Abstract
Campylobacter jejuni is a leading cause of gastrointestinal infections worldwide, due primarily to its ability to asymptomatically colonize the gastrointestinal tracts of agriculturally relevant animals, including chickens. Infection often occurs following consumption of meat that was contaminated by C. jejuni during harvest. Because of this, much interest lies in understanding the mechanisms that allow C. jejuni to colonize the chicken gastrointestinal tract. To address this, we generated a C. jejuni transposon mutant library that is amenable to insertion sequencing and introduced this mutant pool into day-of-hatch chicks. Following deep sequencing of C. jejuni mutants in the cecal outputs, several novel factors required for efficient colonization of the chicken gastrointestinal tract were identified, including the predicted outer membrane protein MapA. A mutant strain lacking mapA was constructed and found to be significantly reduced for chicken colonization in both competitive infections and monoinfections. Further, we found that mapA is required for in vitro competition with wild-type C. jejuni but is dispensable for growth in monoculture.
INTRODUCTION
Campylobacter jejuni is a leading cause of bacterially derived gastroenteritis in the United States, with 13.6 illnesses/100,000 persons annually. The incidence of infection is similar to and often surpasses the annual incidence of similar food-borne infection caused by Salmonella (1). It is reported that C. jejuni costs the United States approximately $1.7 billion annually (1, 2). The high incidence of C. jejuni infections is largely due to its ability to persistently colonize the gastrointestinal tracts of poultry and other agriculturally relevant animals (3). During processing of meat products, bacteria are released from the gastrointestinal tracts of these animals, contaminating the meat. Following consumption of either undercooked poultry or contaminated food, human infection often occurs from inocula as low as 360 organisms and results in moderate to severe and bloody diarrhea (4).
Due to the importance of chicken colonization to the infection cycle of C. jejuni, much emphasis has been placed on identifying and characterizing factors that promote colonization of the chicken gastrointestinal tract. To date, many different classifications of genes have been found to play a role in colonization of the chicken gastrointestinal tract, including those involved in chemotaxis, invasion, iron regulation, motility, and oxidative and nitrosative stress responses (5). Many of these studies identified chicken colonization phenotypes as a consequence of characterizing a factor of interest and often using monoinfections. Only one previous study attempted to identify colonization factors of C. jejuni in the chicken using a genome-wide approach (6). Employing a signature-tagged mutagenesis (STM) screen, this study identified factors involved in motility, N-linked protein glycosylation, and chemotaxis. While these factors have been further confirmed to be involved in chicken colonization, the STM approach identified only 22 new loci and was plagued by a high rate of false-negative identification, presumably due to technical limitations of STM. Not surprisingly, based on the cumulative work in the field, it is postulated that the earlier STM study did not identify a majority of the factors involved in chicken colonization (5).
Recently, development of various methodologies that take advantage of advances in next-generation sequencing technologies has allowed more comprehensive identification of bacterial factors of interest. One such protocol, insertion sequencing (INSeq), relies on a Mariner transposon system with an MmeI site introduced into the Himar1 inverted repeats of the transposon (7). Following construction of a transposon mutant library, extracted genomic DNA can be prepared and digested with MmeI, leaving a 16-bp fragment of flanking genomic DNA attached to the transposon. After ligating sequencing adapters to these fragments, the 16-bp region can be sequenced and mapped to a reference genome, allowing identification and quantification of mutants within a pool. Fortunately, the Mariner transposon system has been developed for use in Campylobacter, making the use of INSeq an attractive approach for identifying determinants of chicken colonization.
Here we describe using INSeq to identify novel colonization determinants of C. jejuni in the chicken ceca (7). We constructed an INSeq-compatible C. jejuni transposon mutant library of several thousand mutants and used it to inoculate day-of-hatch chicks. Analysis of INSeq data from inocula and from C. jejuni recovered from the ceca of infected chickens enabled us to identify mutants that were either lost or enriched during infection. One mutant underrepresented in the ceca contained a transposon insertion in mapA, which encodes a putative outer membrane lipoprotein previously shown to be a species-specific determinant that is immunogenic in both chickens and humans (8, 9). Analysis of a mapA mutant confirmed its importance to C. jejuni fitness in the chicken gastrointestinal tract both in competition with wild-type C. jejuni and during monoinfections. Similarly, mapA was found to be required for in vitro competition with wild-type C. jejuni but was dispensable when the mutant was grown in rich media alone.
MATERIALS AND METHODS
Bacterial strains, plasmids, and oligonucleotides.
Bacterial strains, plasmids, and oligonucleotides used in this study are listed in Table 1. Escherichia coli strains used for subcloning were routinely grown using LB media at 37°C under aerobic conditions. C. jejuni strains were routinely grown under microaerobic conditions (85% N2, 10% CO2, 5% O2) using either Mueller-Hinton (MH) agar containing 10% sheep's blood or MH broth. Cultures were grown at either 37°C or 42°C as noted specifically below. The following antibiotics were used at the given concentrations: carbenicillin at 50 μg/ml, chloramphenicol at 30 μg/ml, kanamycin at 150 μg/ml, and trimethoprim at 10 μg/ml.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Description or sequence (5′–3′)a | Reference or source |
|---|---|---|
| Strains | ||
| C. jejuni DRH212 | Streptomycin-resistant C. jejuni 81-176 (Smr) | 10 |
| C. jejuni ΔrpoN | rpoN deletion mutant of C. jejuni DRH212 (Smr) | 10 |
| C. jejuni kpsM::kan | kpsM insertion mutant of C. jejuni 81-176 | 15 |
| C. jejuni mapA::kan | mapA insertion mutant of C. jejuni DRH212 (Knr Smr) | This study |
| E. coli DH5α | E. coli strain used for cloning | Invitrogen |
| Plasmids | ||
| pRY109 | Campylobacter coli cat cassette plasmid (Cmr Apr) | 26 |
| pGEM-T Easy | Subcloning vector (Apr) | Promega |
| pKinnick | Plasmid containing hawkeye transposon (Cmr Apr) | This study |
| Oligonucleotides | ||
| 5′cat_INSeq | ACGCGTCCTAACAGGTTGGATGATAAGTCCCCGGTCTTCGTATGCCGTCTTCTGCTTGGCGCGCCCTCGAGCAATTGTGCTCGGCGGTGTTCCTTTCCAA | |
| 3′cat_INSeq | ACGCGTCCTAACAGGTTGGATGATAAGTCCCCGGTCTTCGTATGCCGTCTTCTGCTTGGCGCGCCCTGCAGTCTAGTGCGCCCTTTAGTTCCTAAAGGGT | |
| JJ17 | CTAGCGAGCTTCCTCCTGTT | |
| JJ18 | CATCTCTTGCGTCAGGCAAA | |
| JJ19 | GGATCCCCGCATTAAAATTCACATCAAC | |
| JJ20 | GGATCCGCTAGAGGAATAGTTGTGCTT |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Smr, streptomycin resistance.
DNA manipulations were performed using commercially available kits (Qiagen, Valencia, CA) and enzymes (NEB, Ipswich, MA) per the directions of the manufacturers.
Analysis of pseudoread mapping.
