Abstract
Cholangiocarcinomas are biliary tree neoplasms of cholangiocyte origin. Several clinical risk factors are associated with cholangiocarcinogenesis. During the last decade, there has been an increasing interest in the causative molecular mechanisms of cholangiocarcinoma because of its poor prognosis and the lack of effective therapies. A better understanding of cholangiocarcinoma tumor initiation, promotion, and progression, as well as neurotransmitter, neuroendocrine, and endocrine growth effects, may elucidate molecular targets for diagnostic and therapeutic purposes.
Keywords: Cholangiocarcinoma, cholangiocarcinogenesis, gene, molecular, mechanism, Klatskin tumor, review
Cholangiocarcinomas are biliary tree neoplasms that arise from bile duct epithelial cells known as cholangiocytes. Microscopically, their most common feature is a well- to moderately differentiated adenocarcinoma. Cholangiocarcinomas usually occur at biliary duct confluences (Klatskin tumors), but they can also present within the liver or distal to the hilum. Although the incidence of cholangiocarcinoma is low, with approximately 8 cases per million per year in the United States, it is increasing globally (1–3).
Several important clinical risk factors exist for cholangiocarcinoma such as female gender, Caroli’s disease, congenital choledochal cysts, primary sclerosing cholangitis, hepatolithiasis, ampulla of Vater adenomas, Opistorchis viverrini and Clonorchis sinensis liver fluke infestation, Salmonella typhi infection, and obesity. Gallbladder diseases associated with cholangiocarcinoma include symptomatic cholelithiasis, polyps greater than 1 cm, and porcelain gallbladder. Several of the above risk factors play a role in biliary obstruction, chronic inflammation, and consequential cholangiocyte injury, which are well established in cholangiocarcinoma development. Hepatocytes, sinusoidal endothelial cells, hepatic stellate cells, and Kupffer cells in the biliary microenvironment secrete inflammatory cytokines, and it is these cytokines that may induce malignant transformation in cholangiocytes (4, 5).
Current molecular mechanisms of cholangiocarcinogenesis focus on growth regulatory genes and chronic biliary inflammation. Although several studies have clarified the link between chronic cholestasis and endogenous neuroendocrine peptides in the acquisition of a malignant phenotype, a more complete understanding of the genetic profile of cholangiocarcinoma is still needed to develop potentially effective, targeted molecular therapy (6, 7).
Operative intervention is currently the only “curative” treatment for early-stage cholangiocarcinoma; however, the recurrence rate is high. Unfortunately, tumors are usually diagnosed at an advanced stage when the chance of curative resection is very limited (4). Mortality is high and the 5-year survival is less than 5% (8). Chemotherapy and radiation have not yet been proven to prolong long-term survival (9).
Tumor Initiation
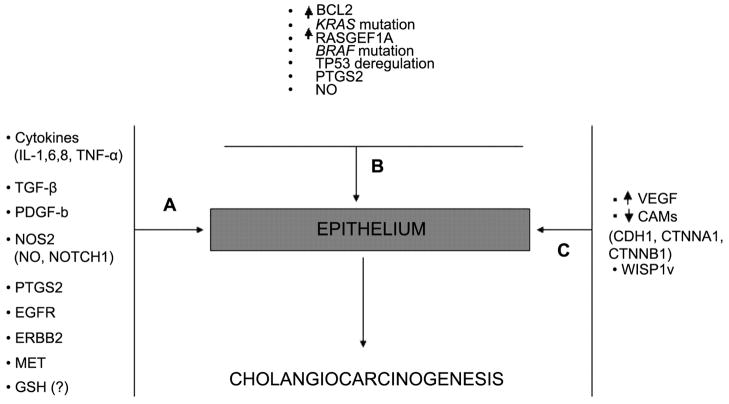
Genetic and molecular abnormalities contribute to cholangiocarcinoma tumor initiation, promotion and progression (Figure 1). A fundamental step in carcinogenesis is the development of autonomous proliferative signaling. A malignant cell phenotype is initiated when mutant cholangiocytes produce mitogens that activate local cellular receptors and intracellular signaling pathways (4, 6). Cholangiocytes secrete cytokines such as IL(Interleukin)6, transforming growth factor-beta (TGF-beta), IL8, tumor necrosis factor-alpha (TNF-alpha), and platelet-derived growth factor (PDGF) beta chain, all of which regulate biliary cell homeostasis through paracrine signaling (10, 11). During carcinogenesis, aberrant cytokine stimuli alter cholangiocyte intracellular signaling, which contributes to the development and growth of biliary tract carcinomas (6, 12).
