Abstract
Successful host cell colonization by the Q fever pathogen, Coxiella burnetii, requires translocation of effector proteins into the host cytosol by a Dot/Icm type 4B secretion system (T4BSS). In Legionella pneumophila, the two-component system (TCS) PmrAB regulates the Dot/Icm T4BSS and several additional physiological processes associated with pathogenesis. Because PmrA consensus regulatory elements are associated with some dot/icm and substrate genes, a similar role for PmrA in regulation of the C. burnetii T4BSS has been proposed. Here, we constructed a C. burnetii pmrA deletion mutant to directly probe PmrA-mediated gene regulation. Compared to wild-type bacteria, C. burnetii ΔpmrA exhibited severe intracellular growth defects that coincided with failed secretion of effector proteins. Luciferase gene reporter assays demonstrated PmrA-dependent expression of 5 of 7 dot/icm operons and 9 of 11 effector-encoding genes with a predicted upstream PmrA regulatory element. Mutational analysis verified consensus sequence nucleotides required for PmrA-directed transcription. RNA sequencing and whole bacterial cell mass spectrometry of wild-type C. burnetii and the ΔpmrA mutant uncovered new components of the PmrA regulon, including several genes lacking PmrA motifs that encoded Dot/Icm substrates. Collectively, our results indicate that the PmrAB TCS is a critical virulence factor that regulates C. burnetii Dot/Icm secretion. The presence of PmrA-responsive genes lacking PmrA regulatory elements also suggests that the PmrAB TCS controls expression of regulatory systems associated with the production of additional C. burnetii proteins involved in host cell parasitism.
INTRODUCTION
The zoonotic disease agent Coxiella burnetii causes human Q fever by invading and replicating within mononuclear phagocytes, such as alveolar macrophages (1, 2). Once internalized by a host cell, this highly infectious intracellular bacterium replicates exclusively within a membrane-bound compartment, or parasitophorous vacuole (PV). Cumulative evidence from studies of infected primary and continuous macrophage cell cultures indicates that the mature PV has phagolysosomal characteristics, including the presence of several late-endosomal/lysosomal membrane markers, an acidic pH, and active cathepsins that correlate with degradative activity capable of destroying Escherichia coli trafficked to the vacuole (2, 3). The PV is a specialized growth-permissive compartment that requires C. burnetii protein synthesis for biogenesis (4–6). C. burnetii actively manipulates vesicular trafficking pathways to sequester structural components for PV expansion and, presumably, nutrients for pathogen growth (5–8). Pronounced PV fusogenicity is associated with recruitment of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) syntaxin-8 and VAMP7 (5, 8). Small interfering RNA (siRNA) studies also suggest important roles for syntaxin-17 and retromer function (9).
New genetic tools have verified that the C. burnetii Dot/Icm type 4B secretion system (T4BSS), analogous to the well-defined Dot/Icm system of Legionella pneumophila, is essential for PV formation. C. burnetii dotA, dotB, icmD, icmL, and icmX mutants all fail to replicate in mammalian host cells (10–13). Intracellular growth of the icmD mutant is rescued if it cooccupies the PV with wild-type bacteria, indicating that dot/icm mutants are capable of intracellular growth if functions of T4BSS effector proteins are provided in trans (11).
Approximately 130 C. burnetii T4BSS substrates have been identified using L. pneumophila as the surrogate host and adenylate cyclase (CyaA)- or β-lactamase-based secretion assays (10, 13–19). Lists of candidate effector genes for screening were assembled based primarily on bioinformatic criteria (13–19). Bioinformatic predictors of effector genes/proteins include the presence of eukaryote-like motifs/domains (14, 16–18), a glutamate-rich C-terminal secretion signal (E block) (13, 15, 20, 21), and/or a regulatory element recognized by the response regulator PmrA (13, 14, 21, 22).
Advances in C. burnetii genetics now allow direct screening of potential Dot/Icm substrates in C. burnetii (12, 23, 24). Cytosolic translocation by C. burnetii has been verified for 27 of the C. burnetii Dot/Icm substrates originally identified using L. pneumophila, and dependency on at least one dot/icm gene has been demonstrated for secretion of 17 substrates (10–14, 17, 25). Furthermore, screening of a C. burnetii Himar1 transposon mutant library recently revealed mutations in five cir (Coxiella effector for intracellular replication) genes among the C. burnetii effector gene pool that have severe replication defects in J774A.1 macrophages (13).
Although progress has been made in identifying substrates translocated by the C. burnetii T4BSS, little information is available on the effector functions of these proteins. The biological roles of Cir proteins remain unresolved (13); however, three C. burnetii T4BSS substrates (AnkG, CeaA, and CeaB) have known antiapoptotic activities (26, 27). Ectopic expression in mammalian cells and gain of function by L. pneumophila demonstrate that the ankyrin repeat-containing protein AnkG inhibits apoptosis through binding of the proapoptotic mitochondrial protein p32 (gClqR) (26). Ectopic expression experiments also show that CaeB blocks apoptotic signals originating from the mitochondria and that CeaA inhibits apoptosis by an unknown mechanism (27). Recently, the effector protein CvpA was demonstrated to subvert clathrin-coated vesicle trafficking by binding to the clathrin adaptor protein AP2 (28). Cooption of clathrin transport processes by CvpA is predicted to promote acquisition of endolysosomal membrane for PV formation (28).
There are also considerable gaps in our knowledge with regard to regulation of C. burnetii type 4B secretion. In L. pneumophila, a complex interplay between the two-component systems (TCSs) PmrAB, CpxRA, LqsRS, and LetAS regulate expression of Dot/Icm effector-encoding genes (29). A prototypical TCS consists of a membrane-localized sensory histidine kinase and a cognate cytosolic response regulator. Environmental signals trigger autophosphorylation of the histidine kinase at a conserved histidine residue. Phosphotransfer to a conserved aspartate on the response regulator then initiates an output response that commonly involves DNA binding and transcriptional regulation (30), although output domains can also have enzymatic activity that directly transduces signals (e.g., diguanylate cyclases and methyltransferases) (31). Most genes encoding TCS kinase-regulator pairs occur in operons. Currently, L. pneumophila PmrAB and CpxRA are known to directly control expression of 43 (22, 29, 32) and 11 (29, 33) effector-encoding genes, respectively, with consensus nucleotide binding sequences identified for the response regulators PmrA (22) and CpxR (33). LqsRS is a quorum-sensing TCS that indirectly regulates expression of 12 effector-encoding genes (29, 34). The sensor kinase LqsS is stimulated by the autoinducer molecule LAI-1, resulting in activation of the response regulator LqsR (35). However, LqsR lacks a DNA-binding motif; consequently, the precise mechanism of gene regulation is unresolved (34). LetAS controls expression of 26 effector-encoding genes by inducing expression of two small RNAs (sRNAs) (RsmY and RsmZ) that inhibit the RNA-binding protein CsrA, a posttranscriptional inhibitor of effector gene expression (29, 36–38). LetS is a hybrid histidine kinase that utilizes a four-step phosphorelay to activate the response regulator LetA (30, 39–41). LetA belongs to the NarL family of response regulators that contain a helix-turn-helix DNA-binding motif (31, 42). letA and letS are unlinked and consequently considered orphan TCS genes (30).
C. burnetii lacks homologs of CpxRA and LqsRS. Clear homologs of LetAS are also not evident; however, C. burnetii encodes four orphan hybrid histidine kinases (39) and four orphan NarL family response regulators (31, 42). Thus, it is conceivable that a TCS derived from this group of proteins functions similarly to LetAS (40). Interestingly, C. burnetii encodes two CsrA proteins but lacks predicted CsrA-regulating sRNAs (43). C. burnetii encodes homologs of L. pneumophila PmrAB (QseBC), with both response regulator PmrAs containing a typical CheY-like receiver domain and an OmpR family winged-helix DNA-binding output domain (42). Based on the presence of the upstream PmrA regulatory element, cTTAATatT-N2-cTTAATatT (where lowercase letters indicate less conserved nucleotides), Zusman et al. (22) predicted 68 PmrA-regulated genes in the C. burnetii genome, including icmD, the CoxigA gene, dotD, icmV, and icmW, encoding components of the secretion apparatus. Furthermore, they showed that the CoxigA gene and icmW are expressed in a PmrA-dependent fashion by wild-type L. pneumophila and by an L. pneumophila pmrA deletion mutant expressing the C. burnetii PmrA homolog (22). A second permutation of the PmrA regulatory element proposed by Chen et al. (14) expanded the number of potential C. burnetii PmrA-regulated genes to 126. Subsequent testing of these candidate genes in L. pneumophila revealed that approximately 27% encode bona fide Dot/Icm substrates (13, 14).
In the current study, we employed a C. burnetii pmrA deletion mutant to evaluate the role of the PmrAB operon in the intracellular replication of C. burnetii and regulation of the T4BSS. C. burnetii ΔpmrA exhibits severe intracellular growth defects compared to wild-type bacteria. Luciferase gene reporter assays, effector translocation assays, RNA sequencing (RNA-seq), and mass spectrometry (MS) confirm that the defective intracellular growth of the ΔpmrA mutant is associated with dysregulation of the Dot/Icm T4BSS. However, transcriptome and proteome data also reveal new Dot/Icm substrates and several PmrA-regulated genes potentially involved in virulence that lack a PmrA regulatory element. Thus, the PmrAB TCS may cross talk with other TCSs and/or indirectly control expression of additional regulatory systems. This study begins to unravel the transcriptional regulatory networks associated with C. burnetii virulence.
MATERIALS AND METHODS
Bacterial strains and mammalian cell lines.
