Abstract
Macromolecular transport by bacterial type IV secretion systems involves regulated uptake of (nucleo)protein complexes by the cell envelope-spanning transport channel. A coupling protein receptor is believed to recognize the specific proteins destined for transfer, but the steps initiating their translocation remain unknown. Here, we investigate the contribution of a complex of transfer initiation proteins, the relaxosome, of plasmid R1 to translocation of competing transferable substrates from mobilizable plasmids ColE1 and CloDF13 or the bacteriophage R17. We found that not only does the R1 translocation machinery engage the R1 relaxosome during conjugative self-transfer and during infection by R17 phage but it is also activated by its cognate relaxosome to mediate the export of an alternative plasmid. Transporter activity was optimized by the R1 relaxosome even when this complex itself could not be transferred, i.e., when the N-terminal activation domain (amino acids 1 to 992 [N1-992]) of TraI was present without the C-terminal conjugative helicase domain. We propose that the functional dependence of the transfer machinery on the R1 relaxosome for initiating translocation ensures that dissemination of heterologous plasmids does not occur at the expense of self-transfer.
INTRODUCTION
Bacterial type IV secretion systems (T4SS) mediate the transfer of macromolecules into various target cells, e.g., the conjugative transfer of DNA into bacteria or the transfer of virulence proteins into eukaryotic host cells (1–3). Another subclass of T4SS release DNA into or mediate its uptake from the extracellular milieu (4). In Gram-negative bacteria, the transfer process requires a multisubunit, cell envelope-spanning machinery comprising a secretion channel and, often, a pilus or cell surface filament (1). The type IV coupling protein (T4CP) is believed to form a gated opening to the transenvelope channel in the donor bacterium that needs to be activated for transfer to start (1, 5, 6). For DNA transfer, an additional complex of DNA-processing initiation proteins, called a relaxosome, is required. The relaxosome assembles at the DNA origin of transfer (oriT) of transferrable DNA (T-DNA) and interacts with the T4CP receptor (6, 7). In addition to functioning as protein substrate receptors, T4CPs have ATPase activity and interact with DNA ligands, giving rise to the proposal that T4CPs translocate DNA substrates through their central channels across the cytoplasmic membrane (8). In addition to the T4CP, one or two other (VirB11-like) or (VirB4-like) ATPases (named for the Agrobacterium tumefaciens paradigm) are located at the cytoplasmic side of the inner membrane (9–13). The available data imply that the T4CP binds the relaxosome or T-DNA transfer intermediate and, assisted by the associated ATPase proteins, delivers the substrate into the translocation channel (5, 9, 11, 12, 14). It is still not known, however, how the type IV channel is activated and what final steps trigger the initiation of transfer.
Understanding of the activation cascade for T4SS generally will not only identify potential targets for inhibition of secretion but also fuel efforts to exploit T4SS for gene therapeutic approaches (15, 16). Accordingly, the initial steps of secretion protein recognition and the DNA processing reactions catalyzed by relaxosomes to prepare plasmid DNA for transfer have been studied intensively (17, 18). F-like plasmids represent one of the most successful plasmid families that encode the complete machinery for conjugative DNA transfer between Gram-negative bacteria. Current knowledge of the transfer initiation process can be illustrated with the F-like paradigm plasmids F and R1. The relaxase enzyme TraI binds to supercoiled oriT DNA and cleaves the T strand at a unique position, nic. This reaction is stimulated by several plasmid and host proteins that bind near nic, including TraM, TraY, and the Escherichia coli integration host factor, IHF. DNA relaxase covalently attached to the 5′ end of a single strand of transferrable DNA represents the secretion substrate for self-transfer (17). TraI proteins of F-like plasmids additionally harbor a DNA helicase that unwinds the T strand to facilitate its transfer to recipients (19, 20).
Secreted proteins, such as relaxases, display translocation signals (TS) that are recognized by the T4CP receptor. TS are either (i) short stretches of positively charged residues at the C terminus or (ii) larger, internally positioned regions of the protein (21–23). The latter type of TS appears to be conserved in conjugative relaxases (24–27). The F-like T4CP TraD also binds to the TraM protein (28, 29), which helps to bridge a stable complex of T4CP and relaxosome (30–32). Interactions with TraD stimulate the relaxase and helicase activities of TraI (33, 34); however, nothing is known about subsequent steps that activate TraD ATPase and enable the TraI-bound T-DNA to enter the transport channel.
The transenvelope channel synthesized by F-like plasmids not only transmits the conjugative plasmid to new bacterial cells, its presence is also co-opted for efficient export or import of other nucleic acids. Coresident mobilizable plasmids that encode their own relaxosome (e.g., ColE1) or even an independent T4CP (e.g., CloDF13) compete for the same transport channel system. In addition, so-called “male-specific” bacteriophages, such as R17, utilize the presence of a conjugative pilus and the underlying T4SS components to gain entry to host cells for viral replication (35). The route of translocation through the T4SS has yet to be defined, but Haft et al. report that the R17 single-stranded RNA (ssRNA)-protein complex directly contacts the T4CP TraD during lytic infection (Haft RJ, Gachelet EG, Traxler B, presented at the International Plasmid Biology Conference, Bariloche, Argentina, 2010). Host cell infection by R17 occurs in the absence of potential recipients for conjugative transfer, i.e., when the channel is not otherwise active for secretion. Thus, mobilizable plasmids and male-specific bacteriophages are also able to activate the transport machinery directly, or, alternatively, they can exploit a secretion channel already primed for self-transfer by the conjugative plasmid.
Since channel activation also occurs during mobilizable plasmid transfer and bacteriophage entry, we sought to identify shared mechanisms of channel activation by comparing the requirements for F-like relaxosome components in these processes under conditions where transfer of the conjugative plasmid itself was blocked. The utility of this approach was demonstrated in our earlier work showing that the N-terminal half (amino acids 1 to 992 [N1-992]) of the R1 relaxase-helicase protein TraI provides a vital mechanism for nucleoprotein entry during phage infection (36). The requirement for a catalytically active relaxase-DNA complex in a transfer process where the plasmid is itself not mobilized implies that the mechanism involved is a general part of the channel activation cascade.
In the current study, we asked whether the relaxosomes of F-like conjugative plasmids and mobilizable plasmids perform exchangeable functions during initiation of transfer activity in whole-cell experiments. The T4SS activities monitored were donor-to-recipient cell transmission of the conjugative plasmid or a mobilizable plasmid or, alternatively, the concomitant transfer of TraI protein fused to a Cre recombinase reporter molecule (illustrated in Fig. 1). We included the mobilizable plasmid ColE1, which expresses its own relaxosome, including the relaxase MbeA (37), which is effectively recognized by T4CP TraD. Mobilization of plasmid CloDF13 was also studied, as this plasmid expresses not only its own relaxosome but also an independent coupling protein, which interacts productively with the F-like T4SS even in the absence of TraD (38). Last, we extended the analysis of relaxosome dependence for R17 nucleoprotein import by these systems. Specifically, we asked whether the substitution of a mobilizable plasmid relaxosome or the CloDF13 coupling protein complements the absence of F-like relaxosomes for T4SS-mediated activities of nucleoprotein uptake or protein secretion. We found that the presence of the mobilizable plasmids did not fulfill the requirement for a relaxosome during R17 infection. The importance of the F-like relaxosomes in secretion system activity was underscored by the complementary finding that transfer of the mobilizable plasmids ranged from low to undetectable levels when the relaxosome of the R1 plasmid was not simultaneously present. Analysis of the mechanisms involved provides evidence that the R1 translocation machinery not only engages the R1 relaxosome during conjugative self-transfer and during infection by R17 phage but is also activated by its cognate relaxosome to mediate the export of an alternative plasmid.
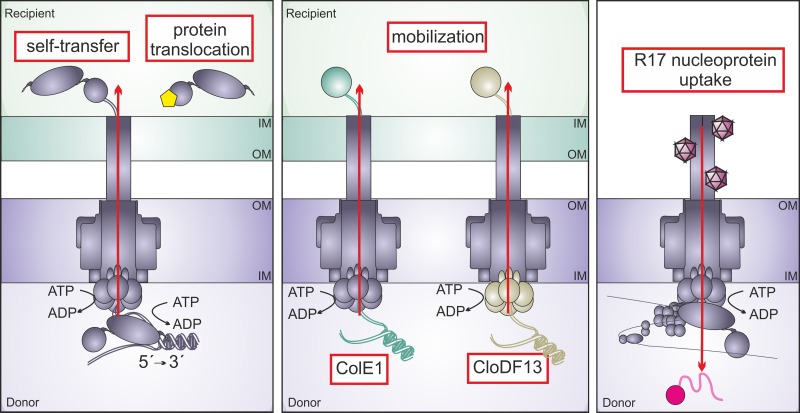
FIG 1.
Type IV macromolecular transfer activities analyzed in this study. Conjugative transfer proteins of F-like plasmids assemble an extracellular pilus and a multiprotein core complex (lavender) spanning the cell envelope of donor cells. The coupling protein ATPase (hexamer) forms the cytoplasmic pore of the transporter and receptor for specific translocation substrates. (Left) Self-transfer of the conjugative plasmid to recipient cells is piloted and energized by the bifunctional relaxase-helicase protein TraI (lavender bilobe). Receptor recognition of TraI also enables translocation of a Cre recombinase-TraI fusion protein (yellow pentagon). (Middle) Plasmid mobilization to recipient cells is mediated by ColE1 relaxase protein MbeA (green) or by CloDF13 relaxase MobC (beige monomer) recognized by its cognate coupling protein (beige hexamer). (Right) T4SS-mediated uptake of the R17 bacteriophage ssRNA-protein A complex (pink) requires the pilus, transporter, and DNA-bound relaxosome (lavender multiprotein complex) docked to the coupling protein (hexamer).
MATERIALS AND METHODS
Strains and plasmids.
All of the E. coli K-12 strains and plasmids used in this study are described in Table 1. A chloramphenicol-resistant derivative of MG1655, MS411Cm, was constructed by chromosomal integration of conditional-replication, integration, and modular (CRIM) plasmid pAR185 at the phage λ attB site as previously described (51).
