Abstract
FXTAS (fragile X-associated tremor/ataxia syndrome) is a late-onset neurodegenerative disorder that affects individuals who are carriers of premutation expansions (55–200 CGG repeats) in the 5′ untranslated region of the FMR1 (fragile X mental retardation 1) gene. The role of MD (mitochondrial dysfunction) in FXTAS was evaluated in fibroblasts and brain samples from premutation carriers with and without FXTAS symptoms, with a range of CGG repeats. This study resulted in several important conclusions: (i) decreased NAD- and FAD-linked oxygen uptake rates and uncoupling between electron transport and synthesis of ATP were observed in fibroblasts from premutation carriers; (ii) a lower expression of mitochondrial proteins preceded both in age and in CGG repeats the appearance of overt clinical involvement; (iii) the CGG repeat size required for altered mitochondrial protein expression was also smaller than that required to produce brain intranuclear inclusions from individuals with the permutation who died, suggesting that MD is an incipient pathological process occurring in individuals who do not display overt features of FXTAS; and (iv) on the basis of the CGG repeats, MD preceded the increase in oxidative/nitrative stress damage, indicating that the latter is a late event. MD in carriers of small CGG repeats, even when the allele size is not sufficient to produce FXTAS, may predispose them to other disorders (e.g. Parkinson’s disease) that are likely to involve MD, and to environmental stressors, which may trigger the development of FXTAS symptoms. Detection of MD is of critical importance to the management of FXTAS, since it opens up additional treatment options for this disorder.
Keywords: fragile X-associated tremor/ataxia syndrome (FXTAS), mitochondrion, neurodegeneration, oxidative stress, trinucleotide repeat expansion, unfolded protein response
INTRODUCTION
FXTAS (fragile X-associated tremor/ataxia syndrome) is a late-onset neurodegenerative disorder [1–5] that affects individuals who are carriers of premutation expansions (55–200 CGG repeats) in the 5′-UTR (untranslated region) of the FMR1 (fragile X mental retardation 1) gene (OMIM *309550). FXTAS typically affects carriers (males>females) over 50 years of age, with core features of action tremor and gait ataxia, but also (more variably) parkinsonism, executive dysfunction, cognitive decline, neuropathy and autonomic dysfunction. The neuropathological feature of FXTAS is the presence of FMR1 mRNA-containing inclusions in the nuclei of neurons and astrocytes of affected individuals [6,7], consistent with the ‘RNA toxicity’ model of pathogenesis (see [8] for a review). A second premutation-specific disorder, also thought to involve RNA toxicity, is POI (primary ovarian insufficiency), which affects approx. 20% of women who carry premutation alleles [9,10]. Larger expansions (>200 CGG repeats; full mutation) generally result in transcriptional silencing and absence of FMRP (fragile X mental retardation protein) [11–14], leading to fragile X syndrome, the most common heritable form of cognitive impairment and leading known form of autism. Individuals with the full mutation are not at risk of FXTAS or POI, as, in these cases, the toxic FMR1 mRNA is absent or present at low levels.
Several symptoms of FXTAS, including gait ataxia, white matter disease, dysautonomia, peripheral neuropathy, weakness/exercise intolerance and neuropsychiatric involvement, overlap those of MRCDs (mitochondrial respiratory enzyme chain enzyme deficiencies).MRCDs are frequently observed in OXPHOS (oxidative phosphorylation) disorders and can give rise to heterogeneous clinical symptoms ranging from isolated organ dysfunction, such as isolated cardiomyopathy and myopathy with exercise intolerance, to multisystem disorders, such as late-onset optic atrophy and ataxia [8].
To address the possible functional connection between FXTAS and MD (mitochondrial dysfunction), both cultured dermal fibroblasts and brain samples from individuals with the premutation with and without FXTAS, with a range of CGG repeats, were tested for the expression of mitochondrial proteins and the occurrence of modified proteins resulting from increased nitrative/oxidative stress. The results of these studies clearly point to an early involvement of MD in the pathogenesis of FXTAS.
MATERIALS AND METHODS
Chemicals and biochemicals
EDTA, EGTA, sodium succinate, mannitol, sucrose and Hepes were all purchased from Sigma. Tris/HCl, glycine, NaCl and KCl were purchased from Fisher. BSA (fatty-acid-free) was obtained from MP Biomedicals. All other reagents were of analytical grade.
