Abstract
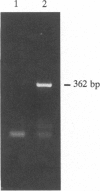
The multicellular obligately photoautotrophic alga Volvox is composed of only two types of cells, somatic and reproductive. Therefore, Volvox provides the simplest model system for the study of multicellularity. Metabolic labeling experiments using radioactive precursors are crucial for the detection of stage- and cell-type-specific proteins, glycoproteins, lipids, and carbohydrates. However, wild-type Volvox lacks import systems for sugars or amino acids. To circumvent this problem, the hexose/H+ symporter (HUP1) gene from the unicellular alga Chlorella was placed under the control of the constitutive Volvox beta-tubulin promoter. The corresponding transgenic Volvox strain synthesized the sugar transporter in a functional state and was able to efficiently incorporate 14C from labeled glucose or glucosamine. Sensitivity toward the toxic glucose/mannose analogue 2-deoxy-glucose increased by orders of magnitude in transformants. Thus we report the successful transformation of Volvox with a gene of heterologous origin. The chimeric gene may be selected for in either a positive or a negative manner, because transformants exhibit both prolonged survival in the dark in the presence of glucose and greatly increased sensitivity to the toxic sugar 2-deoxyglucose. The former trait may make the gene useful as a dominant selectable marker for use in transformation studies, whereas the latter trait may make it useful in development of a gene-targeting system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. R., Stamer K. A., Miller J. K., McNally J. G., Kirk M. M., Kirk D. L. Patterns of organellar and nuclear inheritance among progeny of two geographically isolated strains of Volvox carteri. Curr Genet. 1990 Aug;18(2):141–153. doi: 10.1007/BF00312602. [DOI] [PubMed] [Google Scholar]
- Biely P., Krátký Z., Bauer S. Metabolism of 2-deoxy-D glucose by Baker's yeast. IV. Incorporation of 2-deoxy-D-glucose into cell wall mannan. Biochim Biophys Acta. 1972 Feb 11;255(2):631–639. doi: 10.1016/0005-2736(72)90166-6. [DOI] [PubMed] [Google Scholar]
- Caspari T., Stadler R., Sauer N., Tanner W. Structure/function relationship of the Chlorella glucose/H+ symporter. J Biol Chem. 1994 Feb 4;269(5):3498–3502. [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Ertl H., Hallmann A., Wenzl S., Sumper M. A novel extensin that may organize extracellular matrix biogenesis in Volvox carteri. EMBO J. 1992 Jun;11(6):2055–2062. doi: 10.1002/j.1460-2075.1992.tb05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber H., Goetinck S. D., Kirk D. L., Schmitt R. The nitrate reductase-encoding gene of Volvox carteri: map location, sequence and induction kinetics. Gene. 1992 Oct 12;120(1):75–83. doi: 10.1016/0378-1119(92)90011-d. [DOI] [PubMed] [Google Scholar]
- Hallmann A., Sumper M. An inducible arylsulfatase of Volvox carteri with properties suitable for a reporter-gene system. Purification, characterization and molecular cloning. Eur J Biochem. 1994 Apr 1;221(1):143–150. doi: 10.1111/j.1432-1033.1994.tb18723.x. [DOI] [PubMed] [Google Scholar]
- Hallmann A., Sumper M. Reporter genes and highly regulated promoters as tools for transformation experiments in Volvox carteri. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11562–11566. doi: 10.1073/pnas.91.24.11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Mages W. Organization and structure of Volvox beta-tubulin genes. Mol Gen Genet. 1988 Aug;213(2-3):315–324. doi: 10.1007/BF00339597. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Schmidt M. F., Scholtissek C. Effect of 2-deoxy-D-glucose on the multiplication of Semliki Forest virus and the reversal of the block by mannose. Virology. 1973 Jul;54(1):179–189. doi: 10.1016/0042-6822(73)90127-x. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Inhibition by 2-deoxy-D-glucose of synthesis of glycoprotein enzymes by protoplasts of Saccharomyces: relation to inhibition of sugar uptake and metabolism. J Bacteriol. 1972 Aug;111(2):419–429. doi: 10.1128/jb.111.2.419-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle L., Schwarz R. T. Formation of dolichol monophosphate 2-deoxy-D-glucose and its interference with the glycosylation of mannoproteins in yeast. Eur J Biochem. 1976 Aug 1;67(1):239–245. doi: 10.1111/j.1432-1033.1976.tb10655.x. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Marger M. D., Saier M. H., Jr A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci. 1993 Jan;18(1):13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N., Caspari T., Klebl F., Tanner W. Functional expression of the Chlorella hexose transporter in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7949–7952. doi: 10.1073/pnas.87.20.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiedlmeier B., Schmitt R., Müller W., Kirk M. M., Gruber H., Mages W., Kirk D. L. Nuclear transformation of Volvox carteri. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5080–5084. doi: 10.1073/pnas.91.11.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde O. A., Kindle K. L. Homologous recombination in the nuclear genome of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9199–9203. doi: 10.1073/pnas.90.19.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R. C., Jaenicke L. Purification and characterization of the hormone initiating sexual morphogenesis in Volvox carteri f. nagariensis Iyengar. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1050–1054. doi: 10.1073/pnas.71.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Dotson M., Keen N. T. Plant transformation: a simple particle bombardment device based on flowing helium. Plant Mol Biol. 1992 Feb;18(4):835–839. doi: 10.1007/BF00020031. [DOI] [PubMed] [Google Scholar]
- WICK A. N., DRURY D. R., NAKADA H. I., WOLFE J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957 Feb;224(2):963–969. [PubMed] [Google Scholar]
- Will A., Caspari T., Tanner W. Km mutants of the Chlorella monosaccharide/H+ cotransporter randomly generated by PCR. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10163–10167. doi: 10.1073/pnas.91.21.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Tanner W., Sauer N. The Chlorella H+/hexose cotransporter gene. Curr Genet. 1991 Mar;19(3):215–219. doi: 10.1007/BF00336489. [DOI] [PubMed] [Google Scholar]