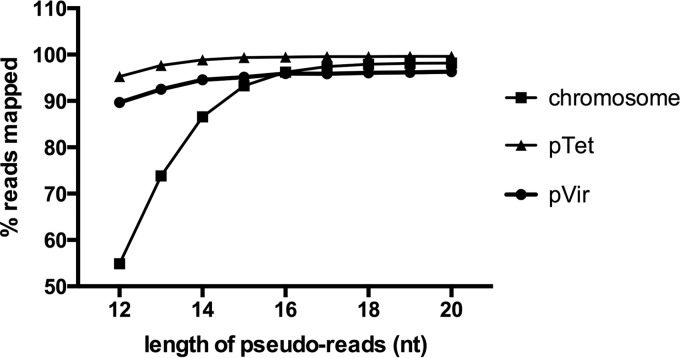
To determine whether the 16-bp genomic fragments flanking the transposons are sufficient to identify the points of insertion, a pseudoread analysis was performed, as previously described (7). Briefly, every TA dinucleotide—the insertion site of mariner transposons—in the C. jejuni 81-176 genome (including all three replicons, namely, chromosome, pTet, and pVir) was identified and reads of various lengths (12 to 20 bp) were generated on each side of the predicted transposon insertion site. These reads were analyzed using our pipeline, and the percentage of those reads that were successfully mapped back to the correct “insertion” locus was determined.
Construction of C. jejuni INSeq library.
A C. jejuni INSeq transposon, termed hawkeye, was generated by amplifying the cat cassette from plasmid pRY109 using oligonucleotides 5′cat_INSeq and 3′cat_INSeq. These primers were adapted from those used by Goodman et al. (7) but included regions complementary to the Campylobacter coli cat gene present in pRY109. This approximately 900-bp amplicon was cloned into the pGEM-T Easy vector (Promega, Madison, WI), resulting in pKinnick, and was sequenced to confirm conservation of required INSeq features, including the Himar1 insertion sequences, the introduced MmeI restriction sites, and the P7 priming sites. Following sequencing, in vitro transposition using purified C. jejuni genomic DNA was performed as previously described (10). Briefly, transposition was performed using 2 μg of purified DRH212 genomic DNA, approximately 500 ng of pKinnick, and 250 ng of purified maltose binding protein-tagged MarC9 transposase (MBP-MarC9) in transposition reaction buffer (10% glycerol, 25 mM HEPES [pH 7.9], 100 mM NaCl, 5 mM MgCl2, 0.25 mg bovine serum albumin [BSA], and 2 mM dithiothreitol [DTT]) at 30°C for 4 h. After repair of the transposon junctions, genomic DNA was purified and introduced into C. jejuni DRH212 via natural competence (11). Successful recombinants were selected under microaerobic conditions on MH agar containing chloramphenicol for approximately 48 h at 37°C.
Prior to en masse collection of the INSeq library, random and single insertion of hawkeye into C. jejuni genomic DNA was determined by Southern blotting. Genomic DNA was isolated from 17 individual mutants and digested with RsaI (Promega). Digested DNA was separated on a 1% Tris-acetate-EDTA agarose gel and transferred to an Amersham Hybond-XL membrane (GE Healthcare, Little Chalfont, United Kingdom). Bound DNA was probed using 32P-labeled hawkeye fragments generated by random priming and visualized by autoradiography.
Mutants were picked to individual wells of 96-well plates containing MH broth and grown an additional 48 h at 37°C microaerobically before being stored at −80°C. To collect the individually stored mutants into a single pool, each 96-well plate was replicated using a 96-pin replicator in a 150-mm-diameter MH agar plate. The plates were grown microaerobically for approximately 48 h at 37°C before the growth from all plates was harvested into a single MH broth–20% glycerol suspension and stored at −80°C.
Infection of day-of-hatch chickens with the INSeq library.
The pooled C. jejuni INSeq mutant library was thawed on ice, and then 100 μl of the stock suspension was placed onto MH agar and grown microaerobically for 4 h at 37°C. Cells were harvested and used to make a suspension of approximately 107 CFU/ml in sterile phosphate-buffered saline (PBS). This suspension was used to inoculate white leghorn chicks (Charles River, Wilmington, MA) by oral gavage with approximately 106 CFU (100 μl of suspension). To profile the input, INSeq libraries were prepared using two different methods. In the first, approximately 109 cells were prepared following 4 h, as previously described (12). In the second, a sample of 106 bacteria that was used to inoculate the day-of-hatch chicks was grown overnight and the input library was generated from that growth. The mutant library was allowed to colonize for 7 days before cecal contents were harvested, and C. jejuni was enumerated following 48 h of microaerobic growth at 37°C on Campylobacter selective media (MH agar with 10% sheep blood containing 40 μg/ml cefoperazone, 100 μg/ml cycloheximide, 30 μg/ml chloramphenicol, 10 μg/ml trimethoprim, and 100 μg/ml vancomycin).
Preparation of INSeq libraries from chickens.
Following harvest of cecal contents, a Campylobacter enrichment step was performed immediately, as previously described (13). Briefly, an equal volume of PBS containing a 1:100 volume of phenol (Life Technologies, Grand Island, NY) was added to PBS suspensions of cecal contents. These suspensions were subjected to low-speed centrifugation at 800 × g for 3 min at 4°C to remove large insoluble particles. The supernatant from this step was removed and subjected to further centrifugation at 9,260 × g for 5 min at 4°C, resulting in a pellet consisting primarily of bacterial cells and small cecal particles (13). Genomic DNA was extracted from these pellets and subjected to the protocol of Goodman et al. (12). Generally, the amount of sample or bacteria from one chick was insufficient to use for this protocol. As a result, genomic DNA from bacteria enriched from the cecal contents of two to five birds was pooled prior to generating INSeq libraries.
Analysis of INSeq results.
To identify mutants present in both input and output pools, DNA fragments were sequenced using an Illumina HiSeq 2000 sequencing system (San Diego, CA) available through the University of Michigan DNA Sequencing Core. Sequence data were analyzed using the following pipeline. Reads were trimmed and aligned to the C. jejuni 81-176 genome (accession no. NC_008787) using the Burrows-Wheeler Aligner (BWA) (14). Reads that (i) did not have a complementary read within 4 bp on the opposite strand, (ii) had a right-left read ratio > 10, or (iii) occurred within the last 10% of the reading frame were eliminated. The reads from each sample were normalized per million total reads. To determine the effect of mutations on colonization, fitness ratios were calculated for each locus by dividing the normalized reads in the output by the number of normalized reads for the same locus in the input.
Construction and characterization of a mapA mutant.
Regions of homology flanking the mapA locus were amplified from DRH212 genomic DNA. The upstream region was amplified using primers JJ17 and JJ19, while the downstream region was amplified using primers JJ18 and JJ20. These fragments were subcloned into pGEM-T Easy and subsequently cloned together, introducing an internal BamHI restriction site into mapA. The Campylobacter kanamycin resistance cassette was excised from pILL600 using BamHI and ligated into mapA in the construct described above. This plasmid was introduced into DRH212 by electroporation and grown on MH agar overnight at 37°C under microaerobic conditions. Cells were plated on MH agar containing kanamycin, and successful integration of mapA::kan into the chromosome was confirmed by PCR.
