Abstract
Objective
GFAP is specific to astrocytes in the central nervous system. We hypothesized that serum GFAP would be increased in neonates with hypoxic-ischemic encephalopathy (HIE) treated with whole body cooling.
Study Design
We measured GFAP at birth and daily for up to 7 days for neonates in the intensive care unit. We compared neonates with HIE treated with whole body cooling to gestational age matched controls without neurologic injury and neonates with HIE by brain abnormalities on MRI.
Results
Neonates with HIE had increased GFAP levels compared to controls. Neonates with HIE and abnormal brain imaging had elevated GFAP levels compared to neonates with HIE and normal imaging.
Conclusions
Serum GFAP levels during the first week of life were increased in neonates with HIE and were predictive of brain injury on MRI. Biomarkers like GFAP could help triage neonates with HIE to treatment, measure treatment efficacy and provide prognostic information.
Keywords: fetal acidosis, glial fibrillary acidic protein, hypoxic-ischemic encephalopathy, neonatal seizures, whole body cooling
Introduction
Obstetricians and neonatologists routinely face the challenging task of identifying the hypoxic-ischemic fetus, as early intervention with head or whole body cooling,(1) or experimental therapies such as erythropoietin,(2) xenon,(3) newer anticonvulsants,(4) and umbilical cord stem cells(5) may ameliorate devastating injuries. Current markers for identification of brain injury due to intrapartum hypoxia-ischemia, such as non-reassuring fetal heart rate tracings, meconium, metabolic acidosis and Apgar scores are imprecise.(6) Newer technologies for intrapartum monitoring such as fetal pulse oximetry and fetal electrocardiogram also lack precision.(7)
Although meta-analyses have shown benefit of hypothermia in reducing death and disability,(8, 9) there are risks, so correct identification of at-risk neonates is critical. In addition, although hypothermia is effective in improving neurodevelopmental outcomes in HIE, we have no means to monitor this therapy or direct it at the neonate with the most severe injury. For example, hypotonia is commonly used to identify clinical HIE; however, most neonates that are hypotonic at the time of birth were not suffering from intrapartum hypoxia-ischemia.(10) Additional means are needed to identify the neonate with true intrapartum hypoxia-ischemia so that therapies with risk are avoided.
A number of circulating proteins, including glial fibrillary acidic protein (GFAP), have been measured in adult patients after stroke, cardiac arrest or traumatic brain injury in an effort to provide prognostic data on survival or residual deficits.(11–14) GFAP is a cytoskeletal intermediate filament protein found in the astroglia of the central nervous system and is a specific marker of differentiated astrocytes. Serum GFAP is specific to brain tissue and is not routinely secreted in blood, but only released after astrocyte death. This makes it an ideal blood marker for brain injury in neonates. We have found GFAP to be a significant predictor of survival and neurologic outcome in neonates and children requiring ECMO.(15) The aims of this study are to determine whether circulating GFAP levels measured at the time of birth and during the first week of life may identify neonates with moderate/severe HIE, and among neonates with moderate/severe HIE that qualified for hypothermia, to determine whether circulating GFAP levels predicted an abnormal brain MRI and were associated with functional outcomes.
Material and methods
Subjects
This is an IRB approved prospective cohort study that examined neonates admitted to the neonatal intensive care unit (NICU) at a single tertiary university hospital. Subjects were liveborn, non-anomalous, non-syndromic infants born at 36–41 weeks gestation. Our study included neonates born at our institution as well as those born within our state and transported to our NICU within 6 hours of birth.
Records were reviewed to abstract clinical information available at the time of maternal and neonatal discharge. Preeclampsia was defined as proteinuria and new onset hypertension. Administration of intravenous magnesium sulfate to the mother prior to delivery was recorded, as this therapy has been linked with a reduced risk of neonatal brain injury.(17) Intrauterine growth restriction was defined as an estimated fetal weight less than the 10th percentile for gestational age.(18) Nonreassuring fetal heart rate tracings were those significant enough to prompt operative vaginal or cesarean delivery. Sepsis was considered present only for neonates with positive blood and/or cerebrospinal fluid cultures.