The bacterial strains used in this study are listed in Table 1. The C. burnetii Nine Mile phase II (clone 4, RSA439) strain was utilized throughout this work. Wild-type C. burnetii and genetic transformants were grown microaerobically in ACCM-2 as previously described (24). For storage, bacteria were pelleted following 6 days of growth, washed 3 times in phosphate-buffered saline (PBS; 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4, pH 7.4), and then suspended in cell-freezing medium (RPMI 1640 medium containing 10% dimethyl sulfoxide and 10% fetal bovine serum [FBS]; Invitrogen, Carlsbad, CA) and frozen at −80°C. E. coli Stellar (BD Clontech, Mountain View, CA) and PIR1 (Invitrogen) cells were used for recombinant DNA procedures and cultivated in Luria-Bertani (LB) broth. E. coli transformants were selected on LB agar plates containing 50 μg of kanamycin/ml or 10 μg of chloramphenicol/ml. African green monkey kidney (Vero) cells (CCL-81; ATCC) and THP-1 cells (TIB-202; ATCC), a human acute monocytic leukemia cell line, were maintained in RPMI 1640 medium containing 10% FBS at 37°C and 5% CO2. C. burnetii replication in host cells or in ACCM-2 was measured by quantitative PCR of genome equivalents (GE) as previously described (3, 44) using a probe specific to dotA.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli Stellar | F− endA1 supE44 thi-1 recA1 relA1 gyrA96 phoA ϕ80ΔlacZΔM15 Δ(lacZYA-argF)U169 Δ(mrr-hsdRMS-mcrBC) ΔmcrA λ− | Clontech |
| E. coli PIR1 | F− Δlac-169 rpoS(Am) robA1 creC510 hsdR514 endA recA1 uidA(ΔMluI)::pir-116 | Invitrogen |
| C. burnetii Nine Mile (RSA439) | Phase II, clone 4 | Beare et al. (12) |
| C. burnetii ΔpmrA | Nine Mile, phase II containing a pmrA (cbu1227) deletion; Kanr | This study |
| C. burnetii ΔdotA | Nine Mile, phase II containing a dotA (cbu1648) deletion; Kanr | Beare et al. (12) |
| Plasmids | ||
| pJB-Kan | pJB2581 containing kan driven by P1169; Kanr | Omsland et al. (24) |
| pJC-CAT | pJC84 containing cat driven by P1169; Cmr | Beare et al. (12) |
| pJC-CAT::cbu1227-5′3′ | 5′- and 3′-end-flanking DNA from cbu1227 cloned into pJC-CAT; Cmr | This study |
| pJC-CAT::cbu1227-5′3′-Kan | P1169-Kan cassette cloned into pJC-CAT::cbu1227-5′3′; Cmr Kanr | This study |
| pTnS2::P1169-tnsABCD | cbu1169 promoter cloned into pTnS2; Ampr R6K ori | Beare et al. (12) |
| pMiniTn7T-CAT | P1169-CAT cloned into pUC18R6K-mini-Tn7T-Gm; Cmr Ampr R6K ori | Beare et al. (12) |
| pMiniTn7T-CAT::cbu1227/1228comp | cbu1227/1228comp fragment cloned into pMiniTn7T-CAT; Cmr Ampr | This study |
| pGSV4 | Vector containing the luxCDABE operon | Warawa et al. (45) |
| pMiniTn7T-CAT::luxCDABE | Promoterless luxCDABE fragment cloned into pMiniTn7-CAT; Cmr Ampr | This study |
| pMiniTn7T-CAT::P0021-luxCDABE | cbu0021 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P0794-luxCDABE | cbu0794 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P0814-luxCDABE | cbu0814 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PenhC-luxCDABE | enhC promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P1213-luxCDABE | cbu1213 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P1226-luxCDABE | cbu1226 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PpmrA-luxCDABE | pmrA promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P1314-luxCDABE | cbu1314 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PgltA-luxCDABE | gltA promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P1556-luxCDABE | cbu1556 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P1636-luxCDABE | cbu1636 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PrpoS-luxCDABE | rpoS promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P1751-luxCDABE | cbu1751 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P1758-luxCDABE | cbu1758 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P1823-luxCDABE | cbu1823 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::P2052-luxCDABE | cbu2052 promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PicmH-luxCDABE | icmH promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PicmW-luxCDABE | icmW promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PicmV-luxCDABE | icmV promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PdotD-luxCDABE | dotD promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PicmT-luxCDABE | icmT promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PcoxigA-luxCDABE | CoxigA gene promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pMiniTn7T-CAT::PicmD-luxCDABE | icmD promoter cloned into pMiniTn7T-CAT-luxCDABE | This study |
| pJB-CAT-CyaA | C. burnetii CyaA fusion vector; Cmr Ampr | Beare et al. (12) |
| pJB-CAT-CyaA-cbu0122 | cbu0122 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu0409 | cbu0409 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu0508 | cbu0508 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu0705 | cbu0705 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu1231 | cbu1231 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu1530 | cbu1530 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu1540 | cbu1540 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu1614 | cbu1614 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu1651 | cbu1651 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu1685 | cbu1685 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu1686 | cbu1686 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cbu1752 | cbu1752 cloned into pJB-CAT-CyaA | This study |
| pJB-CAT-CyaA-cpeD | cbuA0015 cloned into pJB-CAT-CyaA | Beare et al. (12) |
| pJB-CAT-CyaA-cpeE | cbuA0016 cloned into pJB-CAT-CyaA | Beare et al. (12) |
Generation and complementation of a pmrA mutant.
The plasmids and oligonucleotide primers used in this study are listed in Tables 1 and 2, respectively. Restriction enzymes were obtained from New England BioLabs (Ipswich, MA). PCR was performed using Accuprime Pfx or Taq polymerase (Invitrogen). PCR primers were obtained from Integrated DNA Technologies (San Diego, CA). All cloning procedures were conducted using an In-Fusion PCR cloning system (BD Clontech), and DNA was transformed into either E. coli Stellar or PIR1 competent cells. For targeted inactivation of C. burnetii pmrA (cbu1227), the 5′- and 3′-end-flanking regions of the gene were first amplified from genomic DNA by PCR using the upstream and downstream oligonucleotide pairs CBU1227-5′-F/CBU1227-5′-R and CBU1227-3′-F/CBU1227-3′-R, respectively. The 5′ and 3′ fragments were cloned into BamHI/SalI-digested pJC-CAT (12) by In-Fusion PCR, resulting in the formation of an internal AgeI site between the 5′ and 3′ regions and the creation of pJC-CAT::CBU1227-5′3′. The P1169-Kan cassette was amplified from pJB-Kan (24) by PCR with P1169-Kan-AgeI-KO-rev-F and P1169-Kan-AgeI-KO-rev-R oligonucleotides and cloned into AgeI-digested pJC-CAT::CBU1227-5′3′ to create pJC-CAT::CBU1227-5′3′-Kan. For complementation of C. burnetii ΔpmrA, the cbu1227 promoter region, along with cbu1227 and cbu1228, was amplified from genomic DNA by PCR using the oligonucleotides CBU1227comp-F and CBU1227comp-R. The resulting fragment was cloned into EcoRI-digested pMini-Tn7T-CAT (12) by In-Fusion PCR to create pMini-Tn7T-CAT::cbu1227/1228comp.
TABLE 2.
Oligonucleotide primers used in this study
| Category and primer | Sequence (5′ to 3′) |
|---|---|
| Primers for gene deletion, analysis, and complementation | |
| CBU1227-5′-F | CGGTACCCGGGGATCCGCTGCTCACCTACTACGCGCAAC |
| CBU1227-5′-R | CACCACCGGTCGACGCGAGCGTCGAGCCCTGACGGTCTTGGGCATC |
| CBU1227-3′-F | CGTCGACCGGTGGTGCGCATGTACGTCGGTAGAAAAAAAGTCTGAATGACG |
| CBU1227-3′-R | GAACCTGTTTGTCGACTGAACCTCGCTATTGGCTTCAAG |
| P1169-Kan-AgeI-KO-rev-F | CATGCGCACCACCGGTATGGCTTCGTTTCGCAGCG |
| P1169-Kan-AgeI-KO-rev-R | GCTCGCGTCGACCGGTTTATCAGAAGAACTCGTCAAGAAGG |
| CBU1227-F | CTTGTTGAAGATGATGAATTTCTCG |
| CBU1227-R | TTCAGACTTTTTTTCTACCATGTAAC |
| CBU1227-comp-F | TTACTCAATGGAATTCGGTATAGCATCAACTCCCAGTG |
| CBU1227-comp-R | GCTTCTCGAGGAATTCTTATCCACAGAAATTGTGAATAAGGG |
| Primers for luciferase vector construction | |
| miniTn7-Lux-LR-noP-F | GTATCGATAAGCTAGCGGATCCCAGTCTGCAGATGGCAAATATGACTAAAAAAATTTCATTCATTATTAACGG |
| miniTn7-Lux-LR-noP-R | TGCCAACAGATGTACAGATTTACCTTTCGGAAAAGCC |
| Primers for luciferase promoter fusions | |
| PCBU0021-Lux-F | ATAAGCTAGCGGATCCGCATGAACGACCTCCTCATTTAC |
| PCBU0021-Lux-R | GTCATATTTGCCATCGTTTATCTCCAGCGCTTTACG |
| PCBU0794-Lux-F | ATAAGCTAGCGGATCCCATAAAATCAAGATACAAGGTTGGG |
| PCBU0794-Lux-R | GTCATATTTGCCATCTTTTTTATTCCACCTTACAAAAATTTAGAG |
| PCBU0814-Lux-F | ATAAGCTAGCGGATCCGCGGAACACTTATTTTATTGGATGACC |
| PCBU0814-Lux-R | GTCATATTTGCCATCAAAACCATATTCTCATTTTCAATTGCTTTTAATG |
| PenhC-Lux-F | ATAAGCTAGCGGATCCCACGGTTAGATGTAGAAAGCC |
| PenhC-Lux-R | GTCATATTTGCCATCCCATTAATTTTTCCTAGTTAAAATATAGC |
| PCBU1213-Lux-F | ATAAGCTAGCGGATCCTGGTGCCCCAAGGCGATTCTAC |
| PCBU1213-Lux-R | GTCATATTTGCCATCTATAAGCTGCTCACTGGGAAAC |
| PCBU1226-Lux-F | ATAAGCTAGCGGATCCCGAGGTCGAGGATAATAATATC |
| PCBU1226-Lux-R | GTCATATTTGCCATCAATGGATACCCCTGTATTC |
| PpmrA-Lux-F | ATAAGCTAGCGGATCCCGACTTTTTCAACGATTCTATC |
| PpmrA-Lux-R | GTCATATTTGCCATCCACTCTCACCTTACTTACTGCG |
| PCBU1314-Lux-F | ATAAGCTAGCGGATCCGTTATGAAAAATGATTTATTCATC |
| PCBU1314-Lux-R | GTCATATTTGCCATCGAGGTAACCTCCATTTTTATAC |
| PgltA-Lux-F | ATAAGCTAGCGGATCCCCTTATCTTCCTAAACGCC |
| PgltA-Lux-R | GTCATATTTGCCATCTCGTGCTCCTGGCGGGTG |
| PCBU1556-Lux-F | ATAAGCTAGCGGATCCGAAGACGCCTCTCTCATTAC |
| PCBU1556-Lux-R | GTCATATTTGCCATCAAAATACCCTTTTTATTTTCGTTTTCGG |
| PCBU1636-Lux-F | ATAAGCTAGCGGATCCTGAATTATATTTCATCCAAGCTTTAC |
| PCBU1636-Lux-R | GTCATATTTGCCATCCTGTTTTCCCCTCCGAAGTTAGTC |
| PrpoS-Lux-F | ATAAGCTAGCGGATCCCTTCCGATGGAGTTGTTGTATATAG |
| PrpoS-Lux-R | GTCATATTTGCCATCAGTCCTGTCGTCCTTAACCCG |
| PCBU1751-Lux-F | ATAAGCTAGCGGATCCGACAACCCACATAGCGGGAG |