To examine mapA in vivo, equal numbers of the wild-type DRH212 and mapA mutant strains were used in competition assays. For these assays, an inoculum of approximately 5 × 104 CFU of each strain was given to day-of-hatch chicks by oral gavage. Strains were allowed to colonize for 7 days when cecal contents were isolated, and bacterial counts were determined for each strain using the Campylobacter selective media mentioned above. Additionally, the wild-type and the mapA mutant strains were individually introduced into day-of-hatch chicks using the same inocula of approximately 5 × 104. Similarly, at 7 days postinoculation, cecal contents were harvested and C. jejuni numbers were determined.
To examine whether reductions in colonization were due to a general growth defect, competitive and individual growth curves were determined for both wild-type C. jejuni and the mapA mutant. Briefly, each strain was grown overnight on MH agar containing trimethoprim and subsequently passed for a second night on the same media at 37°C. These cultures were used to inoculate 50 ml of MH broth to an A600 optical density (ODA600) of 0.025 and grown under microaerobic conditions at 42°C. At each indicated time point, the ODA600 was recorded (for all growth curves) and the numbers of viable C. jejuni and mapA mutant bacteria were determined using selective media (for in vitro competition).
Motility was examined following growth of wild-type C. jejuni, the mapA mutant, and a nonmotile control, the ΔrpoN mutant, at 37°C under microaerobic conditions. Cells from these plates were inoculated into MH swim agar (0.4% agarose) and incubated overnight at 42°C. This assay was repeated in triplicate, and motility was visualized and measured. Capsule expression was determined by growing wild-type C. jejuni, the mapA mutant, and a nonencapsulated kpsM mutant control at 37°C under microaerobic conditions (15). These strains were passed again onto MH media, as described above, and grown overnight before cell extracts were assayed for capsular material as previously described (16). Briefly, cells were pelleted in 100 μl of lysis buffer (31.25 mM Tris-HCl [pH 6.8], 4% sodium dodecyl sulfate, 0.025% bromophenol blue, and 20% glycerol) and boiled for 5 min. After 20 μl was transferred to a new tube, extracts were treated with 1 μl of a 20 mg/ml proteinase K stock and incubated for 1 h at 50°C. Extracts were separated on a 15% Tris–polyacrylamide gel and stained with alcian blue (0.1% alcian blue, 40% ethanol, 5% acetic acid) prior to visualization.
RESULTS AND DISCUSSION
The genomic fragments flanking the INSeq transposon are sufficient for identifying insertions sites.
To provide a proof of concept that the use of INSeq was possible in C. jejuni, we first determined whether the 16-nucleotide (nt) reads produced by the INSeq protocol were sufficient to identify the locations of insertions in the genome. Pseudoreads of various lengths were generated from every TA dinucleotide in the genome and mapped back to the C. jejuni 81-176 genome. Of all 16-nt pseudoreads generated, 97.21% ± 1.97% were mapped to the correct location in the genome, an efficiency similar to that reported by Goodman et al. for both Bacteroides thetaiotaomicron and Saccharomyces cerevisiae (Fig. 1) (7). While shorter reads could be accurately mapped within the smaller replicons (pTet and pVir), the mapping accuracy of chromosomal reads < 15 nt in length was below 90%. These results demonstrate that, despite the relatively low sequence complexity of an AT-rich organism such as C. jejuni (30% GC content), the 16-mer sequences produced by the INSeq protocol are sufficient for accurate, unambiguous mapping of transposon insertions.
FIG 1.
Analysis of pseudoreads flanking TA dinucleotides in the C. jejuni 81-176 genome. Reads of various lengths are plotted against the percentage of those reads that were successfully mapped to the correct location in each replicon.
The INSeq-compatible hawkeye transposon inserts randomly throughout the C. jejuni genome.
By the use of in vitro transposition reactions, hawkeye was transposed throughout the genome of DRH212, typically yielding 400 to 500 mutants per transposition reaction. Though the randomness of Mariner insertion into the C. jejuni genome had already been demonstrated, adapting the transposon for insertion sequencing made it necessary to confirm that this had not been altered. To determine whether the transposon inserted singly and randomly, RsaI-digested genomic DNA was subjected to Southern blot analysis using radiolabeled probes that hybridized to the hawkeye transposon. This analysis indicated that the transposition conditions that were used resulted in single insertions of hawkeye into the genomic DNA of DRH212, as only a single fragment within each of several isolates was able to hybridize to the probe (Fig. 2). Additionally, hawkeye inserted randomly, as those single fragments were of discrete sizes among the different mutant isolates. To ensure heterogeneity of the library, only 100 to 150 transposon mutants were selected from each individual transposition reaction until approximately 8,500 mutants were collected. At this size, based on the approximately 1,700 genes in the C. jejuni genome, we calculated that the mutant library should represent approximately 5× coverage.
FIG 2.
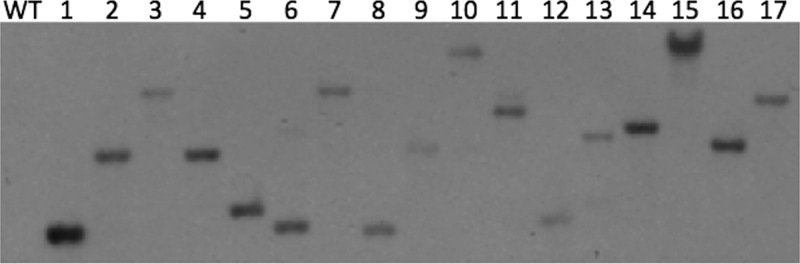
Southern blot analysis of C. jejuni INSeq mutants. Genomic DNA of wild-type C. jejuni (WT) and 17 INSeq mutants was digested with RsaI and separated on a 1% agarose gel. Transposon-containing fragments were detected using 32P-labeled hawkeye sequence.
Preparation of 109 cells from the pooled C. jejuni INSeq mutant library and analysis by our pipeline determined that the library consisted of 3,994 independent insertions into the chromosome, which inserted into 1,002 open reading frames (ORFs) (58.8% of chromosomal ORFs). Many of the ORFs that were missed included those genes encoding ribosomal components or replication factors and other essential functions (Fig. 3A). That C. jejuni can tolerate insertions into only ∼60% of its ORFs is not surprising; C. jejuni likely has to dedicate a relatively large portion of its small genome (1.7 Mbp) to growth. Insertions were mapped to every ORF within the pVir plasmid (52 genes) but only infrequently into pTet (5 genes). The latter observation is likely due to the frequency with which the pTet plasmid is lost from C. jejuni—presumably, pTet would have been mostly absent in genomic preparations for hawkeye mutagenesis. Overall, 1,059 of the 1,758 predicted C. jejuni ORFs were mutagenized, or 60.2% of those in the genome. Additionally, these results were reproducible using separate samples of the library, as a significant correlation between two independently prepared samples was observed (R2 = 0.94) (Fig. 3A and B). This indicates not only that the library is consistent but also that the INSeq protocol provides reproducible results.
FIG 3.