Subject samples
The subjects included in this study were males referred to our centres either because they presented with FXTAS symptoms, including tremor and/or ataxia, or they were either premutation carriers ascertained through families with a fragile X syndrome proband or controls in a similar age group range. All males contributing skin biopsies were participants in a multicentre study to characterize neurological findings in premutation carriers. All studies of post-mortem and fibroblast (biopsy) tissue samples were performed with approved protocols and informed consent in accordance with the Institutional Review Boards of the University of California, Davis, or Rush University Medical Center. Clinical and molecular characteristics of the panel of cultured skin fibroblasts used in this study (Table 1) were cultured and named as described previously [15]. The samples derived from premutation carriers, identified as P6 and P7, have not been described previously.
Table 1.
Characteristics of the cultured primary dermal fibroblasts used in the present study
| Fibroblast ID | Group | Age (years) | CGG repeats | Stage |
|---|---|---|---|---|
| C1 | Control | 60 | 31 | 0 |
| C2 | Control | 75 | 20 | 0 |
| C3 | Control | 57 | 31 | 0 |
| C5 | Control | 65 | 30 | 0 |
| C6 | Control | 58 | 22 | 0 |
| P1 | Pre | 67 | 67 | 0 |
| P2 | Pre | 69 | 74 | 0 |
| P3 | Pre | 67 | 81 | 0 |
| P4 | Pre | 71 | 70 | 0 |
| P5 | Pre | 76 | 67 | 0 |
| P6 | Pre | 75 | 63 | 0 |
| P7 | Pre | 41 | 128 | 0 |
| F1 | FXTAS | 71 | 122 | 3–4 |
| F2 | FXTAS | 70 | 105 | 4 |
| F3 | FXTAS | 60 | 97 | 3 |
| F4 | FXTAS | 75 | 98,113 | 5 |
| F5 | FXTAS | 81 | 78 | 3 |
Cell lines and culture conditions
Skin biopsies were performed with a 3-mm punch under local anaesthesia. The biopsy was diced under sterile conditions and then plated in T25 flasks in AmnioMAX™-C100 basal medium (Gibco) containing 15% AmnioMAX™-C100 supplement (Gibco) at 37°C with a 5% CO2 atmosphere. Longer-term cultures were maintained in RPMI 1640 medium (Gibco) supplemented with 10% (v/v) fetal bovine serum (Gibco) and 1× penicillin/streptomycin medium (100 units/ml penicillin G sodium and 100 µg/ml streptomycin sulfate; Gibco). Protein extracts were obtained from confluent fibroblast cultures (passage number between 7 and 9). Cells were trypsinized (0.25%), resuspended in serum-containing medium, and centrifuged at 200 g for 5 min. Following removal of supernatant, cell pellets were homogenized and resuspended in RIPA buffer (25 mM Mops, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P40, 0.1% SDS and 1% sodium deoxycholate, pH 7.5) at 4 ml/mg (wet pellet weight) with protease and phosphatase inhibitors. Cell viability and cell counts were quantified using Trypan Blue exclusion using a haemocytometer. The viability and number of cells/sample was not analysed for the whole panel before extraction.
Brain samples
Frozen frontal cortex from controls and FXTAS cases were powdered in the presence of liquid N2 and homogenized in a Dounce-style homogenizer with 20 downward strokes of a tight-fitting pestle in RIPA buffer with protease and phosphatase inhibitors. Homogenates were transferred to centrifuge tubes and rotated overnight at 4°C followed by centrifugation at 16000 g at 4°C. RIPA-soluble protein fractions were quantified using the BCA (bicinchoninic acid) protein assay kit (Pierce Biotechnology).
Oxygen uptake
The oxygen uptake of cell suspensions was measured using a Clark-type O2 electrode from Hansatech at 22°C [16]. Intact cells were suspended in 0.5 ml of reaction buffer containing 0.225 M sucrose, 5 mM MgCl2, 20 mM KCl, 10 mM potassium phosphate and 20 mM Hepes/KOH (pH 7.4). Cells were permeabilized by the addition of 60 µg of twice-recrystallized digitonin/5×106 cells for 2 min. The permeabilization was stopped by the addition of 1 mg/ml BSA. Oxygen consumption rates were evaluated in the presence of 1 mM malate and 10 mM glutamate (substrate for NADH oxidase) to record State 4 (without ADP; non-phosphorylating mitochondria), followed by the addition of 1 mM ADP to record State 3 (with ADP; phosphorylating mitochondria) rates. Under these conditions, 5 µM rotenone was then added to inhibit Complex I. The RCR (respiratory control ratio) was obtained by dividing the rate of oxygen consumption in State 3 (expressed as nmol of oxygen/min per mg of protein, obtained with a respiratory substrate and ADP) by that of State 4 (in the presence of a substrate without ADP).