Figure 1.
Molecular basis of cholangiocarcinogenesis. A: Tumor initiation; B: tumor promotion; C: tumor progression.
Cholangiocyte cytokines stimulate inducible nitric oxide synthase (NOS2) to produce nitric oxide (NO), a known DNA mutagen linked to malignant transformation (5, 13). The generation of NO is also important for bile duct development because it induces Notch1 expression (14, 15). The four Notch genes identified in mammals (Notch 1–4) are expressed in a wide variety of cells and play a significant role in cellular differentiation. The activation of Notch by cell-to-cell interaction causes a transcriptional silencing effect that inhibits differentiation in some cells but not in others (16–18). While the Notch pathway is known to be associated with pancreatic carcinogenesis in rats and humans (19), this same pathway may also have a role in cholangiocarcinogenesis via NOS2.
Cyclooxygenase-2 (prostaglandin-endoperoxide synthase 2, PTGS2) is also implicated in the initiation of malignant cholangiocytes (20). PTGS2 is up-regulated in murine and rat models of biliary adenocarcinoma, while the antisense depletion of PTGS2 has been observed to inhibit tumor cell proliferation (21, 22). Oxysterols are the oxidative derivatives of the bile cholesterol present during cholestasis and are also associated with biliary carcinogenesis. Human cholangiocarcinoma cell lines exposed to oxysterols in vitro have elevated PTGS2 expression (23, 24), further supporting the association between inflammation and cholangiocarcinoma.
The oncogenes ERBB2 and MET have also been shown to increase PTGS2 expression, and both are involved in cholangiocyte carcinogenesis (9, 25). Cholangiocarcinoma cell lines strongly overexpress ERBB2, and MET expression is increased in the early phases of cholangiocarcinogenesis (25–27). Normal rat cholangiocytes transfected with Erbb2 underwent malignant transformation with molecular features resembling human cholangiocarcinoma (28). In addition, the MET receptor is bound by hepatocyte growth factor (HGF), and HGF overexpression in cholangiocarcinoma has been shown to have a mitogenic effect on cholangiocytes (29).
The epidermal growth factor receptor (EGFR) is activated by bile acids and has been linked to cholangiocarcinoma growth. The bile acid-dependent activation of EGFR requires metalloproteinase activity and functions with phosphoinositide 3-kinase (PIK3CA) signaling to promote the expression of anti-apoptotic molecules (30). Survival and proliferative signaling are therefore stimulated by EGFR activation through PIK3CA. Furthermore, EGFR expression is prognostic and an indicator of intrahepatic chaolangiocarcinoma recurrence (31).
The acute phase proteins IL6 and TGFB1 affect the growth of biliary epithelial cells (12). IL6 secretion increases during the course of chronic inflammation and biliary duct neoplasia, resulting in sustained proliferation by an autocrine/paracrine mechanism (32). TGFB1 regulates cellular proliferation, differentiation, migration, and apoptosis, thereby acting as a cholangiocyte tumor suppressor (12, 33, 34). However, mutations in TGFBR1 (TGF beta receptor 1) and SMAD4 (alias DPC4) alter TGFB1 signaling in cholangiocarcinoma cells, allowing them to escape from TGFB1 tumor suppression (12, 34, 35). SMAD4 is an important component of the TGFB1 pathway, and mutations causing loss of its expression have been described in biliary malignancies, particularly extrahepatic cholangiocarcinoma (36, 37). SMAD4 and PTEN are tumor suppressor genes that function synergistically in cholangiocarcinogenesis, and their disruption in a mouse model resulted in the development of biliary malignancies (38).
