Abstract
Horizontal gene transfer plays a crucial role in microbial evolution. While much is known about the mechanisms that determine whether physical DNA can be transferred into a new host, the factors determining the utility of the transferred genes are less clear. We have explored this issue using dichloromethane consumption in Methylobacterium strains. Methylobacterium extorquens DM4 expresses a dichloromethane dehalogenase (DcmA) that has been acquired through horizontal gene transfer and allows the strain to grow on dichloromethane as the sole carbon and energy source. We transferred the dcmA gene into six Methylobacterium strains that include both close and distant evolutionary relatives. The transconjugants varied in their ability to grow on dichloromethane, but their fitness on dichloromethane did not correlate with the phylogeny of the parental strains or with any single tested physiological factor. This work highlights an important limiting factor in horizontal gene transfer, namely, the capacity of the recipient strain to accommodate the stress and metabolic disruption resulting from the acquisition of a new enzyme or pathway. Understanding these limitations may help to rationalize historical examples of horizontal transfer and aid deliberate genetic transfers in biotechnology for metabolic engineering.
INTRODUCTION
The recent accumulation of genome sequences from diverse bacterial clades has demonstrated the crucial role of horizontal gene transfer (HGT) in bacterial evolution (1, 2). It has become clear that genes and operons have consistently moved between distant bacterial strains, with important implications for bacterial evolution, physiology, and ecology (3). In appreciating the significant impact of HGT, it is important to also consider the factors that limit transfer (4). If a gene is beneficial when acquired by one strain, why do we not observe that gene transferring into other, closely related strains?
One possible explanation for the rarity of successful horizontal transfers is that recipients that would benefit from the transfer have little opportunity to acquire the corresponding DNA (5), and that factors such as ecological differentiation and barriers to genetic exchange may prevent a strain from encountering a potential donor (4, 6). Additionally, transfer events may be rare even in the presence of a donor (7), since the likelihood of stably integrating and expressing newly acquired DNA is predicted to decrease with increasing genetic distance and will limit the frequency and breadth of transfer (8–10). These factors suggest that ecology and phylogeny should largely determine transfer frequencies, based on how likely a strain is to encounter a donor (ecology) and to acquire, stably integrate, and express the transferred DNA (phylogeny) (11).
Another significant, yet poorly investigated, barrier to HGT depends on how efficiently a recipient can use its new ability. A newly acquired gene or pathway may place novel stresses on the host, either by disrupting existing metabolic and regulatory networks (12, 13) or by producing new toxic metabolites (13, 14). The fitness cost of such stresses is determined by the host physiology. A beneficial ability with costly side effects will preferentially spread to those recipients best able to accommodate its associated stresses, leading to a gene distribution shaped by physiology rather than by phylogeny.
We used dichloromethane catabolism in strains of Methylobacterium to explore factors that limit the functional incorporation of a horizontally transferred gene. Dichloromethane (DCM) is an industrial solvent that has reached significant concentrations in the environment only in the last 50 years. Among several strains isolated for their ability to grow on DCM as the sole carbon and energy source (15, 16), a strain of Methylobacterium extorquens known as DM4 has been investigated in the most detail. Through HGT, this strain has acquired a gene, dcmA, encoding a cytoplasmic glutathione S-transferase that converts DCM to formaldehyde, with the concomitant release of two molecules of hydrochloric acid (17, 18). In M. extorquens DM4, the dcmA gene lies within a 126-kb genomic island that shows clear evidence of horizontal transfer, including a lower GC content (19). Within this genomic island, the dcm cluster contains three other genes, including the transcriptional regulator dcmR and two proteins of unknown function dcmB and dcmC and is flanked on both sides by IS1354 elements. This four-gene dcm islet is conserved within most DCM-degrading strains (18). However, a strain of M. extorquens DM4 with a deletion of the genomic island, known as DM4-2cr (20), requires only the dcmA gene to recover growth on DCM (21). While the other genes in the genomic island may influence growth on DCM, they are not essential.
Growth with DCM is very challenging for the cell (Fig. 1). First, the strain must accommodate the protons and chloride produced intracellularly as a by-product of DCM dehalogenation (22–24). Additionally, the S-chloromethylglutathione intermediate formed during the dehalogenation reaction is highly reactive and mutagenic (25–27). Finally, the cell must quickly channel the formaldehyde product into its native one-carbon metabolic pathways to minimize any resulting toxicity (28). Given these challenges, it is not surprising that deliberate transfer of the dcmA gene to two other strains of M. extorquens, AM1 and CM4, was sufficient to enable growth on DCM only in strain CM4 (21). However, it remains unclear why strain AM1 was unable to grow on DCM, or how prevalent is the ability among methylotrophic bacteria to grow on DCM using dcmA. We have addressed these questions by transferring dcmA into a broad range of Methylobacterium strains, quantifying their success at using their new catabolic potential, and investigating the factors that influence this success.
FIG 1.

Growth on DCM presents several challenges to the host. One molecule of DCM is converted to one molecule of formaldehyde and two molecules of HCl. Stress-inducing compounds are indicated in red. In addition to the stresses resulting from HCl and formaldehyde, the glutathione conjugate intermediate is highly mutagenic.
MATERIALS AND METHODS
Media and chemicals.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Escherichia coli was grown in LB at 37°C with various antibiotic concentrations. Methylobacterium strains were grown at 30°C in liquid culture in M-PIPES (29) supplemented with 3.5 mM succinate or 5 mM DCM as noted. Antibiotics were added to a final concentration of 12.5 μg/ml for tetracycline, 10 μg/ml for streptomycin, or 50 μg/ml for kanamycin. Unless otherwise noted, DCM cultures were grown in 10 ml of medium in gas-tight 50-ml screw-top flasks sealed with Teflon tape and Mininert valves (Supelco, Bellefonte, PA). Valves were surface sterilized with ethanol and dried in a laminar flow hood before use. A freshly prepared 100 mM stock of DCM in water was used to inoculate the flasks.