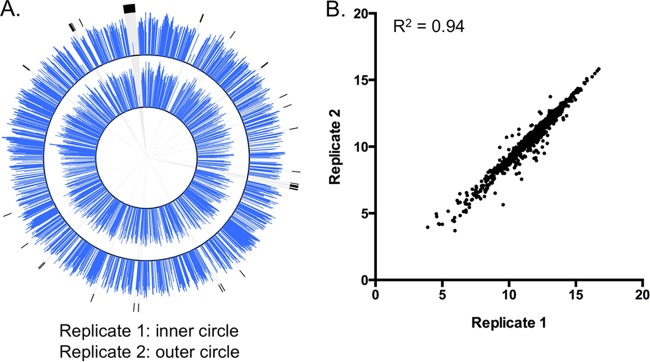
Analysis of C. jejuni INSeq mutant library. (A) Plots of insertion frequencies (log2 of total reads per ORF) in the C. jejuni chromosome generated using Circos (27). The inner and outer plots correspond to 2 independent cultures of ∼109 INSeq mutants. Regions highlighted in black correspond to ORFs encoding ribosomal proteins. (B) The correlation of the locus abundances of the two technical replicates was determined using a Pearson correlation coefficient (R2 = 0.94).
Insertion sequencing identified several determinants that are required for efficient colonization of the chicken gastrointestinal tract.
As stated above, two different methods for characterizing inputs were used. In the first method, an INSeq library was prepared from 109 cells after 4 h of growth at −80°C. In the second method, 106 cells from the inoculum were grown overnight before a sample of 109 cells was used to generate an INSeq library (Fig. 4A). The log2 abundances of reads mapping to each gene from these preparations were plotted against one another and found to be significantly different (R2 = 0.16). This is likely due to both viable and nonviable cells being present in inputs generated using the first method and the sampling error introduced by using only 106 cells in the second method. While the complexity of the input diminishes using the second method, libraries are more likely to resemble the mutant populations that were in the inocula, because the cells were derived directly from those. Using this method, the inoculum was determined to contain a mutant population representing 1,155 independent insertions into 545 chromosomal genes.
FIG 4.
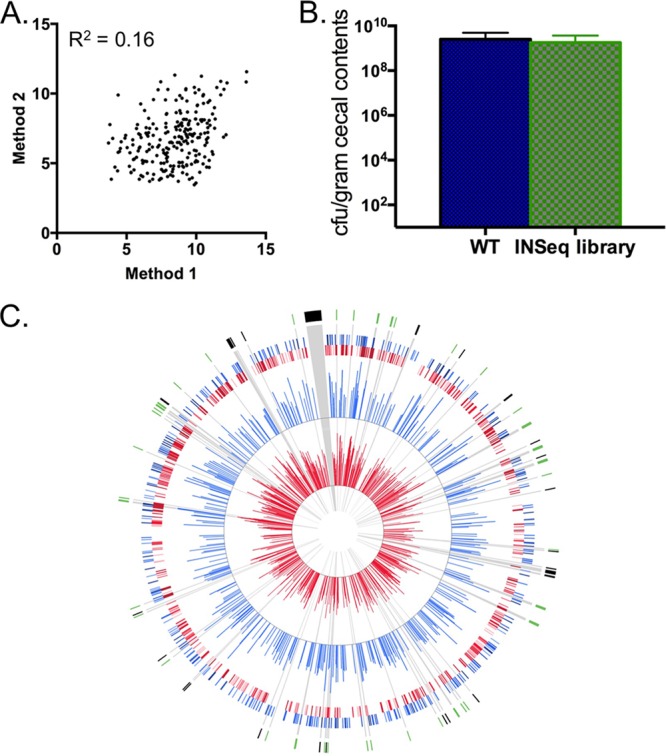
Identification of mutants affected for colonization of the chicken gastrointestinal tract. (A) Correlation between mutant library inputs prepared using two different methods; data were determined using a Pearson correlation coefficient (R2 = 0.16). (B) Cecal colonization of day-of-hatch chicks by either wild-type C. jejuni or mutants from the C. jejuni INSeq mutant library. (C) Plots of insertion frequencies in the C. jejuni chromosome generated using Circos (27). The inner 2 plots correspond to the insertion frequencies (log2 of total reads per ORF) of the input (red) and output (blue) samples, respectively. The outer 2 plots show a heatmap of the inner 2 plots in which the 4 quartiles of insertion frequencies are plotted with increasingly darker shades of blue or red. Regions highlighted in black and green correspond to ORFs encoding ribosomal proteins and motility-associated gene products, respectively.
Following inoculation with 6 ×105 CFU of the INSeq mutant library, day-of-hatch chicks were fully colonized at 7 days postinoculation. Cecal loads of the mutant library had a mean of 1.79 × 109 CFU/gram of cecal content, while the wild-type strain yielded a mean cecal load of 2.52 × 109 CFU/gram (Fig. 4B). These results were found to be statistically indistinguishable (P value = 0.43), indicating that the C. jejuni INSeq library, as a whole, is proficient at colonizing the chicken gastrointestinal tract. Libraries were generated from six genomic samples representing 22 birds in total. Illumina HiSeq 2000 sequencing typically yielded between 500 and 1,500 Mb per sample. The input and each of the individual outputs were analyzed using parameters described in Materials and Methods.
Outputs were pooled and found to contain mutants that represented 565 independent insertions in 329 chromosomal genes. This represents a loss of a slight majority of those mutants present in the input, with 51% of the independent insertions and 40% of the marked genes being lost, which was evidenced by the relative abundance of sequenced loci in the input and the lack of those same loci in the output (Fig. 4C). We reasoned that this was likely due both to a loss of mutants following passage through the chick due to their reduced fitness in vivo and to a large number of the mutants being present at numbers too low to allow efficient colonization of the cecum, regardless of phenotype.
To address the likelihood that some mutants were present at numbers too low to allow colonization, we determined a fitness ratio for each locus that takes into account the abundance of a mutant in the output relative to its abundance in the input. We set a 2-log decrease in abundance within output pools as a standard for those that warranted further investigation. At this level of effect, a mutant would have been present at infectious levels within the inoculum and would have often been uniformly absent in all cecal outputs. Many of the loci identified with this level of stringency were shown to be required for colonization of the chicken gastrointestinal tract in the STM work mentioned previously, including those involved in flagellar motility, N-linked protein glycosylation, and chemotaxis (Table 2) (6). In all, 130 genes exhibited fitness ratios < 0.01, with approximately 15% involved in flagellar motility (flgE, motA, fliS, etc.) and chemotaxis (cheA, cheY, etc.), which have been highlighted (Fig. 4C).
TABLE 2.