Neonates with moderate-severe encephalopathy, defined as per Sarnat,(19) that met criteria for whole body cooling (20) were compared to neonates without neurologic injury admitted to the NICU matched by gestational age within one week in a 1:1 fashion. Neonates ≥ 36 weeks gestation that qualify are cooled using a conductive water-based hypothermia system and hypothermia blankets within 6 hours of birth, and are kept at a rectal temperature of 33.5°C for 72 hours. Routinely, all neonates with HIE treated with whole body cooling have a standard neonatal imaging brain MRI prior to discharge from the NICU. For this study, these images were reviewed by an experienced pediatric neuroradiologist (TAGMH) blinded to the GFAP results.
Neonates with an abnormal brain MRI were compared to those whose brain MRI was normal. The images were reviewed for focal or diffuse lesions related to hypoxic ischemic injury. MRI brain abnormalities were defined as brain swelling; cortical highlighting; focal or global loss of grey-white matter differentiation; abnormal signal intensity in the basal ganglia and thalami; loss of normal signal intensity in the posterior limb of the internal capsule; acute and subacute parenchymal, intraventricular or extracerebral hemorrhage; and acutely evolving focal infarction in an arterial territory or in a parasagittal or watershed distribution. (21)
Specimens
Specimens collected for measurement of GFAP included umbilical cord blood and neonatal serum. For the umbilical cord blood, a small aliquot was taken from the umbilical cord venous blood sample routinely collected at delivery. For neonatal samples, the remaining fraction of serum from daily lab tests was collected after clinically indicated testing was completed. Neonatal serum specimens were collected at the time of admission to the NICU (within 6 hours of birth), and then daily for the first 4 days of life for the non-neurologically injured controls, and daily for 7 days for neonates with HIE that underwent whole body cooling.
GFAP Assay
Using the Mesoscale platform (MesoScale Discovery, Gaithersburg, MD) we developed an electrochemiluminescent sandwich immunoassay for GFAP.(15) This was developed after the method of Petzold (22) using a trio of mouse monoclonal antibodies for capture and a rabbit polyclonal for detection.(15) Serum samples were assayed in duplicate, and the mean concentration used for analysis. The lower limit of quantitation was 0.04 ng/mL; values below this were reported as zero.
Statistical analysis
Comparisons were made using chi-square or Fisher’s exact test for categorical variables and unpaired Student t-test for continuous variables. GFAP levels were compared using the Wilcoxon rank-sum test for non-parametric data. Significance was set at P < 0.05. Linear regression was used to determine the effect of gestational age on GFAP level in the non-neurologically injured control population. Receiver operator characteristic (ROC) curves were constructed to determine the optimal cut-off (as determined by maximal area under the curve) of serum GFAP level at NICU admission to identify HIE qualifying for whole body cooling and to identify neonates with brain abnormalities on MRI. The area under the ROC curve was used to compare the ability of admission GFAP to identify neonates with abnormalities on brain MRI scans compared to other currently used tests to identify these infants: nonreassuring fetal heart rate tracing, meconium, 5 minute Apgar < 7, and umbilical arterial pH<7.0 or base deficit > 12 mM. Statistical analyses were performed with Stata 10 (StataCorp, College Station, TX).
Results
Study Population and Clinical Characteristics
During the period from 4/28/2009 to 7/11/2010 there were 652 admissions to our NICU of which 23 consecutiveneonates were diagnosed with clinical moderate/severe HIE that qualified for whole body cooling. These 23 neonates were matched 1:1 by gestational age at birth within one week to neonates admitted to the NICU for non-neurologic indications. The mean (SD) gestational age for cooled neonates was 38.7 (1.5) weeks and 39 (1.4) weeks for the controls. Maternal and neonatal characteristics are summarized in Table 1. Maternal demographics and incidence of cesarean delivery were not different. The most likely reason for admission for the controls was respiratory distress and to rule out sepsis. The cases weighed 291 g less at birth, which was statistically, but not clinically significant, and were significantly more likely to have had a placental abruption, metabolic acidosis, a 5 minute Apgar < 7 and had a longer length of stay in the NICU.
Table I.