| PCBU1751-Lux-R | GTCATATTTGCCATCATTCCAACTCCTTCACCGAAC |
| PCBU1758-Lux-F | ATAAGCTAGCGGATCCGTCTCGTGTAGATCACTTTACCC |
| PCBU1758-Lux-R | GTCATATTTGCCATCAAAAACCTCCCTTTTAGTAAAGAG |
| PCBU1823-Lux-F | ATAAGCTAGCGGATCCGTTCGCCGGATCTAAATGACCGCC |
| PCBU1823-Lux-R | GTCATATTTGCCATCATTTCCCTCCGCAATAGCTTAGG |
| PCBU2052-Lux-F | ATAAGCTAGCGGATCCCAGGAATGGCGAGATTCTTTC |
| PCBU2052-Lux-R | GTCATATTTGCCATCTTAAGGCCCCTATCAAAGAAATG |
| PicmH-Lux-F | ATAAGCTAGCGGATCCGCCCGCATCCCGTAACCTTCAG |
| PicmH-Lux-R | GTCATATTTGCCATCTACGGCGTATTATACCTGCAATAAATTTG |
| PicmW-Lux-F | ATAAGCTAGCGGATCCCAACGATATTTCTGATGGTTTTG |
| PicmW-Lux-R | GTCATATTTGCCATCGACTTCTCCGCTATTTAGGGTC |
| PicmV-Lux-F | ATAAGCTAGCGGATCCGTTTCGATCTCGGGATTGCCATC |
| PicmV-Lux-R | GTCATATTTGCCATCTCATGCTAGCACCACTTCCTTAAG |
| PdotD-Lux-F | ATAAGCTAGCGGATCCTGGGGCAACGTACGAAGATAC |
| PdotD-Lux-R | GTCATATTTGCCATCTTTGCAGTCGCTTAATCAAGTAAGG |
| PicmT-Lux-F | ATAAGCTAGCGGATCCCTACGGTGGATACATACTGAG |
| PicmT-Lux-R | GTCATATTTGCCATCAAAGACCCTCTTATACTGGTTTAAAATTTAAC |
| PcoxigA-Lux-F | ATAAGCTAGCGGATCCGCCCTCTGAGGAGGAAAGCTCAAC |
| PcoxigA-Lux-R | GTCATATTTGCCATCGTCAATACCCTTATTCGTAGATTAATCC |
| PicmD-Lux-F | ATAAGCTAGCGGATCCGCAGCTCATTCCGCCAATTATTG |
| PicmD-Lux-R | GTCATATTTGCCATCGATGACTAATCTCCAGAAATAATAATTTTTTCTAC |
| Primers for CyaA fusions | |
| CBU0122-CyaA-F | TTCCGGCTATGTCGACATGAGTACGGCTAATAATTGTG |
| CBU0122-CyaA-R | GCATGCCTCAGTCGACCTATTTTTGGGGGTAGAAAAAAAGCTCG |
| CBU0409-CyaA-F | TTCCGGCTATGTCGACATGGCCACAGTCCCAGTCATAGG |
| CBU0409-CyaA-R | GCATGCCTCAGTCGACTTACTGCCCCCCTGCATGTTTATATTTTTTAGG |
| CBU0508-CyaA-F | TTCCGGCTATGTCGACATGCCAACAAATTTAGAAACCG |
| CBU0508-CyaA-R | GCATGCCTCAGTCGACTTAATGAGTAATCAGAAACCGCCGC |
| CBU0705-CyaA-F | TTCCGGCTATGTCGACATGAATAGTCCAACAGGAAGTAGC |
| CBU0705-CyaA-R | GCATGCCTCAGTCGACCTAAATCATTTTCCACGGCTCACC |
| CBU1231-CyaA-F | TTCCGGCTATGTCGACATGTCAAGGCAATCATTTTCCATCACC |
| CBU1231-CyaA-R | GCATGCCTCAGTCGACCTAAAAGAAAAACCCCAATAGTCCACC |
| CBU1530-CyaA-F | TTCCGGCTATGTCGACATGCCTTTATCTAAAGAAGAATTCC |
| CBU1530-CyaA-R | GCATGCCTCAGTCGACTTAAATTAGTCTAGCTCGGAGAAAATCTAC |
| CBU1540-CyaA-F | TTCCGGCTATGTCGACATGTCTTTTCGTTATTTTGCTTCAAAAGG |
| CBU1540-CyaA-R | GCATGCCTCAGTCGACCTATGGATAGTAAAATTTTATTATAAGTG |
| CBU1614-CyaA-F | TTCCGGCTATGTCGACATGCTCAATCTATATTTCCAGAAGC |
| CBU1614-CyaA-R | CATGCCTCAGTCGACTCAATTAAATGTTTTATTGAACAAGAAACAGC |
| CBU1651-CyaA-F | TTCCGGCTATGTCGACATGAATAAATATCTTTTAATAGGAACG |
| CBU1651-CyaA-R | GCATGCCTCAGTCGACTTAAAACCAGTGAATATTAGATGAATC |
| CBU1685-CyaA-F | TTCCGGCTATGTCGACATGAGAACAACCATCTCAACCGTG |
| CBU1685-CyaA-R | GCATGCCTCAGTCGACTTATTTAATTTTAATGCTAGAAATGG |
| CBU1686-CyaA-F | TTCCGGCTATGTCGACATGCCCTCTAGTTCTAAAGATTTAAGAAAAAAGTTAAAAAAC |
| CBU1686-CyaA-R | GCATGCCTCAGTCGACTTACTAGGGTTGATATCCGTTTG |
| CBU1752-CyaA-F | TTCCGGCTATGTCGACATGAGAGATCCAGATCAAGAAATGC |
| CBU1752-CyaA-R | GCATGCCTCAGTCGACTTATGAAGGGCCGAATGCCGGGAC |
Electroporation of C. burnetii was conducted as previously described (24). Selection of C. burnetii “loop-in” pJC-CAT::CBU1227-5′3′-Kan transformants with chromosomal integration of the suicide plasmid was conducted by culture of bacteria in ACCM-2 containing kanamycin (final concentration, 350 μg/ml) and chloramphenicol (final concentration, 3 μg/ml). Resolution of the sacB-bearing plasmid cointegrant was accomplished by subculture of transformants for 4 days in ACCM-2 supplemented with 1% sucrose and kanamycin. C. burnetii ΔpmrA was subsequently expanded by culture in ACCM-2 containing kanamycin. Clonal isolation was achieved by limiting dilution in ACCM-2, and deletion of pmrA was verified by PCR. PCR validation was accomplished by amplifying an internal pmrA gene fragment with specific primer pairs, with wild-type and mutant genomic DNAs as the templates. Generation and verification of a Tn7-based genetic complement of C. burnetii ΔpmrA were performed as previously described (12).
Genes conferring resistance to chloramphenicol, kanamycin, or ampicillin were approved for C. burnetii genetic transformation studies by the Rocky Mountain Laboratories Institutional Biosafety Committee and the Centers for Disease Control and Prevention, Division of Select Agents and Toxins Program.
Construction of luciferase reporter strains.
The operon encoding luciferase (luxCDABE) was amplified by PCR from pGSV4 (45) using the primers miniTn7-Lux-LR-noP-F and miniTn7-Lux-LR-noP-R. The promoterless luxCDABE PCR fragment was cloned into NheI- and EcoRI-digested pMiniTn7T-CAT by using In-Fusion PCR to create pMiniTn7T-CAT::luxCDABE. Promoters for testing in the luciferase assay were amplified by PCR using the primers listed in Table 2. The corresponding fragments were cloned by In-Fusion PCR into BamHI/PstI-digested pMiniTn7T-CAT::luxCDABE to create the plasmids listed in Table 1. These plasmids were used to transform C. burnetii and generate strains expressing single-copy lux promoter fusions.
Luciferase assays.
Individual wells of a 12-well tissue culture plate containing 2 ml of ACCM-2 were inoculated with C. burnetii harboring pMiniTn7T-CAT-luxCDABE promoter constructs at a cell density of 1 × 106 GE/ml. After 4 days of incubation, the growth medium was thoroughly mixed by pipetting and 200 μl of each culture was added to a Microlite 2, white, 96-well plate (Thermo Scientific). Luminescence was measured over 5 s using a Safire2 microplate reader (Tecan).
Indirect immunofluorescence.
Infected Vero cells were fixed for 20 min in 4% paraformaldehyde plus PBS, followed by permeabilization for 5 min in 0.1% saponin in PBS. Cells were stained for indirect immunofluorescence as previously described (4, 46). Rabbit anti-C. burnetii serum and a mouse monoclonal antibody directed against LAMP3 (CD63) (clone H5C6; BD Biosciences) were used as primary antibodies. Alexa Fluor 488 and 594 IgG (Invitrogen) were used as secondary antibodies. Coverslips were mounted using ProLong Gold containing 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) to visualize nuclei. Microscopy was conducted using a modified Perkin-Elmer UltraView spinning-disk confocal system connected to a Nikon Eclipse Ti-E inverted microscope. Images were obtained using MetaMorph software (Molecular Devices, Inc., Downingtown, PA) and processed with ImageJ software (written by W. S. Rasband at the U.S. National Institutes of Health, Bethesda, MD, and available from http://rsb.info.nih.gov/ij/).
Construction of cyaA plasmids and translocation assays.
Genes encoding potential Dot/Icm substrates were amplified by PCR from C. burnetii genomic DNA using the oligonucleotides listed in Table 2. The resulting PCR products were then cloned by In-Fusion PCR into the unique SalI site of pJB-CAT-CyaA to create the plasmids listed in Table 1. THP-1 cells (1 × 105 per well) in 24-well plates were differentiated into macrophage-like cells by incubation overnight in RPMI 1640 medium (Invitrogen) containing 10% fetal bovine serum (FBS) and 200 nM phorbol myristate acetate (PMA) (Sigma-Aldrich). Cells were washed once with RPMI 1640 plus 10% FBS, infected with 1 × 106 C. burnetii transformants expressing CyaA fusion proteins, and incubated in RPMI 1640 plus 10% FBS for 48 h. The concentration of cyclic AMP (cAMP) in lysates from infected cells was determined using the cAMP enzyme immunoassay (GE Healthcare) as previously described (11). Positive secretion of CyaA fusion proteins was scored as a cytosolic cAMP level ≥2.5-fold higher than that for cells infected with C. burnetii expressing CyaA alone (17).
Immunoblotting.
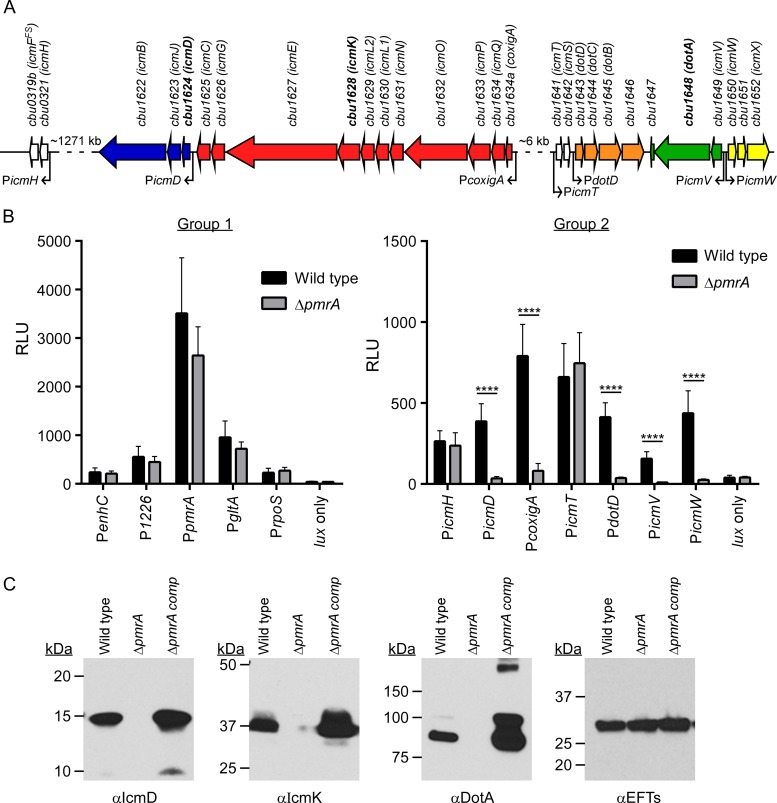
C. burnetii strains were cultivated for 4 days in ACCM-2 and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting to assess expression of IcmD, IcmK, and DotA. As a loading control, blots were also probed with monoclonal antibody against elongation factor Ts (EF-Ts; generously provided by James Samuel, Texas A&M University). Rabbit anti-IcmK and -DotA antibodies were a generous gift of Edward Shaw, Oklahoma State University, and rabbit anti-IcmD antibody has been previously described (11). Following incubation of membranes with primary antibody, reacting proteins were detected using anti-rabbit or anti-mouse IgG secondary antibodies conjugated to horseradish peroxidase (Pierce, Rockford, IL) and chemiluminescence using ECL Pico reagent (Pierce).
RNA-seq.
Wild-type C. burnetii and the ΔpmrA knockout strain were inoculated in triplicate into 20 ml of ACCM-2 at a concentration of 1 × 106 GE/ml. Bacteria were grown in ACCM-2 for 4 days and then harvested by centrifugation (10,000 × g) for 10 min at 4°C. Pellets were suspended in 1 ml of TRIzol and frozen at −80°C. RNA was extracted as previously described (47) prior to rRNA depletion using the MICROBExpress kit (Ambion, Life Technologies). Purified and ribosome-depleted RNAs were sequenced and analyzed by the Oregon State University Center for Genome Research and Biocomputing (OSU CGRB). Samples were processed and multiplexed using an Illumina TruSeq RNA kit, followed by paired-end, 100-bp sequencing using an Illumina HiSeq 2000 system. Three samples each of purified and ribosome-depleted RNAs were sequenced to produce an average of 28.3 million paired-end traces each. Sequences were aligned to the C. burnetii Nine Mile (RSA493) genome (GenBank accession number GI:71066702) using TopHat2 (48). Gene annotations for alignment were created from the GenBank file using custom Perl (practical extraction and reporting language) scripts. Read counts per gene (fragments per kilobase of transcript per million reads mapped) and differential expression were calculated with the Cuffdiff 2 algorithm (49). The R bioconductor package cummeRbund (50) was used to identify differentially expressed genes.
Microcapillary reverse-phase high-performance LC-MS/MS.