The main intracellular defense against oxidative stress during inflammation is reduced glutathione (GSH). GSH maintains proteins and other molecules in the reduced state and participates in the detoxification of many molecules (39). A GSH deficiency can lead to apoptosis deregulation and DNA damage (40). Although the role of GSH in the cholangiocarcinogenic process is not completely understood, reduced GSH levels have been found in cells with chronic biliary diseases and in experimentally induced cholestasis (39).
Tumor Promotion
Apoptosis is the mechanism of programmed cell death allowing organisms to delete cells that are unable to repair DNA damage (41). Abnormalities of this mechanism promote tumorigenesis because mutated cholangiocytes may subsequently result in malignancy (41). The inhibition of apoptosis in cholangiocarcinoma has been linked to increased BCL2 expression, KRAS mutation, and/or TP53 deregulation (40). The anti-apoptotic protein BCL2 is expressed by bile ductules and inhibits cytochrome c release from mitochondria, thereby preventing caspase-3 activation (42, 43). Point mutations of the KRAS proto-oncogene are frequently present in cholangiocarcinoma specimens arising near the hepatic hilum, especially when there is lymph node metastasis (44, 45). Mutations of the tumor suppressor TP53 have also been described in intrahepatic cholangiocarcinomas (6).
In addition to initiating tumor formation via mutagenesis, NO inhibits apoptosis in human cholangiocarcinoma cell lines through the nitrosylation of caspase 9 (5, 46). Interestingly, NOS2 also promotes mouse cholangiocyte growth by up-regulating PTGS2 (47). Administration of the selective PTGS2 inhibitor celecoxib enhances apoptosis in rat cholangiocarcinoma cells (22), suggesting that PTGS2 deregulation may promote carcinogenesis.
BRAF is a RAF family gene activated by KRAS and frequently mutated in cholangiocarcinomas (48). Mutations of either KRAS or BRAF are frequently encountered in cholangiocarcinogenesis (48). RASGEF1A (RasGEF domain family, member 1A) is a novel gene encoding a guanine nucleotide exchange factor for RAS-like small GTPases that has elevated expression in the majority of human intrahepatic cholangiocarcinomas (49). The suppression of RASGEF1A expression by interfering RNA (RNAi) reduces the growth rate of human cholangiocarcinoma cells, demonstrating the potential of RASGEF1A as a therapeutic target in intrahepatic cholangiocarcinoma (49).
Tumor Progression
During tumor progression, neovascularization (de novo formation of functional microvascular networks) and angiogenesis (pre-existing capillary extension) deliver nutrients and oxygen to malignant cells and help prevent the tumor mass from outgrowing the native vascular network. Vascular endothelial growth factor (VEGF) is an important signaling protein for both neovascularization and angiogenesis in cholangiocarcinoma progression (4, 6). Although TGFB1 is a known tumor suppressor, it is coexpressed with VEGF in human cholangiocarcinoma tumors and has been implicated as an angiogenesis activator in an in vitro model (50).
Metastasis is another sign of tumor progression, and VEGF overexpression in intrahepatic cholangiocarcinoma is associated with liver metastasis (31). The highly invasive and metastatic behavior of cholangiocarcinoma is also linked to the expression of matrix metalloproteinases, such as human aspartyl b-hydroxylase, and proteins related to the connective tissue growth factor (CTGF) family (51, 52). WISP1 is a member of the CTGF family encoding the WNT1 inducible signaling pathway (WISP) protein 1. The expression of the splicing variant WISP1v is associated with perineural and lymphatic tumor invasion and is therefore a poor prognosticator in cholangiocarcinoma (53, 54).