Summary statistics for maternal and neonatal characteristics comparing cases with moderate-severe HIE treated with total body cooling to gestational age matched controls admitted to the NICU without neurologic injury. Means and standard deviations are presented for continuous variables; counts, percentages, odds ratios and 95% confidence intervals are presented for categorical variables.
| Cooling | Controls | ||
|---|---|---|---|
| N=23 | N=23 | P value | |
| Maternal Age (years) | 26.2±7.1 | 26.9±6.9 | 0.739 |
| Maternal Race | 0.244 | ||
| White | 9 (39.1%) | 5 (21.7%) | |
| Black | 11 (47.8%) | 17 (73.9%) | |
| Other | 3 (13%) | 1 (4.3%) | |
| Nulliparous | 14 (60.9%) | 8 (34.8%) | 0.078 |
| Cesarean delivery | 15 (65.2%) | 12 (52.2%) | 0.369 |
| Twin gestation | 1 (4.3%) | 0 | 1.00 |
| Preeclampsia | 3 (13%) | 3 (13%) | 1.00 |
| Magnesium exposure | 1 (4.3%) | 3 (13%) | 0.608 |
| Intrauterine growth restriction | 4 (17.4%) | 2 (8.7%) | 0.665 |
| Clinical chorioamnionitis | 3 (13%) | 4 (17.4%) | 1.00 |
| Meconium staining (moderate-thick) | 6 (26.1%) | 7 (30.4%) | 0.743 |
| Nonreassuring FHR tracing | 11 (47.8%) | 6 (21.6%) | 0.127 |
| Abruption | 8 (34.8%) | 1 (4.3%) | 0.022* |
| Birth Weight (grams) | 2978±630 | 3269±655 | 0.132 |
| Umbilical artery pH | 6.92±0.19 | 7.14±0.13 | <0.001* |
| Umbilical artery base deficit (mM) | 15.5±6.7 | 7.8±5.9 | 0.001* |
| Cord pH<7.0 or base deficit > 12 | 16/19 (84.2%) | 3/17 (17.6%) | <0.001* |
| 5 min Apgar < 7 | 21 (91.3%) | 6 (21.6%) | <0.001* |
| Respiratory distress | 11 (47.8%) | 16 (69.6%) | 0.134 |
| Sepsis (+ blood or CSF culture) | 1 (4.3%) | 1 (4.3%) | 1.0 |
| Death | 1 (4.3%) | 0 | 1.0 |
| NICU length of stay (days) | 18.4±11.4 | 6.7±2.7 | <0.001* |
HIE = hypoxic-ischemic encephalopathy
NICU = neonatal intensive care unit
CSF = cerebrospinal fluid
denotes statistical significance with P<0.05
GFAP as a Biomarker of HIE
For the controls we were unable to obtain a GFAP level at 54/138 (39.1%) of the desired time points, and for the cooled cases 50/207 (24.2%), due to an insufficient amount of serum, no blood drawn on that day, or an inability to obtain cord blood because the birth occurred at an outside hospital. When neonates with clinical moderate/severe HIE treated with whole body cooling were compared to controls, mean GFAP levels were significantly elevated in neonatal serum upon admission to the NICU within 6 hours of birth and on days 1, 3 and 4 of life, with P values of 0.032, 0.013, 0.013 and 0.003, respectively. (Figure 1) Persistence of serum GFAP was much greater in the HIE group with 6/23 (26%) controls and 17/23 (74%) of HIE neonates having quantifiable GFAP in the serum from day 1–4 of life (P=0.001). GFAP levels on admission to the NICU in the control neonates did not significantly change with gestational age (R = 0.22, P=0.22). Combining all GFAP values from birth through day 4 of life for the controls showed that the median was 0 ng/mL and the 95th percentile 0.20 ng/mL. None of the controls had a GFAP value above the 95th percentile versus 10/23 (43.4%) cooled neonates (P < 0.001). Of the 23 cooling cases, 4 (17.4%) had abrupt elevations of GFAP the day after the 72 hour cooling regimen was completed.(Figure 1)
Figure 1. Glial fibrillary acidic protein levels for control vs. cooled neonates.

Box plots of glial fibrillary acid protein levels (ng/mL) for control vs. cooled neonates. Number under each box is number of samples available for analysis. *P < .05 compared to controls.
To test GFAP levels upon NICU admission as a screening test for moderate/severe HIE requiring treatment with whole body cooling, ROC curves were generated for various thresholds of GFAP level. NICU admission GFAP ≥ 0.08 ng/ml was the optimal cutoff point to distinguish the groups, and produced an area under the ROC curve of 0.709.
Correlation of GFAP with MRI evidence of brain injury and functional outcome at discharge
All 23 neonates that underwent whole body cooling had a clinical brain MRI during their NICU hospitalization. There was no difference in the time after birth that these MRI scans were performed with a mean (SD) of 7.4 (3.7) days of life in the abnormal MRI group, 7.4 (3.9) days in the normal MRI group (P=0.49). Findings suggestive of HIE were seen in 8/23 (35%) of brain MRI scans. Although those with an abnormal brain MRI were significantly more likely to have been delivered after placental abruption, none of the clinical markers of HIE were associated with an abnormal MRI (Table 2).
