increase in the glycolytic flux is necessary to ensure the demand for reducing equivalents. The downstream response of the cells is related to changes in the lipid membrane and demethylmenaquinone levels of the cell membrane. Steps in the TCA cycle shown in gray correspond to the missing steps in E. faecalis metabolism.
Upregulation of fatty acid biosynthesis and demethylmenaquinone metabolism.
Our metabolomic results indicated that lipid metabolism and changes in the membrane composition could be the key long-term changes induced by E. faecalis exposure to aerobic conditions. Acetate levels decreased 2-fold under aerobic conditions (Fig. 3). Mixed acid fermentation in E. faecalis yields acetate coupled with ATP production by substrate-level phosphorylation. In E. faecalis, acetate can be produced from acetyl-CoA, which is also the main precursor for the biosynthesis of fatty acids. We hypothesize that under aerobic conditions there is a reduction in acetate formation and an upregulation of acetyl-CoA and fatty acid biosynthesis, hence explaining the 2-fold reduction in acetate levels aerobically (Fig. 3). In addition, there was a significant increase in the level of all fatty acids detected in the extracellular medium during aerobic growth (Fig. 3). This supports the hypothesis of an upregulation of fatty acid biosynthesis in response to oxygen despite some of these fatty acids having their levels decreased intracellularly, such as myristate and caproate (Fig. 2). Lower levels of myristate and caproate intracellularly may indicate increased incorporation of these acids into complex lipid molecules such as phospholipids and triglycerides or active excretion. Nonetheless, changes in fatty acid metabolism are likely to result in changes in the fatty acid composition of the bacterial cell membrane. Therefore, to confirm this hypothesis, we have determined the total fatty acid content of the cells grown anaerobically and aerobically. We assumed that most of the measured fatty acids were derived from the cell membrane, as free fatty acids are in fact at a much lower concentration than are the membrane-derived fatty acids. Indeed, the results showed a significant change in the fatty acid composition under aerobic conditions with increased levels of unsaturated fatty acids such as oleic, cis-10-heptadecenoic, myristoleic, and palmitoleic acids (Fig. 6). Nonetheless, some saturated fatty acids, such as lauric, palmitic, myristic, and pentadecanoic acids, also had their levels increased in response to oxygen (Fig. 6).
FIG 6.
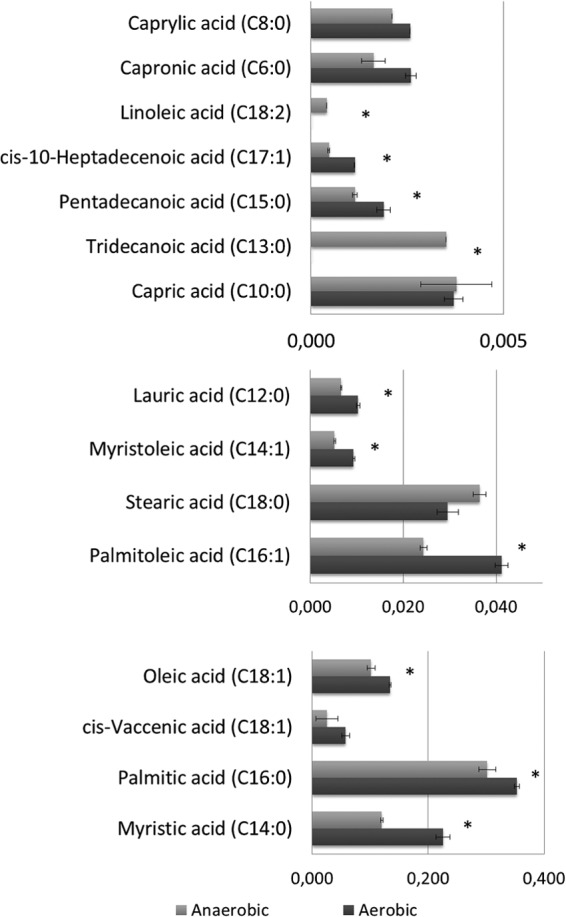
Membrane fatty acid composition of E. faecalis cells grown under different environmental conditions, anaerobic (light gray) versus aerobic (dark gray) growth. The asterisks (*) indicate metabolite levels with statistically significant differences between the two growth conditions.