Citrate synthase activity
The assay was performed at 412 nm following the reduction in 0.1 mM 5,5′-dithiobis-(2-nitrobenzoic acid) in the presence of 2–8 µg of cell lysate and 0.2 mM acetyl-CoA in a medium with 10 mM Tris/HCl (pH 8.1) and 0.2% Triton X-100. The reaction was started by adding 0.5 mM oxaloacetic acid. The absorbance changes were followed in a Molecular Devices Spectramax M2 plate reader using the Soft Max Pro software version 4.7.1. Data points were taken every 34 s for 5 min. Rates were calculated from the linear part of ΔA/min against mg of protein plots and using a molar absorption coefficient of 11400 M−1 · cm−1.
Ca2+ concentrations
Cytosolic and intramitochondrial free Ca2+ concentrations were evaluated as described previously using Fura-2 [17,18].
Western blots
All Western blot procedures were performed essentially as described previously [19] with the modifications indicated below. Protein extracts from brain sample homogenates were concentrated and partly delipidated by acetone precipitation, through the addition of four volumes of −20°C acetone to each homogenate. Acetone-containing mixtures were vortex-mixed and placed at −20°C for 24 h. Samples were then centrifuged at 16000 g for 10 min at 4°C. After pouring off the supernatant, the pellet was resuspended and washed twice more with −20°C acetone, spinning each wash at 16000 g for 10 min at 4°C. After removing the supernatant from the final wash, the samples were placed in a SpeedVac for 15 min to remove residual acetone. Fibroblast protein extracts obtained as described above were used without further extraction. Samples were resuspended in RIPA buffer and the protein concentration was evaluated using the BCA protein assay kit. Proteins from brain and fibroblasts were denatured in SDS/PAGE sample buffer (Bio-Rad Laboratories) plus 1.5% DTT (dithiothreitol) at 100°C for 3 min. For protein normalization, increasing amounts of total cell protein (2–10 µg) were added to successive lanes in the SDS/PAGE, transferred on to membranes and probed with specific antibodies (see Supplementary Table S1 at http://www.BiochemJ.org/bj/429/bj4290545add.htm for details). The loaded protein amounts were plotted against the densitometry readings to ensure that the ECL (enhanced chemiluminescence) response was within a linear range of the protein range. All values were normalized to actin or VDAC1 (voltage-dependent anion channel 1) as a loading control. Membranes were visualized with ECL reagents on a Kodak 2000 MM Imager. Images were analysed with the Kodak Imager 2000 MM software.
Statistical analyses
The experiments were run in duplicate or triplicate and repeated three times in independent experiments unless noted otherwise. Results are expressed as either means±S.E.M. or as means if S.E.M. were ≤12% of mean. Results were evaluated by ANOVA using StatSimple v2.0.5 (Nidus Technologies), considering P≤0.05 as statistically significant, unless indicated otherwise.
RESULTS
Lower coupling between electron transport and ATP production in cultured fibroblasts from FXTAS patients
Primary cultured skin fibroblasts from 12 male premutation carriers, five with FXTAS (identified in Tables as FXTAS) and seven without FXTAS symptoms (asymptomatic; identified in Tables as Pre), and five controls (Table 1) were studied. Considering that measured OXPHOS capacity declines considerably with subject age in human skeletal muscle mitochondria [20–22], subjects of similar age were selected to be able to compare disease and normal subject mitochondrial parameters. There was no statistical difference in age between the control group and the two groups of premutation carriers that donated skin fibroblasts through punch biopsy (controls, 63±3; Pre 67±4; FXTAS 71±3; P>0.05 by ANOVA and by multiple comparisons Bonferroni t test). For the fibroblast samples, there was no significant difference between CGG repeat numbers between premutation carriers with and without FXTAS symptoms (102±7 using a mean CGG repeat value for F4 and 79±9; P>0.05), but both groups were significantly different from controls (CGG repeat for controls 27±2 with P<0.05).Cultured skin fibroblasts from controls (C1–C3 and C5 in Table 1) and patients with FXTAS (F2–F4 in Table 1) were evaluated for biochemical parameters to assess mitochondrial function. The rates of oxygen uptake under State 3 (phosphorylating conditions) and State 4 (non-phosphorylating conditions) of mitochondria from controls and FXTAS fibroblasts were evaluated with an NAD-linked substrate (malate/glutamate) (Table 2).