Wild-type C. burnetii and the ΔpmrA mutant were grown for 4 days in ACCM-2, and then equal numbers of bacteria (based on GE) were pelleted, washed 3 times in PBS, and suspended in 2× Laemmli sample buffer. Samples were boiled prior to one-dimensional SDS-PAGE, conducted by the Research Technologies Branch of the National Institute of Allergy and Infectious Diseases. Each sample lane was cut into 24 pieces for identification of separated proteins. Protein identification was performed on reduced and alkylated, trypsin-digested samples prepared by standard mass spectrometry (MS) protocols. The supernatant and two washes (5% formic acid in 50% acetonitrile) of the gel digests were pooled and dried in 200 μl polypropylene auto-sampler vials (SUN-SRi, Rockwood, TN) using a SpeedVac (Labconco, Kansas City, MO). Recovered peptides were resuspended in 5 μl of solvent A (0.1% formic acid, 2% acetonitrile, and 97.9% water). Prior to mass spectrometry, the resuspended peptides were chromatographed directly on a column without trap cleanup. The bound peptides were separated at 500 nl/min, generating 8 to 12 million Pa of pressure, using a C18 reverse-phase medium packed in a pulled-tip, nano-chromatography column (0.100 mm [internal diameter] by 150 mm [length]) from Precision Capillary Columns (San Clemente, CA). Chromatography was performed in line and at room temperature with an LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific, West Palm Beach, FL) using a mobile phase consisting of a linear gradient prepared from solvent A and solvent B (0.1% formic acid, 2% water, and 97.9% acetonitrile). Nano-electrospray liquid chromatography–tandem MS (LC-MS/MS) was performed with a Proxeon Easy-nLC II multidimensional liquid chromatograph and a temperature-controlled Ion Max Nanospray source (Thermo Fisher Scientific) in line with the LTQ Orbitrap Velos mass spectrometer.
Computer-controlled, data-dependent, automated switching to MS/MS by Xcalibur 2.1 software was used for data acquisition and provided the peptide sequence information. UniProt KB/Swiss-Prot (August 2013) and the UniProtKB/TrEMBL (August 2013) databases containing C. burnetii genomic sequences were searched using Mascot software (Matrix Science, Beachwood, OH), with one allowed missed cleavage and mass tolerances of 10 ppm and 0.8 Da for the precursor and fragment ions, respectively. Carbamidomethylation of cysteine was set as a fixed modification, while oxidation of methionine, deamidation of asparagine and glutamine, and N-terminal acetylation of protein were searched as dynamic modifications. The resulting search files were reclustered against the same sequence database for further analysis using ProteoIQ software (PREMIER Biosoft, Palo Alto, CA). Assignments were filtered using the Protein Prophet algorithm as implemented within ProteoIQ, with cutoff filters set to 95% for proteins. Normalization was carried out by both total sampling and normalized spectral abundance factors (NSAF) (51). Two spectra per peptide and two peptides per protein minimum were used to refine the results, as were protein and peptide probability filters of 0.95 and 0.5, respectively. Protein NSAF are calculated as the number of spectral counts for protein X (SpC) divided by the number of amino acids (L) in protein X divided by the sum of SpC/L for all proteins in the experimental data set.
Statistical analysis.
Statistical analyses were performed using a one-way analysis of variance (ANOVA) and Prism software (GraphPad Software, Inc., La Jolla, CA).
RESULTS
C. burnetii requires PmrA for intracellular replication.
Previous studies using L. pneumophila as a surrogate host indicated that the response regulator PmrA has an important role in the transcription of C. burnetii Dot/Icm genes. Furthermore, Dot/Icm function is necessary for the intracellular growth of C. burnetii (10, 11, 13). To directly examine the importance of PmrA in the replication of C. burnetii in mammalian host cells, we generated a pmrA mutant by targeted gene deletion (12). Using Tn7, the mutant was genetically complemented in cis with a single copy of pmrAB under the control of its native promoter. Levels of growth of wild-type C. burnetii, the ΔpmrA mutant, and the complemented mutant were indistinguishable in the axenic medium, ACCM-2 (Fig. 1A). However, C. burnetii ΔpmrA replication was significantly reduced in Vero cells at 6 days postinfection, as shown by a 3-fold increase in GE, relative to a 300-fold increase for wild-type C. burnetii (Fig. 1B). As in C. burnetii Dot/Icm mutants (12), defective intracellular replication of C. burnetii ΔpmrA correlated with the formation of small, tight-fitting LAMP3-positive vacuoles in Vero cells (Fig. 1C). Complementation by Tn7::pmrAB rescued both intracellular replication and PV biogenesis, which confirmed that the altered phenotypes were due to the absence of pmrA (Fig. 1B and C). These data indicate that PmrA is essential for the intracellular growth of C. burnetii.
FIG 1.
C. burnetii ΔpmrA has severe intracellular growth defects. Replication of wild-type C. burnetii, the ΔpmrA mutant, and the complemented mutant (comp) in ACCM-2 (A) and Vero cells (B). Fold increases in C. burnetii genome equivalents (GE) after 6 days of growth are depicted. Results are expressed as the means of results from two biological replicates from four independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (P < 0.0001) compared to values for wild-type C. burnetii. (C) Confocal fluorescence micrographs of Vero cells infected for 4 days with wild-type C. burnetii, the ΔpmrA mutant, or the complemented mutant. LAMP3 (green) and C. burnetii (red) are stained by indirect immunofluorescence, and DNA (blue) is stained with DAPI. Bar, 5 μm.
Transcription of pmrA declines following entry into stationary phase.
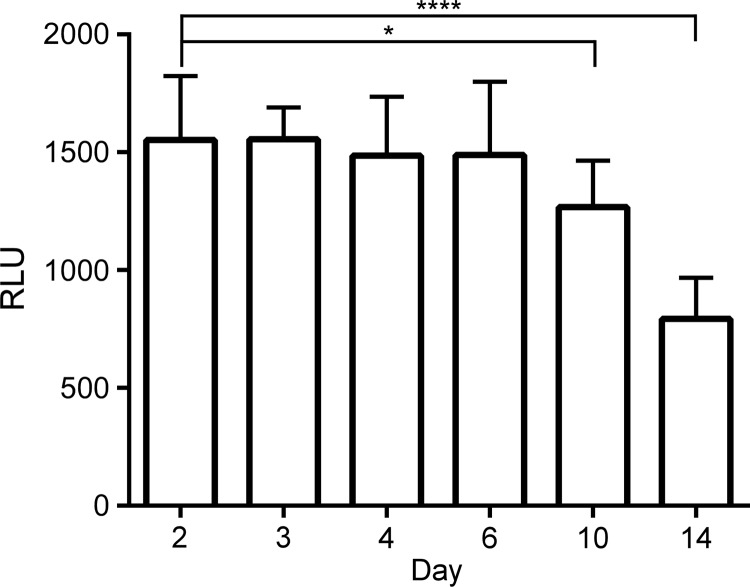
We next employed the luciferase bioluminescent reporter system to explore PmrA function in C. burnetii. The plasmid pMiniTn7T-CAT::luxCDABE was constructed and used to generate strains expressing a single copy of luxCDABE (lux) transcriptionally fused to selected promoter regions. To gain insight into the temporal regulation of pmrA, luciferase assays were first conducted on wild-type C. burnetii carrying lux fused to the pmrA promoter region. Luciferase activity was measured after 2, 3, 4, 6, 10, and 14 days of growth in axenic medium. Luciferase activity was maximal and roughly the same through 6 days of growth (Fig. 2). Significant reductions in activity occurred during stationary-phase growth at 10 and 14 days (24). Accordingly, a 4-day time point, representing the late exponential phase of C. burnetii's growth cycle, was used throughout this study to characterize PmrA-mediated gene regulation.
FIG 2.
Transcription of pmrA declines following entry of C. burnetii into stationary phase. Luciferase assays were conducted after 2, 3, 4, 6, 10, and 14 days of growth in axenic medium of wild-type C. burnetii carrying lux fused to the pmrA promoter region. Bioluminescent readings are expressed as relative light units (RLU). Results are expressed as the means of results from two biological replicates from three independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (*, P < 0.05; ****, P < 0.0001).
Deletion of PmrA reduces expression of the Dot/Icm apparatus and substrate-encoding genes.
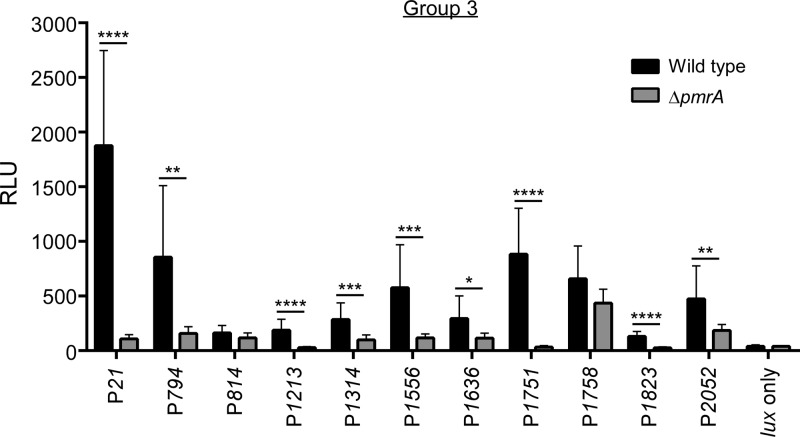
Based on the presence of consensus regulatory elements, Zusman et al. (22) predicted a C. burnetii PmrA regulon and demonstrated in L. pneumophila that two C. burnetii dot/icm genes are regulated by PmrA. To explore PmrA's regulation of the Dot/Icm system directly in C. burnetii, we constructed lux fused to the promoter regions of the Dot/Icm apparatus and substrate-encoding genes. C. burnetii and the ΔpmrA mutant were transformed with Tn7 constructs bearing lux fusions, and then luciferase assays were conducted following 4 days of growth in axenic medium. Tested genes fell into three groups. Group 1 is comprised of five control genes that lack a PmrA regulatory element: cbu1136 (enhC), cbu1226, cbu1227 (pmrA), cbu1410 (gltA), and cbu1669 (rpoS). cbu1226, encoding an NAD-specific glutamate dehydrogenase, was included in this group to determine if deletion of closely linked pmrA affected its transcription. Group 2 consists of icmH (cbu0321), icmD (cbu1624), the CoxigA gene (cbu1634a), icmT (cbu1641), dotD (cbu1643), icmV (cbu1649), and icmW (cbu1650), which are the first structural genes of the seven predicted dot/icm operons (Fig. 3A) (52). With the exception of icmT and icmH, these genes contain an upstream PmrA regulatory element (22). Group 3 contains 11 genes with an upstream PmrA regulatory element that encodes proteins that are positive for Dot/Icm secretion by L. pneumophila: cbu0021 (cig2), cbu0794 (cig20), cbu0814 (cig22), cbu1213 (cig33, ankI), cbu1314 (cig37), cbu1556 (cig50), cbu1636 (cig55), cbu1751 (cig57), cbu1758 (cig58, ankM), cbu1823 (cig61), and cbu2052 (cirD) (13, 15, 16, 18, 22). Furthermore, cbu1636 and cbu1823 have been demonstrated to be regulated by PmrA in L. pneumophila (22).
FIG 3.
The C. burnetii dot/icm locus is regulated by PmrA. (A) Linkage and predicted operon structures of C. burnetii dot/icm genes. Operons with upstream PmrA regulatory elements are colored. Operon-specific promoters are indicated with an arrow below the first gene of each predicted operon. icmF is truncated due to a frameshift (icmFFS). Antibodies specific for proteins encoded by boldface genes were used in immunoblotting, described below. (B) Luciferase activities of the lux operon transcriptionally fused to the promoter regions of control genes (left) and dot/icm genes (right). Assays were conducted after 4 days of growth in axenic medium of wild-type C. burnetii and the ΔpmrA mutant expressing lux fusions. Bioluminescent readings are expressed as relative light units (RLU). Results are expressed as the means of results from two biological replicates from three independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (P < 0.0001) between wild-type C. burnetii and the ΔpmrA mutant. (C) Immunoblots of lysates of wild-type C. burnetii (lane 1), the ΔpmrA mutant (lane 2), and the complemented mutant (lane 3) probed with anti-IcmD (αIcmD), -IcmK, and -DotA antibodies. Probing for EF-Ts was conducted as a loading control.