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Descriptiona (reference or source) |
|---|---|
| Strains | |
| MG1655 | ilvG rfb-50 thi (39) |
| MS411 | ilvG rfb-50 thi (M. Schembri, DTU, Denmark) |
| MS411Cm | Cmr MG1655 attBλ::pAR185 (this study) |
| MS614 | Smr ilvG rfb-50 thi rpsL (M. Schembri, DTU, Denmark) |
| CSH26Cm::LTL | Tcr CSH26 galK::cat::loxP-Tet-loxP (25) |
| DY330 | W3110 ΔlacU169 gal 490(Ts) λcI857 Δ(cro-bioA) (40) |
| DH5α | endA1 recA1 gyrA96 thi-l hsdR17 supE44 λ− relA1 deoR Δ(lacZYA-argF)U169 ϕ80dlacZΔM15 (41) |
| Conjugative plasmids | |
| R1-16 | Kmr IncFII Δfin (42) |
| R1-16ΔtraI | Kmr Tcr traI::tetRA (25) |
| R1-16ΔtraM | Kmr R1-16 carrying traM null allele, identical to R1-16M0 (43) |
| R1-16Δnic | Kmr Δnicb (25) |
| R1-16ΔoriT | Kmr ΔoriTb (25) |
| R1-16ΔtraC | Kmr ΔtraCb (this study) |
| R1-16ΔtraD | Kmr ΔtraDb (this study) |
| R1-16ΔtraCΔoriT | Kmr ΔtraCb ΔoriT (this study) |
| R1-16ΔtraDΔoriT | Kmr ΔtraDb ΔoriT (this study) |
| R1-16M13 | Kmr R1-16 carrying mutant allele traM13 (43) |
| pOX38 | Kmr IncFI, derivative of F (44) |
| pOX38ΔtraC | Kmr ΔtraCb (this study) |
| pOX38ΔtraCΔoriT | Kmr ΔtraCb ΔoriT (this study) |
| pOX38traD411 | Kmr ΔtraD (45) |
| pOX38 traD411ΔoriT | Kmr ΔtraD ΔoriT (this study) |
| pOX38ΔtraI | Kmr Tcr traI::tetRA (25) |
| pOXMK3 | Kmr TraM− (46) |
| pOX38Δnic | Kmr Δnicb (this study; sequence positions 66153 to 66186 deletedc) |
| pOX38ΔoriT | Kmr ΔoriTb (this study; sequence positions 66153 to 66256 deletedc) |
| pOX38ΔIM | Kmr Tcr TraM− traI::tetRA (this study) |
| Mobilizable plasmids | |
| CloDF13 | Ampr (47) |
| pUIV209 | Ampr pUIV208::MbeA− (37) |
| pUIV208 | Ampr pUC19::ColE1 (37) |
| RSF1010 | Smr Sur IncQ (48) |
| pBR111M0 | Ampr pBR322 with R1 traM null mutant and oriT (49) |
| poriT-traM0 | Ampr pBR322 with F traM null mutant and oriT (this study) |
| Expression vectorsd | |
| CFP-B | Ampr pBR322 expressing Cre recombinase (26) |
| CFP-B Sm | Smr pBR322 expressing Cre recombinase (25) |
| CreTraI(3-1756) | Ampr CFP-B with R1 traI, encoding residues 3 to 1756 (25) |
| CreTraI(3-1756) Sm | Smr CFP-B Sm with R1 traI, encoding residues 3 to 1756 (25) |
| CreTraI F | Ampr CFP-B with wild-type F traI (25) |
| CreTraI F Sm | Smr CFP-B Sm with wild-type F traI (25) |
| pKD-cre | Ampr temperature-sensitive pKD46 expressing Cre recombinase (this study) |
| pTraIrel | Cmr pGZ119EH with R1 traI residues 1 to 309 (25) |
| pCG02 | Cmr pGZ119EH with R1 traI residues 1 to 992 (25) |
| pRelTSAY16FY17F | Cmr pGZ119EH with R1 traI residues 1 to 922 and Tyr 16/17 replaced with Phe to yield a nic cleavage-deficient variant of TraI N1-992 (36) |
| pRelTSAFR100 | Cmr pGZ119EH with R1 traI residues 1 to 922 and E153D/Q193R/R201Q, with reduced binding affinity for oriT (36) |
| pAR179Cm | Cmr dsRed expression plasmid (50) |
| pMS-traC | Ampr pMS119EH with R1 traC (this study) |
Construction of expression vectors.
Primer sequences are shown in Table 2. Two-step PCR was used to generate plasmid poriT-traM0. In the first step, primer sets 1 (EcoForiT and FM0rev) and 2 (FM0fwd and PstForiT) were used to amplify two pOX38 sequences, creating the desired exchange of the traM start for a stop codon. In the second step, the two products were annealed and amplified with primer set 3 (EcoForiT and PstoriT). The product was cut with EcoRI/PstI and ligated with plasmid pBR322. To construct plasmid pKD-cre, the EcoRI/KpnI fragment harboring cre was first excised from plasmid CFP-B and religated with plasmid pMS119EH to generate the intermediate vector pMS-cre. The EcoRI/XmaI fragment from pMS-cre was then ligated with EcoRI/XmaI-digested pKD46 (52). Plasmid pMS-traC was constructed by digestion of plasmid R1-16 with EcoRI and SmaI. The 2.9-kb gene traC was cloned into pMS119EH.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′–3′)a |
|---|---|
| EcoForiT | GGGAATTCACATCAGGCAGATGGCTA |
| PstForiT | GTTCTGCAGCCTGAATAACTGCCGTCA |
| FM0rev | CACCTTAGCCTCTAGAGATTACC |
| FM0fwd | GGTAATCTCTAGAGGCTAAGGTG |
| oriTko1_FW | CAAAAAGGCTCAACAGGTTGGTGGTTCTCACCACCAAAA GGAGAAAAAAATCACTATAACTTCGTATAG |
| oriTko1_FW-UCAS | CAAAAAGGCTCAACAGGTTGGTGGTTCTCACCACCAAAAGCAAGAATTGCCGGCGGAT |
| oriTko2_RevDCAS | TTGCGTTAAATTCATTGGTGAATCATATGCGATTCACCAATGAAGGTATTTCACACCGCATAGC |
| ForiTko2_RevDCAS | CCTACAAAACGGTGTCGGCGCGTTGTTGTAGCCGCGCCGACACCGGTATTTCACACCGCATAGC |
| ForiTko1_Rev | AGAAACTAATTTTTCATAACACTCTATTTATAAAGAAAAA ATCAACGGTGGTATATCCGGATAACTTCGTATAA |
| ForiTko2_Rev | CCTACAAAACGGTGTCGGCGCGTTGTTGTAGCCGCGCCGACACCATCAACGGTGGTATATCCGGATAACTTCGTATAA |
| ar042 | GGAGCGTGGGGAGGATGTTGAGCCGGGAGATGATTTCTG ATCAAGAATTGCCGGCGGAT |
| traC_KO_fw | GAATTCATGTGAATAACCCACTTGAGGCCGTCACTCAGGCGGTTAACTCCGAGAAAAAAATCACTATAACTTCGTATAG |
| traC_K_Orev | TCATGCCACACTCCTGTATTTCTCATGTTCTTCCAGCCAGGCTTCCAGCGATATCAACGGTGGTATATCCGGATAACTTCGTATAA |
| traD_KOLOX_fw | ATTGCGTCCATGCGTATCCGCATGTTCAGCCAGATCGCCAAGAGAAAAAAATCACTATAACTTCGTATAG |
| traD_KOLOX_Rev | ATCTGCTGCTGGATGTCCGGATGATTTTCCTGTTGCCATGTATCAACGGTGGTATATCCGGATAACTTCGTATAA |
| DtraIDcas | CATTGTTAATGGCATCCGTCCAGCCCTGACGGTTATCGGT GGTATTTCACACCGCATAGC |
Boldface indicates site-specific base pair exchanges; underlining indicates homologous regions.
Construction of pOX38 and R1-16 mutant derivatives.
To construct plasmid R1-16ΔtraC, pOX38ΔtraC, R1-16ΔtraD, pOX38Δnic, and pOX38ΔoriT, a loxP-tetRA-loxP cassette was amplified from strain CSH26Cm::LTL (25) using primer pairs traC_KO_FW and traC_KO_Rev (ΔtraC), traD_KOLOX_FW and traD_KOLOX_Rev (ΔtraD), oriTko1_FW and ForiTko1_Rev (ΔoriT), and oriTko1_FW and ForiTko2_Rev (Δnic), respectively. To generate plasmid pOX38ΔIM, the primer pair ar042 and DtraIDcas was used to amplify a TetRA resistance cassette from pAR183 (53). The amplified fragments were introduced into E. coli DY330(R1-16), DY330(pOX38), or DY330(pOX38MK3) and integrated via λ-Red-mediated recombination as described previously (54). To remove the loxP-tetRA-loxP cassette via Cre/loxP-mediated recombination, a temperature-sensitive plasmid expressing the Cre recombinase from phage P1 (pKD-cre) was brought into strains carrying the mutant plasmids. Cells were grown overnight at 30°C, analyzed for the loss of the tetRA cassette via selection plating and PCR, and then propagated twice overnight at 37°C to cure the cells of plasmid pKD-cre.
To generate the double-deletion plasmids R1-16ΔtraCΔoriT, R1-16ΔtraDΔoriT, pOX38ΔtraCΔoriT, and pOX38traD411ΔoriT, E. coli DY330 cells carrying the respective single-knockout plasmids R1-16ΔtraC, pOX38ΔtraC, R1-16ΔtraD, and pOX38traD411 (45) were transformed with ΔoriT targeting DNA amplified from CSH26Cm::LTL with primer pair oriTko1_FW_UCAS and oriTko2_Rev_DCAS (R1-16) or oriTko1_FW_UCAS and ForiTko2_Rev_DCAS (pOX38). Following integration into the respective single-deletion plasmids as described above, the loxP-tetRA-loxP was not removed.