Table 2.
Rates of oxygen uptake in State 3 and State 4 and RCR in primary FXTAS and control fibroblasts
| Oxygen uptake (nmol of O2/min per 106 cells) |
|||
|---|---|---|---|
| State 4 | State 3 | RCR | |
| Controls | 0.9 ± 0.2 | 9 ± 2 | 10 ± 2 |
| FXTAS | 2.6 ± 0.6 | 5.1 ± 0.9 | 2.0 ± 0.4 |
| P value | 0.04 | 0.007 | 10−5 |
Results are means ± S.E.M. obtained with control samples C1–C6 and FXTAS samples F1–F5 by supplementing mitochondria with malate–glutamate as a respiratory substrate. Experiments were performed in triplicate. The P values were obtained using Student’s t test. Other experimental details are described in the Materials and methods section.
The process responsible for mitochondrial oxygen consumption during State 4 respiration, a state when ADP is limited and its phosphorylation has ceased [23], is the proton leak or leakage [24]. In the FXTAS fibroblasts, the oxygen uptake in State 4 was 2.7-fold that of controls (Table 2), indicating that proton leak at the inner membrane is an important process in these cells. The rate of oxygen uptake in State 3 in FXTAS cells was 57% of controls, indicating a lower OXPHOS capacity with NAD-linked substrates. Similar results were obtained with an FAD-linked substrate (i.e. succinate), suggesting that the defect was located at the OXPHOS pathway shared by NAD- and FAD-linked substrates (i.e. Complexes III–V).
The RCR evaluates the coupling between electron transfer and OXPHOS, and remains the best indicator of the membrane integrity of mitochondria [25,26]. Fully uncoupled mitochondria have RCR values of 1 [23,27]. The RCR of FXTAS cells was 2, whereas that of control cells was 10, indicating that the mitochondria from FXTAS cells were ~90% uncoupled (Table 2). Given that uncoupling can be promoted by Ca2+ overload, the free Ca2+ concentration in the cytosol and mitochondria of controls and FXTAS cells was evaluated as indicated in the Materials and methods section. In controls, the free Ca2+ concentrations were higher in both cytosol and mitochondria than those found in FXTAS cells (cytosolic free Ca2+: control cells, 221±34 pmol of Ca2+/106 cells; FXTAS, 108±6 pmol of Ca2+/106 cells; P=0.023; mitochondrial free Ca2+: control cells, 9±1 pmol of Ca2+/106 cells; FXTAS, 3±2 pmol of Ca2+/106 cells; P=0.04). The lower level of Ca2+ in mitochondria supports the concept that the functional defect observed in FXTAS cells was not a consequence of Ca2+ overload, despite the fact that there seems to be a Ca2+ dysregulation when compared with controls.
The uncoupling, caused mainly by an increase in State 4 oxygen uptake rate in addition to an inhibition of respiration in State 3, suggested that some of the Complexes could be deficient (e.g. III–V) in FXTAS causing an increase in ROS and decrease in ATP production via OXPHOS.
Expansion repeat thresholds for clinical diagnosis, presence of intranuclear inclusions in CNS (central nervous system) tissue and MD in fibroblasts and CNS
The levels of protein expression of three mitochondrial proteins were each determined for control and FXTAS samples indicated in Table 1. These were ATPB (ATPase β-subunit) from Complex V, CCOIV (cytochrome c oxidase subunit IV) from Complex IV and MnSOD (manganese superoxide dismutase) as part of the mitochondrial antioxidant defence. The results indicated that all three proteins were decreased in FXTAS samples when compared with controls, although to different extents, with CCOIV being the most affected (63% decrease; Table 3), followed by ATPB (25% decrease; Table 3) and MnSOD (13% decrease; Table 3). The decline of ATPB and CCOIV proteins was consistent with the oxygen uptake data obtained with cells derived from patients with FXTAS (Table 2).