Additional factors exhibiting at least a 2-log decrease in abundance from chicken cecal samples relative to the input
| Locus | Annotation/predicted functiona | Fitness ratiob |
|---|---|---|
| CJJ81176_1449 | Hypothetical protein | 0.00027 |
| CJJ81176_0310 | cheA | 0.00028 |
| CJJ81176_1411 | Hypothetical protein | 0.00034 |
| CJJ81176_0025 | flgE flagellar hook protein | 0.00037 |
| CJJ81176_0311 | cheV | 0.00037 |
| CJJ81176_1452 | Hypothetical protein | 0.00053 |
| CJJ81176_0283 | Sulfatase, putative | 0.00056 |
| CJJ81176_0305 | carB carbamoyl phosphate synthase large subunit | 0.00056 |
| CJJ81176_0246 | Pathogenicity domain-containing protein | 0.00072 |
| CJJ81176_0573 | fliD flagellar capping protein | 0.00095 |
| CJJ81176_0343 | dxs 1-deoxy-d-xylulose-5-phosphate synthase | 0.00096 |
| CJJ81176_0038 | rbr rubrerythrin | 0.00119 |
| CJJ81176_1567 | Peptide ABC transporter, permease protein | 0.00133 |
| CJJ81176_0290 | zupT zinc transporter | 0.00144 |
| CJJ81176_0764 | Putative outer membrane protein | 0.00148 |
| CJJ81176_0098 | fliM flagellar motor switch protein | 0.00155 |
| CJJ81176_0356 | Antioxidant AhpCTSA family protein | 0.00157 |
| CJJ81176_0918 | Hypothetical protein | 0.00168 |
| CJJ81176_1062 | Hypothetical protein | 0.00168 |
| CJJ81176_1087 | Hypothetical protein | 0.00175 |
| CJJ81176_1242 | htrA protease DO | 0.00178 |
| CJJ81176_0389 | cmeB RND efflux system, inner membrane transporter CmeB | 0.00184 |
| CJJ81176_1214 | 2OG-Fe(II) oxygenase family oxidoreductase | 0.00188 |
| CJJ81176_1457 | Hypothetical protein | 0.00237 |
| CJJ81176_1341 | Hypothetical protein | 0.00238 |
| CJJ81176_0671 | cbrR response regulator/GGDEF domain-containing protein | 0.00246 |
| CJJ81176_0444 | Hypothetical protein | 0.00248 |
| CJJ81176_0079 | Hypothetical protein | 0.00265 |
| CJJ81176_0083 | Hypothetical protein | 0.00272 |
| CJJ81176_0357 | flhB flagellar biosynthesis protein | 0.00276 |
| CJJ81176_1598 | rpsT 30S ribosomal protein S20 | 0.00277 |
| CJJ81176_1061 | Putative transmembrane transport protein | 0.00296 |
| CJJ81176_0813 | Hypothetical protein | 0.00298 |
| CJJ81176_1206 | Methyl-accepting chemotaxis protein | 0.00302 |
| CJJ81176_1548 | Methyl-accepting chemotaxis protein | 0.00303 |
| CJJ81176_0508 | rplL 50S ribosomal protein L7/L12 | 0.00305 |
| CJJ81176_1433 | Putative sugar transferase | 0.00307 |
| CJJ81176_0040 | Inner membrane protein YagU | 0.00311 |
| CJJ81176_0215 | Ser/Thr protein phosphatase family protein | 0.00313 |
| CJJ81176_1438 | kpsD capsular polysaccharide ABC transporter, periplasmic polysaccharide-binding protein | 0.00316 |
| CJJ81176_1675 | gltA citrate synthase | 0.00324 |
| CJJ81176_0414 | Hypothetical protein | 0.00346 |
| CJJ81176_0080 | flgD flagellar basal body rod modification protein | 0.00347 |
| CJJ81176_0109 | Hypothetical protein | 0.00352 |
| CJJ81176_0574 | fliS flagellar protein | 0.00356 |
| CJJ81176_0294 | Hypothetical protein | 0.00365 |
| CJJ81176_0906 | pheS phenylalanyl-tRNA synthetase subunit alpha | 0.00367 |
| CJJ81176_1415 | Putative sugar-1-phosphate nucleotidyltransferase | 0.00367 |
| CJJ81176_0589 | Hypothetical protein | 0.00374 |
| CJJ81176_0236 | OPT family oligopeptide transporter | 0.00379 |
| CJJ81176_0359 | motA flagellar motor protein | 0.00390 |
| CJJ81176_1462 | Hypothetical protein | 0.00391 |
| CJJ81176_1602 | Hemin ABC transporter, permease protein, putative | 0.00399 |
| CJJ81176_1541 | Hypothetical protein | 0.00400 |
| CJJ81176_1050 | Hypothetical protein | 0.00402 |
| CJJ81176_1203 | gidA tRNA uridine 5-carboxymethylaminomethyl modification enzyme | 0.00416 |
| CJJ81176_0307 | tal transaldolase | 0.00435 |
| CJJ81176_1413 | kpsC capsule polysaccharide export protein | 0.00451 |
| CJJ81176_0299 | Hypothetical protein | 0.00454 |
| CJJ81176_0273 | MCP-domain-containing signal transduction protein | 0.00459 |
| CJJ81176_1335 | Motility accessory factor | 0.00462 |
| CJJ81176_1529 | Integral membrane protein | 0.00462 |
| CJJ81176_0602 | ilvB acetolactate synthase 3 catalytic subunit | 0.00463 |
| CJJ81176_1455 | flgI flagellar basal body P-ring protein | 0.00468 |
| CJJ81176_1459 | flgK flagellar hook-associated protein | 0.00468 |
| CJJ81176_0032 | Na+/H+ antiporter family protein | 0.00479 |
| CJJ81176_0641 | ftn nonheme iron-containing ferritin | 0.00493 |
| CJJ81176_1337 | pseE | 0.00493 |
| CJJ81176_1012 | argF ornithine carbamoyltransferase | 0.00495 |
| CJJ81176_0219 | Hypothetical protein | 0.00519 |
| CJJ81176_1416 | Class I glutamine amidotransferase, putative | 0.00525 |
| CJJ81176_0590 | Hypothetical protein | 0.00537 |
| CJJ81176_0892 | rpsO 30S ribosomal protein S15 | 0.00542 |
| CJJ81176_1652 | ABC transporter, permease protein | 0.00550 |
| CJJ81176_0251 | argB acetylglutamate kinase | 0.00559 |
| CJJ81176_0296 | ilvE branched-chain amino acid aminotransferase | 0.00564 |
| CJJ81176_0674 | rlpA rare lipoprotein A | 0.00566 |
| CJJ81176_0005 | ksgA dimethyladenosine transferase | 0.00571 |
| CJJ81176_1238 | Hypothetical protein | 0.00576 |
| CJJ81176_1606 | kgtP alpha-ketoglutarate permease | 0.00587 |
| CJJ81176_1143 | pglB general glycosylation pathway protein | 0.00588 |
| CJJ81176_1679 | Major facilitator superfamily protein | 0.00590 |
| CJJ81176_1436 | Putative glycosyl transferase | 0.00593 |
| CJJ81176_0245 | Hypothetical protein | 0.00596 |
| CJJ81176_1136 | cheY chemotaxis protein | 0.00603 |
| CJJ81176_1147 | wlaB ABC transporter, permease/ATP-binding protein | 0.00668 |
| CJJ81176_1605 | Hypothetical protein | 0.00668 |
| CJJ81176_0366 | uvrA excinuclease ABC subunit A | 0.00674 |
| CJJ81176_0585 | MATE efflux family protein, authentic frameshift | 0.00677 |
| CJJ81176_0801 | napA nitrate reductase catalytic subunit | 0.00702 |
| CJJ81176_0386 | Coproporphyrinogen III oxidase | 0.00713 |
| CJJ81176_1654 | ABC transporter, ATP-binding protein | 0.00720 |
| CJJ81176_0185 | hom homoserine dehydrogenase | 0.00724 |
| CJJ81176_0274 | Hypothetical protein | 0.00728 |
| CJJ81176_0730 | waaA 3-deoxy-d-manno-octulosonic-acid transferase | 0.00728 |
| CJJ81176_0119 | cydB cytochrome d ubiquinol oxidase, subunit II | 0.00736 |
| CJJ81176_1417 | Hypothetical protein | 0.00737 |
| CJJ81176_1392 | metC cystathionine beta-lyase | 0.00752 |
| CJJ81176_1547 | MerR family transcriptional regulator | 0.00752 |
| CJJ81176_1154 | Beta-1,4-N-acetylgalactosaminyltransferase | 0.00756 |
| CJJ81176_1405 | Hypothetical protein | 0.00760 |
| CJJ81176_0252 | argD acetylornithine aminotransferase | 0.00764 |
| CJJ81176_1550 | pflA paralyzed flagellar protein | 0.00766 |
| CJJ81176_0543 | Hypothetical protein | 0.00769 |
| CJJ81176_0572 | flaG flagellar protein | 0.00777 |
| CJJ81176_1458 | Hypothetical protein | 0.00786 |