Table II.
Summary statistics for neonates with moderate-severe HIE treated with total body cooling comparing neonates with abnormal versus normal MRI brain scans. Means and standard deviations are presented for continuous variables; counts and percentages are presented for categorical variables.
| Abnormal MRI | Normal MRI | P value | |
|---|---|---|---|
| N=8 | N=15 | ||
| Gestational age (weeks) | 37.9±1.3 | 39.0±1.4 | 0.073 |
| Birth weight (grams) | 2807±643 | 3069±625 | 0.353 |
| Nulliparous | 6 (75%) | 8 (53.3%) | 0.400 |
| Cesarean delivery | 7 (87.5%) | 8 (53.3%) | 0.176 |
| IUGR | 0 | 4 (26.7%) | 0.257 |
| Clinical chorioamnionitis | 1 (12.5%) | 2 (13.3%) | 1.00 |
| Nonreassuring FHR tracing | 5 (62.5%) | 3 (20%) | 0.400 |
| Abruption | 6 (75%) | 2 (13.3%) | 0.006* |
| pH | 6.85±0.20 | 6.95±0.19 | 0.322 |
| Base Deficit (mM) | 19.6±4.3 | 13.8±6.9 | 0.104 |
| Cord pH<7.0 and base deficit > 12 | 7/7 (100%) | 9/12 (75%) | 0.263 |
HIE = hypoxic-ischemic encephalopathy
MRI = magnetic resonance imaging
IUGR = intrauterine growth restriction
FHR = fetal heart rate
denotes statistical significance with P<0.05
Serum GFAP levels were consistently elevated in neonates treated with whole body cooling that had abnormal brain MRI scans.(Figure 2) These comparisons were statistically significant on days 1–2 and 4–7 of life, with P values of 0.02, 0.007, <0.001, <0.001 and 0.007, respectively. In 4/8 (50%) of neonates with an abnormal MRI, a significant increase in GFAP levels the day after cooling ended was observed.
Figure 2. Glial fibrillary acidic protein levels for cooled neonates.
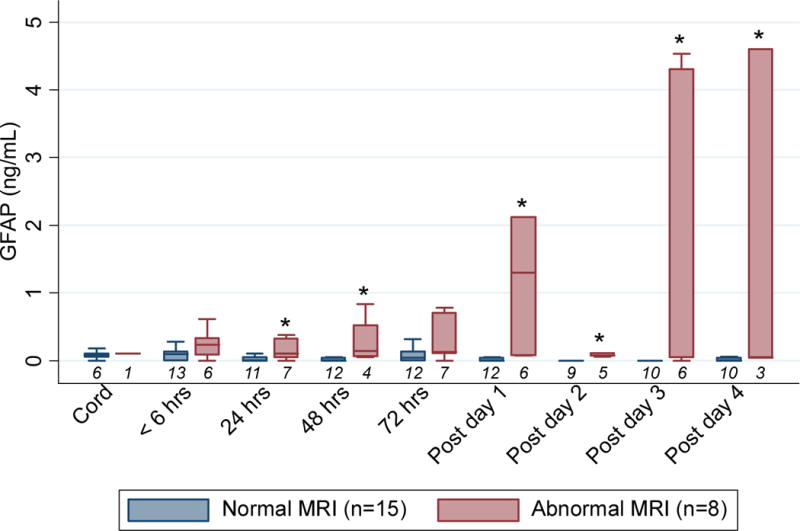
Box plots of glial fibrillary acidic protein levels (ng/mL) for cooled neonates compared by results of brain magnetic resonance imaging. Number under each box is number of samples available for analysis. *P < .05 compared to neonates with normal MRI.
Using ROC analysis a GFAP ≥ 0.15 ng/mL upon NICU admission was the optimal cutoff to identify neonates with abnormal brain MRI scans among all neonates that underwent whole body cooling (area under ROC curve 0.718). This was superior to indicators currently used to identify intrapartum HIE such as nonreassuring fetal heart rate traing (area under ROC curve 0.613), meconium (0.508), 5 minute Apgar < 7 (0.500), and umbilical arterial pH<7.0 or base deficit > 12 mM (0.500).