Table 3.
Mitochondrial protein expression in FXTAS and control primary fibroblasts
| Protein expression | Percentage of controls |
|---|---|
| CCOIV (Complex IV) | 37 ± 2 (0.004)* |
| ATPB (Complex V) | 75 ± 3 (0.002)* |
| MnSOD | 87 ± 43 (0.05)* |
Results are expressed as percentages of control values (means ± S.E.M.) performed with three to five replicates. Numbers with asterisks were significantly different from controls. The individual P values obtained using Student’s t test are indicated in parentheses. Western blots were performed on controls C1–C6 and FXTAS F1–F5 (Table 1).
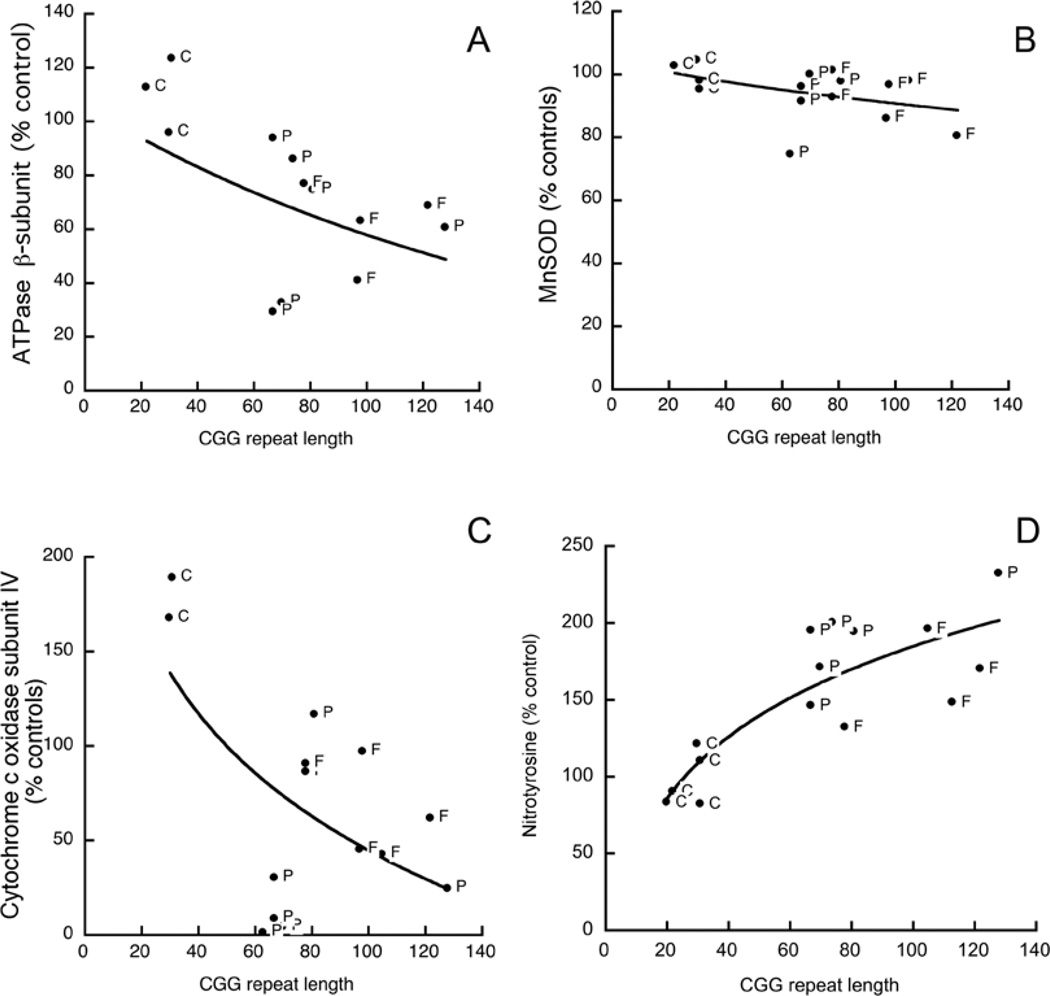
The decline observed with all three proteins in premutation carriers with FXTAS was also observed in premutation carriers without FXTAS symptoms (Figures 1A–1C). If individual outcomes for each parameter (ATPB and CCOIV) were plotted against the CGG repeat size, the protein contents followed exponential decay functions (Figures 1A and 1C; r2 = 0.87 and 0.70 respectively). It could be argued that the lower expression of mitochondrial proteins indicated a simple decrease in mitochondrial numbers. To address this issue, citrate synthase activity, which is considered to be a relatively stable enzyme activity proportional to mitochondrial numbers [28], was not significantly different among the three experimental groups (10±1, 10±1 and 7.4±0.69; P>0.127).