Increased levels of unsaturated and saturated fatty acids indicate a modulation of the lipid membrane under oxidative stress. These data therefore support the metabolomics hypothesis that oxygen affects fatty acid metabolism and subsequent membrane composition. The effect of a stress condition on the membrane lipid composition is usually dependent on the imposed stress. Marr and Ingraham showed that an increase in temperature or glucose limitation was coupled to an increased content of unsaturated fatty acids, while a limitation of ammonium salts was coupled with an increase in saturated fatty acids (39). When Lactobacillus helveticus was exposed to a varied combination of stress conditions such as NaCl, H2O2, temperature, and pH, the main consequence was an increase desaturation of the fatty acids in the membrane (40). Although no previous studies have been reported for E. faecalis, our overall data indicate that there was an increase in membrane unsaturation due to the presence of oxygen (see Table S1 in the supplemental material). We observed a 55% increase of the total unsaturated fatty acids and a 32% increase of the saturated fatty acids in response to oxygen. A deeper analysis of only the statistically significant fatty acid alterations indicated a 40.7% increase of unsaturated fatty acids, against a 36.4% increase of saturated fatty acids in response to oxygen tension. Heptadecenoic (C17:1) and myristoleic (C14:1) acids highly contributed to the unsaturation levels while myristic (C14:0) and pentadecanoic (C15:0) acids contributed to the saturation pools. Our data clearly suggest that under oxidative stress the fatty acid composition of the cell changes with an overall trend toward increased unsaturation. Studies on other organisms have observed similar results (40–42). On the other hand, a study from Henry et al. (43) tested different fatty acids and their antioxidant effect over cyclooxygenase I and cyclooxygenase II. Results showed that saturated fatty acids such as myristic and lauric acid displayed antioxidant activities (43), and our results suggest that the reshaping of the membrane structure in E. faecalis may follow a strategy that combines the most effective antioxidant fatty acids among saturated and unsaturated fatty acids.
Extracellular benzoate levels (and hydroxybenzoate) increased significantly under aerobic growth in parallel with a significant decrease in intracellular benzoate levels (Fig. 2 and 3). Benzoate is an intermediate metabolite involved in the synthesis and catabolism of aromatic compounds, including aromatic amino acids (44). Oxidative phosphorylation requires quinones (e.g., ubiquinone [UQ]) which are derivatives of either ubiquinone [2,3-dimethoxy-5-methyl-6-(prenyl)-n-1,4-benzoquinone] or menaquinone (MQ) [2-methy-13-(prenyl)-n-1,4-naphthoquinones]. E. coli synthesizes ubiquinone-8 and therefore requires benzoate, hydroxybenzoate, and other related compounds (45). In E. faecalis, many elements of the MQ operon have been identified, such as menF, -D, -E, and -B genes, (46), and code for a derivative termed demethylmenaquinone (DMK), which appears to be constitutively expressed (38). Isochorismate is the precursor for DMK biosynthesis in E. faecalis as well as for aromatic amino acids such as phenylalanine, tyrosine, and tryptophan. The level of these amino acids increased in the extracellular medium (with the exception of tyrosine) in response to oxygen, in addition to 2-phenylaminoacetate, another aromatic amino acid intermediate that could be a catabolic product of aromatic compounds. Therefore, we hypothesize that DMK metabolism (biosynthesis or catabolism) is altered in response to oxygen, which would also affect the cell membrane composition under aerobic growth. To validate this hypothesis, we quantified the level of DMK in E. faecalis cells growing aerobically and anaerobically. Indeed, the concentration of demethylmenaquinone found in bacterial cells grown anaerobically was 5 times higher than that in cells grown aerobically (Fig. 5 to 7). This has been also observed for Lactococcus lactis (47). A consequence of demethylmenaquinone expression by E. faecalis is the production of extracellular O2−. If, on one hand, this strategy could mean a potential threat to the host, on the other hand it can be suicidal to the bacterial cell due to the accumulation of toxic free radicals when grown in a chemostat, which may explain the downregulation of its biosynthesis under the aerobic condition as confirmed by its lower level aerobically. Additionally, DMKs are located in the lipid membrane of E. faecalis (Fig. 5); thus, we hypothesize that changes in the synthesis or degradation of DMK in parallel with modulation of membrane lipid composition in response to a stress condition could be the ultimate response of E. faecalis to oxidative stress (46).
FIG 7.