Altered cell adhesion molecule (CAM) expression is an additional factor contributing to tumor progression. Down-regulation of CDH1 (E-cadherin), CTNNA1 (alpha-catenin) and CTNNB1 (beta-catenin) is associated with high-grade cholangiocarcinomas but not with vascular invasion or metastatic behavior (55). Nevertheless, the interaction of CTNNB1 with MUC1 and MET has been shown to enhance the invasive and metastatic properties of cholangiocarcinoma (56).
Neurotransmitter, Neuroendocrine and Endocrine Growth Effects
Certain cholangiocarcinoma cell lines express several alpha-adrenergic receptor subtypes, and stimulation of the alpha2-adrenoreceptors in vitro up-regulates cAMP, inhibits EGF-induced MAPK1 activity, and reduces cell proliferation (57). Muscarinic acetylcholine receptors are located on the surface of the same cell lines; however, the effect of the parasympathetic nervous system on cholangiocarcinoma growth is still unknown (58). Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter synthesized and metabolized in the central nervous system and liver. GABA inhibits both cholangiocarcinoma cell migration in vitro and xenograft tumor growth in mice via GABA(A), GABA(B), and GABA(C) receptors on cholangiocytes (59).
Gastrin, cholecystokinin octapeptide (CCK8), and the longer-acting somatostatin analogs octreotide and lanreotide are all neuroendocrine peptides known to regulate cholangiocarcinoma growth (7). The activation of gastrin receptors expressed by cholangiocarcinoma cells leads to inhibition of proliferation and acceleration of apoptosis through a Ca2+-dependent signaling pathway (60). However, gastrin’s inhibitory or stimulatory effect on cancer cell growth is specific to the predominant intracellular isoform of cAMP-dependent protein kinase A (61).
Several therapeutic models have demonstrated the efficacy of neuropeptide drugs to alter malignant growth. Chronic CCK8 treatment reduced the growth of human cholangiocarcinoma xenografts in nude mice (62). In cholangiocarcinoma models expressing the somatostatin receptor 2, octreotide attenuates in vitro cholangiocyte proliferation and lanreotide inhibits in vivo tumor growth of human cholangiocarcinoma xenografts (63, 64).
The endocrine system influences cholangiocarcinogenesis via the estrogen receptor (ESR1), a ligand-activated transcription factor with multiple domains for hormone binding, DNA binding, and transcription activation. The ESR1 agonist 17-beta-estradiol stimulates cholangiocarcinoma cell growth in vitro, and the ESR1 antagonist tamoxifen inhibits the growth of human cholangiocarcinoma cells both in vitro and in vivo (65). Moreover, tamoxifen administered to human cholangiocarcinoma cells following pre-treatment with interferon-gamma induces in vitro apoptosis and inhibits tumor growth in mouse xenografts (66, 67). Further in vivo studies are required to clearly define the association between estrogens and cholangiocarcinoma.
Conclusion
Continued genetic and molecular research is crucial because of the rising incidence of cholangiocarinoma and the lack of effective treatments. The high frequency of late-stage diagnosis is a major difficulty facing surgeons treating cholangiocarcinoma because this limits the possibility of a curative resection. The development of novel therapeutic approaches based on tumor biology is among the goals of modern medicine. Putative molecular targets such as PTGS2 and NOS2 inhibitors can potentially affect the incidence and growth of cholangiocarcinoma when used as prophylactic and therapeutic options, respectively. Future studies focusing on the discussed genetic and molecular targets will help develop more effective therapies to treat cholangiocarcinoma when curative resection is unlikely.