Figure 1. Mitochondrial protein expression and nitrotyrosine content in ATPB in cultured primary dermal fibroblasts from control, premutation carriers and FXTAS patients.
Changes in ATPB (A), MnSOD (B), CCO IV (C) and nitrotyrosine in ATPB (D) as a function of CGG repeat length in fibroblasts from premutation carriers with (F) or without (P) FXTAS symptoms, or controls (C; controls with <40 CGG repeats). All results are expressed as the percentage of mean control values. All samples from Table 1 were tested for these parameters and those excluded were due to limited sample amount, lack of linear response between protein loading and ECL response, or because they were not different from controls. Actin was used as a loading control.
On the basis of these results, it was important to estimate the CGG threshold required to detect MD relative to the corresponding thresholds for clinical (FXTAS) diagnosis and for the appearance of intranuclear inclusions, to ascertain the probable causal (and temporal) relationships between MD and disease phenotype. The CGG repeat number required to obtain a 50% reduction in protein expression (CCOIV and ATPB) was 58±2.
The expression of MnSOD, an antioxidant enzyme that dismutates superoxide anion to hydrogen peroxide, was also decreased in FXTAS fibroblasts, but to a lesser extent than the other two mitochondrial proteins (14%; P=0.05; Figure 1B). Considering that a decline in MnSOD could lead to an increase in superoxide anion steady-state concentration, and a consequent increase in peroxynitrite or other nitrating agents, the fibroblast content of nitrotyrosine was evaluated in all samples from Table 1. Tyrosine nitration, dityrosine and tyrosine oxidation products are considered to be markers for protein damage during increased oxidative/nitrative stress in biological systems [29]. In the current instance, principally five proteins (molecular masses 70, 58, 54, 47 and 40 kDa) were nitrated in both controls and FXTAS fibroblasts. However, the extent of tyrosine nitration was increased an average 1.7-fold (P<0.05) in the FXTAS fibroblasts relative to control cells. The nitrated protein at 54 kDa was identified by MS as ATPB. Recently, we have found that nitration of specific tyrosine residues in the ATPB could be used as a biomarker for nitrative/oxidative stress because of the low pKa of the phenolic group, the abundance of ATPB and the correlation between nitration and activity loss [30,31]. Therefore we evaluated the nitration of the ATPB in samples from Table 1 (Figure 1D). The [nitrotyrosine]/[ATPB] was elevated approx. 2-fold in both premutation and FXTAS cells when compared with controls (P=0.05), but was not different from each other (Figure 1D). The content of nitrotyrosine from these cells and the CGG repeat length fitted a rectangular hyperbola (r2 = 0.70; Figure 1D). The CGG repeat number required to double the nitrotyrosine content was 84±10 (Figure 1D). These results indicated that, in FXTAS cells, there is increased protein nitration due to an increased oxidative/nitrative stress.