Plasmid construction.
Plasmids and strains used in this study are listed in Tables 1 and 2. Plasmid pJM10 was constructed by cloning the AatII-SacI fragment containing kanR2 from pCM184 (30) into pME8220 (21) digested with AatII-SacI to remove the 5′ end of tetA. Plasmid pJM40 was constructed by amplifying superecliptic pHluorin (31) and using Gibson assembly to clone it into pHC08 (32) as a translational fusion to mCherry, yielding pJM25. The entire mCherry-pHluorin expression cassette was amplified from pJM25 by PCR to add BamHI and SacI restriction sites. The digested PCR fragment was then cloned into pME8220 and pCM62 to yield pJM40 and pJM41, respectively. Plasmid pJM53 was constructed by amplifying 500-bp regions upstream and downstream of the hypoxanthine phosphoribosyl transferase (hpt) from the genome of M. extorquens DM4. Using Gibson assembly, these fragments were combined with a Venus expression cassette in pPS04 (P. Swanson and C. J. Marx, unpublished results), a kanamycin resistance derivative of pCM433 (33).
TABLE 1.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pCM433 | oriTRP4 cat bla tetA sacB | 33 |
| pJM10 | dcmA kanR2 | This work |
| pJM40 | PTac-mCherry-pHluorin dcmA tetA | This work |
| pJM41 | PTac-mCherry-pHluorin tetA | This work |
| pJM53 | pPS04 hpt::Venus | This work |
| pPS04 | kanR2 replaces cat, bla, and tetA of pCM433 | P. Swanson and C. J. Marx, unpublished data |
TABLE 2.
Strains used in this study
| Designation | Strain | Genotype | Reference |
|---|---|---|---|
| AM1 | CM3120 | M. extorquens AM1 Δcel katA::mCherry | L. Chubiz and C. J. Marx, unpublished data |
| PA1 | CM3839 | M. extorquens PA1 Δcel hpt::mCherry | D. Nayak and C. J. Marx, unpublished data |
| DM4 ΔdcmA | CM4250 | M. extorquens DM4 ΔdcmA hpt::Venus | This work |
| DM4-2cr | DM4-2cr | M. extorquens DM4 with a deletion spanning dcmABC | 20 |
| CM4 | CM4 | M. extorquens CM4 | 45 |
| BJ001 | BJ001 | M. extorquens BJ001 | 41 |
| M. nodulans | ORS 2060 | M. nodulans ORS 2060 | 42 |
| M. radiotolerans | JCM 2831 | M. radiotolerans JCM 2831 | 43 |
Plasmid matings.
Plasmids were transferred to recipient Methylobacterium strains using triparental matings as described previously (34). The dcmA gene was deleted from M. extorquens DM4 using a derivative of pCM433, which resulted in a mutant unable to grow on DCM (F. Bringel and S. Vuilleumier, unpublished results). M. extorquens DM4 ΔdcmA-Venus (strain CM4250) was constructed from M. extorquens DM4 ΔdcmA using pJM53 and following established protocols (33). Integration of Venus into the hpt locus of M. extorquens DM4 ΔdcmA was selectively neutral during growth on DCM.
Growth rate measurements.
Methylobacterium strains were grown to saturation in 48-well plates under each of the conditions being tested. Cultures were then diluted 64× into 640 μl of fresh medium. Over 48 h, optical densities at 600 nm (OD600) were measured every 30 to 45 min using an automated system (29). Growth rates were calculated using CurveFitter (29).
Competitive fitness assays.
Competitive fitness for growth on DCM was measured by competing each strain against M. extorquens DM4 ΔdcmA-Venus(pJM10) generally following a previous protocol (32). In brief, each strain was grown to saturation in M-PIPES–succinate–kanamycin and then diluted 100× into M-PIPES–DCM. The exception was M. extorquens AM1 Δcel-mCherry(pJM10), which cannot grow on DCM alone. This strain was grown in M-PIPES–DCM–tetracycline, where the tetracycline is dissolved in 80% ethanol and therefore provides 14 mM ethanol for growth. After 3 days, the cultures were diluted and mixed in fresh M-PIPES–DCM. M. extorquens DM4 ΔdcmA-Venus(pJM10) was added to each flask at an OD of 0.001. The test strain was then added at an OD of 0.005. A 450-μl portion of each culture was removed, mixed with 50 μl of dimethyl sulfoxide (DMSO), and frozen at −80°C. The mixed cultures were then grown for 3 days.
At the end of the growth phase, the population ratios of the samples, before and after growth on DCM, were determined using flow cytometry. Postgrowth samples were diluted into fresh M-PIPES to a final OD of ∼0.015. Fluorescence was measured on an LSRII flow cytometer (BD, Franklin Lakes, NJ). Venus was excited at 488 nm and measured at 530 nm with a 30-nm bandpass filter. When available, mCherry was excited at 561 nm and measured at 620/40 nm. The competitive fitness was calculated as log[(R1*N)/R0]/log[(1 − R1)*N/(1 − R0)], where R0 and R1 represent the population fraction of the test strain before and after mixed growth and N represents the fold increase in the population density. When the test strain was labeled with mCherry, the population fraction was calculated as the ratio of mCherry-positive cells to Venus-positive cells. When the test strain was unlabeled, the population fraction was the ratio of Venus-negative cells to Venus-positive cells.
Chloride measurements.
Chloride released by dehalogenation was measured using the method of Jörg and Bertau (35), comparing the absorbance against that obtained with M-PIPES without added carbon.