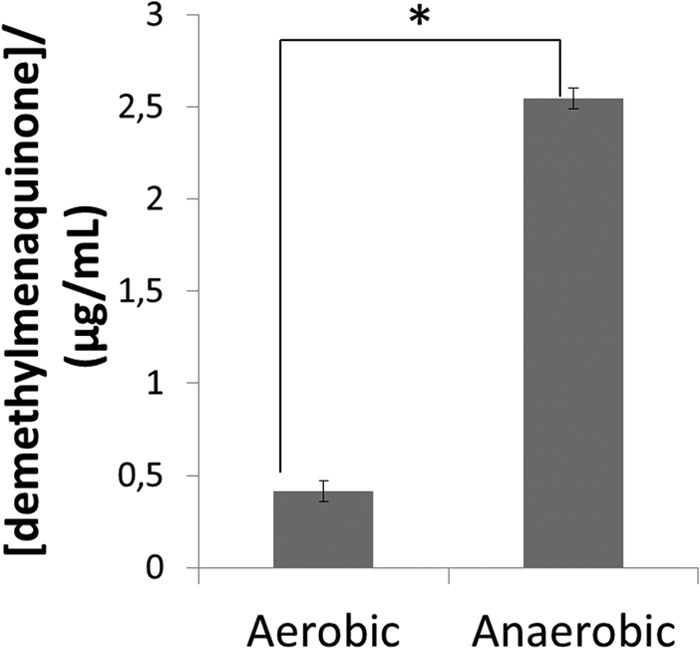
Demethylmenaquinone levels of E. faecalis under aerobic versus anaerobic conditions. The asterisk (*) indicates metabolite levels with statistically significant differences between the two growth conditions.
Pathway Activity Profiling (PAPi) analysis supports our metabolomics hypotheses.
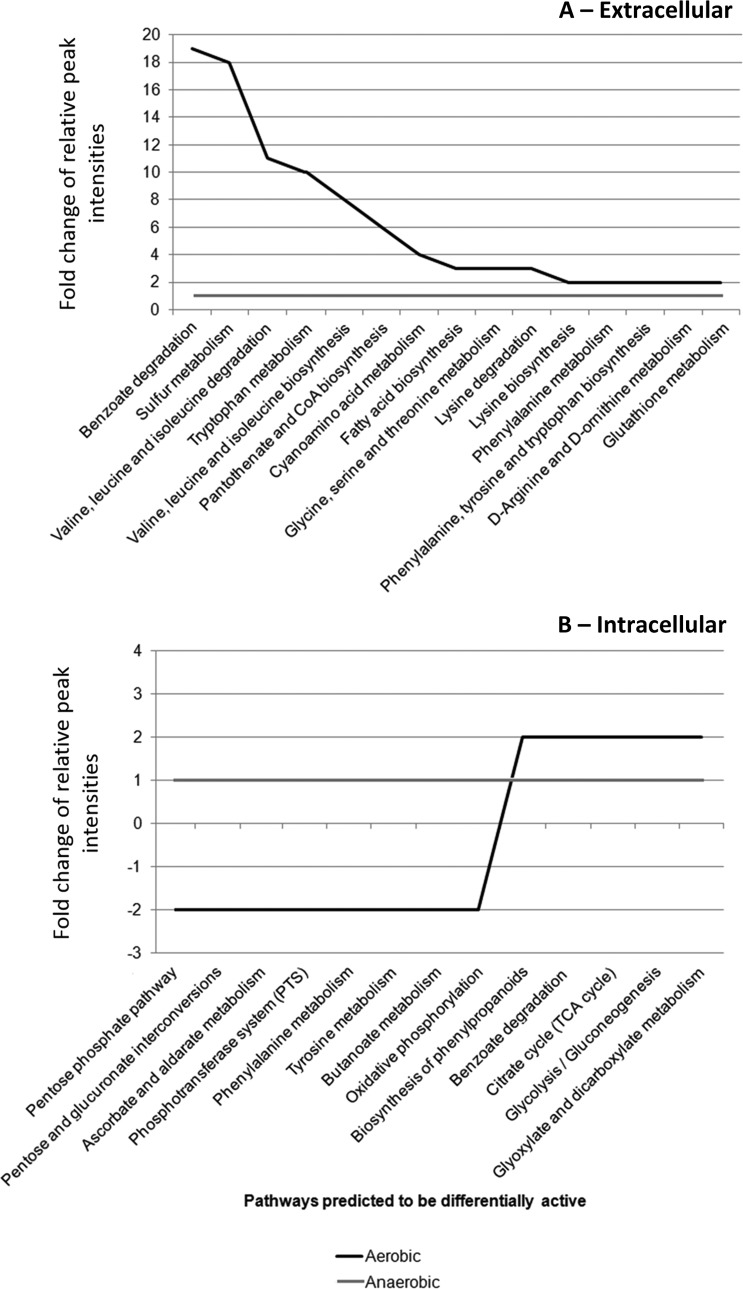
Based on the identified metabolites in the different samples, we used a software program developed in-house (29) to determine which metabolic pathways were more likely to be up- or downregulated when comparing the two experimental conditions (aerobic versus anaerobic). Figure 8 shows the Pathway Activity Profiling (PAPi) profile obtained. Based on the profile of extracellular metabolites (Fig. 8A), there were five metabolic pathways that could be responsible for the greatest differences in extracellular metabolite profiles when comparing anaerobic and aerobic growth: benzoate degradation; sulfur metabolism; valine, leucine, and isoleucine degradation; fatty acid biosynthesis; and glycine, serine, and threonine metabolism. Prediction of an upregulation of benzoate degradation aerobically parallel to an upregulation of phenylalanine, tyrosine, and tryptophan metabolism is in agreement with our hypothesis of a possible interconversion of aromatic amino acids and other aromatic compounds such as menaquinones into benzoate and its derivatives, such as hydroxybutyrate. Valine, leucine, and isoleucine degradation and, in particular, isoleucine degradation yield propanoyl-CoA and acetyl-CoA, compounds involved in fatty acid biosynthesis. This could be linked to the observed changes in fatty acid metabolism discussed above. Additionally, sulfur metabolism and glycine, serine, and threonine metabolism are important in the production of precursors for glutathione biosynthesis, which seems to be the first-line response of E. faecalis to oxidative stress and is also in perfect agreement with our hypotheses discussed above. Moreover, glycolysis, benzoate degradation, and biosynthesis of phenylpropanoids are metabolic pathways that appear to be upregulated aerobically compared to the anaerobic condition based on the intracellular metabolite profile (Fig. 8B), further supporting our hypotheses.
FIG 8.