Decreased mitochondrial protein levels in frontal cortex from post-mortem FXTAS cases mirrors the findings in skin fibroblasts
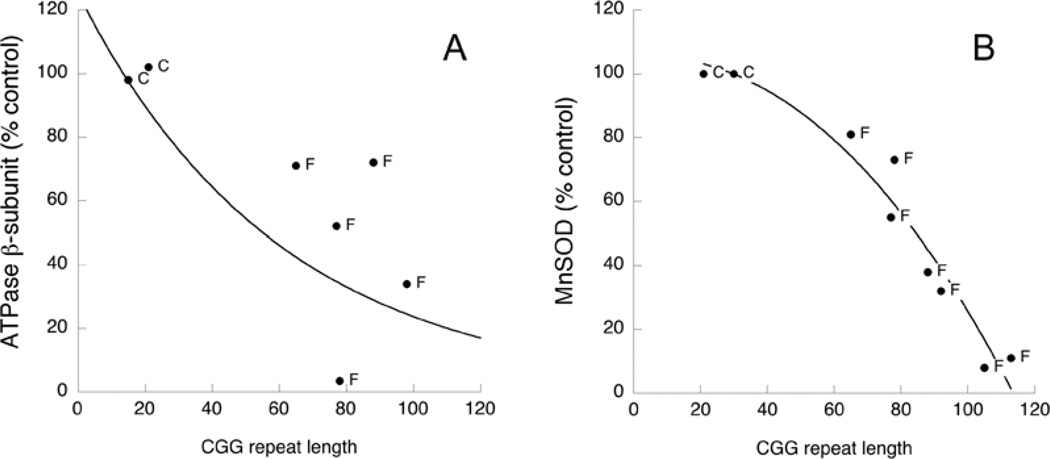
We tested whether mitochondrial protein expression is altered in frontal cortex with increased CGG repeat lengths (as observed previously with dermal fibroblasts). To address this possibility, we evaluated by Western blotting the expression of both ATPB and MnSOD in brain cortex samples from controls (n=4; 58±4-years-old) and FXTAS patients (n=8; 75±2-years-old) (Table 4 and Figure 2). Brain samples from control patients had 24±3 CGG repeats and those from the FXTAS group had 90±6 (P<0.05). The expression of the mitochondrial proteins ATPB and MnSOD both decreased linearly with increasing CGG repeat lengths (Figure 2; r2 = 0.56 and 0.88 respectively). The CGG repeat length to obtain 50% protein expression was 67±3 CGG repeats, similar to the number obtained with skin fibroblasts (58±2 CGG repeats; see above).
Table 4.
Characteristics of the brain samples used in the present study
| Brain ID | Group | Age (years) | CGG repeats |
|---|---|---|---|
| BC1 | Control | 69 | 30 |
| BC2 | Control | 57 | 28 |
| BC3 | Control | 53 | 21 |
| BC4 | Control | 54 | 15 |
| Case 1 | FXTAS | 70 | 113 |
| Case 3 | FXTAS | 75 | 78 |
| Case 4 | FXTAS | 87 | 65 |
| Case 5 | FXTAS | 66 | 105 |
| Case 6 | FXTAS | 77 | 77 |
| Case 8 | FXTAS | 75 | 92 |
| Case 9 | FXTAS | 81 | 88 |
| Case 10 | FXTAS | 68 | 98 |
Clinical and molecular characteristics of the panel of post-mortem brain samples (frontal cortex) used in the present study correspond in case number to those presented previously [15], except for the control sample BC4. Case BC4 was obtained from the NICHD Brain and Tissue Bank. The post-mortem intervals for cases affected with FXTAS were shorter (approx. 12 h or less, except for BC4 which was 17 h) than control cases (between 12 and 17 h). Brain samples were kept at −80°C until the protein samples were obtained. The mean CGG repeat size for FXTAS samples was significantly different from controls (24 ± 7 for controls and 90 ± 16 for FXTAS; P =1.4 × 10−5).
Figure 2. Mitochondrial protein expression in frontal brain cortex from controls and FXTAS patients.
Changes in ATPB (A) and MnSOD (B) as a function of CGG repeat length in fibroblasts from premutation carriers with (F) or without (P) FXTAS symptoms, or controls (C; controls with <40 CGG repeats). All samples from Table 4 were tested for these parameters and those excluded were due to limited sample amount, lack of linear response between protein loading and ECL response, or because they were not different from controls. All results are expressed as the percentage of mean control values. VDAC1 was used as a loading control.
The CGG repeat required to achieve a 50% decrease in mitochondrial protein expression in fibroblasts (~58 CGG repeats; Figure 1) was lower than the lower limit for premutation expansions in our cohort (63–128 CGG repeats; Table 1), indicating that mitochondrial changes are observed at lower expansion repeats than those detected by existing clinical criteria.