Total protein measurements.
Total protein was measured by growing the desired culture in 10 ml of M-PIPES–DCM for 3 days. The cultures were concentrated by centrifugation and resuspended in 1 ml of PBS plus 1 mM EDTA. Cells were lysed using a FastPrep-24 (MP Bio, Santa Ana, CA) and lysing matrix B (MP Bio). Lysates were centrifuged, and total protein in the supernatant was quantified using the Bradford quick-start assay (Bio-Rad, Hercules, CA) with bovine serum albumin (BSA) as a standard.
Dehalogenase activity measurements in whole cells.
Strains were grown at 30°C in 50 ml of M-PIPES in 300-ml screw-top Erlenmeyer flasks fitted with Mininert stoppers (Supelco), using 5 mM DCM with or without the addition of 3.5 mM succinate as the carbon and energy source. Cultures were harvested in exponential phase after measurement of their ODs and centrifuged for 15 min at 8,000 rpm. The final pH of the cultures was measured in the spent medium supernatant. The cell pellets were resuspended in 2 ml of M-PIPES, and the protein concentration in cell suspensions was determined by a commercial bicinchoninic acid protein assay kit (Sigma) adapted to microplate format (0 to 10 μg protein, using BSA as a reference). Chloride concentration in the final spent medium was determined as described above, adapting the assay to microplate format. DCM dehalogenase activity was determined by the Nash method as described previously (36), with minor modifications. Briefly, concentrated cell suspensions of the different strains (200 to 500 μl in M-PIPES) were incubated at 30°C in a total volume of 1 ml M-PIPES containing 7.5 mM potassium sulfite, 2 mM reduced glutathione, and 20 mM DCM added last to the mixture from a 100 mM stock in M-PIPES. Aliquots (60 μl) were taken at different times, added to 540 μl Nash reagent (4 ml 30% [wt/vol] ammonium acetate with 0.4% acetylacetone, 1 ml 1% [wt/vol] iodine in acetone, 10 ml H2O), and incubated for 30 min at 65°C, and absorbance was measured at 412 nm. Activity was expressed as μmol formaldehyde produced per minute per mg protein, using an ε412 value of 7,812 M−1 cm−1 for the produced dimethyldihydropyridine derivative.
Internal pH measurements.
Strains were grown to saturation in M-PIPES–succinate–tetracycline, diluted 100× in M-PIPES–DCM, and grown for 3 days. Strain AM1 Δcel(pJM40) was the exception, as it cannot grow on DCM alone. Consequently, this strain was grown in M-PIPES–DCM–tetracycline, where the tetracycline was dissolved in 80% ethanol and provided 14 mM ethanol. A negative control, M. extorquens DM4 ΔdcmA(pJM41), was inoculated into M-PIPES–succinate–tetracycline and grown to saturation overnight.
Cultures were diluted to 2 ml of fresh M-PIPES at an OD of 0.015. Fluorescence was measured on an LSRII flow cytometer (BD). pHluorin was excited at 488 nm and measured at 530/30 nm. mCherry was excited at 561 nm and measured at 620/40 nm. Cultures were gated on forward and side scatter to isolate cells and then gated to remove events with low mCherry (pH-independent) fluorescence.
For each sample, a time zero measurement was made by collecting 50,000 cells. A 100-μl portion of a 100 mM DCM stock was added to the tube and briefly vortexed, and the tube was put back on the flow cytometer for continuous sampling. Approximately 20 s elapsed between the addition of DCM and stable measurements of the population fluorescence.
To calibrate the pH biosensor, cultures were diluted into a solution containing 20 mM buffer, 50 mM NaCl, 3 mM KCl, 10 μM valinomycin, and 10 μM nigericin. Buffers used for calibration were MES at pH 5.1, 5.3, 5.5, 5.7, and 5.9 and PIPES at pH 6.1, 6.3, 6.5, 6.7, 6.9, 7.1, and 7.3. Three technical replicates were performed for each combination of strain and pH. Four calibrations were performed for each experiment, using M. extorquens DM4-2cr(pJM40) (20), M. extorquens DM4 ΔdcmA(pJM41), M. radiotolerans(pJM40), and M. nodulans(pJM40).
Internal pH values were calculated by comparison to the calibration curve. The ratio of green (pH-dependent) to red (pH-independent) fluorescence was calculated for each cell. For the calibration and time zero samples, the population geometric mean was calculated for this ratio. For the time course samples, the ratio and timestamp of each cell were exported as comma-separated values and imported into Matlab. Further processing in Matlab using a custom script removed outliers, divided the cells into 5-s bins, calculated the geometric mean of the fluorescence ratio for each bin, and converted that mean fluorescence into a measured pH by comparison to the appropriate calibration curve.
Phylogenetic analyses.
Phylogenetic trees using 16S rRNA gene sequences were constructed in the Ribosomal Database Project (37). Phylogenetic trees based on 400 conserved proteins were constructed with PhyloPhlAn (38).
RESULTS
To determine the prevalence of the capacity to grow on DCM, we constitutively expressed dcmA from its native promoter on a broad-host-range plasmid (21). The plasmid was transferred by conjugation into seven Methylobacterium strains, including five that belong to the same species, M. extorquens (Fig. 2; also, see Fig. S1 in the supplemental material). As recipients, we tested the previously studied strains M. extorquens AM1, CM4, and DM4 (21) as well as the recently sequenced strains M. extorquens PA1 (39, 40), M. extorquens BJ001 (formerly M. populi) (40, 41), M. nodulans (42), and M. radiotolerans (43). To aid in accurate growth rate and fitness measurements, some recipients were modified by genomic insertions of fluorescent markers and gene disruption to prevent cell clumping, as previously described for strain M. extorquens AM1. The strains AM1 Δcel-mCherry (CM3120, referred to here as AM1) and PA1 Δcel-mCherry (CM3839, referred to here as PA1) lack the cellulose biosynthetic cluster (30) and express a red fluorescent protein (32), and DM4 ΔdcmA-Venus (CM4250, referred to here as DM4 ΔdcmA) lacks the chromosomal dcmA gene and expresses a yellow fluorescent protein.