Pathway Activity Profiling (PAPi) analysis based on extracellular metabolite data (A) and intracellular metabolite data (B) from E. faecalis grown under different environmental conditions (anaerobic and aerobic). Only pathways with statistical significance (P < 0.05) and with more than a 2-fold change are shown. The relative activity of pathways under the anaerobic condition has been set to 1 and compared to their comparative activity under the aerobic condition.
We observed 13C enrichment in free glutamate from aerobic samples, indicating that under aerobic conditions glutamate was (at least in part) de novo synthesized from glucose despite the fact that E. faecalis lacks citrate dehydrogenase and isocitrate dehydrogenase (Fig. 4B). Similarly, 13C enrichment was also observed in pyroglutamate, which is produced from glutamate (Fig. 4C). The amino acid glutamate is synthesized from the TCA cycle intermediate 2-ketoglutarate. Among TCA cycle intermediate metabolites, only citrate, succinate, and fumarate were detected in E. faecalis samples (Fig. 2 and 3), which begs the question of how E. faecalis synthesizes glutamate. On the other hand, we have not found 13C-labeling enrichment in the amino acids proline, valine, isoleucine, and leucine under either growth condition, which demonstrates that these amino acids were primarily derived from the peptone in the medium rather than synthesized de novo from glucose.
In summary, oxygen had a significant impact on the cellular metabolism of E. faecalis. The overall mechanism by which cells regulate their metabolism in order to cope with oxidative stress based on our study is summarized in Fig. 5. Our study yielded three key insights into E. faecalis response to oxidative stress: (i) upregulation of sulfur metabolism and glutathione biosynthesis, which seems to be the immediate response of E. faecalis to oxidative stress; (ii) increased glycolytic flux to attend to the metabolic demand for critical metabolite precursors and reducing power; and (iii) upregulation of fatty acid metabolism and benzoate degradation, which is linked to important changes in the bacterial membrane composition as evidenced by changes in membrane fatty acid composition and a decrease in the demethylmenaquinone level associated with the membrane.