Plate assay for phage sensitivity.
R17 lysate preparation and soft agar plaque assays were performed as described previously (36).
Cre recombinase assay for translocation (CRAfT) and mating procedure.
Protein translocation was measured using the Cre fusion reporter assay as described previously (25). E. coli MS411 and CSH26Cm::LTL cells were used as donor and recipient cells, respectively. Donors were selected on plates containing appropriate antibiotics (Table 1) and recombinants with chloramphenicol. Frequencies of protein translocation were calculated as recombinants per donor cell.
Conjugative self-transfer and mobilization of the coresident plasmid were measured in a parallel experiment using MS614 or MS411Cm as the recipient. Overnight cultures of donor strains were diluted to an optical density at 600 nm (OD600) of 0.005 in 900 μl antibiotic-free LB medium and incubated for 1 h at 37°C, and then 100 μl of recipient (OD600 of 2) was added. Conjugation was allowed to proceed for 30 min at 37°C and then stopped by vortexing for 1 min. Transconjugants were selected on plates containing appropriate antibiotics (Table 1). Conjugation and mobilization frequencies were expressed as transconjugants per donor cell. The presence of all plasmids in the donor was confirmed by PCR before and after the CRAfT was performed.
Western analysis.
One OD600 unit of mid-log-phase culture grown with or without 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was pelleted, resolved on a 10% polyacrylamide gel, and transferred to a nitrocellulose membrane. Separated proteins were visualized by development of blots with anti-TraD antiserum and anti-rabbit antibodies conjugated to horseradish peroxidase (Sigma). Detection was performed with ECL Western blotting detection reagents (GE Healthcare) according to the manufacturer's recommendations. The relative intensity of specific protein signals was measured using ImageJ (http://imagej.nih.gov/ij/; National Institutes of Health, Bethesda, Maryland, USA).
R17 phage propagation and labeling.
Amounts of 4 liters of E. coli MS411(R1-16) were grown in LB medium supplemented with 40 μg/ml kanamycin and 0.2 mM CaCl2 at 37°C with shaking. Cultures at an OD600 of 0.6 were infected with R17 phage lysates prepared according to a previously described method (36), and incubation continued for 5 h. Cells were harvested, and the supernatant was treated with 1 μg/ml DNase I. After 1 h of incubation at room temperature (RT), the supernatant was adjusted to 0.5 M NaCl and 6% polyethylene glycol (PEG) 6000 and stirred for 10 min. The suspension was allowed to settle for 12 h at 4°C. PEG-precipitated phage was harvested by centrifugation at 15,000 × g for 10 min, and the pellet was dissolved in 5 ml P buffer (50 mM Tris-HCl, pH 7.6, 0.1 M NaCl, 0.1 mM MgCl2, and 0.1 mM EDTA). The total volume was applied to a discontinuous CsCl gradient (ρ = 1.5, 1.4, 1.3, and 1.2 g/ml) and concentrated by centrifugation at 100,000 × g for 22 h at RT. R17 phage sedimented in a well-defined band at a ρ of ∼1.5 g/ml CsCl. Phage were harvested, dialyzed into P buffer, and stored at 4°C. The titer was 1.02 × 1014 PFU/ml. Phage were labeled with Alexa Fluor 488 (Invitrogen) as described previously (55), except that phages were harvested from a discontinuous CsCl gradient at a ρ of ∼1.45 g/ml and the addition of glycerol was omitted. After labeling, phage were treated with chloroform to dissolve detached R1-16 pili still present in the preparation, dialyzed in P buffer, and then filtered through a 0.2-μm filter and stored at 4°C. The final phage titer was 2.60 × 1013 PFU/ml.
Fluorescence microscopy.
E. coli MS411 cells carrying R1-16, R1-16ΔtraC, or R1-16ΔtraC plus pMS-traC were freshly transformed with dsRed-harboring plasmid pAR179 (50). Cells were grown to an OD600 of ∼0.4 to 0.6 and diluted with 1× phosphate-buffered saline (PBS), pH 7.5, to an OD600 of 0.2. Suspended cells (200 μl) were applied to coated glass bottom dishes (MatTek Corporation) and incubated at RT for 10 min. Medium was removed by aspiration, 100 μl of a 1/100 dilution of Alexa Fluor 488-labeled R17 phage in 1× PBS, pH 7.5, was added, and the mixture incubated for 10 min. Microscopy was done with a Leica SP5. The excitation wavelengths were 488 nm and 561 nm. Emissions were recorded between 500 and540 nm for dsRed and between 610 and 700 nm for Alexa Fluor 488.
Biofilm and planktonic growth.
To measure biofilm development in vitro, overnight cultures of E. coli MS411 cells carrying R1-16, R1-16ΔtraC, or R1-16ΔtraC plus pMS-traC were used to inoculate 100 μl of LB with antibiotics to an OD600 of 0.05. The bacteria grew for 24 h at 37°C without shaking in U-bottom 96-well plates (BioSterilin). After cultivation, plates were washed and stained with crystal violet as described previously (56). Comparable growth of these strains in planktonic culture was confirmed in parallel. For this control, 1 ml LB with antibiotics was inoculated with the respective strains to an OD600 of 0.05 in 24-well plates (Greiner). Bacteria grew for 6 h at 37°C with shaking. The absorption at 600 nm was measured every 30 min.
RESULTS
pOX38 relaxosome dependence for R17 nucleoprotein uptake.
Our earlier analysis of the R17 nucleoprotein uptake process supported by E. coli cells carrying the plasmid R1 derivative R1-16 demonstrated that, in addition to an ATPase-proficient T4CP, the nicking-closing activity of the TraI protein within the relaxosome was key to efficient infection (36). To test the relevance of relaxosome dependence for R17 uptake via F-like conjugation systems generally, we prepared a collection of mutant derivatives of pOX38 (a laboratory version of plasmid F) matching those studied with R1-16 (Fig. 2), including pOX38Δnic (lacking 34 bp of oriT spanning nic), pOX38ΔoriT (lacking 104 bp of oriT, including nic and ihfA for IHF binding and sby for TraY binding), and derivatives lacking relaxosome components traM (pOX38MK3) and both traM and traI (pOX38ΔIM). Verification of normal transfer (tra) gene expression and function with self-transfer-deficient mutants pOX38ΔoriT and pOX38Δnic was done by measuring the wild-type frequencies of mobilization of a coresident recombinant plasmid carrying the pOX38 oriT (not shown). The self-transfer of mutants pOX38MK3 and pOX38ΔIM was fully complemented by the wild-type genes in trans (not shown).
FIG 2.

Schematic representation of the F-like oriT and deletion variants. DNA sequences important to TraI binding and strand cleavage include nic (black triangle) and the inverted repeat (arrows). The binding sites for the accessory proteins IHF (ihfA and ihfB), TraY (sbyA), and TraM (sbmA, sbmB, and sbmC) are illustrated. The Δnic deletions in R1-16 and pOX38 remove TraI relaxase binding sites. The ΔoriT derivatives lack binding sites for TraI, IHF, and TraY.
To reveal whether the T4CP-dependent uptake of R17 RNA-protein A complexes by the F system depends in any way on the F relaxosome, E. coli cells carrying the pOX38 derivatives were infected with phage lysates. The number, size, and clarity of plaques produced in a bacterial lawn through cell lysis were used as the indication for the efficiency of phage infection. The screen of mutant pOX38 derivatives verified our previous findings that, unlike R1-16-carrying hosts, the absence of TraI or a functional nic site is still compatible with effective phage R17 infection (Table 3) (36). The opaque appearance of the plaques produced with these defective plasmids compared to plaques produced with the wild-type system suggests that the efficiency of the infection is somewhat diminished; however, the numbers of PFU detected were equivalent to the numbers obtained with the wild type. In contrast, hosts lacking a larger region of oriT were no longer able to propagate phage detectably. The sequences removed from pOX38ΔoriT include specific binding sites for the TraI relaxase, the adjacent IHF binding site, and a site bound by TraY (Fig. 2). TraM binding sites remain, but clearly, the formation of a complete relaxosome is blocked in this variant. The absence of TraM alone was compatible with phage infection (pOX38MK3) (Table 3), but hosts lacking both TraI and TraM (pOX38ΔIM) became completely resistant to R17 under these conditions (Table 3). These findings demonstrate that the ability of R17 to exploit hosts carrying the pOX38 T4SS is less dependent on the presence of individual factors, such as TraI or its nicking cleaving activity (Δnic), than is the case with R1-16-carrying hosts. Nonetheless, further disruption of the relaxosome by the combined loss of TraM and TraI (pOX38ΔIM) or the inability of TraI, IHF, and TraY to bind a wild-type oriT (ΔoriT) eliminated detectable plaque formation (Table 3). These results underscored that relaxosome reconstitution is a general requirement of F-like T4SS for effective phage R17 infection.
TABLE 3.
Sensitivity of E. coli(pOX38) to R17 requires at least partial relaxosome assembly
| Plasmid | Infection levela | Plaque morphology | PFU/ml (mean ± SD)b |
|---|---|---|---|
| pOX38 | +++ | Clear | 8.8 × 1011 ± 3.0 × 1011 |
| pOX38MK3 | +++ | Clear | 3.8 × 1011 ± 3.5 × 1011 |
| pOX38ΔtraI | ++ | Opaque | 6.7 × 1011 ± 2.2 × 1011 |
| pOX38Δnic | ++ | Opaque | 5.2 × 1011 ± 3.0 × 1011 |
| pOX38ΔoriT | − | None | ND |
| pOX38ΔIM | − | None | ND |
+++, wild type; ++, opaque but countable; −, none.
ND, not detected.
Mobilizable plasmids cannot complement R17 sensitivity of E. coli carrying R1-16ΔoriT.