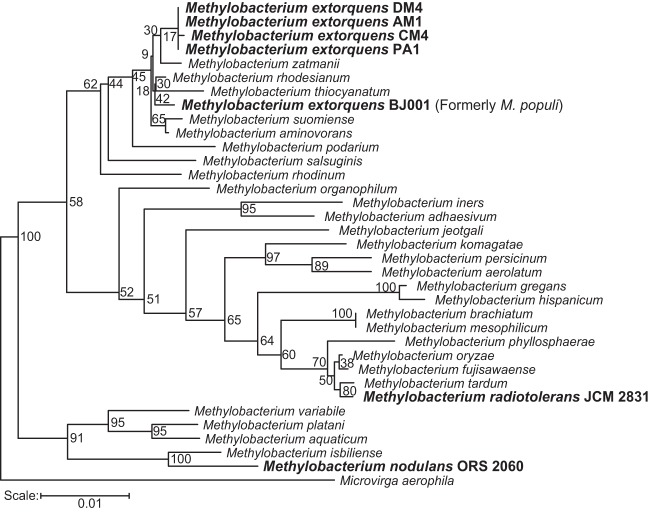
FIG 2.
The DCM degradation pathway can be transferred to diverse Methylobacterium species. Strains used in this work are in bold. The phylogenetic tree was built using the Ribosomal Database Project (37), based on 16S rRNA genes.
Six of the strains, with the notable exception of AM1, were able to use pJM10 to grow on DCM as the sole carbon and energy source (Fig. 3). Four of the strains grew planktonically and showed a linear relationship between the final optical density and the amount of chloride released, indicating that chloride generation is linked to cell growth to the same extent across these strains. The remaining three strains, M. extorquens CM4(pJM10), M. extorquens BJ001(pJM10), and M. radiotolerans(pJM10), grew as clumpy particulates, rendering measurements of optical density impractical. When we grew these strains on DCM, lysed the cells, and measured total protein, we observed a linear relationship between total protein and chloride released (see Fig. S2 in the supplemental material).
FIG 3.
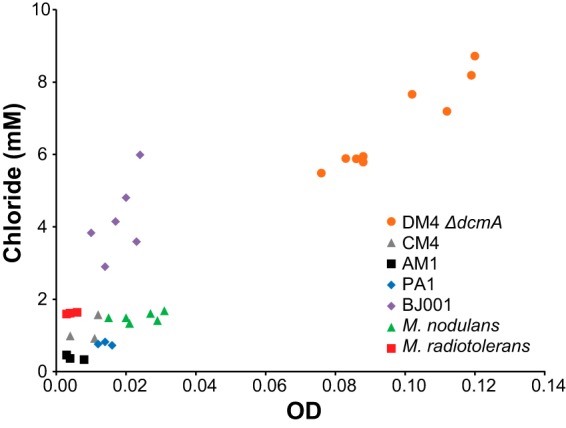
Transconjugant Methylobacterium strains vary in their ability to grow on DCM as the sole carbon and energy source. All strains contain the pJM10 plasmid expressing DcmA. Cultures were grown in sealed flasks with M-PIPES medium containing 5 mM DCM. After 3 days' growth, the optical density and supernatant chloride concentrations were measured using a spectrophotometer. Each point represents a biological replicate. The experiment was repeated at least three times for each strain, with results similar to those shown.
We next asked whether these differences in yield would translate into differences in competitive fitness. Most strains that grew poorly on DCM in pure culture were also less fit in competition (Fig. 4). However, M. radiotolerans(pJM10) and M. extorquens BJ001(pJM10) were more and less fit, respectively, than their individual yields would predict (see Fig. S3A in the supplemental material). The phylogenetic distance between a test strain and M. extorquens DM4 did not predict the competitive fitness during growth on DCM (see Fig. S3B in the supplemental material).
FIG 4.
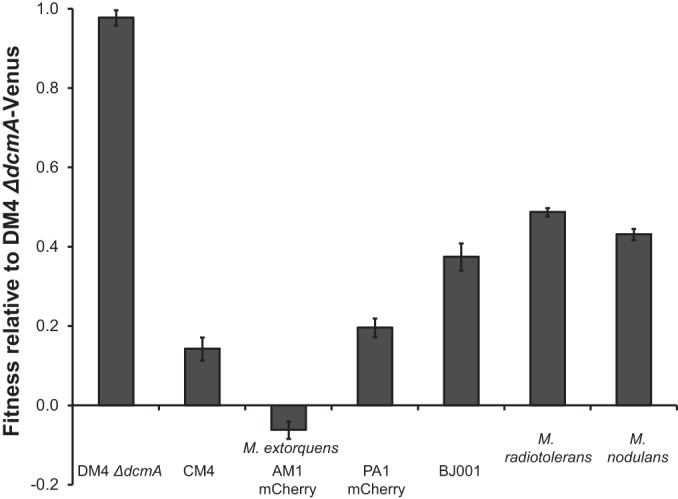
Competitive fitness of transconjugant Methylobacterium strains containing pJM10. Fitness is measured relative to DM4 ΔdcmA-Venus. Cultures were grown in pure culture using DCM as the sole carbon and energy source. The strains were then individually competed against DM4 ΔdcmA-Venus. Error bars show one standard deviation, calculated from three biological replicates. Each competition was repeated at least three times, with results similar to those shown here.