| CJJ81176_0223 | clpP ATP-dependent Clp protease proteolytic subunit | 0.00791 |
| CJJ81176_0295 | SPFH domain-containing protein | 0.00795 |
| CJJ81176_0076 | Hypothetical protein | 0.00800 |
| CJJ81176_0107 | Hypothetical protein | 0.00805 |
| CJJ81176_1421 | Putative glycosyltransferase | 0.00814 |
| CJJ81176_1336 | pseD | 0.00816 |
| CJJ81176_0534 | alaS alanyl-tRNA synthetase | 0.00829 |
| CJJ81176_1519 | Bacterioferritin, putative | 0.00829 |
| CJJ81176_0856 | Hypothetical protein | 0.00839 |
| CJJ81176_0802 | napG quinol dehydrogenase periplasmic component | 0.00865 |
| CJJ81176_0661 | Hypothetical protein | 0.00882 |
| CJJ81176_1431 | Putative sugar transferase | 0.00882 |
| CJJ81176_0202 | Hypothetical protein | 0.00893 |
| CJJ81176_0893 | Cell division protein FtsK, putative | 0.00936 |
| CJJ81176_0110 | Hypothetical protein | 0.00955 |
| CJJ81176_1352 | ceuC enterochelin ABC transporter, permease protein | 0.00955 |
| CJJ81176_1387 | katA catalase | 0.00962 |
| CJJ81176_1530 | Flavodoxin-like fold domain-containing protein | 0.00962 |
| CJJ81176_0024 | Hypothetical protein | 0.00969 |
| CJJ81176_0339 | hisC histidinol-phosphate aminotransferase | 0.00969 |
| CJJ81176_0483 | miaB (dimethylallyl)adenosine tRNA methylthiotransferase | 0.00969 |
| CJJ81176_0210 | Iron ABC transporter, permease protein | 0.00983 |
| CJJ81176_1569 | Peptide ABC transporter, periplasmic peptide-binding protein | 0.00983 |
| CJJ81176_1063 | AraC family transcriptional regulator | 0.00997 |
MATE, multiantimicrobial extrusion protein; OPT, oligopeptide transporter; RND, resistance-nodulation-cell division.
Fitness ratio data were calculated by dividing normalized sequence abundance in output by normalized input abundance. All genes listed are significantly decreased in abundance (P < 0.05).
These results also confirm several other processes required for colonization or survival within the chicken gastrointestinal tract. For example, INSeq mutants of the GGDEF domain-containing protein CbrR were uniformly absent from cecal samples (17). This determinant has previously been shown to be required for resistance of C. jejuni to the bile acid sodium deoxycholate, making it required for survival within the chicken gastrointestinal tract (17). Additionally, insertions in genes required for capsule biosynthesis were absent from cecal samples, including mutations in genes required for capsule transport, kpsC and kpsD (18). Insertions in genes required for stress response—catalase (katA), for example—or energy production and metabolism (such as cytochrome d ubiquinol oxidase subunit II [cydB]) were also absent from cecal samples. Based on transcriptome sequencing (RNA-Seq) analysis, transcripts from genes involved in these processes are increased in abundance during infection of the chicken ceca compared to their abundance in in vitro cultures (19).
In addition to those mutants with decreased abundance in the output pools, insertion mutants of several genes were enriched following passage through the chicken (Table 3). Of these, more than 25% encode hypothetical proteins, making it difficult to interpret their biological significance. Several might be predicted to be at a disadvantage during colonization of the chicken gastrointestinal tract, rather than being enriched, including mutations within trpB, encoding a product required for tryptophan biosynthesis, and waaF, encoding lipopolysaccharide heptosyltransferase, a component of the C. jejuni lipooligosaccharide (LOS). Previous work demonstrated that hyperosmotic stress caused elevated trpB expression, which may be required for osmotic stress resistance and efficient colonization of the chicken gastrointestinal tract (20). Mutation of waaF resulted in a truncated core oligosaccharide, which resulted in increased sensitivity to serum killing and decreased binding to cellular ligands (21). Notwithstanding the presumed effects of mutations in these genes, neither has been previously shown to be required for chicken colonization, underscoring the need to repeat colonization studies with isogenic mutants.
TABLE 3.
Factors exhibiting at least a 3-log increase in abundance from chicken cecal samples relative to the input
| Locus | Annotation/predicted functiona | Fitness ratiob |
|---|---|---|
| CJJ81176_0636 | macB macrolide-specific efflux protein | 137,828.30 |
| CJJ81176_1116 | pyrB aspartate carbamoyltransferase catalytic subunit | 113,410.73 |
| CJJ81176_0372 | trpB tryptophan synthase subunit beta | 87,386.48 |
| CJJ81176_0159 | Hypothetical protein | 47,536.15 |
| CJJ81176_1393 | PurB-2 adenylosuccinate lyase | 43,154.53 |
| CJJ81176_0043 | exsB protein ExsB | 34,172.52 |
| CJJ81176_1608 | Hypothetical protein | 30,149.72 |
| CJJ81176_1344 | Hypothetical protein | 27,510.78 |
| CJJ81176_0041 | Membrane protein, putative, degenerate | 27,237.86 |
| CJJ81176_0015 | leuC isopropylmalate isomerase large subunit | 22,670.49 |
| CJJ81176_0190 | Tetrapyrrole methylase family protein | 15,887.70 |
| CJJ81176_0626 | Hypothetical protein | 6,533.57 |
| CJJ81176_1668 | eno phosphopyruvate hydratase | 6,111.82 |
| CJJ81176_1655 | Thiredoxin-like protein | 5,811.00 |
| CJJ81176_1164 | waaF ADP-heptose LPS heptosyltransferase II | 5,388.21 |
| CJJ81176_1008 | Hypothetical protein | 5,142.55 |
| CJJ81176_0437 | Hypothetical protein | 4,427.12 |
| CJJ81176_1325 | Formyl transferase domain-containing protein | 4,020.13 |
| CJJ81176_0738 | Transthyretin-like protein | 3,743.10 |
| CJJ81176_0863 | psd phosphatidylserine decarboxylase | 3,261.48 |
| CJJ81176_1527 | Hypothetical protein | 3,023.00 |
| CJJ81176_0973 | Peptidyl-arginine deiminase family protein | 2,437.90 |
| CJJ81176_0503 | nusG transcription antitermination protein | 2,430.53 |
| CJJ81176_0402 | Hypothetical protein | 1,963.88 |
| CJJ81176_0363 | ydfJ inner membrane metabolite transport protein | 1,635.86 |
| CJJ81176_1552 | nuoM NADH-quinone oxidoreductase, M subunit | 1,633.78 |
| CJJ81176_1553 | nuoL NADH dehydrogenase subunit L | 1,633.78 |
| CJJ81176_0588 | Hypothetical protein | 1,313.33 |
| CJJ81176_1447 | yliG putative tRNA modifying protein | 1,309.45 |
| CJJ81176_1188 | SMR family multidrug efflux pump | 1,244.10 |
| CJJ81176_0815 | Hypothetical protein | 1,242.03 |
| CJJ81176_0072 | Hypothetical protein | 1,216.87 |
| CJJ81176_1270 | Hypothetical protein | 1,142.36 |
| CJJ81176_1521 | galU UTP-glucose-1-phosphate uridylyltransferase | 1,119.73 |
| CJJ81176_1311 | Aminotransferase | 1,098.35 |
| CJJ81176_0163 | Inositol monophosphatase family protein | 1,032.00 |
| CJJ81176_1700 | rpsS 30S ribosomal protein S19 | 1,016.91 |
| CJJ81176_0576 | efp elongation factor P | 1,012.63 |
LPS, lipopolysaccharide; SMR, small multidrug resistance.