References
- 1.Shaib YA, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 3.Taylor-Robinson SD, Toledano MB, Arora S, Keegan J, Hargreaves S, Beck A, Khan SA, Elliott P, Thomas HC. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut. 2001;48:816–820. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 5.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 6.Berthiaume EP, Wands J. The molecular pathogenesis of cholangiocarcinoma, Semin. Liver Dis. 2004;24:127–137. doi: 10.1055/s-2004-828890. [DOI] [PubMed] [Google Scholar]
- 7.Marzioni M, Fava G, Benedetti A. Nervous and neuroendocrine regulation of the pathophysiology of cholestasis and of biliary carcinogenesis. World J Gastroenterology. 2006;12:3471–3480. doi: 10.3748/wjg.v12.i22.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 9.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 10.Yasoshima M, Kono N, Sugawara H, Katayanagi K, Harada K, Nakanuma Y. Increased expression of interleukin- 6 and tumor necrosis factor-alpha in pathologic biliary epithelial cells: in situ and culture study. Lab Invest. 1998;78:89–100. [PubMed] [Google Scholar]
- 11.Grappone C, Pinzani M, Parola M, Pellegrini G, Caligiuri A, DeFranco R, Marra F, Herbst H, Alpini G, Milani S. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol. 1999;31:100–109. doi: 10.1016/s0168-8278(99)80169-x. [DOI] [PubMed] [Google Scholar]
- 12.Tadlock L, Yamagiwa Y, Hawker J, Marienfeld C, Patel T. Transforming growth factor-beta inhibition of proteasomal activity: a potential mechanism of growth arrest. Am J Physiol Cell Physiol. 2003;285:C277–C285. doi: 10.1152/ajpcell.00550.2002. [DOI] [PubMed] [Google Scholar]
- 13.Spirli C, Fabris L, Duner E, Fiorotto R, Ballardini G, Roskams T, Larusso NF, Sonzogni A, Okolicsanyi L, Strazzabosco M. Cytokine-stimulated nitric oxide production inhibits adenylyl cyclase and cAMP-dependent secretion in cholangiocytes. Gastroenterology. 2003;124:737–753. doi: 10.1053/gast.2003.50100. [DOI] [PubMed] [Google Scholar]
- 14.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 15.Louis AA, Van Eyken P, Haber BA, Hicks C, Weinmaster G, Taub R, Rand EB. Hepatic jagged 1 expression studies. Hepatology. 1999;30:1269–1275. doi: 10.1002/hep.510300512. [DOI] [PubMed] [Google Scholar]
- 16.Milner LA, Bigas A, Kopan R. Inhibition of granulocytic differentiation by mNotch1. Proc Natl Acad Sci USA. 1996;93:13014–13019. doi: 10.1073/pnas.93.23.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature. 2000;405:966–970. doi: 10.1038/35016111. [DOI] [PubMed] [Google Scholar]
- 18.Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: evicence and speculation. Blood. 1999;93:243–248. [PubMed] [Google Scholar]
- 19.Miyamoto Y, Maitr A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 20.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 21.Kiguchi K, Carbajal S, Chan K, Beltrán L, Ruffino L, Shen J, Matsumoto T, Yoshimi N, DiGiovanni J. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61:6971–6976. [PubMed] [Google Scholar]
- 22.Zhang Z, Lai GH, Sirica AE. Celecoxib-induced apoptosis in rat cholangiocarcinoma cells mediated by Akt inactivation and Bax translocation. Hepatology. 2004;39:1028–1037. doi: 10.1002/hep.20143. [DOI] [PubMed] [Google Scholar]
- 23.Yoon JH, Canbay AE, Werneburg NW, Lee SP, Gores GJ. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology. 2004;39:732–738. doi: 10.1002/hep.20125. [DOI] [PubMed] [Google Scholar]