Given the observed variation in competitive fitness on DCM, we sought to identify physiological traits that differed between the transconjugants and correlated with fitness. We first tested whether the strains expressed functional DcmA, as this enzyme is essential for growth on DCM. All transconjugants expressed active DcmA (see Fig. S4 in the supplemental material), in agreement with previous results (21). However, AM1(pJM10) showed significantly lower dehalogenase activity than the other transconjugants. Next we compared the ability of the transconjugants to respond to the predicted stresses of hydrochloric acid and formaldehyde production. None of the transconjugant strains was able to grow on 2 mM formaldehyde as the sole carbon and energy source (data not shown). For comparison, despite being unable to grow on DCM, AM1 can grow on 0.5 mM formaldehyde (data not shown). We next used an automated system to measure growth rates under various medium conditions (29). Somewhat unexpectedly, DM4 ΔdcmA(pJM10) was the most sensitive to growth in high external chloride or low external pH (Fig. 5). Altogether, the measured growth rates at high external chloride or low pH did not correlate to competitive fitness on DCM (see Fig. S5 in the supplemental material).
FIG 5.
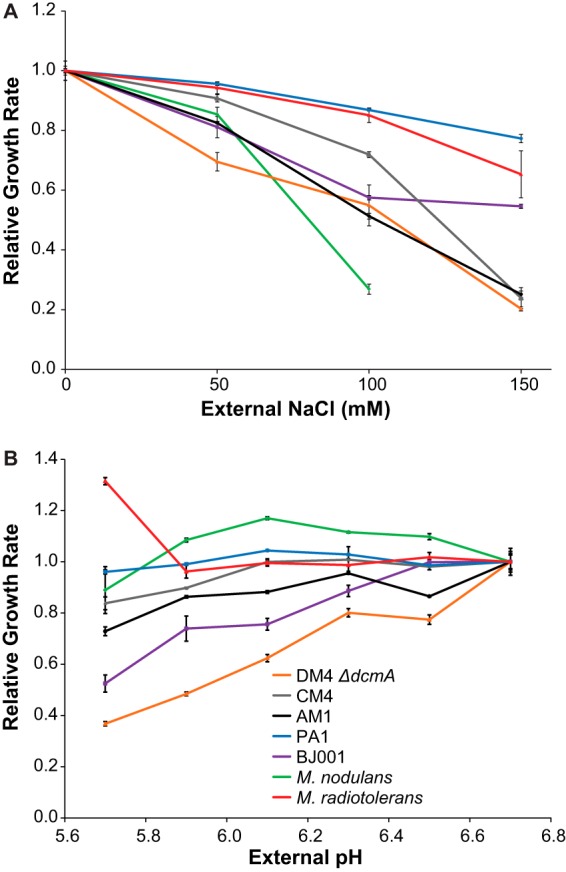
Transconjugant Methylobacterium strains containing pJM10 vary in their sensitivity to high external concentrations of NaCl (A) and to low external pH (B). Cultures were grown in M-PIPES containing succinate as the carbon and energy source. Growth rates were measured in 48-well plates using an automated system. M. nodulans grows as a biofilm at 150 mM NaCl, making growth rates difficult to measure. Error bars show one standard deviation, calculated from three biological replicates. The experiment was repeated three times, with similar results.
Adding hydrochloric acid to the growth medium does not exactly reflect the stress associated with intracellular production of hydrochloric acid upon DCM dehalogenation. We therefore sought to measure how the transconjugant strains responded to the dehalogenation of DCM. We used a fluorescent biosensor to measure internal pH on a rapid, approximately 5-s time scale (44). When this biosensor was coexpressed with DcmA, we could measure the change in internal pH upon addition of DCM (Fig. 6; also, see Fig. S6 in the supplemental material). We found only a weak correlation between smaller transient decreases in intracellular pH and increased fitness of the strain on DCM (Fig. S7 in the supplemental material).
FIG 6.
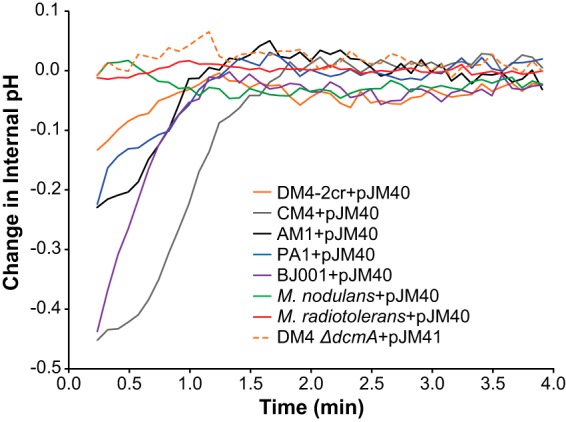
Internal pH decreases transiently upon addition of DCM. pJM40 expresses both the pH biosensor and DcmA. pJM41 contains only the biosensor and serves as a negative control. Internal pH upon addition of 5 mM DCM was measured in cell suspensions of dcmA-containing transconjugants using a pH-sensitive GFP translationally fused to a pH-insensitive mCherry (see Materials and Methods; also, see Fig. S5 in the supplemental material) (dead time before reliable fluorescence measurements, approximately 20 s).