All group 1 control genes showed the same level of expression in wild-type C. burnetii and the ΔpmrA mutant. Of note, equal levels of transcription of the PpmrA::luxCDABE reporter fusion indicated that PmrA does not regulate itself. Within group 2, icmD, the CoxigA gene, dotD, icmV, and icmW were all expressed in a PmrA-dependent fashion (Fig. 3A and B). The icmTS and icmHF operons were transcribed independently of PmrA (Fig. 3A and B). Immunoblotting confirmed that production of IcmD, IcmK, and DotA was PmrA dependent (Fig. 3C). Nine group 3 Dot/Icm substrate-encoding genes were regulated by PmrA, while two genes (cbu0814 and cbu1758) were not (Fig. 4). Thus, lux assays collectively show that 14 of 16 genes with predicted PmrA regulatory elements are regulated in C. burnetii in a PmrA-dependent fashion.
FIG 4.
Expression of C. burnetii Dot/Icm substrates is regulated by PmrA. Luciferase activities of the lux operon transcriptionally fused to the promoter regions of Dot/Icm substrate-coding genes with predicted pmrA regulatory elements. Assays were conducted after 4 days of growth in axenic medium of wild-type C. burnetii and the ΔpmrA mutant expressing lux fusions. Bioluminescent readings are expressed as relative light units (RLU). Results are expressed as the means of results from two biological replicates from three independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference between wild-type C. burnetii and the ΔpmrA mutant (*, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001).
PmrA is required for secretion by the T4BSS.
To confirm that PmrA is required for assembly of a functional Dot/Icm secretion apparatus, CyaA translocation assays were performed on wild-type C. burnetii and the ΔpmrA mutant expressing adenylate cyclase fused to the defined C. burnetii effector proteins CpeD and CpeE (17). Expression of CyaA alone was used as a negative control. With these constructs, fusion protein expression is driven by the C. burnetii hsp20 promoter (17). CpeD and CpeE fusion proteins were secreted in THP-1 macrophages by wild-type C. burnetii, as indicated by the ≥2.5-fold increase in cAMP levels relative to those in organisms expressing CyaA alone (Fig. 5). Conversely, cAMP levels generated by the ΔpmrA mutants did not exceed the CyaA-alone negative-control level (Fig. 5). Negative secretion was not due to a lack of CyaA fusion protein, as immunoblotting revealed equal amounts of fusion protein produced by wild-type C. burnetii and the ΔpmrA mutant (data not shown). Collectively, these data agree with lux assays and suggest that PmrA-dependent transcriptional activation is essential for secretion by the Dot/Icm T4BSS.
FIG 5.
PmrA is required for secretion by the Dot/Icm T4BSS. Cytosolic levels of cAMP were measured following infection of THP-1 macrophages for 2 days with wild-type C. burnetii or the ΔpmrA mutant expressing CyaA alone or CyaA fused to the previously defined Dot/Icm substrates CpeD and CpeE. Elevated levels of cAMP indicating secretion were observed only with wild-type C. burnetii expressing CyaA-CpeD or -CpeE fusion proteins. Results shown are from one experiment conducted in duplicate and are representative of three independent experiments. Error bars indicate the standard deviations from the means.
Mutational analysis defines nucleotides required for PmrA-regulated expression.
Consensus PmrA regulatory element sequences have been proposed for C. burnetii based on experiments conducted with L. pneumophila (22). To define nucleotides recognized by PmrA in C. burnetii, promoter regions of pmrA-regulated cbu0021 and the CoxigA gene were mutated. Two conserved TTAA nucleotide regions in the predicted PmrA-binding regions (22) were changed individually to TACA. In addition, the predicted −10 promoter regions TAGAAT (cbu0021) and TATAAT (CoxigA gene) were changed to CTGAAT and CTTTCT, respectively. Luciferase assays were conducted after 4 days of growth in axenic medium of wild-type C. burnetii expressing lux fused to mutated or nonmutated promoter regions. As depicted in Fig. 6, all regulatory element/promoter mutations resulted in dramatically reduced levels of expression compared to that of lux fused to the nonmutated promoter. Diminished expression levels were comparable to those associated with the ΔpmrA mutant expressing lux fused to nonmutated cbu0021 or the CoxigA gene promoter regions.
FIG 6.
Mutational analysis defines regulatory element nucleotides required for PmrA-driven expression. (A) Regions upstream of pmrA-regulated cbu0021 and the CoxigA gene. Boldface nucleotides show mutational changes of the two TTAA regions within the predicted PmrA regulatory element (MUT1 and MUT2), while underlined nucleotides show mutational changes within the −10 promoter region (MUT3). (B) Luciferase activity of wild-type (WT) C. burnetii expressing transcriptional proteins of the lux operon fused to nonmutated promoter regions or regions containing the MUT1, MUT2, or MUT3 mutation. Luciferase activity was also assessed for the ΔpmrA mutant expressing lux fused to nonmutated promoters and wild-type C. burnetii and the ΔpmrA mutant expressing lux alone. Assays were conducted after 4 days of growth in axenic medium. Bioluminescent readings are expressed as relative light units (RLU). Results are expressed as the means of results from two biological replicates from three independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (P < 0.0001) with respect to wild-type C. burnetii expressing a nonmutated promoter lux fusion.
RNA-seq and whole bacterial cell mass spectrometry reveal constituents of the C. burnetii PmrA regulon and new Dot/Icm substrates.
To obtain an extended view of the PmrA regulon, transcriptional profiling of wild-type C. burnetii and the ΔpmrA mutant cultivated for 4 days in medium was performed using RNA-seq. This procedure identified 49 genes that were downregulated greater than 2-fold in C. burnetii ΔpmrA relative to levels in wild-type bacteria (Table 3 and see Data Set S1 in the supplemental material). No genes were upregulated greater than 2-fold. Twenty-one downregulated genes contained previously identified upstream PmrA regulatory elements (14, 22), including icmD, icmQ, dotD, icmV, and icmW. Accordingly, 17 additional genes were downregulated due to the polycistronic nature of dot/icm transcription. Eight downregulated genes with PmrA motifs were previously identified as T4BSS substrates (10, 13, 15, 28). The remaining eight downregulated genes with PmrA elements were tested elsewhere for Dot/Icm secretion in L. pneumophila, with negative results (13, 15). Twelve downregulated genes (not including dot/icm genes) did not contain discernible PmrA motifs. Four of these genes encoded hypothetical proteins with a predicted C-terminal E block translocation signal, one of which (cbu1686) encodes a substrate secreted by the L. pneumophila Dot/Icm system (13, 15). Replacement of pmrA with a kanamycin cassette in the ΔpmrA mutant correlated with decreased expression of downstream pmrB, a result consistent with the predicted operon structure of pmrAB (52).
TABLE 3.
Genes and proteins downregulated ≥2-fold by C. burnetii ΔpmrA compared to their levels of expression in wild-type bacteria
| Category and identifier | Product | Gene(s) | Size (aa)a | Motif (reference[s]) | Dot/Icm substrate (reference[s])b | Fold change |
|
|---|---|---|---|---|---|---|---|
| RNA-seq | MSc | ||||||
| Downregulated by RNA-seq and MS | |||||||
| CBU0021 | Hypothetical protein | cig2 | 809 | PmrA (13, 22) | Yes (15) | −5.1 | −14.0 |
| CBU0077 | Hypothetical membrane-spanning protein | 263 | PmrA (13) | Yes (10, 25),d no (13) | −2.4 | −2.5 | |
| CBU0505 | Ribosomal protein-alanine acetyltransferase | cig14 | 205 | PmrA (13, 22) | No (13) | −2.9 | −8.2 |
| CBU0560 | Hypothetical cytosolic protein | 410 | No (15) | −2.8 | −3.5 | ||
| CBU1103 | Membrane-bound lytic murein transglycosylase D precursor | cig29 | 426 | PmrA (13, 22) | No (13) | −4.7 | NP |
| CBU1228 | Sensor protein PmrB | pmrB | 478 | −3.4 | NP | ||
| CBU1366 | Hypothetical exported proteine | cig40 | 110 | PmrA (14, 22) | No (14) | −3.1 | NP |
| CBU1622 | IcmB protein | icmB | 1,003 | −3.2 | −8.0 | ||
| CBU1623 | IcmJ protein | icmJ | 212 | −3.2 | NP | ||
| CBU1624 | IcmD protein | icmD | 138 | PmrA (13, 22) | No (13) | −3.1 | −5.7 |
| CBU1626 | IcmG protein | icmG | 244 | −2.6 | −5.6 | ||
| CBU1627 | IcmE protein | icmE | 1,039 | −2.7 | −5.3 | ||
| CBU1628 | IcmK protein | icmK | 351 | −2.8 | −6.3 | ||
| CBU1629 | IcmL protein | icmL | 218 | −3.1 | −10.5 | ||
| CBU1630 | IcmL protein | icmL | 207 | −3.0 | −4.4 | ||
| CBU1631 | IcmN protein | icmN | 212 | −3.5 | −16.8 | ||
| CBU1632 | IcmO protein | icmO | 792 | −3.7 | NP | ||
| CBU1633 | IcmP protein | icmP | 387 | −4.1 | NP | ||
| CBU1634 | IcmQ protein | icmQ | 238 | PmrA (13, 22) | −5.0 | −18.1 | |
| CBU1643 | DotD protein | dotD | 169 | PmrA (13, 22) | No (13) | −2.3 | −6.3 |
| CBU1644 | DotC protein | dotC | 274 | −2.5 | −6.6 | ||
| CBU1648 | DotA protein | dotA | 814 | −4.0 | −594.3 | ||
| CBU1649 | IcmV protein | icmV | 164 | PmrA (13, 22) | No (13) | −5.5 | NP |
| CBU1650 | IcmW protein | icmW | 149 | PmrA (13, 22) | No (13) | −6.1 | NP |
| CBU1651 | Hypothetical membrane-associated protein | 152 | −4.7 | NP | |||
| CBU1685 | Hypothetical protein | 473 | PmrA (13) | Yes (13), no (15) | −4.3 | −9.6 | |
| CBU1686 | Hypothetical protein | 773 | E block (13) | Yes (15), no (13) | −4.0 | −8.1 | |
| CBU1751 | Hypothetical protein | cig57 | 420 | PmrA (13, 22) | Yes (13) | −3.0 | −7.2 |
| CBU1752 | Hypothetical protein | 412 | −3.0 | −7.8 | |||
| Downregulated by RNA-seq | |||||||
| CBU0122 | Hypothetical membrane-associated protein | 92 | E block (13) | No (13) | −5.2 | — | |
| CBU0273 | Hypothetical protein | cig9 | 248 | PmrA (13, 22) | No (13, 15) | −2.2 | — |
| CBU0409 | Hypothetical protein | 51 | −3.5 | — | |||
| CBU0410 | Hypothetical membrane-spanning protein | cig12 | 578 | PmrA (13, 22) | Yes (13) | −2.5 | — |
| CBU0436 | Hypothetical membrane-spanning protein | cig13 | 258 | PmrA (13, 22) | No (13) | −2.2 | — |
| CBU0508 | Hypothetical membrane-spanning protein | 237 | PmrA (14) | No (13) | −3.8 | — | |
| CBU0665 | Hypothetical protein | cig18, cvpA | 328 | PmrA (13, 22) | Yes (28) | −2.6 | — |
| CBU0705 | Hypothetical protein | 109 | E block (13) | No (13) | −2.2 | — | |
| CBU0706 | Hypothetical protein | 236 | E block (13) | No (13) | −2.5 | — | |
| CBU0860 | Hypothetical protein | 153 | PmrA (13) | No (13) | −2.8 | — | |
| CBU1231 | Hypothetical membrane-associated protein | 232 | −2.6 | — | |||
| CBU1530 | Hypothetical membrane-spanning protein | cig47 | 645 | PmrA (13, 22) | No (10, 13) | −2.1 | — |
| CBU1540 | Hypothetical protein | 109 | −2.6 | — | |||
| CBU1543 | Hypothetical protein | cig49 | 188 | PmrA (13, 22) | Yes (13) | −2.3 | — |
| CBU1614 | Hypothetical protein | 139 | −2.2 | — | |||
| CBU1625 | IcmC protein | icmC | 169 | −2.0 | — | ||
| CBU1634a | CoxigA protein | CoxigA gene | 49 | −6.8 | — | ||
| CBU1645 | ATP-binding protein DotB | dotB | 372 | −2.1 | −1.9 | ||
| CBU1652 | IcmX protein | icmX | 376 | −4.8 | — | ||
| CBU2052 | Hypothetical protein | cirD | 300 | PmrA (13) | Yes (10, 13) | −2.3 | — |
| Downregulated by MS | |||||||
| CBU0027 | Acyltransferase family protein | 296 | 0.3 | −2.3 | |||
| CBU0044 | Hypothetical exported protein | 251 | −0.3 | −4.7 | |||
| CBU0049 | Hypothetical protein | 436 | −1.7 | −9.7 | |||