- 24.Haigh WG, Lee SP. Identification of oxysterols in human bile and pigment gallstones. Gastroenterology. 2001;121:118–123. doi: 10.1053/gast.2001.25513. [DOI] [PubMed] [Google Scholar]
- 25.Aishima SI, Taguchi KI, Sugimachi K, Shimada M, Sugimachi K, Tsuneyoshi M. c-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology. 2002;40:269–278. doi: 10.1046/j.1365-2559.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 26.Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439–450. doi: 10.1053/jhep.2002.34435. [DOI] [PubMed] [Google Scholar]
- 27.Sirica AE, Lai GH, Endo K, Zhang Z, Yoon BI. Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma: potential therapeutic targets. Semin Liver Dis. 2002;22:303–313. doi: 10.1055/s-2002-34507. [DOI] [PubMed] [Google Scholar]
- 28.Lai GH, Zhang Z, Shen XN, Ward DJ, Dewitt JL, Holt SE, Rozich RA, Hixson DC, Sirica AE. ErbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;129:2047–2057. doi: 10.1053/j.gastro.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Lai GH, Radaeva S, Nakamura T, Sirica AE. Unique epithelial cell production of hepatocyte growth factor/scatter factor by putative precancerous intestinal metaplasias and associated “intestinal-type” biliary cancer chemically induced in rat liver. Hepatology. 2000;31:1257–1265. doi: 10.1053/jhep.2000.8108. [DOI] [PubMed] [Google Scholar]
- 30.Werneburg NW, Yoon JH, Higuchi H, Gores GJ. Bile acids activate EGF receptor via a TGF-alpha-dependent mechanism in human cholangiocyte cell lines. Am J Physiol Gastrointest Liver Physiol. 2003;285:G31–G36. doi: 10.1152/ajpgi.00536.2002. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada K, Shimizu Y, Nambu S, Higuchi K, Watanabe A. Interleukin-6 functions as an autocrine growth factor in a cholangiocarcinoma cell line. J Gastroenterol Hepatol. 1994;9:462–467. doi: 10.1111/j.1440-1746.1994.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 33.Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 34.Yazumi S, Ko K, Watanabe N, Yoshikawa K, Chiba T, Takahashi R. Disrupted transforming growth factor-beta signaling and deregulated growth in human biliary tract cancer cells. Int J Cancer. 2000;86:782–789. doi: 10.1002/(sici)1097-0215(20000615)86:6<782::aid-ijc5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 35.Zen Y, Harada K, Sasaki M, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y. Intrahepatic cholangiocarcinoma escapes from growth inhibitory effect of transforming growth factor-beta1 by overexpression of cyclin D1. Lab Invest. 2005;85:572–581. doi: 10.1038/labinvest.3700236. [DOI] [PubMed] [Google Scholar]
- 36.Chuang SC, Lee KT, Tsai KB, Sheen PC, Nagai E, Mizumoto K, Tanaka M. Immunohistochemical study of DPC4 and p53 proteins in gallbladder and bile duct cancers. World J Surg. 2004;28:995–1000. doi: 10.1007/s00268-004-7447-8. [DOI] [PubMed] [Google Scholar]
- 37.Hahn SA, Bartsch D, Schroers A, Galehdari H, Becker M, Ramaswamy A, Schwarte-Waldhoff I, Maschek H, Schmiegel W. Mutations of the DPC4/Smad4 gene in biliary tract carcinoma. Cancer Res. 1998;58:1124–1126. [PubMed] [Google Scholar]
- 38.Xu X, Kobayashi S, Qiao W, Li C, Xiao C, Radaeva S, Stiles B, Wang RH, Ohara N, Yoshino T, LeRoith D, Torbenson MS, Gores GJ, Wu H, Gao B, Deng CX. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. J Clin Invest. 2006;116:1843–1852. doi: 10.1172/JCI27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celli A, Que FG, Gores GJ, LaRusso NF. Glutathione depletion is associated with decreased Bcl-2 expression and increased apoptosis in cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1998;275:G749–G757. doi: 10.1152/ajpgi.1998.275.4.G749. [DOI] [PubMed] [Google Scholar]