DISCUSSION
DCM is a very challenging substrate, as its catabolism imposes multiple types of stress on the host cell: protons and chloride ions need to be extruded from the cell interior; the reactive intermediate of DCM dehalogenation, S-chloromethylglutathione, is mutagenic; and formaldehyde, the product of DCM dehalogenation, must be efficiently transformed to minimize its toxic effects. The combination of these stresses may explain why transferring dcmA into naive Methylobacterium strains allowed only poor growth on DCM. In contrast to previous work, however, we found that the ability to use DcmA to grow on DCM was widespread and that only M. extorquens AM1 remained unable to grow on DCM after dcmA transfer. Most evidently, phylogeny was a poor predictor of a strain's ability to exploit the dehalogenase DcmA and grow on DCM under the investigated conditions. M. extorquens strains AM1 and CM4 have 16S rRNA gene sequences identical to those of the natural isolate M. extorquens DM4 but are the least successful at growing on DCM. Meanwhile, the organisms most distantly related to M. extorquens DM4, M. nodulans and M. radiotolerans, are among the most successful.
In this study, we were able to set aside many of the factors predicted to limit productive HGT in nature. We know that the necessary dcmA gene was introduced into the new host, can stably replicate, and is functionally expressed. Despite these facts, all of the transconjugants are much less fit that the original donor, M. extorquens DM4, and some strains show little or no growth. Other factors clearly limit the growth of these transconjugants on DCM, and we used a series of physiological assays in an attempt to identify these factors.
We expected that transconjugant growth on DCM would be limited by one or more of the following: DcmA expression, tolerance to intracellular production of HCl, efficient use of formaldehyde, and the mutagenic effects of the glutathione-conjugant intermediate. None of the assays that we used precisely replicates the overall stress involved in growing on DCM. That a single physiological parameter was not predictive of fitness on DCM may simply reflect the limitations of these assays. However, we hypothesize that this inconsistency stems from the multiple stresses imposed by growth on DCM, combined with differing abilities of the recipients to cope with these stresses. For example, some strains may be more sensitive to intracellular chloride production, while others are limited by the intracellular production of protons. In such a scenario, no single physiological parameter could predict fitness on DCM.
All of the strains showed a linear relationship between chloride produced and the final optical density or total protein of the culture. We conclude that dehalogenation is productive in each of these strains; if DCM is dehalogenated to formaldehyde, the cells use that formaldehyde to grow. Consequently, we do not expect that inefficient metabolism of formaldehyde limits growth in the transconjugants.
The natural isolate, M. extorquens DM4, grows slowly in media with low external pH but maintains its internal pH when dechlorinating DCM. We hypothesize that DM4 has adapted to growth on DCM by becoming more permeable to protons, a trait that would be beneficial when the dechlorination of DCM produces intracellular protons but detrimental when the extracellular pH is low. Similarly, the poor growth of DM4 on medium containing high concentrations of chloride may reflect a selection for chloride excretion mechanisms that result in detrimental leaky import under these laboratory conditions.
Two other M. extorquens isolates, AM1 and CM4, struggle to maintain their internal pH upon addition of DCM and are also the least fit when growing on DCM. This result with CM4 is surprising, as it naturally has the ability to catabolize chloromethane, which also involves release of a proton and a chloride ion (45). While these results suggest that strains AM1 and CM4 grow poorly on DCM at least partly because of their inability to tolerate the protons released during dechlorination, we also note that M. extorquens BJ001(pJM10) has a relatively high fitness on DCM yet also has a transient decrease in pH upon addition of DCM. While maintaining a stable internal pH during dechlorination is clearly an important factor in determining fitness, it therefore cannot be the only one.
Conclusions.
We have transferred the challenging, one-gene pathway for dichloromethane catabolism from M. extorquens DM4 into a range of naive recipient strains. All but one of the transconjugants could use the new pathway to grow on dichloromethane, but no strain could use this new ability as effectively as the donor. Among the strains tested, those with a negligible evolutionary distance from the donor were less successful at exploiting their new ability than the more distant relatives. Moreover, physiological measurements suggested that the type of stress limiting growth on DCM varied between transconjugants. These results demonstrate the necessity for evolutionary refinement following the horizontal acquisition of new genes (46). In the future, experimental laboratory evolution of HGT recipients may help to unravel the factors limiting the utility of a newly acquired gene as well as the biochemical mechanisms available to overcome those limitations.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge financial support from the National Institutes of Health (F32 GM106629 to J.K.M.).
We thank the Marx laboratory for helpful comments on the manuscript, Paige Swanson for providing pPS04, Lon Chubiz for CM3120, and Dipti Nayak for CM3839.
Footnotes
Published ahead of print 28 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00034-14.