In addition, labeling in free glutamate was an unprecedented finding, as it indicates that glutamate was de novo synthesized from glucose despite no previous evidence being found of E. faecalis being capable of expressing citrate and isocitrate dehydrogenase. Therefore, E. faecalis is capable of synthesizing glutamate, probably via a yet-undescribed metabolic pathway. Moreover, differences in the lipid compositions and DMK contents of the cell membrane may represent potential targets that can synergistically enhance the action of bactericidal drugs by weakening the bacterial resistance to oxidative stress. Ultimately, this represents a step change in the development of new drugs that can act on a specific target of the cell (e.g., cell wall and DNA synthesis) while also potentiating the oxidative stress in the cell, making it more vulnerable. These strategies may be an alternative way to control E. faecalis survival or to increase its susceptibility toward bactericidal drugs.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the HRC (Health and Research Council of New Zealand) and the FCT (Portuguese Foundation for Science and Technology), with grant reference SFRH/BD/47016/2008.
We thank Ting-Li Han for assisting with figure graphics.
Footnotes
Published ahead of print 21 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01354-13.
REFERENCES
- 1.Fisher K, Phillips C. 2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology 155:1749–1757. 10.1099/mic.0.026385-0 [DOI] [PubMed] [Google Scholar]
- 2.Franz CMAP, Huch M, Abriouel H, Holzapfel W, Gálvez A. 2011. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 151:125–140. 10.1016/j.ijfoodmicro.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 3.Balciunas EM, Castillo Martinez FA, Todorov SD, Franco BDGDM, Converti A, Oliveira RPDS. 2013. Novel biotechnological applications of bacteriocins: a review. Food Control 32:134–142. 10.1016/j.foodcont.2012.11.025 [DOI] [Google Scholar]
- 4.Foulquié Moreno MR, Sarantinopoulos P, Tsakalidou E, De Vuyst L. 2006. The role and application of enterococci in food and health. Int. J. Food Microbiol. 106:1–24. 10.1016/j.ijfoodmicro.2005.06.026 [DOI] [PubMed] [Google Scholar]
- 5.Ferrieri P. 2002. Unique features of infective endocarditis in childhood. Circulation 105:2115–2126. 10.1161/01.CIR.0000013073.22415.90 [DOI] [PubMed] [Google Scholar]
- 6.Levine DP. 2006. Vancomycin: a history. Clin. Infect. Dis. 42:S5–S12. 10.1086/491709 [DOI] [PubMed] [Google Scholar]
- 7.Poh CH, Oh HML, Tan AL. 2006. Epidemiology and clinical outcome of enterococcal bacteraemia in an acute care hospital. J. Infect. 52:383–386. 10.1016/j.jinf.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 8.Livermore DM. 2009. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 64(Suppl 1):i29–i36. 10.1093/jac/dkp255 [DOI] [PubMed] [Google Scholar]
- 9.Neely AN, Maley MP. 2000. Survival of enterococci and staphylococci on hospital fabrics and plastic. J. Clin. Microbiol. 38:724–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, Kolonay JF, Shetty J, Killgore GE, Tenover FC. 2003. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302:1569–1571. 10.1126/science.1090956 [DOI] [PubMed] [Google Scholar]
- 11.Riboulet E, Verneuil N, La Carbona S, Sauvageot N, Auffray Y, Hartke A, Giard J-C. 2007. Relationships between oxidative stress response and virulence in Enterococcus faecalis. J. Mol. Microbiol. Biotechnol. 13:140–146. 10.1159/000103605 [DOI] [PubMed] [Google Scholar]
- 12.Albesa I, Becerra MC, Battán PC, Páez PL. 2004. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem. Biophys. Res. Commun. 317:605–609. 10.1016/j.bbrc.2004.03.085 [DOI] [PubMed] [Google Scholar]
- 13.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. 10.1016/j.cell.2007.06.049 [DOI] [PubMed] [Google Scholar]