To relate GFAP levels to functional outcome, time to oral feeding was used.(Table 3) For the 15 neonates that underwent whole body cooling whose MRI was normal, the time to full oral feeds was a mean (SD) of 9.1 (5.4) days. For the 8 neonates with abnormal MRI scans, 1 died at day of life 7, 5 required a gastric tube at a mean (SD) of 114 (81) days of life, and 2 were on full oral feeds at mean (SD) of 17 (9) days of life. Serum GFAP levels on NICU admission were 0.61 ng/mL for the neonate who died, a mean (SD) of 0.26 (0.10) ng/mL for the neonates discharged with gastric feeding assistance (both of which are above the 95th percentile of GFAP level among non-neurologically injured neonates), and a mean (SD) of 0.04 (0.06) ng/mL for the neonates discharged on full oral feeds (P=0.03).
Table 3.
GFAP Levels on Admission and Neurologic Outcomes in Cooled Neonates with Hypoxic-Ischemic Encephalopathy
| N | Age (Days) | GFAP on NICU Admission (ng/mL) | |
|---|---|---|---|
| Abnormal MRI (N=8) | |||
| Death | 1 | 7 | 0.61 |
| G-tube feeding | 5 | 114±81 | 0.26±0.10 |
| Full oral feeding | 2 | 17±9 | 0.04±0.06* |
| Normal MRI (N=15) | |||
| Death | – | – | – |
| G-tube feeding | – | – | – |
| Full oral feeding | 15 | 9±5 | 0.08±0.09 |
P = 0.03 compared to normal MRI.
Among all neonates treated with cooling for moderate/severe HIE, serum GFAP level at NICU admission had an area under the ROC curve of 0.731 in predicting an abnormal brain MRI with sensitivity 50.0%, specificity 84.6%, positive predictive value 60.0% and negative predictive value 78.6%.
Comment
In the present study we describe the first use of a brain-specific protein, GFAP, as a serum biomarker correlating with clinical moderate/severe HIE, development of MRI evidence of brain injury and functional outcome. Although HIE occurs in 2.5/1000 live births at term, and 14.5% of all cases of cerebral palsy are associated with intrapartum hypoxia-ischemia,(23) the timing, duration and outcomes of these injuries are poorly defined. Biomarkers serve as surrogates of disease injury, evolution and outcome. Authors of a systematic review of 110 publications in newborns (> 36 weeks) that had HIE strictly defined, concluded that without biomarkers, the timing, duration, and effectiveness of therapies are ascertained in a relatively blind fashion.(24),(25) They also concluded that prospective studies should focus on determining which biomarkers best identify neonates who will benefit from intervention.
One study assayed cerebrospinal fluid GFAP levels and found them predictive of neonatal death only, not of abnormal outcomes in survivors; however, blood levels of GFAP were not explored.(26) In the present study, using a new sensitive assay for GFAP, we found that GFAP was an early biomarker of HIE. Our control group for this study did not include normal neonates, but was representative of the general NICU population of non-premature ill neonates; therefore the conclusions regarding GFAP as a diagnostic biomarker of HIE may be conservative and highlight the specificity of GFAP for neonatal brain injury in the context of potentially confounding failure of other organ systems.
Several postnatal biomarkers in blood, CSF, and urine have been studied to detect HIE, but most have limitations. The urine lactate:creatinine ratio requires specialized nuclear magnetic resonance technology,(27) CSF is not amenable to serial sampling,(24, 26) and other protein biomarkers, e.g neuron-specific enolase and S100B, have limitations in tissue specificity.(24)
Mild hypothermia immediately after hypoxic-ischemic brain injury preserves cerebral energy metabolism, reduces cytotoxic edema, and improves histologic and functional outcome.(28) A study of term infants with HIE treated with selective head cooling (1) or whole body cooling (29) found that both modes were associated with a decrease in basal ganglia and thalamic lesions, which are predictive of abnormal outcome, and a decrease in severe cortical lesions was seen with selective head cooling. (30) Although cooling in infants with clinical HIE is associated with a reduction in death and neurological impairment,(9) we use it as a “one size fits all” therapy as we have no means of monitoring efficacy while it is being delivered. Hypothermia therapy has been shown not to alter serum GFAP levels after severe traumatic brain injury in children.(31)
We report here that elevated serum GFAP during cooling is significantly associated with evidence of brain injury by MRI. The ability to monitor the level of GFAP during cooling may allow triage of neonates to an escalation of therapy with cooling plus other therapies such as erythropoietin,(2) anti-epileptic drugs (4) and xenon.(3) 50% of neonates with brain injury on MRI in this study had a marked increase in levels of GFAP after completion of the 72 hour cooling protocol. These were also the only neonates that had daily increasing levels of GFAP during cooling making this pattern 100% predictive of an abnormal MRI. This increase after cooling could result from continued brain injury that was suppressed by cooling or could be evidence of rewarming injury. The dramatic increase in GFAP levels seen at the conclusion of the 72 hour cooling period also raises the question as to what is the optimal duration. At this point we have no surrogate markers of therapeutic success, and there are no benchmarks to compare new treatments as they are developed. It is possible that circulating brain protein biomarkers such as GFAP will fill this gap.