To date, the only generally established function for relaxosomes is the processing of plasmid T-DNA to prepare a single strand for export to recipients. Since plasmid DNA transfer is not occurring in our experiments observing R17 RNA transfer, a logical explanation for the TraI dependence of efficient phage infection is that TraI bound to both nic and the T4CP TraD has a key function at transfer start regardless of whether the nucleoprotein substrates are imported (protein A-R17 RNA) or exported (TraI-plasmid T-DNA). To better understand this putative activation step, we asked whether the relaxosomes expressed and assembled by the small mobilizable (Mob) plasmids ColE1, RSF1010, and CloDF13 on their own oriT regions could complement the F-like relaxosome dependence for R17 sensitivity of host cells. ColE1 and RSF1010 represent Mob plasmids encoding their own relaxase with, respectively, high (10−1 transconjugants per donor) and low (10−5 transconjugants per donor) efficiencies of mobilization by F-like conjugative plasmids. CloDF13 encodes MobB, a T4CP that replaces TraD in mediating efficient docking interactions between its own relaxosome and the F-like T4SS (47). CloDF13 is mobilized by F-like plasmids with moderate efficiency (10−4 transconjugants per donor).
We tested whether any of these Mob relaxosomes could replace the relaxosome-dependent activity required for R17 uptake by the R1-16 T4SS. The plaque formation efficiencies on host cells carrying either wild-type R1-16, R1-16ΔoriT, or the Δnic variant harboring in addition one of the three Mob plasmids were compared. Cells carrying R1-16 supported equivalent levels of plaque formation in the presence or absence of any of the coresident Mob plasmids (Table 4). The plaques showed a clear morphology, except for hosts carrying CloDF13. The opaque appearance of their plaques implies that a component of CloDF13 interferes with function(s) of the T4SS assembled with TraD and an R1-16 relaxosome. This negative effect was not apparent during conjugation. None of the Mob plasmids influenced the efficiency of F-like plasmid self-transfer under our experimental conditions (not shown). However, inhibition of F-like plasmid transfer by an elevated-copy-number mutant of CloDF13 has been reported in the past (57). No plaques were detected with either the Δnic or ΔoriT mutant derivatives of R1-16, as expected (36). Notably, the R17-resistant phenotype was not complemented by the additional presence of any of the Mob plasmids.
TABLE 4.
Mobilizable plasmids cannot complement R17 phage sensitivity of E. coli cells carrying R1-16ΔoriT mutant derivatives
| Plasmid | Coresident MOB plasmid | Infection levela | Plaque morphology | PFU/ml (mean ± SD)b |
|---|---|---|---|---|
| R1-16 | − | +++ | Clear | 1.7 × 1010 ± 2.9 × 109 |
| R1-16ΔoriT | − | − | None | ND |
| R1-16Δnic | − | − | None | ND |
| R1-16 | ColE1 | +++ | Clear | 1.6 × 1010 ± 2.0 × 109 |
| R1-16ΔoriT | ColE1 | − | None | ND |
| R1-16Δnic | ColE1 | − | None | ND |
| R1-16 | RSF1010 | +++ | Clear | 1.5 × 1010 ± 3.2 × 109 |
| R1-16ΔoriT | RSF1010 | − | None | ND |
| R1-16Δnic | RSF1010 | − | None | ND |
| R1-16 | CloDF13 | ++ | Opaque | 3.5 × 1010 ± 2.0 × 109 |
| R1-16ΔoriT | CloDF13 | − | None | ND |
| R1-16Δnic | CloDF13 | − | None | ND |
+++, wild type; ++, opaque but countable; +, very opaque and not countable; −, none.
ND, not detected.
We conclude that the relaxosome of R1-16 contributes function(s) to the R17 infection process that cannot be provided by the Mob plasmids evaluated here. This appears to be the case even when the Mob plasmid provides a relaxosome that, under normal conjugation conditions, is transferred efficiently by the T4SS, i.e., ColE1 and CloDF13.
Mob plasmid transfer and TraI translocation are impaired by the absence of a conjugative plasmid oriT.
The failure of the Mob plasmids to complement R17 infection in the absence of the R1-16 relaxosome provides evidence that the F-like relaxosome components contribute unique (still undefined) mechanisms to the R17 nucleoprotein uptake process. We therefore asked whether a functional R1 relaxosome is also required for (i) mobilization of plasmids that encode their own relaxosome or (ii) translocation of the protein TraI into recipients. The plasmid mobilization frequencies reported in the literature are typically restricted to wild-type conjugation systems, and thus, there is little general knowledge about the contribution of a conjugative relaxosome to the transfer of a competing Mob plasmid relaxosome. We therefore tested whether plasmid mobilization occurs normally in the absence of the F-like oriT region. Surprisingly, we observed no detectable mobilization of ColE1 by R1-16ΔoriT (Fig. 3). Similarly, R1-16ΔoriT failed to transfer CloDF13 at detectable levels, in contrast to a mobilization frequency of 10−4 transconjugants per donor mediated by wild-type R1-16. This result is particularly striking as it implies that efficient activity of the T4SS assembled with the alternative T4CP and relaxosome of CloDF13 still relies on a function involving the R1-16 relaxosome.
FIG 3.

Type IV macromolecular transfer activities supported by R1-16ΔoriT. Activities tested are indicated below the graph and expressed relative to the value for R1-16 wild type (100%). Coresidence of a second plasmid is indicated as follows: R1 oriT plasmid, recombinant plasmid control; E1, ColE1; F13, CloDF13. The values for oriTR1 mobilization represent the means of at least 3 experiments. Standard deviation is shown. <Det, results were below the detection limit.
We next asked whether interaction of the Mob plasmid relaxosomes with the T4SS was sufficient to support Cre-TraI translocation in hosts carrying R1-16ΔoriT. To answer this question, we used the Cre recombinase assay for translocation, which was developed to monitor T4SS-mediated transfer of protein substrates (58). In F-like systems, the T4SS recognizes and translocates TraI fused with reporter protein Cre (25, 59). The Cre-TraI fusion does not need to be attached to T-DNA, but so far in our previous work, translocation has not been observed under conditions where no DNA transfer takes place (60). Here, we founnd that protein transfer was not complemented at all by either Mob plasmid from hosts carrying R1-16ΔoriT (Fig. 3). Since no Mob plasmid transfer occurs under these conditions, we cannot conclude whether the oriT R1-16 dependence observed for protein translocation is indirectly due to the absence of a DNA ligand or whether an active role for the R1 relaxosome is additionally involved.
The results of the R17 phage assays presented in Table 4 are included again in Fig. 3 to help summarize the effects of oriT deletion on the different T4SS activities. Taken together, we found that the loss of R1-16 oriT eliminated activity in every case. These findings argue strongly that the R1-16 relaxosome is commonly involved in several T4SS-mediated transfer processes. The contribution clearly goes beyond conjugative DNA processing.
Partial assembly of the relaxosome on R1-16Δnic restores some T4SS activities.
To gain insight into the role of specific relaxosome components in the various T4SS-mediated processes, we next asked which of these activities could be restored when only single components of the relaxosome were disrupted. The Δnic derivative of R1-16 not only carries specific binding sites for TraM but also both consensus sequences for IHF and the site of TraY binding (Fig. 2). The deletion removes only recognition sequences for TraI binding and nic, which eliminates relaxase catalytic activity. As mentioned above, the tra gene expression from R1-16Δnic appears normal, as the mobilization of the control plasmid carrying oriT of R1 (oriTR1) from these donor cells was equivalent to the mobilization by wild-type R1-16 (Fig. 4). In contrast to E. coli(R1-16ΔoriT), hosts carrying R1-16Δnic were able to mobilize ColE1, albeit at <1% of the efficiency of wild-type R1-16 (Fig. 4). The combination of R1-16Δnic and ColE1 also supported detectable translocation of Cre-TraI of R1 (Cre-TraIR1). Replacing ColE1 with a deletion mutant with the relaxase gene mbeA deleted eliminated both mobilization and Cre-TraIR1 secretion, as expected for relaxosome-dependent activities.
FIG 4.

Type IV macromolecular transfer activities supported by R1-16Δnic. Activities tested are indicated below the graph and expressed relative to the value for R1-16 wild type (100%). Carriage of a second plasmid is indicated as follows: R1 oriT plasmid, recombinant plasmid control; E1, ColE1; E1 ΔMbeA, relaxase-deficient ColE1; F13, CloDF13. Values represent the means of at least 3 experiments. Standard deviations are shown. <Det, results were below the detection limit.
R1-16Δnic also supported mobilization of CloDF13 DNA with wild-type efficiency.
Cre-TraIR1 transfer was not detected concomitantly with CloDF13 DNA transfer. It is important to note that both the cognate T4CP and the heterologous CloDF13 T4CP are present in these cells. Therefore, this result implies that the R1-16 type IV secretion channels interacting with the CloDF13 T4CP engage and transfer the CloDF13 relaxosome but are unable to recruit Cre-TraI. Conversely, the type IV secretion channels interacting with T4CP TraD may bind the specific TS of Cre-TraI but fail to be active in the absence of a normal F-like relaxosome. Finally, as shown by the data in Table 4, R1-16Δnic-carrying hosts are defective for R17 propagation.
In conclusion, the deletion derivative R1-16Δnic, lacking only nic and the site of TraI binding, supported more efficient plasmid mobilization and protein transfer with the coresident ColE1 than the ΔoriT mutant additionally lacking IHF and TraY binding sites. This result may indicate that oriT binding by TraY and occupation of the adjacent IHF binding site have a positive impact on T4SS-mediated activity despite the lack of DNA processing by the R1-16Δnic relaxosome. TraY and IHF may contribute surfaces involved in binding or stabilizing protein-protein interactions. Since both factors induce strong bending of oriT DNA (61, 62), topological effects may be important to the enhanced activity as well.
The activation domain TraI N1-992 complements ColE1 mobilization.