- 14.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. 2013. Killing by bactericidal antibiotics. Science 339:1213–1216. 10.1126/science.1232688 [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Imlay JA. 2013. Cell death from antibiotics without the involvement of reactive oxygen species. Science 339:1210–1213. 10.1126/science.1232751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezraty B, Vergnes A, Banzhaf M, Duverger Y, Huguenot A, Brochado AR, Su S, Espinosa L, Loiseau L, Py B, Typas A, Barras F. 2013. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 340:1583–1587. 10.1126/science.1238328 [DOI] [PubMed] [Google Scholar]
- 17.Paulsen IT, Banerjei L, Myers GSA, Nelson KE, Seshadri R, Read TD, Fouts DE, Eisen JA, Gill SR, Heidelberg JF, Tettelin H, Dodson RJ, Umayam L, Brinkac L, Beanan M, Daugherty S, DeBoy RT, Durkin S, Kolonay J, Madupu R, Nelson W, Vamathevan J, Tran B, Upton J, Hansen T, Shetty J, Khouri H, Utterback T, Radune D, Ketchum KA, Dougherty BA, Fraser CM. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071–2074. 10.1126/science.1080613 [DOI] [PubMed] [Google Scholar]
- 18.Bizzini A, Zhao C, Auffray Y, Hartke A. 2009. The Enterococcus faecalis superoxide dismutase is essential for its tolerance to vancomycin and penicillin. J. Antimicrob. Chemother. 64:1196–1202. 10.1093/jac/dkp369 [DOI] [PubMed] [Google Scholar]
- 19.Verneuil N, Sanguinetti M, Le Breton Y, Posteraro B, Fadda G, Auffray Y, Hartke A, Giard J-C. 2004. Effects of the Enterococcus faecalis hypR gene encoding a new transcriptional regulator on oxidative stress response and intracellular survival within macrophages. Infect. Immun. 72:4424–4431. 10.1128/IAI.72.8.4424-4431.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giard JC, Laplace JM, Rincé A, Pichereau V, Benachour A, Leboeuf C, Flahaut S, Auffray Y, Hartke A. 2001. The stress proteome of Enterococcus faecalis. Electrophoresis 22:2947–2954. [DOI] [PubMed] [Google Scholar]
- 21.Winstedt L, Frankenberg L, Hederstedt L, von Wachenfeldt C. 2000. Enterococcus faecalis V583 contains a cytochrome bd-type respiratory oxidase. J. Bacteriol. 182:3863–3866. 10.1128/JB.182.13.3863-3866.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flahaut S, Laplace JM, Frère J, Auffray Y. 1998. The oxidative stress response in Enterococcus faecalis: relationship between H2O2 tolerance and H2O2 stress proteins. Lett. Appl. Microbiol. 26:259–264. 10.1046/j.1472-765X.1998.00325.x [DOI] [PubMed] [Google Scholar]
- 23.Manson JM, Keis S, Smith JMB, Cook GM. 2003. Characterization of a vancomycin-resistant Enterococcus faecalis (VREF) isolate from a dog with mastitis: further evidence of a clonal lineage of VREF in New Zealand. J. Clin. Microbiol. 41:3331–3333. 10.1128/JCM.41.7.3331-3333.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31:426–428. 10.1021/ac60147a030 [DOI] [Google Scholar]
- 25.Smart KF, Aggio RBM, Van Houtte JR, Villas-Bôas SG. 2010. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography-mass spectrometry. Nat. Protoc. 5:1709–1729. 10.1038/nprot.2010.108 [DOI] [PubMed] [Google Scholar]
- 26.Christensen B, Nielsen J. 1999. Isotopomer analysis using GC-MS. Metab. Eng. 1:282–290 [DOI] [PubMed] [Google Scholar]
- 27.Villas-Bôas SG, Bruheim P. 2007. Cold glycerol-saline: the promising quenching solution for accurate intracellular metabolite analysis of microbial cells. Anal. Biochem. 370:87–97. 10.1016/j.ab.2007.06.028 [DOI] [PubMed] [Google Scholar]
- 28.Suvarna K, Stevenson D, Meganathan R, Hudspeth ME. 1998. Menaquinone (vitamin K2) biosynthesis: localization and characterization of the menA gene from Escherichia coli. J. Bacteriol. 180:2782–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aggio RBM, Ruggiero K, Villas-Bôas SG. 2010. Pathway activity profiling (PAPi): from the metabolite profile to the metabolic pathway activity. Bioinformatics 26:2969–2976. 10.1093/bioinformatics/btq567 [DOI] [PubMed] [Google Scholar]