Fitness ratio data were calculated by dividing normalized sequence abundance in output by normalized input abundance
A C. jejuni mapA mutant exhibits decreased colonization of the chicken gastrointestinal tract.
We chose one representative locus, mapA, which exhibited a >3-log decrease in its fitness ratio, for further analysis. It encodes a species-specific outer membrane lipoprotein that is immunogenic in both chickens and humans but has not been previously shown to play a role in colonization of the chicken gastrointestinal tract (8, 9, 22). We reconstructed a mapA mutation using allelic exchange mutagenesis and carried out two separate colonization studies. In one, a mapA mutant was introduced at a 1:1 ratio with wild-type C. jejuni DRH212 (4.1 × 104 and 5.3 × 104, respectively) into day-of-hatch white leghorn chicks by oral gavage, and the mixture was allowed to colonize for 7 days before bacterial loads from the ceca were determined. In these infections, the mapA mutant was at a significant competitive disadvantage, with a median competitive index of 2.2 × 10−3 (P value < 0.0001) (Fig. 5A). We also carried out an infection with the mapA mutant alone (i.e., not in competition with the wild type). For this, the mutant and wild-type DRH212 strains were individually introduced into day-of-hatch chicks. In these infections, the mapA mutant was recovered with a median colonization level of 5.3 × 108 CFU/gram of cecal contents, while DRH212 colonized at a median of 1.3 × 109 CFU/gram of cecal contents, a decrease of approximately 0.5-log (P value = 0.03) (Fig. 5B). Thus, while it is required for competing against wild-type C. jejuni in the chicken gastrointestinal tract, the mapA gene product is less critical during monoinfections. Further, the difference in colonization proficiency following assays of competitive infection and monoinfection demonstrated the value of genome-wide approaches such as INSeq because protocols focusing on monoinfection phenotypes likely would have been unable to identify MapA as a fitness determinant.
FIG 5.
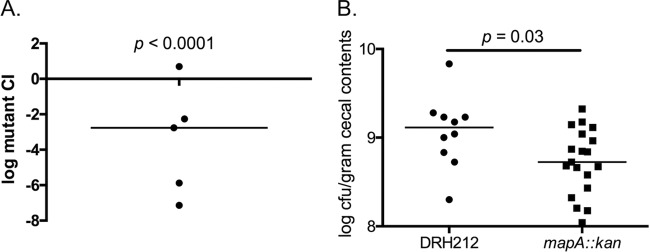
Cecal colonization of a mapA mutant. (A) Competitive index (CI) calculated by determining ratio of wild-type C. jejuni to mapA mutant in cecal samples from chicks infected with equivalent amounts of each strain. Significance was determined using a Wilcoxon signed-rank test (P < 0.0001). (B) Colonization loads in the cecum following monoinfections with either wild-type C. jejuni or the mapA mutant. Significance was determined using a Mann-Whitney test (P = 0.03).
To examine whether the competitive colonization defect of the mapA mutant is due to a general growth defect, growth curves were determined for individually and competitively grown wild-type DRH212 and mapA mutant strains (Fig. 6A and B, respectively). During competitive growth with wild-type C. jejuni, the mapA mutant exhibited decreased growth potential, with a peak concentration of 5 × 108 CFU/ml at 20 h of growth compared to 4.9 × 109 CFU/ml for the wild type (Fig. 6B). Also, the mapA mutant exhibited a more rapid decrease in viability as cultures continued into stationary phase. The mapA mutant did not exhibit any growth defect as a monoculture, consistent with the observation that mapA is more critical during competition with the wild-type strain in the chicken gastrointestinal tract than in monoinfections. We tried without success to complement this phenotype, as a plasmid encoding mapA appears to alter the viability of C. jejuni (data not shown); this may be due to gene dosage effects. We examined transcript levels of downstream genes in both wild-type and mapA mutant backgrounds and observed that transcription was not significantly affected by the insertion mutation (data not shown).
FIG 6.
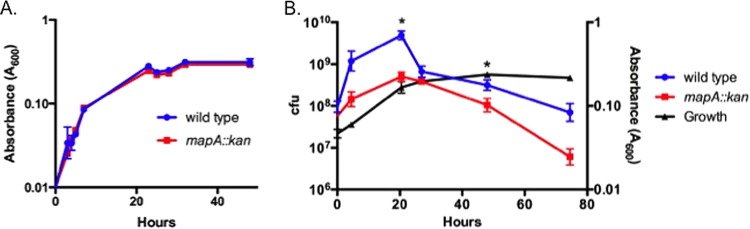
Growth phenotypes of the mapA mutant. (A) Growth curves for wild-type C. jejuni and the mapA mutant in monocultures. (B) Competitive growth assay of wild-type C. jejuni and the mapA mutant. CFU counts for each strain are plotted against the growth curve for the combined culture. Student's t test was used to determine significance (P < 0.05).
As motility and capsule production have previously been shown to be required for colonization of the chicken gastrointestinal tract, the ability of the mapA mutant to swim and produce capsule was determined (6, 18). The mutant exhibited motility in semisolid agar similar to that of the wild type (see Fig. S1A and B in the supplemental material). Additionally, the mapA mutant produced capsule at levels similar to wild-type levels, and both strains produced much more than a strain lacking KpsM, encoding a capsular polysaccharide transporter (see Fig. S1C).