Although the use of imaging has greatly improved diagnostic accuracy in brain injury, these tests are limited in the early hours after hypoxic-ischemic injury, and are difficult to perform in critically ill neonates. Consequently, there are settings in which a rapid blood test for brain injury would be invaluable. MRI is the most common clinical test used for evaluation of neonates with HIE and is associated with outcomes.(32) We demonstrated that levels of GFAP upon NICU admission and during cooling are predictive of an abnormal MRI. At this time, because of our small study sample, it is not possible to correlate the degree of brain injury to levels of GFAP, or sensitivity of circulating GFAP for detecting injury in specific brain segments. These questions need to be addressed with larger and more diverse groups of neonatal brain injury patients.
This study has several strengths. The assay used to measure GFAP is reliable, reproducible and highly sensitive. One radiologist using stringent criteria reviewed the MRI studies. There are, however, limitations. We were unable to obtain serum samples on some days for some neonates. This was due to the practical nature of using only serum remaining from clinical samples; this method meant that there was occasionally no or inadequate volume left for testing. The number of missing samples was similar for the cases and controls, mitigating any effect on the conclusions. The first blood draw in the NICU for cooled neonates may have happened after cooling was started, and it is possible that the initial GFAP value could have been suppressed by the hypothermia. We have no historical data to determine how GFAP levels may change in neonates with clinical HIE prior to the standard use of whole body hypothermia.
In summary, GFAP may serve as a biomarker to identify and monitor neonates with clinical HIE receiving cooling therapy. Its predictive ability to identify neonates with brain abnormalities due to HIE is better than currently used clinical indicators. Future studies are necessary to correlate elevated GFAP levels with neurologic disability, as assessed by long term follow up and degree of injury on brain MRI. Based on the results of this study, GFAP could be used to more specifically and sensitively diagnose brain injury at birth, facilitate triage of infants into HIE treatment protocols with hypothermia plus adjuvant treatments, serve as an intermediate outcome to benchmark evolving HIE therapies, and give prognostic information to the parents of these at risk children.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
Reprints not available from authors
References
- 1.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 2.Kim SS, Lee KH, Sung DK, Shim JW, Kim MJ, Jeon GW, et al. Erythropoietin attenuates brain injury, subventricular zone expansion, and sensorimotor deficits in hypoxic-ischemic neonatal rats. J Korean Med Sci. 2008;23:484–91. doi: 10.3346/jkms.2008.23.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke. 2008;39:1307–13. doi: 10.1161/STROKEAHA.107.499822. [DOI] [PubMed] [Google Scholar]
- 4.Glass HC, Ferriero DM. Treatment of hypoxic-ischemic encephalopathy in newborns. Curr Treat Options Neurol. 2007;9:414–23. doi: 10.1007/s11940-007-0043-0. [DOI] [PubMed] [Google Scholar]
- 5.Pimentel-Coelho PM, Mendez-Otero R. Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev. 2010;19:299–310. doi: 10.1089/scd.2009.0403. [DOI] [PubMed] [Google Scholar]
- 6.Larma JD, Silva AM, Holcroft CJ, Thompson RE, Donohue PK, Graham EM. Intrapartum electronic fetal heart rate monitoring and the identification of metabolic acidosis and hypoxic-ischemic encephalopathy. Am J Obstet Gynecol. 2007;197:301.e1–301.e8. doi: 10.1016/j.ajog.2007.06.053. [DOI] [PubMed] [Google Scholar]
- 7.East CE, Chan FY, Colditz PB, Begg LM. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database Syst Rev. 2007;(2):CD004075. doi: 10.1002/14651858.CD004075.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Shah PS, Ohlsson A, Perlman M. Hypothermia to treat neonatal hypoxic ischemic encephalopathy: systematic review. Arch Pediatr Adolesc Med. 2007;161:951–8. doi: 10.1001/archpedi.161.10.951. [DOI] [PubMed] [Google Scholar]