| CBU0084 | Phosphoglycerol transferase MdoB | mdoB, cig3 | 638 | PmrA (13, 22) | No (13) | −1.4 | NP |
| CBU0138 | Cell division protein FtsQ | ftsQ | 243 | −0.4 | −2.7 | ||
| CBU0156 | Type 4 major prepilin protein PilA | pilA | 140 | 0.3 | NP | ||
| CBU0280 | DNA polymerase IV | dinP | 372 | −0.1 | −2.3 | ||
| CBU0378 | Hypothetical membrane-associated protein | 140 | −0.9 | −4.5 | |||
| CBU0388 | Hypothetical protein | 1,392 | PmrA (13) | Yes (13, 15) | −1.4 | NP | |
| CBU0432 | Transporter, MFS superfamily | 428 | 0.5 | −2.7 | |||
| CBU0447 | Ankyrin repeat protein | ankF | 184 | Yes (10, 18) | 0.3 | −2.7 | |
| CBU0459 | Potassium/proton antiporter RosB | rosB | 406 | 0.4 | −2.0 | ||
| CBU0644 | Plasmid stabilization system toxin protein | 112 | −0.4 | −2.3 | |||
| CBU0787 | Peptide synthetase | 514 | −1.5 | −4.5 | |||
| CBU0794 | Hypothetical protein | cig20 | 464 | PmrA (13, 22) | Yes (13) | −0.8 | NP |
| CBU0881 | Hypothetical cytosolic protein | cig23 | 221 | PmrA (13, 22) | Yes (13) | −0.8 | NP |
| CBU0970 | Hypothetical exported membrane-spanning protein | 351 | E block (13) | No (13) | −1.1 | −2.6 | |
| CBU1098 | Hypothetical cytosolic protein | cig28 | 274 | PmrA (13, 22) | No (13) | −1.6 | −5.3 |
| CBU1179 | Multidrug resistance transporter, Bcr family | 398 | 0.1 | −2.5 | |||
| CBU1370 | Hypothetical membrane-associated protein | 328 | PmrA (13) | No (13) | −1.6 | −5.9 | |
| CBU1439 | NADH-quinone oxidoreductase chain J | nuoJ | 201 | 0.1 | −3.2 | ||
| CBU1493 | Hypothetical protein | cig46 | 557 | PmrA (22) | −0.4 | NP | |
| CBU1636 | Hypothetical protein | cig55 | 391 | PmrA (13) | Yes (13) | −1.0 | NP |
| CBU1641 | IcmT protein | icmT | 85 | −0.3 | NP | ||
| CBU1719 | 10-kDa chaperonin GroES | groES | 116 | 1.2 | −2.8 | ||
| CBU1768 | Hypothetical exported protein | 153 | No (10) | −0.2 | NP | ||
| CBU1794 | Hypothetical protein | cig59 | 272 | PmrA (13, 22) | No (13) | −1.6 | NP |
| CBU1823 | Hypothetical protein | cig61 | 349 | PmrA (13, 22) | Yes (10, 13) | −0.9 | NP |
| CBU1843 | Hypothetical exported protein | 134 | 0.5 | NP | |||
| CBU1850 | Hypothetical protein | 161 | 0.2 | −2.7 | |||
| CBU1863 | Hypothetical membrane-spanning protein | cig62 | 603 | PmrA (13, 22) | No (13) | −1.4 | −2.9 |
| CBU1896 | Macrolide efflux protein | 412 | 0.5 | NP | |||
| CBU2032 | GGDEF family protein | 254 | 0.1 | NP | |||
aa, amino acids.
CBU0122, CBU1530, CBU1614, CBU1685, CBU1686, and CBU1752 were subsequently shown to be positive for Dot/Icm-mediated secretion by C. burnetii (Fig. 7). CBU0409, CBU0508, CBU0705, CBU1213, CBU1540, and CBU1651 were subsequently shown to be negative for Dot/Icm-mediated secretion by C. burnetii (Fig. 7).
NP, no peptide fragments were detected in the ΔpmrA mutant; —, no peptide fragments were detected in wild-type C. burnetii or the ΔpmrA mutant.
Carey et al. (10) and Newton et al. (25) demonstrated that CBU0077 is a Dot/Icm substrate of L. pneumophila and C. burnetii, respectively.
Zusman et al. (22) demonstrated that cbu1366 is regulated by PmrA in L. pneumophila.
To determine the degree to which transcription correlated with protein synthesis, we performed LC-MS/MS on whole cells of wild-type C. burnetii and the ΔpmrA mutant cultivated for 4 days in axenic medium. Compared to proteins in wild-type bacteria, 39 proteins were ≥2-fold less abundant and 23 were undetected in C. burnetii ΔpmrA. Twenty-nine of these proteins (46.8%) are encoded by genes transcriptionally downregulated ≥2-fold in the ΔpmrA mutant according to RNA-seq results, including 17 and 5 Dot/Icm apparatus proteins and substrates, respectively (Table 3 and see Data Set S2 in the supplemental material). Transcript levels of genes encoding the 33 remaining proteins did not vary more than 2-fold, although 11 of these genes contained PmrA motifs (13, 22). Moreover, 6 proteins within this group are previously described Dot/Icm substrates (10, 13, 15). Proteins encoded by 19 PmrA-regulated genes were not detected in wild-type C. burnetii or the ΔpmrA mutant. Possible explanations for this result include posttranscriptional inhibition of translation and/or protein production below the level of detection by mass spectrometry. Overall, transcriptome and proteome data correspond with the bioinformatically based PmrA regulon (13, 22) but also reveal new regulon genes without PmrA regulatory elements.
RNA-seq revealed six genes (cbu0409, cbu1231, cbu1540, cbu1614, cbu1651, and cbu1752) downregulated in the ΔpmrA mutant that encode hypothetical proteins, lack PmrA regulatory elements, and have not been previously tested for Dot/Icm-mediated secretion (Table 3). We were curious whether any of these proteins are secreted by the C. burnetii Dot/Icm T4BSS. CyaA translocation assays were performed on wild-type C. burnetii and a ΔdotA mutant (11) expressing adenylate cyclase fused to the encoded proteins. Expression of CyaA alone was used as a negative control. As depicted in Fig. 7, CBU1614 and CBU1752 fusion proteins were secreted in THP-1 macrophages by wild-type C. burnetii, as indicated by the ≥2.5-fold increase in cAMP levels relative to levels in organisms expressing CyaA alone. Levels of cAMP generated by the ΔdotA mutant expressing Cya fusion proteins did not exceed the CyaA-alone negative-control level, confirming Dot/Icm-dependent translocation. We extended this analysis to proteins within our PmrA-regulated list with an associated PmrA (CBU0508 and CBU1530) or E block (CBU0122 and CBU0705) motif that were reported as negative for Dot/Icm secretion by L. pneumophila (13). Secretion was also assessed for CBU1685 (PmrA motif), and CBU1686 (E block motif), for which there are conflicting reports on secretion by L. pneumophila (13, 15). As shown Fig. 7, CBU0122, CBU1530, CBU1685, and CBU1686 fusion proteins were secreted in a Dot/Icm-dependent fashion by C. burnetii. Thus, comparative transcriptomics and proteomics of wild-type C. burnetii and the ΔpmrA mutant can be used to identify new candidate substrates of the C. burnetii Dot/Icm system and to clarify disparate secretion results obtained with the surrogate L. pneumophila system.
FIG 7.
RNA-seq reveals new Dot/Icm substrates. Cytosolic levels of cAMP were measured following infection of THP-1 macrophages for 2 days with wild-type C. burnetii or the ΔdotA mutant expressing CyaA alone or CyaA fused to possible Dot/Icm substrates. Elevated levels of cAMP indicating secretion were observed for CBU0122, CBU1530, CBU1614, CBU1685, CBU1686, and CBU1752. Results shown are from one experiment conducted in duplicate and are representative of three independent experiments. Error bars indicate the standard deviations from the means.
DISCUSSION
Due to a historic lack of molecular methods, genetic analysis of C. burnetii regulatory systems required for host cell parasitism and disease pathogenesis has not been possible. For this study, we developed a luciferase-based gene reporter system for use in C. burnetii and showed, using a pmrA deletion mutant, that the PmrA response regulator of the PmrAB TCS is a critical positive regulator of the C. burnetii Dot/Icm T4BSS. Consequently, the severe growth defect of C. burnetii ΔpmrA in mammalian cells is similar to that of dot/icm mutants, with little to no replication occurring in LAMP3-positive PVs. Mutation of predicted regulatory elements confirmed nucleotides required for promoter recognition by PmrA, and differential gene expression between wild-type C. burnetii and the isogenic ΔpmrA mutant revealed new Dot/Icm substrates.
There are notable differences between PmrA regulation of L. pneumophila and C. burnetii Dot/Icm systems. In L. pneumophila, dot/icm genes lack PmrA motifs and are not directly transcriptionally regulated by PmrA (22). However, dot/icm genes are downregulated in an L. pneumophila ΔpmrA mutant (22, 32), suggesting indirect regulation, possibly through PmrAB cross talk with CpxR (22). In C. burnetii, operons initiating with icmD, the CoxigA gene, dotD, icmV, and icmW, all of which contain a PmrA motif, are expressed in a PmrA-dependent fashion. icmTS and icmHF do not require PmrA for expression, which is consistent with the lack of PmrA motifs. The reason for this regulatory disconnect is unclear. icmHF is located over 1 megabase from the linked dot/icm locus. Moreover, icmF is annotated as a pseudogene because of an internal stop codon and is unlikely to produce a functional protein. Unlike most Dot/Icm proteins that are evolutionarily related to proteins involved in IncI plasmid conjugation, homologs of IcmH and IcmF are common among pathogenic bacteria, and they have an origin different from that of Dot/Icm proteins (53). In L. pneumophila, IcmH and IcmF apparently function together and mutation of either coding region results in severe growth defects in amoebae but only moderate growth defects in human macrophages (53). C. burnetii does not use amoebae as an environmental replication niche (54), and the truncation of IcmF presumably results in a nonfunctional IcmHF complex. Thus, icmHF may not be required for C. burnetii Dot/Icm function. IcmT is necessary for the intracellular growth of L. pneumophila and mediates pore formation, which enables pathogen egress from macrophages (55, 56). A concerted lytic event is not associated with C. burnetii release from host cells (57); however, whether IcmT serves an essential role in C. burnetii Dot/Icm secretion is unknown. L. pneumophila IcmS and IcmW form a heterodimer that serves as a chaperone for translocation of a subset of substrates (15, 58). C. burnetii IcmS also interacts with IcmW, and the complex is predicted to have chaperone-like activity (59). The lack of PmrA regulation of C. burnetii icmS may indicate that the protein is constitutively expressed. Consequently, PmrA interactions with temporally regulated icmW and other undefined chaperone-like proteins might promote secretion of distinct subsets of effectors at different time points during the infectious cycle (60). L. pneumophila icmS is also not directly or indirectly regulated by PmrA (22).
The L. pneumophila PmrA regulon appears to be more complex than the C. burnetii counterpart. Microarray transcriptome analysis of wild-type L. pneumophila and an isogenic ΔpmrA mutant in the exponential and postexponential growth phases revealed PmrA regulation of 279 genes, including genes encoding the Dot/Icm apparatus and substrates, type II secretion substrates, flagellar components, and multiple metabolic enzymes (32). We found 82 C. burnetii genes/proteins regulated by PmrA during late-exponential-phase growth that are associated primarily with type IV secretion. Interestingly, one of these genes, cbu1103, encodes a predicted membrane-bound lytic murine transglycosylase. Specialized lytic transglycosylases generate gaps in the peptidoglycan that allow assembly of both type III and type IV secretion systems (61). Thus, it is intriguing to speculate that cbu1103 is PmrA regulated to ensure coexpression with the dot/icm locus. The C. burnetii genome is roughly 60% of the size of L. pneumophila's, with a less complex repertoire of Dot/Icm effectors; thus, a reduced PmrA regulon is not unexpected. Moreover, we recognize the caveat that the single late-exponential-phase time point used in our study provides only a snapshot of the PmrA regulon. Several time points over the complete C. burnetii growth cycle are required to gain a comprehensive view of the PmrA regulon. Nonetheless, these data suggest that the C. burnetii PmrAB TCS primarily regulates the T4BSS.