Analysis of MapA amino acid sequence using the protein structure and activity predictor I-TASSER provided few clues about its function, although weak homologies to various zinc-containing endopeptidases, e.g., endothelin-converting enzyme I, were detected (23, 24). Thus, it is possible that MapA functions as an endopeptidase, but it is currently unknown what the consequence of this activity might be for in vivo or in vitro competition. Whatever its precise role in chicken colonization, mapA sequences are highly conserved among several sequenced strains of C. jejuni, most with different origins. Despite the relatively high rate of genomic sequence variation in C. jejuni strains, mapA typically contains few nucleotide changes in the sequences of 100 strains, representing greater than 97% identity. This may indicate that MapA serves an important role in C. jejuni, preventing significant variation in the coding sequences.
This study demonstrated the value of deep sequencing analysis with transposon mutagenesis for identifying fitness determinants encoded by C. jejuni. Of the 130 mutations with relative abundances of at least 0.01, 37 are in genes annotated as hypothetical proteins, though some hypothetical determinants have been shown to encode colonization factors. These include Cjj81176_0083 and Cjj81176_0414, which encode a new class of flagellar coexpressed determinants (Feds) required for full colonization of the chicken gastrointestinal tract (25). Thus, approximately 28% of those genes identified as required for colonization of the chicken gastrointestinal tract exhibit no significant primary homology to known protein domains, signaling that an immense amount of information still remains to be uncovered about C. jejuni colonization factors.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Allergy and Infectious Diseases to V.J.D. (R01 AI069383) and J.G.J. (T32 AI007528) as well as awards for J.G.J. from the U.S. Department of Agriculture's National Institute for Food and Agriculture (awards 2010-65201-20594 and 2013-67012-21136).
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272200900018C.
We thank Patricia Guerry for kindly providing the C. jejuni 81-176 kpsM::kan mutant.
Footnotes
Published ahead of print 14 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01395-13.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). 2011. Vital signs: incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 1996–2010. MMWR Morb. Mortal. Wkly. Rep. 60:749–755 [PubMed] [Google Scholar]
- 2.Hoffmann S, Batz MB, Morris JG., Jr 2012. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J. Food Prot. 75:1292–1302. 10.4315/0362-028X.JFP-11-417 [DOI] [PubMed] [Google Scholar]
- 3.Hale CR, Scallan E, Cronquist AB, Dunn J, Smith K, Robinson T, Lathrop S, Tobin-D'Angelo M, Clogher P. 2012. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin. Infect. Dis. 54(Suppl 5):S472–S479. 10.1093/cid/cis051 [DOI] [PubMed] [Google Scholar]
- 4.Hara-Kudo Y, Takatori K. 2011. Contamination level and ingestion dose of foodborne pathogens associated with infections. Epidemiol. Infect. 139:1505–1510. 10.1017/S095026881000292X [DOI] [PubMed] [Google Scholar]
- 5.Hermans D, Van Deun K, Martel A, Van Immerseel F, Messens W, Heyndrickx M, Haesebrouck F, Pasmans F. 2011. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 42:82. 10.1186/1297-9716-42-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendrixson DR, DiRita VJ. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52:471–484. 10.1111/j.1365-2958.2004.03988.x [DOI] [PubMed] [Google Scholar]
- 7.Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, Knight R, Gordon JI. 2009. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6:279–289. 10.1016/j.chom.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoaf-Sweeney KD, Larson CL, Tang X, Konkel ME. 2008. Identification of Campylobacter jejuni proteins recognized by maternal antibodies of chickens. Appl. Environ. Microbiol. 74:6867–6875. 10.1128/AEM.01097-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnens A, Stucki U, Nicolet J, Frey J. 1995. Identification and characterization of an immunogenic outer membrane protein of Campylobacter jejuni. J. Clin. Microbiol. 33:2826–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendrixson DR, Akerley BJ, DiRita VJ. 2001. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol. Microbiol. 40:214–224. 10.1046/j.1365-2958.2001.02376.x [DOI] [PubMed] [Google Scholar]
- 11.Wiesner RS, Hendrixson DR, DiRita VJ. 2003. Natural transformation of Campylobacter jejuni requires components of a type II secretion system. J. Bacteriol. 185:5408–5418. 10.1128/JB.185.18.5408-5418.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman AL, Wu M, Gordon JI. 2011. Identifying microbial fitness determinants by insertion sequencing using genome-wide transposon mutant libraries. Nat. Protoc. 6:1969–1980. 10.1038/nprot.2011.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerome JP, Bell JA, Plovanich-Jones AE, Barrick JE, Brown CT, Mansfield LS. 2011. Standing genetic variation in contingency loci drives the rapid adaptation of Campylobacter jejuni to a novel host. PLoS One 6:e16399. 10.1371/journal.pone.0016399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40:769–777. 10.1046/j.1365-2958.2001.02431.x [DOI] [PubMed] [Google Scholar]
- 16.Karlyshev AV, Wren BW. 2001. Detection and initial characterization of novel capsular polysaccharide among diverse Campylobacter jejuni strains using alcian blue dye. J. Clin. Microbiol. 39:279–284. 10.1128/JCM.39.1.279-284.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raphael BH, Pereira S, Flom GA, Zhang Q, Ketley JM, Konkel ME. 2005. The Campylobacter jejuni response regulator, CbrR, modulates sodium deoxycholate resistance and chicken colonization. J. Bacteriol. 187:3662–3670. 10.1128/JB.187.11.3662-3670.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones MA, Marston KL, Woodall CA, Maskell DJ, Linton D, Karlyshev AV, Dorrell N, Wren BW, Barrow PA. 2004. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect. Immun. 72:3769–3776. 10.1128/IAI.72.7.3769-3776.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taveirne ME, Theriot CM, Livny J, Dirita VJ. 2013. The complete Campylobacter jejuni transcriptome during colonization of a natural host determined by RNAseq. PLoS One 8:e73586. 10.1371/journal.pone.0073586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron A, Frirdich E, Huynh S, Parker CT, Gaynor EC. 2012. Hyperosmotic stress response of Campylobacter jejuni. J. Bacteriol. 194:6116–6130. 10.1128/JB.01409-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldfield NJ, Moran AP, Millar LA, Prendergast MM, Ketley JM. 2002. Characterization of the Campylobacter jejuni heptosyltransferase II gene, waaF, provides genetic evidence that extracellular polysaccharide is lipid A core independent. J. Bacteriol. 184:2100–2107. 10.1128/JB.184.8.2100-2107.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stucki U, Frey J, Nicolet J, Burnens AP. 1995. Identification of Campylobacter jejuni on the basis of a species-specific gene that encodes a membrane protein. J. Clin. Microbiol. 33:855–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy A, Kucukural A, Zhang Y. 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5:725–738. 10.1038/nprot.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y. 2008. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9:40. 10.1186/1471-2105-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrero-Tobon AM, Hendrixson DR. 2012. Identification and analysis of flagellar coexpressed determinants (Feds) of Campylobacter jejuni involved in colonization. Mol. Microbiol. 84:352–369. 10.1111/j.1365-2958.2012.08027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao R, Alm RA, Trust TJ, Guerry P. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127–130. 10.1016/0378-1119(93)90355-7 [DOI] [PubMed] [Google Scholar]
- 27.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res. 19:1639–1645. 10.1101/gr.092759.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.