To better understand the contribution of TraI to different T4SS functions, we next investigated the T4SS transfer activities supported by R1-16ΔtraI (Fig. 5). Self-transfer was eliminated, as expected without the relaxase-helicase. The ColE1 mobilization by R1-16ΔtraI was slightly higher (5-fold) than that observed with R1-16Δnic but remained about 30-fold lower than the wild-type efficiency. To know whether the nicking-joining activity of the enzyme is important, we expressed in trans a fragment of traI encoding the protein relaxase domain (TraI1-309). The relaxase domain alone failed to complement. However, wild-type levels of ColE1 mobilization were observed with the larger TraI N1-992 activation domain (30-fold enhancement). This variant includes the relaxase and extends to include the N-terminal TS to mediate recognition by the T4CP (25). DNA-processing activity by the TraI N1-992 protein proved to be dispensable, however, since an exchange mutation replacing the catalytic tyrosine with phenylalanine was equally competent for ColE1 mobilization. Similarly efficient mobilization was also observed with a mutant allele creating three amino acid exchanges, E153D/Q193R/R201Q, that should lower the protein's high-affinity interactions with single-stranded DNA (ssDNA) (36, 63).
FIG 5.

Type IV macromolecular transfer activities supported by R1-16ΔtraI. Activities tested are indicated below the graph and expressed relative to the value for R1-16 wild type (100%). Carriage of a second plasmid is indicated as follows: E1, ColE1; F13, CloDF13. Complementation of R1-16ΔtraI is indicated as follows: +TraI, full-length TraI protein; +Rel, TraI N1-309 relaxase domain; +N992, TraI N1-992; +TyrtoPhe, cleavage-deficient TraI N1-992 mutant; +FR100, TraI N1-992 mutant with reduced affinity for oriT-specific sequences. Values represent the means of at least 3 experiments. Standard deviations are shown. <Det, results were below the detection limit.
We next asked whether the TraI N1-992 domain was sufficient to complement Cre-TraI translocation by R1-16ΔtraI-carrying hosts. Although protein transfer activity was again observed with the wild-type traI allele, the expression of the TraI N1-992 activation domain in trans did not restore activity. This result is consistent with the observed DNA dependency for protein transfer by the F-like systems (60). Self-transfer of R1-16ΔtraI in that case would require in addition the helicase activity in the TraI fragment comprising residues 310 to 1256 (36).
We conclude that the N-terminal residues of TraI1-992 are sufficient to fulfill the requirement for TraI in mediating ColE1 mobilization by R1-16ΔtraI. This finding is in very good agreement with our previous observation that the identical TraI fragment is sufficient to activate R17 nucleoprotein uptake in R1-16ΔtraI-carrying hosts (36). Interestingly, however, while the nicking-joining activity of TraI is required to support R17 ssRNA uptake, this property of TraI was not necessary to detect transporter function when the heterologous substrate, the ColE1 relaxosome, provides its own DNA-processing activity.
In a final point, although it would also be logical in this study to test the effect of single gene deletions for each of the remaining relaxosome components—TraM, TraY, and IHF—disruptions of each of these loci have substantial negative effects on tra gene expression (43, 64, 65). Since phenotypes observed in that case could be due to the absence of secretion channels, the experiments were not included.
Test for relaxosome-interacting proteins.
Our results show that reconstitution of the R1-16 relaxosome has a positive effect on transfer of the ColE1 and CloDF13 plasmids even when the R1-16 plasmid itself cannot be transferred. To better understand how binding interactions between the R1-16 relaxosome and receptor proteins of the type IV secretion machinery might control transfer of the Mob plasmids, we tried to identify the interacting proteins. R1-16ΔoriT cannot mobilize ColE1 detectably (Fig. 3), whereas the T4SS of pOX38 mobilizes ColE1 with 10% of the wild-type efficiency despite the identical oriT deletion. We hypothesized that if the traD allele determines the efficiency of ColE1 mobilization, then expression of the traD allele from pOX38 in trans should eliminate the dependence of ColE1 mobilization on the R1-16 relaxosome. We found that ColE1 transfer by R1-16ΔoriTΔtraD was not detectable regardless of whether the wild-type traD allele provided in trans was from R1-16 or pOX38 (Fig. 6A). We also observed that ColE1 transfer by pOX38ΔoriTΔtraD did not acquire dependence on the F relaxosome by providing traD from R1-16. Immunochemical detection of TraD in these cell extracts using anti-TraD antibodies indicated that the uninduced levels of traD expression from the complementation vectors were equivalent to the amount of traD expressed from a wild-type copy of R1-16 or pOX38 (not shown).
FIG 6.
ColE1 mobilization by R1-16ΔtraDΔoriT is restored by traD overexpression. Complementation of ColE1 (+E1) transfer activities by the mutant derivatives shown above the graphs was tested with either traD from R1-16 (TraD) or pOX38 (TraDF) in trans. Results in the absence (A) or presence (B) of IPTG-induced traD expression are shown. Values shown are expressed relative to the value for the wild type (100%). n ≥ 3 experiments. Standard deviations are shown. <Det, results were below the detection limit.
This experiment was then repeated under conditions of traD overexpression (Fig. 6B). Western analyses showed that the TraD concentrations after induction exceeded those with the wild type by more than 20-fold (not shown). High levels of TraD led to lower ColE1 mobilization frequencies with the pOX38ΔoriTΔtraD derivative than were observed without overexpression. Inhibition of the pOX38 transfer system by an imbalance of traD and traI expression has been reported earlier (66, 67). Importantly, however, increased TraD concentrations eliminated the requirement for oriTR1 in ColE1 transfer such that wild-type frequencies of mobilization by the R1-16ΔoriTΔtraD derivative were observed regardless of the source of traD. The observed gain of function suggests that higher-than-wild-type levels of TraD eliminate a form of negative interference when the transporter engages the relaxosome of ColE1. This effect might reflect binding and, possibly, sequestering of F-like relaxosome proteins, which are not properly localized to plasmid DNA in the absence of oriT.
The inability to switch the oriT dependence in mobilization based on the plasmid-specific source of TraD suggests that other plasmid proteins are involved in the activation mechanism. F-like transfer systems lack a virB11 homolog (68), and thus, the remaining ATPase, the VirB4-like protein TraC, was a good candidate. To investigate this hypothesis, both the traC genes and oriT of the F-like plasmids were deleted and the efficiency of transfer of the Mob plasmids was compared with that of wild-type traC genes in trans. No complementation of either ColE1 or CloDF13 transfer was observed without chemical induction of traC expression (Fig. 7). Since pOX38 ΔoriT transfers both ColE1 and CloDF13 at detectable levels (not shown), the lack of translocation activity by pOX38ΔoriTΔtraC was apparently due to lower-than-wild-type levels of TraC production in trans. We do not have antibodies to TraC protein to be able to measure expression levels in these cells by Western analyses; however, we do know that F-like plasmids lacking traC alone require IPTG-induced expression in trans to reach wild-type efficiencies of conjugative self-transfer (not shown). Overexpression of traC (Fig. 7B) restored transfer of ColE1 and CloDF13 by both the R1-16 and pOX38 double mutant derivatives. The mobilization by the pOX38 system under these complementation conditions was comparable to the frequencies of mobilization supported by pOX38ΔoriT.
FIG 7.
Plasmid mobilization by R1-16ΔtraCΔoriT is restored by traC overexpression. Complementation of ColE1 (+E1) and CloDF13 (+F13) transfer activities by the mutant derivatives shown above the graphs was tested with either traC from R1-16 (TraC) or pOX38 (TraCF) in trans. Results in the absence (A) or presence (B) of IPTG-induced traC expression are shown. Values shown are expressed relative to the value for the wild type (100%). n ≥ 3 experiments. Standard deviations are shown. <Det, results were below the detection limit.
These results suggest that traD and traC alleles do not determine the plasmid specificity of mobilization; however, altered expression of TraD or TraC caused a significant change in the mobilization frequencies of coresident plasmids in the presence of a transfer-deficient F-like plasmid lacking oriT. One possible explanation for this modulation would be that, like TraD, TraC may normally bind one or many proteins in the R1-16 relaxosome. In that case, similar to TraD overexpression, high TraC concentrations could aid transporter function by sequestering the R1-16 relaxosome factors mislocalized by the absence of oriT. In support of this notion, TraD-relaxosome interactions are well established (28–32, 36). Similar studies have not been published for TraC, but characterized VirB4-like proteins of Gram-negative and Gram-positive systems were shown to bind T4CP (9, 10, 12, 13), DNA (69, 70), and also, in the case of Enterococcus faecalis pCF10, components of the relaxosome (11).
Cells are not hyperpiliated by TraC overexpression.
A second possible explanation could account for the ability of high TraC levels to complement the defect in ColE1 and CloDF13 transfer. Since TraC is involved in assembly of the F conjugative pilus (71), overexpression might drive abundant pilus biogenesis. With more T4SS available, the negative effects of incomplete relaxosome assembly could be effectively diluted. To test the latter possibility, we visualized the conjugative pili produced by the host using fluorescent R17 phage binding and laser-scanning confocal microscopy (Fig. 8). Bacteriophage R17 was conjugated with the fluorescent dye Alexa Fluor 488 and purified as described in Materials and Methods. E. coli cells were labeled internally by expression of the fluorophore dsRed (Fig. 8A to D). The addition of the R17 phage to cultures identified the pili as long fluorescent filaments when cells carried the wild-type R1-16 (Fig. 8B) but not in its absence (Fig. 8A). Fluorescent R1-16 pili were also not observed on cells carrying R1-16ΔtraC, consistent with the essential role of TraC in pilus biogenesis (Fig. 8C). The expression in trans of traC under inducing conditions restored the detection of phage-bound pili (Fig. 8D) but did not reveal hyperpiliated cells. Complementation of the host's ability to conjugate with wild-type frequency was verified in a parallel experiment (not shown).
FIG 8.