- 40.Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 2: molecular pathology and treatment. J Gastroenterol Hepatol. 2002;17:1056–1063. doi: 10.1046/j.1440-1746.2002.02780.x. [DOI] [PubMed] [Google Scholar]
- 41.Torok NJ, Larusso EM, McNiven MA. Alterations in vesicle transport and cell polarity in rat hepatocytes subjected to mechanical or chemical cholestasis. Gastroenterology. 2001;121:1176–1184. doi: 10.1053/gast.2001.28652. [DOI] [PubMed] [Google Scholar]
- 42.Skopelitou A, Hadjiyannakis M, Alexopoulou V, Krikoni O, Kamina S, Agnantis N. Topographical immunohistochemical expression of bcl-2 protein in human liver lesions. Anticancer Res. 1996;16:975–978. [PubMed] [Google Scholar]
- 43.Harnois DM, Que FG, Celli A, LaRusso NF, Gores GJ. Bcl-2 is overexpressed and alters the threshold for apoptosis in a cholangiocarcinoma cell line. Hepatology. 1997;26:884–890. doi: 10.1002/hep.510260413. [DOI] [PubMed] [Google Scholar]
- 44.Ohashi K, Nakajima Y, Kanehiro H, Tsutsumi M, Taki J, Aomatsu Y, Yoshimura A, Ko S, Kin T, Yagura K. Ki-ras mutations and p53 protein expressions in intrahepatic cholangiocarcinomas: relation to gross tumor morphology. Gastroenterology. 1995;109:1612–1617. doi: 10.1016/0016-5085(95)90650-9. [DOI] [PubMed] [Google Scholar]
- 45.Isa T, Tomita S, Nakachi A, Miyazato H, Shimoji H, Kusano T, Muto Y, Furukawa M. Analysis of microsatellite instability, K-ras gene mutation and p53 protein overexpression in intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2002;49:604–608. [PubMed] [Google Scholar]
- 46.Torok NJ, Higuchi H, Bronk S, Gores GJ. Nitric oxide inhibits apoptosis downstream of cytochrome c release by nitrosylating caspase 9. Cancer Res. 2002;62:1648–1653. [PubMed] [Google Scholar]
- 47.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates cyclooxygenase-2 in mouse cholangiocytes promoting cell growth. Am J Physiol Gastrointest Liver Physiol. 2004;287:G88–G95. doi: 10.1152/ajpgi.00539.2003. [DOI] [PubMed] [Google Scholar]
- 48.Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, Hauss J, Wittekind C. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–712. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ura K, Obama K, Satoh S, Sakai Y, Nakamura Y, Furukawa Y. Enhanced RASGEF1A expression is involved in the growth and migration of intrahepatic cholangiocarcinoma. Clin Cancer Res. 2006;12:6611–6616. doi: 10.1158/1078-0432.CCR-06-0783. [DOI] [PubMed] [Google Scholar]
- 50.Benckert C, Jonas S, Cramer T, Schäfer G, Peters M, Wagner K, Radke C, Wiedenmann B, Neuhaus P, Höcker M, Rosewicz S. Transforming growth factor beta 1 stimulates vascular endothelial growth factor gene transcription in human cholangiocellular carcinoma cells. Cancer Res. 2003;63:1083–1092. [PubMed] [Google Scholar]
- 51.Terada T, Okada Y, Nakanuma Y. Expression of immunoreactive matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human normal livers and primary liver tumors. Hepatology. 1996;23:1341–1344. doi: 10.1053/jhep.1996.v23.pm0008675149. [DOI] [PubMed] [Google Scholar]
- 52.Maeda T, Lahousse S, Tamaki S, Tamaki S, Enjoji M, Wands JR, de la Monte SM. Antisense oligodeoxynucleotides directed against aspartyl (asparaginyl) beta-hydroxylase suppress migration of cholangiocarcinoma cells. J Hepatol. 2003;38:615–622. doi: 10.1016/s0168-8278(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 53.Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka S, Sugimachi K, Kameyama T, Maehara S, Shirabe K, Shimada M, Wands JR, Maehara Y. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003;37:1122–1129. doi: 10.1053/jhep.2003.50187. [DOI] [PubMed] [Google Scholar]