- 9.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva AM, Cootauco AC, Aina-Mumuney A, Donohue PK, Graham EM. The association of hypotonia and depression in the term and near-term neonate with metabolic acidemia. J Perinat Med. 2008;36:151–6. doi: 10.1515/JPM.2008.023. [DOI] [PubMed] [Google Scholar]
- 11.Pelinka LE, Kroepfl A, Leixnering M, Buchinger W, Raabe A, Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J Neurotrauma. 2004;21:1553–61. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko T, Kasaoka S, Miyauchi T, Fujita M, Oda Y, Tsuruta R, et al. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009;80:790–4. doi: 10.1016/j.resuscitation.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Lumpkins KM, Bochicchio GV, Keledjian K, Simard JM, McCunn M, Scalea T. Glial fibrillary acidic protein is highly correlated with brain injury. J Trauma. 2008;65:778–82. doi: 10.1097/TA.0b013e318185db2d. discussion 782–4. [DOI] [PubMed] [Google Scholar]
- 14.Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62:1303–10. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- 15.Bembea MM, Savage WJ, Strouse JJ, Schwartz J, Graham EM, Thompson C, E AD. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. doi: 10.1097/PCC.0b013e3181fe3ec7. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelinka LE, Kroepfl A, Schmidhammer R, Krenn M, Buchinger W, Redl H, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004;57:1006–12. doi: 10.1097/01.ta.0000108998.48026.c3. [DOI] [PubMed] [Google Scholar]
- 17.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadlock FP, Harrist RB, Martinez Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 19.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 20.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 21.Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–42. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 22.Petzold A, Keir G, Green AJ, Giovannoni G, Thompson EJ. An ELISA for glial fibrillary acidic protein. J Immunol Methods. 2004;287:169–77. doi: 10.1016/j.jim.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 24.Ramaswamy V, Horton J, Vandermeer B, Buscemi N, Miller S, Yager J. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatr Neurol. 2009;40:215–26. doi: 10.1016/j.pediatrneurol.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 25.American College of Obstetricians and Gynecologists, American Academy of Pediatricians. Neonatal encephalopathy and cerebral palsy. Washington DC: ACOG; 2003. Chapter 8: Criteria required to define an acute intrapartum hypoxic event as sufficient to cause cerebral palsy; pp. 73–80. [Google Scholar]
- 26.Blennow M, Savman K, Ilves P, Thoresen M, Rosengren L. Brain-specific proteins in the cerebrospinal fluid of severely asphyxiated newborn infants. Acta Paediatr. 2001;90:1171–5. doi: 10.1080/080352501317061594. [DOI] [PubMed] [Google Scholar]
- 27.Oh W, Perritt R, Shankaran S, Merritts M, Donovan EF, Ehrenkranz RA, et al. Association between urinary lactate to creatinine ratio and neurodevelopmental outcome in term infants with hypoxic-ischemic encephalopathy. J Pediatr. 2008;153:375–8. doi: 10.1016/j.jpeds.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoresen M. Cooling the newborn after asphyxia – physiological and experimental background and its clinical use. Semin Neonatol. 2000;5:61–73. doi: 10.1053/siny.1999.0118. [DOI] [PubMed] [Google Scholar]
- 29.Inder TE, Hunt RW, Morley CJ, Coleman L, Stewart M, Doyle LW, et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145:835–7. doi: 10.1016/j.jpeds.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 30.Rutherford MA, Azzopardi D, Whitelaw A, Cowan F, Renowden S, Edwards AD, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116:1001–6. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 31.Fraser DD, Close TE, Rose KL, Ward R, Mehl M, Farrell C, et al. Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum. Pediatr Crit Care Med. 2010 doi: 10.1097/PCC.0b013e3181e8b32d. [DOI] [PubMed] [Google Scholar]
- 32.Barnett A, Mercuri E, Rutherford M, Haataja L, Frisone MF, Henderson S, et al. Neurological and perceptual-motor outcome at 5–6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics. 2002;33:242–8. doi: 10.1055/s-2002-36737. [DOI] [PubMed] [Google Scholar]