TraC overexpression does not result in hyperpiliated cells, Piliation status of E. coli MS411, MS411(R1-16), MS411(R1-16ΔtraC), and MS411(R1-16ΔtraC pMS-traC) was evaluated qualitatively by fluorescence microscopy (A to D) and quantitatively by measuring biofilm formation (E). (A to D) MS411 cells (A) and MS411 cells harboring R1-16ΔtraC (C) show no piliation, while MS411(R1-16) cells have abundant pili (visualized by adherent fluorescently labeled R17 phage [green]) (B) and overexpression of traC in MS411(R1-16ΔtraC pMS-traC) cells partially restores piliation (D). (E) Corresponding biofilm data using E. coli MS411 in the absence (−) or presence of plasmids show that MS411 and MS411(R1-16ΔtraC) (ΔtraC) cells do not develop a biofilm, while MS411(R1-16) cells develop a strong biofilm. Again, partial complementation of biofilm formation is observed when traC is overexpressed in MS411(R1-16ΔtraC pMS-traC) cells (ΔtraC+traC).
To assess the level of pilus production more quantitatively, we also measured the level of biofilm formation by the host. The capacity for F-like pili to support biofilm formation by E. coli in vitro has been well documented (72, 73). The levels of biofilm biomass adhering to the wells of a microtiter tray after 24 h of growth were compared for plasmid-carrying E. coli cells. Strain MS411 forms very poor biofilm in the absence of R1-16 pili in this assay (Fig. 8E). In contrast, strong biofilm is observed when cells carry R1-16. Poor biofilm production in the absence of traC was complemented via overexpression of traC in trans. The amount of biofilm measured for the complementation strain was lower than the wild-type level. No difference in the growth properties of these strains was observed in planktonic cultures (not shown); therefore, the variations in the biofilm results are due to differences in surface adherence.
Taken together, these findings rule out the explanation that traC overexpression results in more pilus production by donor cells.
DISCUSSION
F-like relaxosomes have been intensively studied for the DNA-processing reactions that prepare plasmid DNA for conjugative transfer to recipient cells. In this report, we provide evidence that F-like relaxosomes perform additional functions that are centrally important to T4SS-mediated transport of diverse macromolecular substrates. We show that the F plasmid oriT is required for the T4CP-dependent process of R17 RNA uptake. Consistent with our earlier results with R1-16, the mechanisms stimulating nucleoprotein uptake also involve proteins within the relaxosome complex. The R17-resistant phenotype observed with R1-16Δnic and the single-gene-deletion mutant R1-16ΔtraI was not observed with equivalent mutations in pOX38. Although the F T4SS retained nucleoprotein uptake activity when single components were missing, the loss of additional proteins or disruption of oriT binding eliminated plaque formation. We conclude that relaxosome reconstitution is a general requirement of F-like T4SS for effective phage R17 infection.
We next asked whether any Mob plasmid relaxosome that productively docks with the T4CP can provide this key function and found that replacing the R1-16 relaxosome with any of the alternative Mob plasmids ColE1, CloDF13, and RSF1010 did not complement the R17 sensitivity of the host. The mechanism(s) involved are thus specific to the cognate F-like relaxosome.
Last, we also show that R1-16 relaxosomes are commonly required in the T4SS-mediated transport of the Mob plasmids ColE1 and CloDF13 to recipient cells. Mutations disrupting the normal assembly or function of the R1-16 relaxosome (ΔoriT, Δnic, and ΔtraI) reduced the efficiency of ColE1 mobilization most severely. The R1-16 relaxosome dependence for plasmid mobilization by the T4SS was quite stringent compared to the F system. Nonetheless, deletion of oriT from pOX38 lowered the mobilization efficiency of ColE1 and CloDF13 by 1 or 2 orders of magnitude, respectively, compared to the efficiency with the wild type. Thus, the stimulating activity contributed by the F-like relaxosomes is apparently conserved.
During both the phage nucleoprotein uptake process and heterologous plasmid mobilization by the T4SS, self-transfer of the F-like plasmid is dispensable. It follows that key transfer-promoting functions provided by F-like relaxosomes are not limited to T-DNA processing. We anticipated that relaxosome binding by receptor proteins at the conjugative pore is integrally involved in the activation mechanisms, and therefore, we explored in this study a role for the T4CP and VirB4-like ATPase. VirB4 proteins are the most conserved component of T4SS (74). They promote pilus assembly and are also proposed to play a direct role in substrate translocation (9, 71, 75, 76). T4CP-relaxosome interactions are well established (1, 17). Direct interactions between the T4CP and both of the motor proteins VirB4 and VirB11 have also been demonstrated in some systems (9–13). Recent advances in characterizing VirB4-like proteins of Gram-negative and Gram-positive systems have shown direct interactions with DNA (69, 70, 77) and also, in the case of E. faecalis pCF10, components of the relaxosome (11). The latter observation is particularly intriguing given that the E. faecalis pCF10 Prg/Pcf system, like the F T4SS, lacks a VirB11-like ATPase (1, 68). Li and colleagues propose a model where the T4CP and VirB4-like PrgJ ATPase act sequentially and through direct interactions with the plasmid substrate to mediate its uptake by the Prg/Pcf translocation apparatus (11).
Here, we showed that overexpression of both TraD and TraC affected ColE1 mobilization when F-like relaxosome assembly was disrupted by oriT deletion. TraC is essential for pilus biogenesis, and yet our results rule out the possibility that overproduction of TraC results in detectably higher than normal numbers of pili per cell. Instead the data best support the simple explanation that TraD and TraC normally act as relaxosome receptor proteins at the donor cell opening of F-like T4SS. In that case, TraD and TraC would be expected to also recruit the ColE1 relaxosome. It is further conceivable that both plasmid complexes normally localize to the same T4SS.
Mutations disrupting the normal assembly or function of the R1-16 relaxosome (ΔoriT, Δnic, and ΔtraI) reduced the efficiency of ColE1 mobilization. We propose that, under normal conditions, docking of the R1-16 relaxosome supports an activating mechanism that is important during mobilization of the second docked relaxosome, ColE1. The relative drop in ColE1 transfer efficiency in the absence of the F-like relaxosome implies that transporter activity is optimally initiated by the R1 relaxosome even when this complex itself cannot be transferred. The most severe transfer defect was observed with the ΔoriT derivatives, where TraI, TraY, and IHF cannot bind normally on the plasmid DNA. The ability of the T4SS to mobilize ColE1 improved when more complete assembly of the R1-16 relaxosome was possible, i.e., with the Δnic and ΔtraI derivatives. We also observed a gain of function in ColE1 mobilization in the complete absence of oriT when traC and traD were overexpressed. It appears that, when the R1-16 proteins are unable to form a complete relaxosome without oriT, they exert a negative effect on ColE1 mobilization. We propose that under these conditions, excess levels of the interaction partners are needed to sequester the remaining factors away from their binding sites at the entrance of the secretion channel. In that case, despite the high concentrations of TraC and TraD, the properly assembled ColE1 relaxosome must support higher-affinity interactions with the T4SS than the incompletely assembled R1-16 complex. A complete absence of the partial R1 assembly “frees” the receptor proteins at the conjugative pore to be efficiently activated by the alternative ColE1 relaxosome.
This conclusion seems at first to contradict our major finding that the R1 relaxosome has an active role in promoting translocation of not only Mob plasmids but also R17 RNA. We argue, however, that both models are compatible and neither alone is sufficient to account for all our data. The key evidence derives from the traI deletion. If the absence of T4SS activity observed thus far was solely due to negative interference, then neither nucleoprotein uptake nor plasmid export should be affected by the complete absence of TraI. In that case, a wild-type oriT is present to permit the assembly of all factors except TraI. Since TraI is absent and the remaining relaxosome proteins should bind DNA normally, no proteins would be available to mediate a negative effect. In contrast, however, we observed no phage infection in the absence of traI, and ColE1 was mobilized 30-fold less efficiently than with the wild type. T4SS activity in both processes was fully restored by expression of the TraI N1-992 (relaxase plus TS) fragment. We conclude that transporter activity is normally optimized by the R1 relaxosome. This positive regulation is apparent even when the R1 relaxosome itself cannot be transferred, i.e., when the activation domain of TraI N1-992 is present but the C-terminal conjugative helicase is missing.
The regulatory activity contributed by the R1 relaxosome remains unspecified. In our experiments, transporter activation seemed to be optimized by binding to the conjugative relaxosome even when the substrate actually transferred was a competing nucleoprotein complex. In a natural setting, relaxosomes of the conjugative and Mob plasmid will typically be present in the same donor cells, since mutations disrupting the conjugative relaxosome also eliminate lateral dissemination of this plasmid in the population. We propose that the biological sense underlying this mechanism is to ensure that the T4SS supports self-transfer most efficiently. This functional dependence would favor the fitness of the host and conjugative and mobilizable plasmids alike. First, R17 infection of the host is possible only when a conjugative relaxosome is docked to the transporter. This ensures that the T4SS renders its host sensitive to phage predation only when the machinery is capable of self-dissemination. A similar requirement to colocalize a functional R1 relaxosome to a conjugative pore bound by the mobilizable plasmid ColE1 lowers the ability of the Mob plasmid to spread to recipients at the expense of the conjugative plasmid. Remarkably, this also seems to be the case for the Mob plasmid CloDF13, which is uniquely adapted with its own T4CP. Outcompeting the conjugative relaxosome for access to the transfer machinery would lower the probability that cells receiving the Mob plasmid also acquire the conjugation system. The development of a functional dependence on colocalization of both substrates lowers the chances that the new host becomes a dead end for further lateral transfer of the Mob plasmid. Finally, a conjugative T4SS optimized for self-transfer should benefit the host cells most, since advantageous genes expressing, e.g., antibiotic resistances and virulence factors are typically carried only by the large conjugative plasmid.
In conclusion, it appears that the conjugative pore is a crowded place, where the initiation of substrate transfer follows a reaction pathway optimally initiated by an idealized docking with the conjugative relaxosome. Additional substrates may utilize the activated channel, but evolutionary pressure seems to restrict T4SS engagement to favor its own dissemination.
ACKNOWLEDGMENTS
This research was supported by Austrian Science Fund (FWF) grants P-24016 and W901, DK Molecular Enzymology, and the European Fund for Regional Development and the province of Styria (grant A3-11.B-38/2011-6).