- 55.Ashida K, Terada T, Kitamura Y, Kaibara N. Expression of E-cadherin, alpha-catenin, beta-catenin, and CD44 (standard and variant isoforms) in human cholangiocarcinoma: an immunohistochemical study. Hepatology. 1998;27:974–982. doi: 10.1002/hep.510270412. [DOI] [PubMed] [Google Scholar]
- 56.Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–1332. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 57.Kanno N, LeSage G, Phinizy JL, Glaser S, Francis H, Alpini G. Stimulation of alpha2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology. 2002;35:1329–1340. doi: 10.1053/jhep.2002.33330. [DOI] [PubMed] [Google Scholar]
- 58.Elsing C, Hubner C, Fitscher BA, Kassner A, Stremmel W. Muscarinic acetylcholine receptor stimulation of biliary epithelial cells and its effect on bile secretion in the isolated perfused liver. Hepatology. 1997;25:804–813. doi: 10.1002/hep.510250404. [DOI] [PubMed] [Google Scholar]
- 59.Fava G, Marucci L, Glaser S, Francis H, De Morrow S, Benedetti A, Alvaro D, Venter J, Meininger C, Patel T, Taffetani S, Marzioni M, Summers R, Reichenbach R, Alpini G. Gamma-aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437–11446. doi: 10.1158/0008-5472.CAN-05-1470. [DOI] [PubMed] [Google Scholar]
- 60.Kanno N, Glaser S, Chowdhury U, Phinizy JL, Baiocchi L, Francis H, LeSage G, Alpini G. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284–291. doi: 10.1016/s0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 61.Bold RJ, Alpard V, Ishizuka J, Townsend CM., Jr Growth-regulatory effect of gastrin on human colon cancer cell lines is determined by protein kinase a isoform content. Regul Pept. 1994;53:61–70. doi: 10.1016/0167-0115(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 62.Hudd C, Euhus DM, LaRegina MC Herbold DR, Palmer DC, Johnson FE. Effect of cholecystokinin on human cholangiocarcinoma xenografted into nude mice. Cancer Res. 1985;45:1372–1377. [PubMed] [Google Scholar]
- 63.Zhao B, Zhao H, Zhao N, Zhu XG. Cholangiocarcinoma cells express somatostatin receptor subtype 2 and respond to octreotide treatment. J Hepatobiliary Pancreat Surg. 2002;9:497–502. doi: 10.1007/s005340200062. [DOI] [PubMed] [Google Scholar]
- 64.Tan CK, Podila PV, Taylor JE, Nagorney DM, Wiseman GA, Gores GJ, LaRusso NF. Human cholangiocarcinomas express somatostatin receptors and respond to somatostatin with growth inhibition. Gastroenterology. 1995;108:1908–1916. doi: 10.1016/0016-5085(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 65.Sampson LK, Vickers SM, Ying W, Phillips JO. Tamoxifen-mediated growth inhibition of human cholangiocarcinoma. Cancer Res. 1997;57:1743–1749. [PubMed] [Google Scholar]
- 66.Ahn EY, Pan G, Oh JH, Tytler EM, McDonald JM. The combination of calmodulin antagonists and interferongamma induces apoptosis through caspase-dependent and -independent pathways in cholangiocarcinoma cells. Am J Pathol. 2003;163:2053–2063. doi: 10.1016/s0002-9440(10)63563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vickers SM, Jhala NC, Ahn EY, McDonald JM, Pan G, Bland KI. Tamoxifen (TMX)/Fas-induced growth inhibition of human cholangiocarcinoma (HCC) by gamma interferon (IFN-gamma) Ann Surg. 2002;235:872–878. doi: 10.1097/00000658-200206000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]