REFERENCES
- 1.Gogarten JP, Doolittle WF, Lawrence JG. 2002. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 19:2226–2238. 10.1093/oxfordjournals.molbev.a004046 [DOI] [PubMed] [Google Scholar]
- 2.Ochman H, Lawrence JG, Groisman EA. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299–304. 10.1038/35012500 [DOI] [PubMed] [Google Scholar]
- 3.Wiedenbeck J, Cohan FM. 2011. Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol. Rev. 35:957–976. 10.1111/j.1574-6976.2011.00292.x [DOI] [PubMed] [Google Scholar]
- 4.Popa O, Dagan T. 2011. Trends and barriers to lateral gene transfer in prokaryotes. Curr. Opin. Microbiol. 14:615–623. 10.1016/j.mib.2011.07.027 [DOI] [PubMed] [Google Scholar]
- 5.Thomas CM, Nielsen KM. 2005. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 3:711–721. 10.1038/nrmicro1234 [DOI] [PubMed] [Google Scholar]
- 6.Shapiro BJ, Friedman J, Cordero OX, Preheim SP, Timberlake SC, Szabó G, Polz MF, Alm EJ. 2012. Population genomics of early events in the ecological differentiation of bacteria. Science 336:48–51. 10.1126/science.1218198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marraffini LA, Sontheimer EJ. 2008. CRISPR interference limits horizontal gene transfer in Staphylococci by targeting DNA. Science 322:1843–1845. 10.1126/science.1165771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser C, Hanage WP, Spratt BG. 2007. Recombination and the nature of bacterial speciation. Science 315:476–480. 10.1126/science.1127573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popa O, Hazkani-Covo E, Landan G, Martin W, Dagan T. 2011. Directed networks reveal genomic barriers and DNA repair bypasses to lateral gene transfer among prokaryotes. Genome Res. 21:599–609. 10.1101/gr.115592.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuller T, Girshovich Y, Sella Y, Kreimer A, Freilich S, Kupiec M, Gophna U, Ruppin E. 2011. Association between translation efficiency and horizontal gene transfer within microbial communities. Nucleic Acids Res. 39:4743–4755. 10.1093/nar/gkr054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smillie CS, Smith MB, Friedman J, Cordero OX, David LA, Alm EJ. 2011. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480:241–244. 10.1038/nature10571 [DOI] [PubMed] [Google Scholar]
- 12.Sorek R, Zhu Y, Creevey CJ, Francino MP, Bork P, Rubin EM. 2007. Genome-wide experimental determination of barriers to horizontal gene transfer. Science 318:1449–1452. 10.1126/science.1147112 [DOI] [PubMed] [Google Scholar]
- 13.Chou HH, Chiu HC, Delaney NF, Segre D, Marx CJ. 2011. Diminishing returns epistasis among beneficial mutations decelerates adaptation. Science 332:1190–1192. 10.1126/science.1203799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Copley SD. 2012. Inhibitory cross-talk upon introduction of a new metabolic pathway into an existing metabolic network. Proc. Natl. Acad. Sci. U. S. A. 109:E2856–E2864. 10.1073/pnas.1208509109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gälli R, Leisinger T. 1985. Specialized bacterial strains for the removal of dichloromethane from industrial waste. Conserv. Recycling 8:91–100. 10.1016/0361-3658(85)90028-1 [DOI] [Google Scholar]
- 16.Muller EE, Bringel F, Vuilleumier S. 2011. Dichloromethane-degrading bacteria in the genomic age. Res. Microbiol. 162:869–876. 10.1016/j.resmic.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 17.La Roche SD, Leisinger T. 1990. Sequence analysis and expression of the bacterial dichloromethane dehalogenase structural gene, a member of the glutathione S-transferase supergene family. J. Bacteriol. 172:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid-Appert M, Zoller K, Traber H, Vuilleumier S, Leisinger T. 1997. Association of newly discovered IS elements with the dichloromethane utilization genes of methylotrophic bacteria. Microbiol. 143:2557–2567. 10.1099/00221287-143-8-2557 [DOI] [PubMed] [Google Scholar]
- 19.Vuilleumier S, Chistoserdova L, Lee M-C, Bringel F, Lajus A, Zhou Y, Gourion B, Barbe V, Chang J, Cruveiller S, Dossat C, Gillett W, Gruffaz C, Haugen E, Hourcade E, Levy R, Mangenot S, Muller E, Nadalig T, Pagni M, Penny C, Peyraud R, Robinson DG, Roche D, Rouy Z, Saenampechek C, Salvignol G, Vallenet D, Wu Z, Marx CJ, Vorholt JA, Olson MV, Kaul R, Weissenbach J, Médigue C, Lidstrom ME. 2009. Methylobacterium genome sequences: a reference blueprint to investigate microbial metabolism of C1 compounds from natural and industrial sources. PLoS One 4:e5584. 10.1371/journal.pone.0005584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gälli R, Leisinger T. 1988. Plasmid analysis and cloning of the dichloromethane-utilization genes of Methylobacterium sp. DM4. J. Gen. Microbiol. 134:943–952 [DOI] [PubMed] [Google Scholar]
- 21.Kayser MF, Ucurum Z, Vuilleumier S. 2002. Dichloromethane metabolism and C1 utilization genes in Methylobacterium strains. Microbiology 148:1915–1922 http://mic.sgmjournals.org/content/148/6/1915.long [DOI] [PubMed] [Google Scholar]
- 22.Torgonskaya ML, Doronina NV, Hourcade E, Trotsenko YA, Vuilleumier S. 2011. Chloride-associated adaptive response in aerobic methylotrophic dichloromethane-utilising bacteria. J. Basic Microbiol. 51:296–303. 10.1002/jobm.201000280 [DOI] [PubMed] [Google Scholar]
- 23.Muller EE, Hourcade E, Louhichi-Jelail Y, Hammann P, Vuilleumier S, Bringel F. 2011. Functional genomics of dichloromethane utilization in Methylobacterium extorquens DM4. Environ. Microbiol. 13:2518–2535. 10.1111/j.1462-2920.2011.02524.x [DOI] [PubMed] [Google Scholar]
- 24.Evans GJ, Ferguson GP, Booth IR, Vuilleumier S. 2000. Growth inhibition of Escherichia coli by dichloromethane in cells expressing dichloromethane dehalogenase/glutathione S-transferase. Microbiology 146:2967–2975 http://mic.sgmjournals.org/content/146/11/2967.long [DOI] [PubMed] [Google Scholar]