L. Frost, C. Drainas, R. Meyer, and M. Bamberger provided plasmids. We gratefully acknowledge P. Silverman's helpful suggestions in R17 labeling and purification and J. Schmiedhofer's assistance with graphics.
Footnotes
Published ahead of print 28 March 2014
REFERENCES
- 1.Alvarez-Martinez CE, Christie PJ. 2009. Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 73:775–808. 10.1128/MMBR.00023-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatty M, Laverde Gomez JA, Christie PJ. 2013. The expanding bacterial type IV secretion lexicon. Res. Microbiol. 164:620–639. 10.1016/j.resmic.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zechner EL, Lang S, Schildbach JF. 2012. Assembly and mechanisms of bacterial type IV secretion machines. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367:1073–1087. 10.1098/rstb.2011.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton HL, Dominguez NM, Schwartz KJ, Hackett KT, Dillard JP. 2005. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol. Microbiol. 55:1704–1721. 10.1111/j.1365-2958.2005.04521.x [DOI] [PubMed] [Google Scholar]
- 5.Cascales E, Christie PJ. 2004. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304:1170–1173. 10.1126/science.1095211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomis-Rüth FX, Moncalían G, Pérez-Luque R, González A, Cabezón E, de la Cruz F, Coll M. 2001. The bacterial conjugation protein TrwB resembles ring helicases and F1-ATPase. Nature 409:637–641. 10.1038/35054586 [DOI] [PubMed] [Google Scholar]
- 7.Clewell DB, Helinski DR. 1969. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an open circular DNA form. Proc. Natl. Acad. Sci. U. S. A. 62:1159–1166. 10.1073/pnas.62.4.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llosa M, Gomis-Rüth FX, Coll M, de la Cruz F. 2002. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 45:1–8. 10.1046/j.1365-2958.2002.03014.x [DOI] [PubMed] [Google Scholar]
- 9.Atmakuri K, Cascales E, Christie PJ. 2004. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 54:1199–1211. 10.1111/j.1365-2958.2004.04345.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draper O, Middleton R, Doucleff M, Zambryski PC. 2006. Topology of the VirB4 C terminus in the Agrobacterium tumefaciens VirB/D4 type IV secretion system. J. Biol. Chem. 281:37628–37635. 10.1074/jbc.M606403200 [DOI] [PubMed] [Google Scholar]
- 11.Li F, Alvarez-Martinez C, Chen Y, Choi KJ, Yeo HJ, Christie PJ. 2012. Enterococcus faecalis PrgJ, a VirB4-like ATPase, mediates pCF10 conjugative transfer through substrate binding. J. Bacteriol. 194:4041–4051. 10.1128/JB.00648-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ripoll-Rozada J, Zunzunegui S, de la Cruz F, Arechaga I, Cabezon E. 2013. Functional interactions of VirB11 traffic ATPases with VirB4 and VirD4 molecular motors in type IV secretion systems. J. Bacteriol. 195:4195–4201. 10.1128/JB.00437-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward DV, Draper O, Zupan JR, Zambryski PC. 2002. Peptide linkage mapping of the Agrobacterium tumefaciens vir-encoded type IV secretion system reveals protein subassemblies. Proc. Natl. Acad. Sci. U. S. A. 99:11493–11500. 10.1073/pnas.172390299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cascales E, Atmakuri K, Sarkar MK, Christie PJ. 2013. DNA substrate-induced activation of the Agrobacterium VirB/VirD4 type IV secretion system. J. Bacteriol. 195:2691–2704. 10.1128/JB.00114-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron C. 2013. A novel strategy to target bacterial virulence. Future Microbiol. 8:1–3. 10.2217/fmb.12.120 [DOI] [PubMed] [Google Scholar]
- 16.Llosa M, de la Cruz F. 2005. Bacterial conjugation: a potential tool for genomic engineering. Res. Microbiol. 156:1–6. 10.1016/j.resmic.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 17.de la Cruz F, Frost LS, Meyer RJ, Zechner EL. 2010. Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol. Rev. 34:18–40. 10.1111/j.1574-6976.2009.00195.x [DOI] [PubMed] [Google Scholar]
- 18.Wong JJ, Lu J, Glover JN. 2012. Relaxosome function and conjugation regulation in F-like plasmids—a structural biology perspective. Mol. Microbiol. 85:602–617. 10.1111/j.1365-2958.2012.08131.x [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Monem M, Durwald H, Hoffmann-Berling H. 1976. Enzymic unwinding of DNA. 2. Chain separation by an ATP-dependent DNA unwinding enzyme. Eur. J. Biochem. 65:441–449 [DOI] [PubMed] [Google Scholar]
- 20.Matson SW, Sampson JK, Byrd DR. 2001. F plasmid conjugative DNA transfer: the TraI helicase activity is essential for DNA strand transfer. J. Biol. Chem. 276:2372–2379. 10.1074/jbc.M008728200 [DOI] [PubMed] [Google Scholar]
- 21.Hohlfeld S, Pattis I, Puls J, Plano GV, Haas R, Fischer W. 2006. A C-terminal translocation signal is necessary, but not sufficient for type IV secretion of the Helicobacter pylori CagA protein. Mol. Microbiol. 59:1624–1637. 10.1111/j.1365-2958.2006.05050.x [DOI] [PubMed] [Google Scholar]
- 22.Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. 2005. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl. Acad. Sci. U. S. A. 102:826–831. 10.1073/pnas.0406239101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulein R, Guye P, Rhomberg TA, Schmid MC, Schröder G, Vergunst AC, Carena I, Dehio C. 2005. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc. Natl. Acad. Sci. U. S. A. 102:856–861. 10.1073/pnas.0406796102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alperi A, Larrea D, Fernandez-Gonzalez E, Dehio C, Zechner EL, Llosa M. 2013. A translocation motif in relaxase TrwC specifically affects recruitment by its conjugative type IV secretion system. J. Bacteriol. 195:4999–5006. 10.1128/JB.00367-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang S, Gruber K, Mihajlovic S, Arnold R, Gruber CJ, Steinlechner S, Jehl MA, Rattei T, Frohlich KU, Zechner EL. 2010. Molecular recognition determinants for type IV secretion of diverse families of conjugative relaxases. Mol. Microbiol. 78:1539–1555. 10.1111/j.1365-2958.2010.07423.x [DOI] [PubMed] [Google Scholar]
- 26.Parker C, Meyer RJ. 2007. The R1162 relaxase/primase contains two, type IV transport signals that require the small plasmid protein MobB. Mol. Microbiol. 66:252–261. 10.1111/j.1365-2958.2007.05925.x [DOI] [PubMed] [Google Scholar]
- 27.Redzej A, Ilangovan A, Lang S, Gruber CJ, Topf M, Zangger K, Zechner EL, Waksman G. 2013. Structure of a translocation signal domain mediating conjugative transfer by type IV secretion systems. Mol. Microbiol. 89:324–333. 10.1111/mmi.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beranek A, Zettl M, Lorenzoni K, Schauer A, Manhart M, Koraimann G. 2004. Thirty-eight C-terminal amino acids of the coupling protein TraD of the F-like conjugative resistance plasmid R1 are required and sufficient to confer binding to the substrate selector protein TraM. J. Bacteriol. 186:6999–7006. 10.1128/JB.186.20.6999-7006.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Disque-Kochem C, Dreiseikelmann B. 1997. The cytoplasmic DNA-binding protein TraM binds to the inner membrane protein TraD in vitro. J. Bacteriol. 179:6133–6137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J, Frost LS. 2005. Mutations in the C-terminal region of TraM provide evidence for in vivo TraM-TraD interactions during F-plasmid conjugation. J. Bacteriol. 187:4767–4773. 10.1128/JB.187.14.4767-4773.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, Wong JJ, Edwards RA, Manchak J, Frost LS, Glover JN. 2008. Structural basis of specific TraD-TraM recognition during F plasmid-mediated bacterial conjugation. Mol. Microbiol. 70:89–99. 10.1111/j.1365-2958.2008.06391.x [DOI] [PubMed] [Google Scholar]
- 32.Wong JJ, Lu J, Edwards RA, Frost LS, Glover JN. 2011. Structural basis of cooperative DNA recognition by the plasmid conjugation factor, TraM. Nucleic Acids Res. 39:6775–6788. 10.1093/nar/gkr296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mihajlovic S, Lang S, Sut MV, Strohmaier H, Gruber CJ, Koraimann G, Cabezón E, Moncalián G, de la Cruz F, Zechner EL. 2009. Plasmid R1 conjugative DNA processing is regulated at the coupling protein interface. J. Bacteriol. 191:6877–6887. 10.1128/JB.00918-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sut MV, Mihajlovic S, Lang S, Gruber CJ, Zechner EL. 2009. Protein and DNA effectors control the TraI conjugative helicase of plasmid R1. J. Bacteriol. 191:6888–6899. 10.1128/JB.00920-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loeb T. 1960. Isolation of a bacteriophage specific for the F plus and Hfr mating types of Escherichia coli K-12. Science 131:932–933. 10.1126/science.131.3404.932 [DOI] [PubMed] [Google Scholar]
- 36.Lang S, Kirchberger PC, Gruber CJ, Redzej A, Raffl S, Zellnig G, Zangger K, Zechner EL. 2011. An activation domain of plasmid R1 TraI protein delineates stages of gene transfer initiation. Mol. Microbiol. 82:1071–1085. 10.1111/j.1365-2958.2011.07872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varsaki A, Lucas M, Afendra AS, Drainas C, de la Cruz F. 2003. Genetic and biochemical characterization of MbeA, the relaxase involved in plasmid ColE1 conjugative mobilization. Mol. Microbiol. 48:481–493. 10.1046/j.1365-2958.2003.03441.x [DOI] [PubMed] [Google Scholar]