As predicted, a high percentage of C. burnetii PmrA-regulated genes contain PmrA regulatory elements, and several code for previously defined Dot/Icm substrates. However, several regulated genes do not contain a PmrA regulatory element, implicating indirect regulation via PmrAB cross talk with other TCSs and/or control of expression of other regulatory factors (22, 29, 62). We demonstrated that, among the hypothetical proteins encoded by genes lacking PmrA motifs, CBU0122, CBU1686, CBU1614, and CBU1752 are secreted by C. burnetii in a Dot/Icm-dependent fashion. Secretion of CBU1686 and CBU1752 was not altogether unexpected, as their encoding genes are downstream (in predicted two-gene operons [52]) from PmrA motif-containing and Dot/Icm substrate-coding cbu1685 and cbu1751, respectively (13). Moreover, CBU1686, along with secreted CBU0122, contain an E block motif; however, both proteins were previously reported by Weber et al. (13) as negative for secretion by L. pneumophila by the β-lactamase translocation assay. We also demonstrate that CBU1530 (PmrA motif), which is negative for secretion by L. pneumophila (10, 13), is secreted by the C. burnetii Dot/Icm system. Our data show that, as previously reported for L. pneumophila (63), transcriptome data can be used to identify new Dot/Icm substrates. Furthermore, our secretion results that conflict with published data using the surrogate L. pneumophila system reinforce the idea that secretion assays should be conducted directly in C. burnetii, which is now amenable to genetic manipulation.
Relative to free-living bacteria, C. burnetii encodes few TCSs. This likely reflects the stable environment encountered by C. burnetii as a natural obligate intracellular bacterium requiring few adaptive responses (30). C. burnetii encodes, in addition to PmrAB, PhoBR and an EnvZ-like histidine kinase paired with an OmpR family response regulator (42). In E. coli, PhoBR senses environmental phosphate concentrations (64), while EnvZ/OmpR responds to environmental osmolarity (65). As mentioned above, C. burnetii also encodes four orphan hybrid histidine kinases (CBU0634, CBU0789, CBU1084, and CBU1761) (31, 39, 42) and four orphan response regulators with output domains containing a DNA-binding motif (CBU0712, CBU0780, CBU0955, and CBU1043) (31, 39, 42). Only CBU1761 contains a histidine phosphotransferase domain required for phosphoryl group transfer to a cognate response regulator (30, 39). However, CBU0351 contains this domain (39) and consequently may catalyze phosphotransfer from CBU0634, CBU0789, and/or CBU1084 (39). A fifth unclassified orphan response regulator, CBU0760, contains a phosphoryl group receiver domain but lacks an output domain (39). Following activation, this type of response regulator engages downstream effectors via direct protein-protein interactions (66).
The presence of csrA-1 and csrA-2 in the C. burnetii genome suggests that some combination of C. burnetii orphan histidine kinases and response regulators functions analogously to L. pneumophila LetAS. The LetAS-RsmYZ-CsrA regulatory cascade is intricately woven into L. pneumophila stationary-phase physiology, controlling expression of transmissive-phase traits in addition to Dot/Icm effector proteins (67). Both LetAS and RpoS (σs) positively respond to the alarmone ppGpp generated during periods of low amino acid abundance (67). RpoS positively regulates expression of RsmYZ, thereby potentiating LetAS-mediated relief of posttranscriptional repression by csrA (36). Interestingly, the majority of LetAS-RsmYZ-CsrA-regulated Dot/Icm substrates are known, or predicted, effectors of eukaryotic vesicular trafficking (29). For productive infection, L. pneumophila must escape the default endolysosomal pathway and generate a specialized endoplasmic reticulum-derived replication vacuole within minutes of internalization (68). Thus, preloading the stationary-phase, transmissive form of L. pneumophila with these effectors is an elegant pathogenic strategy to ensure proper trafficking immediately upon host cell internalization. Unlike L. pneumophila, C. burnetii requires transit through the canonical endolysosomal pathway for productive infection. Nonetheless, C. burnetii undergoes a biphasic developmental cycle superficially similar to that of L. pneumophila by generating cell types specifically adapted for intracellular replication and extracellular survival (67, 69). Indeed, the nonreplicating and environmentally stable C. burnetii small-cell variant that develops during stationary phase is biologically analogous to the L. pneumophila transmissive form (70). A stationary-phase regulatory network with an integrated TCS comprised of an orphan sensor/regulator pair may therefore control C. burnetii developmental transitions.
This work further expands the C. burnetii genetics toolbox to include a reporter system based on Photorhabdus luminescens luciferase. This luciferase system has advantages over other reporter systems in being highly sensitive and responsive, detectable in living cells without addition of exogenous substrate, and capable of generating a signal that does not stably accumulate (71, 72). Luciferase is comprised of LuxAB, which oxidizes cellular substrates to generate visible light of approximately 490 nm. LuxCDE comprises a reductase complex that generates a reduced substrate for LuxAB (71, 72). Expression of the five Lux subunits required for luciferase activity does not negatively affect C. burnetii growth (P. A. Beare and R. A. Heinzen, unpublished data). Thus, the lux system should have broad utility in the study of C. burnetii-host interactions, including assessment of gene expression during various stages of macrophage invasion, intracellular survival of mutant strains, and bioluminescent imaging of C. burnetii during animal infection (73).
This research sets the stage for identification of the environmental stimuli that activate the C. burnetii PmrAB TCS and production of the T4BSS. In Salmonella enterica, PmrAB is activated by submillimolar Fe3+ and low pH (∼5.8) (74). Low pH has also been implicated as an activator of L. pneumophila PmrAB in L. pneumophila (32). It is logical to suspect that acidification of the C. burnetii PV as it transits through the endolysosomal cascade also stimulates the PmrAB TCS, although this effect would have to be distinguished from the global activation of C. burnetii metabolism that occurs under acidic conditions (75, 76). Nonetheless, the increasing genetic tractability of C. burnetii now allows elucidation of the roles that TCSs and other regulatory factors play in Q fever pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Renee Olano of the Research Technologies Branch, National Institute of Allergy and Infectious Diseases, for mass spectrometry.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print 7 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01532-14.
REFERENCES
- 1.Stein A, Louveau C, Lepidi H, Ricci F, Baylac P, Davoust B, Raoult D. 2005. Q fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infect. Immun. 73:2469–2477. 10.1128/IAI.73.4.2469-2477.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham JG, MacDonald LJ, Hussain SK, Sharma UM, Kurten RC, Voth DE. 2013. Virulent Coxiella burnetii pathotypes productively infect primary human alveolar macrophages. Cell. Microbiol. 15:1012–1025. 10.1111/cmi.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe D, Shannon JG, Winfree S, Dorward DW, Heinzen RA. 2010. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect. Immun. 78:3465–3474. 10.1128/IAI.00406-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howe D, Melnicakova J, Barak I, Heinzen RA. 2003. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell. Microbiol. 5:469–480. 10.1046/j.1462-5822.2003.00293.x [DOI] [PubMed] [Google Scholar]
- 5.Campoy EM, Zoppino FC, Colombo MI. 2011. The early secretory pathway contributes to the growth of the Coxiella-replicative niche. Infect. Immun. 79:402–413. 10.1128/IAI.00688-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI. 2007. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol. 9:891–909. 10.1111/j.1462-5822.2006.00838.x [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, Colombo MI. 2005. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell. Microbiol. 7:981–993. 10.1111/j.1462-5822.2005.00527.x [DOI] [PubMed] [Google Scholar]
- 8.Ghigo E, Colombo MI, Heinzen RA. 2012. The Coxiella burnetii parasitophorous vacuole. Adv. Exp. Med. Biol. 984:141–169. 10.1007/978-94-007-4315-1_8 [DOI] [PubMed] [Google Scholar]
- 9.McDonough JA, Newton HJ, Klum S, Swiss R, Agaisse H, Roy CR. 2013. Host pathways important for Coxiella burnetii infection revealed by genome-wide RNA interference screening. mBio 4(1):e00606–00612. 10.1128/mBio.00606-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey KL, Newton HJ, Luhrmann A, Roy CR. 2011. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog. 7:e1002056. 10.1371/journal.ppat.1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beare PA, Gilk SD, Larson CL, Hill J, Stead CM, Omsland A, Cockrell DC, Howe D, Voth DE, Heinzen RA. 2011. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. mBio 2(4):e00175–00111. 10.1128/mBio.00175-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beare PA, Larson CL, Gilk SD, Heinzen RA. 2012. Two systems for targeted gene deletion in Coxiella burnetii. Appl. Environ. Microbiol. 78:4580–4589. 10.1128/AEM.00881-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber MM, Chen C, Rowin K, Mertens K, Galvan G, Zhi H, Dealing CM, Roman VA, Banga S, Tan Y, Luo ZQ, Samuel JE. 2013. Identification of Coxiella burnetii type IV secretion substrates required for intracellular replication and Coxiella-containing vacuole formation. J. Bacteriol. 195:3914–3924. 10.1128/JB.00071-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Banga S, Mertens K, Weber MM, Gorbaslieva I, Tan Y, Luo ZQ, Samuel JE. 2010. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 107:21755–21760. 10.1073/pnas.1010485107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lifshitz Z, Burstein D, Peeri M, Zusman T, Schwartz K, Shuman HA, Pupko T, Segal G. 2013. Computational modeling and experimental validation of the Legionella and Coxiella virulence-related type-IVB secretion signal. Proc. Natl. Acad. Sci. U. S. A. 110:707–715. 10.1073/pnas.1215278110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR. 2008. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320:1651–1654. 10.1126/science.1158160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voth DE, Beare PA, Howe D, Sharma UM, Samoilis G, Cockrell DC, Omsland A, Heinzen RA. 2011. The Coxiella burnetii cryptic plasmid is enriched in genes encoding type IV secretion system substrates. J. Bacteriol. 193:1493–1503. 10.1128/JB.01359-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, Heinzen RA. 2009. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J. Bacteriol. 191:4232–4242. 10.1128/JB.01656-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maturana P, Graham JG, Sharma UM, Voth DE. 2013. Refining the plasmid-encoded type IV secretion system substrate repertoire of Coxiella burnetii. J. Bacteriol. 195:3269–3276. 10.1128/JB.00180-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Boyd D, Amyot WM, Hempstead AD, Luo ZQ, O'Connor TJ, Chen C, Machner M, Montminy T, Isberg RR. 2011. The E Block motif is associated with Legionella pneumophila translocated substrates. Cell. Microbiol. 13:227–245. 10.1111/j.1462-5822.2010.01531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burstein D, Zusman T, Degtyar E, Viner R, Segal G, Pupko T. 2009. Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog. 5:e1000508. 10.1371/journal.ppat.1000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zusman T, Aloni G, Halperin E, Kotzer H, Degtyar E, Feldman M, Segal G. 2007. The response regulator PmrA is a major regulator of the icm/dot type IV secretion system in Legionella pneumophila and Coxiella burnetii. Mol. Microbiol. 63:1508–1523. 10.1111/j.1365-2958.2007.05604.x [DOI] [PubMed] [Google Scholar]