- 25.Kayser MF, Vuilleumier S. 2001. Dehalogenation of dichloromethane by dichloromethane dehalogenase/glutathione S-transferase leads to formation of DNA adducts. J. Bacteriol. 183:5209–5212. 10.1128/JB.183.17.5209-5212.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayser MF, Stumpp MT, Vuilleumier S. 2000. DNA polymerase I is essential for growth of Methylobacterium dichloromethanicum DM4 with dichloromethane. J. Bacteriol. 182:5433–5439. 10.1128/JB.182.19.5433-5439.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gisi D, Leisinger T, Vuilleumier S. 1999. Enzyme-mediated dichloromethane toxicity and mutagenicity of bacterial and mammalian dichloromethane-active glutathione S-transferases. Arch. Toxicol. 73:71–79. 10.1007/s002040050589 [DOI] [PubMed] [Google Scholar]
- 28.Vorholt JA, Marx CJ, Lidstrom ME, Thauer RK. 2000. Novel formaldehyde-activating enzyme in Methylobacterium extorquens AM1 required for growth on methanol. J. Bacteriol. 182:6645–6650. 10.1128/JB.182.23.6645-6650.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaney NF, Kaczmarek ME, Ward LM, Swanson PK, Lee MC, Marx CJ. 2013. Development of an optimized medium, strain and high-throughput culturing methods for Methylobacterium extorquens. PLoS One 8:e62957. 10.1371/journal.pone.0062957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marx CJ, Lidstrom ME. 2002. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques 33:1062–1067 http://www.biotechniques.com/multimedia/archive/00010/02335rr01_10092a.pdf [DOI] [PubMed] [Google Scholar]
- 31.Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. 2000. The use of pHluorins for optical measurements of presynaptic activity. Biophys. J. 79:2199–2208. 10.1016/S0006-3495(00)76468-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MC, Chou HH, Marx CJ. 2009. Asymmetric, bimodal trade-offs during adaptation of Methylobacterium to distinct growth substrates. Evolution 63:2816–2830. 10.1111/j.1558-5646.2009.00757.x [DOI] [PubMed] [Google Scholar]
- 33.Marx CJ. 2008. Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res. Notes 1:1. 10.1186/1756-0500-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulton GL, Nunn DN, Lidstrom ME. 1984. Molecular cloning of a malyl coenzyme A lyase gene from Pseudomonas sp. strain AM1, a facultative methylotroph. J. Bacteriol. 160:718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jörg G, Bertau M. 2004. Thiol-tolerant assay for quantitative colorimetric determination of chloride released from whole-cell biodehalogenations. Anal. Biochem. 328:22–28. 10.1016/j.ab.2004.01.027 [DOI] [PubMed] [Google Scholar]
- 36.Vuilleumier S, Leisinger T. 1996. Protein engineering studies of dichloromethane dehalogenase/glutathione S-transferase from Methylophilus sp. strain DM11. Ser12 but not Tyr6 is required for enzyme activity. Eur. J. Biochem. 239:410–417 [DOI] [PubMed] [Google Scholar]
- 37.Cole JR, Chai B, Farris RJ, Wang Q, Kulam SA, McGarrell DM, Garrity GM, Tiedje JM. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33:D294–D296. 10.1093/nar/gki038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segata N, Bornigen D, Morgan XC, Huttenhower C. 2013. PhyloPhlAn is a new method for improved phylogenetic and taxonomic placement of microbes. Nat. Commun. 4:2304. 10.1038/ncomms3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knief C, Frances L, Vorholt JA. 2010. Competitiveness of diverse Methylobacterium strains in the phyllosphere of Arabidopsis thaliana and identification of representative models, including M. extorquens PA1. Microb. Ecol. 60:440–452. 10.1007/s00248-010-9725-3 [DOI] [PubMed] [Google Scholar]
- 40.Marx CJ, Bringel F, Chistoserdova L, Moulin L, Farhan Ul Haque M, Fleischman DE, Gruffaz C, Jourand P, Knief C, Lee MC, Muller EE, Nadalig T, Peyraud R, Roselli S, Russ L, Goodwin LA, Ivanova N, Kyrpides N, Lajus A, Land ML, Medigue C, Mikhailova N, Nolan M, Woyke T, Stolyar S, Vorholt JA, Vuilleumier S. 2012. Complete genome sequences of six strains of the genus Methylobacterium. J. Bacteriol. 194:4746–4748. 10.1128/JB.01009-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Aken B, Peres CM, Doty SL, Yoon JM, Schnoor JL. 2004. Methylobacterium populi sp. nov., a novel aerobic, pink-pigmented, facultatively methylotrophic, methane-utilizing bacterium isolated from poplar trees (Populus deltoides×nigra DN34). Int. J. Syst. Evol. Microbiol. 54:1191–1196. 10.1099/ijs.0.02796-0 [DOI] [PubMed] [Google Scholar]
- 42.Sy A, Giraud E, Jourand P, Garcia N, Willems A, de Lajudie P, Prin Y, Neyra M, Gillis M, Boivin-Masson C, Dreyfus B. 2001. Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes. J. Bacteriol. 183:214–220. 10.1128/JB.183.1.214-220.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders SW, Maxcy RB. 1979. Patterns of cell division, DNA base compositions, and fine structures of some radiation-resistant vegetative bacteria found in food. Appl. Environ. Microbiol. 37:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valkonen M, Mojzita D, Penttilä M, Benčina M. 2013. Noninvasive high-throughput single-cell analysis of the intracellular pH of Saccharomyces cerevisiae by ratiometric flow cytometry. Appl. Environ. Microbiol. 79:7179–7187. 10.1128/AEM.02515-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald IR, Doronina NV, Trotsenko YA, McAnulla C, Murrell JC. 2001. Hyphomicrobium chloromethanicum sp. nov. and Methylobacterium chloromethanicum sp. nov., chloromethane-utilizing bacteria isolated from a polluted environment. Int. J. Syst. Evol. Microbiol. 51:119–122. 10.1099/00207713-51-1-119 [DOI] [PubMed] [Google Scholar]
- 46.Quandt EM, Deatherage DE, Ellington AD, Georgiou G, Barrick JE. 2014. Recursive genomewide recombination and sequencing reveals a key refinement step in the evolution of a metabolic innovation in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 111:2217–2222. 10.1073/pnas.1314561111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.