- 38.van de Pol H, Veltkamp E, Nijkamp HJJ. 1978. Genetic analysis of the mobilization of the non-conjugative plasmid Clo DF13. Mol. Gen. Genet. 160:139–149. 10.1007/BF00267475 [DOI] [Google Scholar]
- 39.Miranda RL, Conway T, Leatham MP, Chang DE, Norris WE, Allen JH, Stevenson SJ, Laux DC, Cohen PS. 2004. Glycolytic and gluconeogenic growth of Escherichia coli O157:H7 (EDL933) and E. coli K-12 (MG1655) in the mouse intestine. Infect. Immun. 72:1666–1676. 10.1128/IAI.72.3.1666-1676.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 97:5978–5983. 10.1073/pnas.100127597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodcock DM, Crowther PJ, Doherty J, Jefferson S, DeCruz E, Noyer-Weidner M, Smith SS, Michael MZ, Graham MW. 1989. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 17:3469–3478. 10.1093/nar/17.9.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goebel W, Lindenmaier W, Schrempf H, Kollek R, Blohm D. 1977. Dissociation and recombination of fragments with defined functions of the antibiotic resistance factor R1, p 261–275 In Drews J, Ḧogenauer G. (ed), Topics in infectious diseases, vol 2 Springer Verlag, New York, NY [Google Scholar]
- 43.Pölzleitner E, Zechner EL, Renner W, Fratte R, Jauk B, Högenauer G, Koraimann G. 1997. TraM of plasmid R1 controls transfer gene expression as an integrated control element in a complex regulatory network. Mol. Microbiol. 25:495–507. 10.1046/j.1365-2958.1997.4831853.x [DOI] [PubMed] [Google Scholar]
- 44.Chandler M, Galas DJ. 1983. Cointegrate formation mediated by Tn9. II. Activity of IS1 is modulated by external DNA sequences. J. Mol. Biol. 170:61–91 [DOI] [PubMed] [Google Scholar]
- 45.Maneewannakul K, Kathir P, Endley S, Moore D, Manchak J, Frost L, Ippen-Ihler K. 1996. Construction of derivatives of the F plasmid pOX-tra715: characterization of traY and traD mutants that can be complemented in trans. Mol. Microbiol. 22:197–205. 10.1046/j.1365-2958.1996.00087.x [DOI] [PubMed] [Google Scholar]
- 46.Penfold SS, Simon J, Frost LS. 1996. Regulation of the expression of the traM gene of the F sex factor of Escherichia coli. Mol. Microbiol. 20:549–558. 10.1046/j.1365-2958.1996.5361059.x [DOI] [PubMed] [Google Scholar]
- 47.Cabezón E, Sastre JI, de la Cruz F. 1997. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol. Gen. Genet. 254:400–406. 10.1007/s004380050432 [DOI] [PubMed] [Google Scholar]
- 48.Scholz P, Haring V, Wittmann-Liebold B, Ashman K, Bagdasarian M, Scherzinger E. 1989. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene 75:271–288. 10.1016/0378-1119(89)90273-4 [DOI] [PubMed] [Google Scholar]
- 49.Karl W, Bamberger M, Zechner EL. 2001. Transfer protein TraY of plasmid R1 stimulates TraI-catalyzed oriT cleavage in vivo. J. Bacteriol. 183:909–914. 10.1128/JB.183.3.909-914.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherlock O, Schembri MA, Reisner A, Klemm P. 2004. Novel roles for the AIDA adhesin from diarrheagenic Escherichia coli: cell aggregation and biofilm formation. J. Bacteriol. 186:8058–8065. 10.1128/JB.186.23.8058-8065.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haldimann A, Wanner B. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 183:6384–6393. 10.1128/JB.183.21.6384-6393.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reisner A, Holler BM, Molin S, Zechner EL. 2006. Synergistic effects in mixed Escherichia coli biofilms: conjugative plasmid transfer drives biofilm expansion. J. Bacteriol. 188:3582–3588. 10.1128/JB.188.10.3582-3588.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reisner A, Molin S, Zechner EL. 2002. Recombinogenic engineering of conjugative plasmids with fluorescent marker cassettes. FEMS Microbiol. Ecol. 42:251–259. 10.1111/j.1574-6941.2002.tb01015.x [DOI] [PubMed] [Google Scholar]
- 55.Daehnel K, Harris R, Maddera L, Silverman P. 2005. Fluorescence assays for F-pili and their application. Microbiology 151:3541–3548. 10.1099/mic.0.28159-0 [DOI] [PubMed] [Google Scholar]
- 56.Reisner A, Krogfelt KA, Klein BM, Zechner EL, Molin S. 2006. In vitro biofilm formation of commensal and pathogenic Escherichia coli strains: impact of environmental and genetic factors. J. Bacteriol. 188:3572–3581. 10.1128/JB.188.10.3572-3581.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willetts N. 1980. Interactions between the F conjugal transfer system and CloDF13::TnA plasmids. Mol. Gen. Genet. 180:213–217. 10.1007/BF00267372 [DOI] [PubMed] [Google Scholar]
- 58.Vergunst AC, Schrammeijer B, den Dulk-Ras A, de Vlaam CM, Regensburg-Tuink TJ, Hooykaas PJ. 2000. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290:979–982. 10.1126/science.290.5493.979 [DOI] [PubMed] [Google Scholar]
- 59.Dostal L, Shao S, Schildbach JF. 2011. Tracking F plasmid TraI relaxase processing reactions provides insight into F plasmid transfer. Nucleic Acids Res. 39:2658–2670. 10.1093/nar/gkq1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang S, Zechner EL. 2012. General requirements for protein secretion by the F-like conjugation system R1. Plasmid 67:128–138. 10.1016/j.plasmid.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo Y, Gao Q, Deonier RC. 1994. Mutational and physical analysis of F plasmid traY protein binding to oriT. Mol. Microbiol. 11:459–469. 10.1111/j.1365-2958.1994.tb00327.x [DOI] [PubMed] [Google Scholar]
- 62.Rice PA, Yang S, Mizuuchi K, Nash HA. 1996. Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn. Cell 87:1295–1306. 10.1016/S0092-8674(00)81824-3 [DOI] [PubMed] [Google Scholar]
- 63.Harley MJ, Schildbach JF. 2003. Swapping single-stranded DNA sequence specificities of relaxases from conjugative plasmids F and R100. Proc. Natl. Acad. Sci. U. S. A. 100:11243–11248. 10.1073/pnas.2035001100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dempsey WB, Fee BE. 1990. Integration host factor affects expression of two genes at the conjugal transfer origin of plasmid R100. Mol. Microbiol. 4:1019–1028. 10.1111/j.1365-2958.1990.tb00674.x [DOI] [PubMed] [Google Scholar]
- 65.Silverman PM, Wickersham E, Harris R. 1991. Regulation of the F plasmid traY promoter in Escherichia coli by host and plasmid factors. J. Mol. Biol. 218:119–128. 10.1016/0022-2836(91)90878-A [DOI] [PubMed] [Google Scholar]
- 66.Haft RJ, Palacios G, Nguyen T, Mally M, Gachelet EG, Zechner EL, Traxler B. 2006. General mutagenesis of F plasmid TraI reveals its role in conjugative regulation. J. Bacteriol. 188:6346–6353. 10.1128/JB.00462-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kienesberger S, Trummler CS, Fauster A, Lang S, Sprenger H, Gorkiewicz G, Zechner EL. 2011. Interbacterial macromolecular transfer by the Campylobacter fetus subsp. venerealis type IV secretion system. J. Bacteriol. 193:744–758. 10.1128/JB.00798-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawley TD, Klimke WA, Gubbins MJ, Frost LS. 2003. F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 224:1–15. 10.1016/S0378-1097(03)00430-0 [DOI] [PubMed] [Google Scholar]
- 69.Chen Y, Zhang X, Manias D, Yeo HJ, Dunny GM, Christie PJ. 2008. Enterococcus faecalis PcfC, a spatially localized substrate receptor for type IV secretion of the pCF10 transfer intermediate. J. Bacteriol. 190:3632–3645. 10.1128/JB.01999-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pena A, Matilla I, Martin-Benito J, Valpuesta JM, Carrascosa JL, de la Cruz F, Cabezon E, Arechaga I. 2012. The hexameric structure of a conjugative VirB4 protein ATPase provides new insights for a functional and phylogenetic relationship with DNA translocases. J. Biol. Chem. 287:39925–39932. 10.1074/jbc.M112.413849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schandel KA, Muller MM, Webster RE. 1992. Localization of TraC, a protein involved in assembly of the F conjugative pilus. J. Bacteriol. 174:3800–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghigo JM. 2001. Natural conjugative plasmids induce bacterial biofilm development. Nature 412:442–445. 10.1038/35086581 [DOI] [PubMed] [Google Scholar]
- 73.Reisner A, Haagensen JAJ, Schembri MA, Zechner EL, Molin S. 2003. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48:933–946. 10.1046/j.1365-2958.2003.03490.x [DOI] [PubMed] [Google Scholar]
- 74.Fernández-López R, Garcillán-Barcia MP, Revilla C, Lázaro M, Vielva L, de la Cruz F. 2006. Dynamics of the IncW genetic backbone imply general trends in conjugative plasmid evolution. FEMS Microbiol. Rev. 30:942–966. 10.1111/j.1574-6976.2006.00042.x [DOI] [PubMed] [Google Scholar]
- 75.Kerr JE, Christie PJ. 2010. Evidence for VirB4-mediated dislocation of membrane-integrated VirB2 pilin during biogenesis of the Agrobacterium VirB/VirD4 type IV secretion system. J. Bacteriol. 192:4923–4934. 10.1128/JB.00557-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rabel C, Grahn AM, Lurz R, Lanka E. 2003. The VirB4 family of proposed traffic nucleoside triphosphatases: common motifs in plasmid RP4 TrbE are essential for conjugation and phage adsorption. J. Bacteriol. 185:1045–1058. 10.1128/JB.185.3.1045-1058.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Durand E, Oomen C, Waksman G. 2010. Biochemical dissection of the ATPase TraB, the VirB4 homologue of the Escherichia coli pKM101 conjugation machinery. J. Bacteriol. 192:2315–2323. 10.1128/JB.01384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]