- 23.Beare PA, Sandoz KM, Omsland A, Rockey DD, Heinzen RA. 2011. Advances in genetic manipulation of obligate intracellular bacterial pathogens. Front. Microbiol. 2:97. 10.3389/fmicb.2011.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omsland A, Beare PA, Hill J, Cockrell DC, Howe D, Hansen B, Samuel JE, Heinzen RA. 2011. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl. Environ. Microbiol. 77:3720–3725. 10.1128/AEM.02826-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newton HJ, McDonough JA, Roy CR. 2013. Effector protein translocation by the Coxiella burnetii Dot/Icm type IV secretion system requires endocytic maturation of the pathogen-occupied vacuole. PLoS One 8:e54566. 10.1371/journal.pone.0054566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luhrmann A, Nogueira CV, Carey KL, Roy CR. 2010. Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc. Natl. Acad. Sci. U. S. A. 107:18997–19001. 10.1073/pnas.1004380107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klingenbeck L, Eckart RA, Berens C, Luhrmann A. 6 November 2012. The Coxiella burnetii type IV secretion system substrate CaeB inhibits intrinsic apoptosis at the mitochondrial level. Cell. Microbiol. 10.1111/cmi.12066 [DOI] [PubMed] [Google Scholar]
- 28.Larson CL, Beare PA, Howe D, Heinzen RA. 2013. Coxiella burnetii effector protein subverts clathrin-mediated vesicular trafficking for pathogen vacuole biogenesis. Proc. Natl. Acad. Sci. U. S. A. 110:E4770–E4779. 10.1073/pnas.1309195110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal G. 2013. The Legionella pneumophila two-component regulatory systems that participate in the regulation of Icm/Dot effectors. Curr. Top. Microbiol. Immunol. 376:35–52. 10.1007/82_2013_346 [DOI] [PubMed] [Google Scholar]
- 30.Capra EJ, Laub MT. 2012. Evolution of two-component signal transduction systems. Annu. Rev. Microbiol. 66:325–347. 10.1146/annurev-micro-092611-150039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galperin MY. 2010. Diversity of structure and function of response regulator output domains. Curr. Opin. Microbiol. 13:150–159. 10.1016/j.mib.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Khodor S, Kalachikov S, Morozova I, Price CT, Abu Kwaik Y. 2009. The PmrA/PmrB two-component system of Legionella pneumophila is a global regulator required for intracellular replication within macrophages and protozoa. Infect. Immun. 77:374–386. 10.1128/IAI.01081-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altman E, Segal G. 2008. The response regulator CpxR directly regulates expression of several Legionella pneumophila icm/dot components as well as new translocated substrates. J. Bacteriol. 190:1985–1996. 10.1128/JB.01493-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiaden A, Spirig T, Weber SS, Bruggemann H, Bosshard R, Buchrieser C, Hilbi H. 2007. The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell. Microbiol. 9:2903–2920. 10.1111/j.1462-5822.2007.01005.x [DOI] [PubMed] [Google Scholar]
- 35.Tiaden A, Spirig T, Sahr T, Walti MA, Boucke K, Buchrieser C, Hilbi H. 2010. The autoinducer synthase LqsA and putative sensor kinase LqsS regulate phagocyte interactions, extracellular filaments and a genomic island of Legionella pneumophila. Environ. Microbiol. 12:1243–1259. 10.1111/j.1462-2920.2010.02167.x [DOI] [PubMed] [Google Scholar]
- 36.Rasis M, Segal G. 2009. The LetA-RsmYZ-CsrA regulatory cascade, together with RpoS and PmrA, post-transcriptionally regulates stationary phase activation of Legionella pneumophila Icm/Dot effectors. Mol. Microbiol. 72:995–1010. 10.1111/j.1365-2958.2009.06705.x [DOI] [PubMed] [Google Scholar]
- 37.Sahr T, Bruggemann H, Jules M, Lomma M, Albert-Weissenberger C, Cazalet C, Buchrieser C. 2009. Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol. Microbiol. 72:741–762. 10.1111/j.1365-2958.2009.06677.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hovel-Miner G, Pampou S, Faucher SP, Clarke M, Morozova I, Morozov P, Russo JJ, Shuman HA, Kalachikov S. 2009. SigmaS controls multiple pathways associated with intracellular multiplication of Legionella pneumophila. J. Bacteriol. 191:2461–2473. 10.1128/JB.01578-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulrich LE, Zhulin IB. 2010. The MiST2 database: a comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res. 38:D401–D407. 10.1093/nar/gkp940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards RL, Jules M, Sahr T, Buchrieser C, Swanson MS. 2010. The Legionella pneumophila LetA/LetS two-component system exhibits rheostat-like behavior. Infect. Immun. 78:2571–2583. 10.1128/IAI.01107-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nevo O, Zusman T, Rasis M, Lifshitz Z, Segal G. 2014. Identification of Legionella pneumophila effectors regulated by the LetAS-RsmYZ-CsrA regulatory cascade, many of which modulate vesicular trafficking. J. Bacteriol. 196:681–692. 10.1128/JB.01175-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galperin MY, Higdon R, Kolker E. 2010. Interplay of heritage and habitat in the distribution of bacterial signal transduction systems. Mol. Biosyst. 6:721–738. 10.1039/b908047c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulkarni PR, Cui X, Williams JW, Stevens AM, Kulkarni RV. 2006. Prediction of CsrA-regulating small RNAs in bacteria and their experimental verification in Vibrio fischeri. Nucleic Acids Res. 34:3361–3369. 10.1093/nar/gkl439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omsland A, Cockrell DC, Howe D, Fischer ER, Virtaneva K, Sturdevant DE, Porcella SF, Heinzen RA. 2009. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 106:4430–4434. 10.1073/pnas.0812074106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warawa JM, Long D, Rosenke R, Gardner D, Gherardini FC. 2011. Bioluminescent diagnostic imaging to characterize altered respiratory tract colonization by the Burkholderia pseudomallei capsule mutant. Front. Microbiol. 2:133. 10.3389/fmicb.2011.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beare PA, Howe D, Cockrell DC, Omsland A, Hansen B, Heinzen RA. 2009. Characterization of a Coxiella burnetii ftsZ mutant generated by Himar1 transposon mutagenesis. J. Bacteriol. 191:1369–1381. 10.1128/JB.01580-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Virtaneva K, Porcella SF, Graham MR, Ireland RM, Johnson CA, Ricklefs SM, Babar I, Parkins LD, Romero RA, Corn GJ, Gardner DJ, Bailey JR, Parnell MJ, Musser JM. 2005. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc. Natl. Acad. Sci. U. S. A. 102:9014–9019. 10.1073/pnas.0503671102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14:R36. 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. 2013. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31:46–53. 10.1038/nbt.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goff LA, Trapnell C, Kelly D. 2012. CummeRbund: visualization and exploration of Cufflinks high-throughput sequencing data. R package version 2.2.0. http://www.bioconductor.org/packages/release/bioc/vignettes/cummeRbund/inst/doc/cummeRbund-manual.pdf.
- 51.Zybailov B, Mosley AL, Sardiu ME, Coleman MK, Florens L, Washburn MP. 2006. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J. Proteome Res. 5:2339–2347. 10.1021/pr060161n [DOI] [PubMed] [Google Scholar]
- 52.Mao F, Dam P, Chou J, Olman V, Xu Y. 2009. DOOR: a database for prokaryotic operons. Nucleic Acids Res. 37:D459–D463. 10.1093/nar/gkn757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zusman T, Feldman M, Halperin E, Segal G. 2004. Characterization of the icmH and icmF genes required for Legionella pneumophila intracellular growth, genes that are present in many bacteria associated with eukaryotic cells. Infect. Immun. 72:3398–3409. 10.1128/IAI.72.6.3398-3409.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.La Scola B, Raoult D. 2001. Survival of Coxiella burnetii within free-living amoeba Acanthamoeba castellanii. Clin. Microbiol. Infect. 7:75–79. 10.1046/j.1469-0691.2001.00193.x [DOI] [PubMed] [Google Scholar]
- 55.Segal G, Shuman HA. 1999. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 67:2117–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molmeret M, Alli OA, Zink S, Flieger A, Cianciotto NP, Kwaik YA. 2002. icmT is essential for pore formation-mediated egress of Legionella pneumophila from mammalian and protozoan cells. Infect. Immun. 70:69–78. 10.1128/IAI.70.1.69-78.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voth DE, Heinzen RA. 2009. Coxiella type IV secretion and cellular microbiology. Curr. Opin. Microbiol. 12:74–80. 10.1016/j.mib.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cambronne ED, Roy CR. 2007. The Legionella pneumophila IcmSW complex interacts with multiple Dot/Icm effectors to facilitate type IV translocation. PLoS Pathog. 3:e188. 10.1371/journal.ppat.0030188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zusman T, Yerushalmi G, Segal G. 2003. Functional similarities between the icm/dot pathogenesis systems of Coxiella burnetii and Legionella pneumophila. Infect. Immun. 71:3714–3723. 10.1128/IAI.71.7.3714-3723.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vincent CD, Friedman JR, Jeong KC, Buford EC, Miller JL, Vogel JP. 2006. Identification of the core transmembrane complex of the Legionella Dot/Icm type IV secretion system. Mol. Microbiol. 62:1278–1291. 10.1111/j.1365-2958.2006.05446.x [DOI] [PubMed] [Google Scholar]
- 61.Koraimann G. 2003. Lytic transglycosylases in macromolecular transport systems of Gram-negative bacteria. Cell. Mol. Life Sci. 60:2371–2388. 10.1007/s00018-003-3056-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laub MT, Goulian M. 2007. Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41:121–145. 10.1146/annurev.genet.41.042007.170548 [DOI] [PubMed] [Google Scholar]
- 63.Faucher SP, Mueller CA, Shuman HA. 2011. Legionella pneumophila transcriptome during intracellular multiplication in human macrophages. Front. Microbiol. 2:60. 10.3389/fmicb.2011.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baek JH, Lee SY. 2006. Novel gene members in the Pho regulon of Escherichia coli. FEMS Microbiol. Lett. 264:104–109. 10.1111/j.1574-6968.2006.00440.x [DOI] [PubMed] [Google Scholar]
- 65.Cai SJ, Inouye M. 2002. EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J. Biol. Chem. 277:24155–24161. 10.1074/jbc.M110715200 [DOI] [PubMed] [Google Scholar]
- 66.Jenal U, Galperin MY. 2009. Single domain response regulators: molecular switches with emerging roles in cell organization and dynamics. Curr. Opin. Microbiol. 12:152–160. 10.1016/j.mib.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molofsky AB, Swanson MS. 2004. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53:29–40. 10.1111/j.1365-2958.2004.04129.x [DOI] [PubMed] [Google Scholar]
- 68.Roy CR, Berger KH, Isberg RR. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663–674. 10.1046/j.1365-2958.1998.00841.x [DOI] [PubMed] [Google Scholar]
- 69.Heinzen RA, Hackstadt T, Samuel JE. 1999. Developmental biology of Coxiella burnettii. Trends Microbiol. 7:149–154. 10.1016/S0966-842X(99)01475-4 [DOI] [PubMed] [Google Scholar]
- 70.Swanson MS, Fernandez-Moreira E, Fernandez-Moreia E. 2002. A microbial strategy to multiply in macrophages: the pregnant pause. Traffic 3:170–177. 10.1034/j.1600-0854.2002.030302.x [DOI] [PubMed] [Google Scholar]
- 71.Hakkila K, Maksimow M, Karp M, Virta M. 2002. Reporter genes lucFF, luxCDABE, gfp, and dsred have different characteristics in whole-cell bacterial sensors. Anal. Biochem. 301:235–242. 10.1006/abio.2001.5517 [DOI] [PubMed] [Google Scholar]
- 72.Vannini A, Agriesti F, Mosca F, Roncarati D, Scarlato V, Danielli A. 2012. A convenient and robust in vivo reporter system to monitor gene expression in the human pathogen Helicobacter pylori. Appl. Environ. Microbiol. 78:6524–6533. 10.1128/AEM.01252-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun Y, Connor MG, Pennington JM, Lawrenz MB. 2012. Development of bioluminescent bioreporters for in vitro and in vivo tracking of Yersinia pestis. PLoS One 7:e47123. 10.1371/journal.pone.0047123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perez JC, Groisman EA. 2007. Acid pH activation of the PmrA/PmrB two-component regulatory system of Salmonella enterica. Mol. Microbiol. 63:283–293. 10.1111/j.1365-2958.2006.05512.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Omsland A, Cockrell DC, Fischer ER, Heinzen RA. 2008. Sustained axenic metabolic activity by the obligate intracellular bacterium Coxiella burnetii. J. Bacteriol. 190:3203–3212. 10.1128/JB.01911-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hackstadt T, Williams JC. 1981. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 78:3240–3244. 10.1073/pnas.78.5.3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.