- 30.Aggio R, Villas-Bôas SG, Ruggiero K. 2011. Metab: an R package for high-throughput analysis of metabolomics data generated by GC-MS. Bioinformatics 27:2316–2318. 10.1093/bioinformatics/btr379 [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Nauta A, Francke C, Siezen RJ. 2008. Comparative genomics of enzymes in flavor-forming pathways from amino acids in lactic acid bacteria. Appl. Environ. Microbiol. 74:4590–4600. 10.1128/AEM.00150-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elias RJ, McClements DJ, Decker EA. 2005. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase beta-lactoglobulin in oil-in-water emulsions. J. Agric. Food Chem. 53:10248–10253. 10.1021/jf0521698 [DOI] [PubMed] [Google Scholar]
- 33.Park J-H, Dorrestein PC, Zhai H, Kinsland C, McLafferty FW, Begley TP. 2003. Biosynthesis of the thiazole moiety of thiamin pyrophosphate (vitamin B1). Biochemistry 42:12430–12438. 10.1021/bi034902z [DOI] [PubMed] [Google Scholar]
- 34.Zhang SG, Sanyal I, Bulboaca GH, Rich A, Flint DH. 1994. The gene for biotin synthase from Saccharomyces cerevisiae: cloning, sequencing, and complementation of Escherichia coli strains lacking biotin synthase. Arch. Biochem. Biophys. 309:29–35. 10.1006/abbi.1994.1079 [DOI] [PubMed] [Google Scholar]
- 35.Carmel-Harel O, Storz G. 2000. Roles of the glutathione and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54:439–461. 10.1146/annurev.micro.54.1.439 [DOI] [PubMed] [Google Scholar]
- 36.Imlay JA. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395–418. 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- 37.Patel MP, Marcinkeviciene J, Blanchard JS. 1998. Enterococcus faecalis glutathione reductase: purification, characterization and expression under normal and hyperbaric O2 conditions. FEMS Microbiol. Lett. 166:155–163 [DOI] [PubMed] [Google Scholar]
- 38.Gilmore MS. 2002. The enterococci: pathogenesis, molecular biology, and antibiotic resistance, p 133–175 ASM Press, Washington, DC [Google Scholar]
- 39.Marr AG, Ingraham JL. 1962. Effect of temperature on the composition of fatty acids in Escherichia coli. J. Bacteriol. 84:1260–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerzoni ME, Lanciotti R, Cocconcelli PS. 2001. Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiology 147:2255–2264 [DOI] [PubMed] [Google Scholar]
- 41.Quivey RG, Faustoferri R, Monahan K, Marquis R. 2000. Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans. FEMS Microbiol. Lett. 189:89–92. 10.1111/j.1574-6968.2000.tb09211.x [DOI] [PubMed] [Google Scholar]
- 42.Pesakhov S, Benisty R, Sikron N, Cohen Z, Gomelsky P, Khozin-Goldberg I, Dagan R, Porat N. 2007. Effect of hydrogen peroxide production and the Fenton reaction on membrane composition of Streptococcus pneumoniae. Biochim. Biophys. Acta 1768:590–597. 10.1016/j.bbamem.2006.12.016 [DOI] [PubMed] [Google Scholar]
- 43.Henry GE, Momin RA, Nair MG, Dewitt DL. 2002. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 50:2231–2234. 10.1021/jf0114381 [DOI] [PubMed] [Google Scholar]
- 44.Fernández M, Zúñiga M. 2006. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 32:155–183. 10.1080/10408410600880643 [DOI] [PubMed] [Google Scholar]
- 45.Knoell HE. 1979. Isolation of a soluble enzyme complex comprising the ubiquinone-8 synthesis apparatus from the cytoplasmic membrane of Escherichia coli. Biochem. Biophys. Res. Commun. 91:919–925. 10.1016/0006-291X(79)91967-3 [DOI] [PubMed] [Google Scholar]
- 46.Huycke MM, Moore D, Joyce W, Wise P, Shepard L, Kotake Y, Gilmore MS. 2001. Extracellular superoxide production by Enterococcus faecalis requires demethylmenaquinone and is attenuated by functional terminal quinol oxidases. Mol. Microbiol. 42:729–740. 10.1046/j.1365-2958.2001.02638.x [DOI] [PubMed] [Google Scholar]
- 47.Brooijmans R, Smit B, Santos F, van Riel J, de Vos WM, Hugenholtz J. 2009. Heme and menaquinone induced electron transport in lactic acid bacteria. Microb. Cell Fact. 8:28. 10.1186/1475-2859-8-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.