The number of CGG repeats required for decreased brain mitochondrial protein expression was also lower (58 from Figure 2 compared with ≥70 from [6,7]) than those required to produce significant numbers of intranuclear inclusions in the brains of individuals with FXTAS who had died [7], as is the CGG repeat number for nitrotyrosine content, which is doubled at approx. 84 CGG repeats. In addition, the curve of mitochondrial protein expression against CGG repeat number (Figures 1A–1C) suggests that inclusion formation [7] appears at a repeat number where more than 80% of mitochondrial protein expression had already been lost. Thus inclusions may be a very late event in the course of the disease.
The loss of several mitochondrial proteins in brain samples (Figure 2) was consistent with the observations made with skin fibroblasts (Figure 1) and in skeletal muscle biopsies from FXTAS and premutation carriers (results not shown), indicating that the severity of the phenotype follows either the relevance of OXPHOS for that particular tissue or the tissue-specific expression of critical components of mitochondrial function.
It is important to note that not all samples from each panel followed this pattern of MD. For the fibroblasts, two samples out of 12 (17%) presented some or all properties that were similar to controls; for the brain samples, only one exhibited characteristics similar to the control brains (one out of eight or 12.5%) for all tested parameters. This suggests that the MD could be present as a mosaic, as observed with other mitochondrial diseases [32], or that, under culture conditions (i.e. for fibroblasts), the phenotype is not evident [33]. The current observations nevertheless indicate that the preponderance of FXTAS and premutation carriers with/without FXTAS (approx. 83%) presented a MD.
Decreased protein translation in FXTAS
Accumulated proteins present in inclusions with overexpressed FMR1 mRNA had been observed in the CNS of FXTAS patients at late stages of the disease [34]. In the present study, we have observed accumulation of oxidatively modified mitochondrial proteins. We reasoned that accumulation of misfolded/unprocessed proteins could lead to the activation of the UPR (unfolded protein response) [35]. Recent reports show that dysregulation of the UPR is implicated in several pathologies, including some neurodegenerative diseases and cerebral ischaemia [36]. Considering that, upon activation of the UPR, PERK [PKR (RNA-dependent protein kinase)-like ER (endoplasmic reticulum) kinase] displays two known activities, autophosphorylation and phosphorylation of its physiological substrate, the α subunit of the heterotrimeric eIF2 (eukaryotic translational initiation factor 2), inhibiting protein synthesis at the level of initiation [37], we tested the hypothesis that the activation of the UPR in premutation cells results in an increase in eIF2α phosphorylation, leading to a decrease in protein translation. To this end, the phosphorylation of eIF2α was assessed in samples from Table 1. The phosphorylation of eIF2α in FXTAS samples (F3 and F6–F8) was significantly increased relative to controls (216% of control values; P=0.0398). Premutation carrier samples without FXTAS symptoms (P1 and P3–P5) had an eIF2α phosphorylation equal to 271% of control values (P=0.018), but not different from FXTAS (P=0.78). These results indicate that phosphorylation of eIF2α in premutation cells (regardless of the presence of FXTAS symptoms in patients) might result in a decreased protein translation in an attempt to overcome the accumulation of misfolded/unprocessed proteins.
DISCUSSION
In the present study, we have shown that abnormal mitochondrial function and protein expression was observed in both CNS and non-CNS (dermal fibroblasts) tissues from patients with the neurodegenerative disorder FXTAS. We observed decreased NAD- and FAD-linked oxygen uptake rates, uncoupling between electron transport and synthesis of ATP (Table 2), decreased mitochondrial protein expression (Figures 1A–1C and 2), and increased oxidative/nitrative stress (increased mitochondrial protein nitration and decreased MnSOD protein; Figure 1D). All of these biochemical characteristics point to a lower ATP production that would particularly affect brain because of its high dependence on OXPHOS of glucose, although other tissues could be affected as well [38]. The lower OXPHOS capacity would be expected to add to the co-morbidity of the disease progression, resulting in the decline in energy-dependent processes.
The lower ATP production by FXTAS mitochondria, especially in highly aerobic tissues such as brain [38], will have an impact on several cellular functions, among them the activity of SERCAs (sarcoplasmic/endoplasmic reticulum Ca2+-ATPases), thus probably resulting in the depletion of ER Ca2+ stores. This mechanism may explain the difference in matrix Ca2+ found between FXTAS and control mitochondria. Although mitochondria and ER have been viewed as independent subcellular compartments, and the functions of both structures have been investigated in isolated systems, imaging techniques have provided evidence that the ER has close physical and functional contact to mitochondria [39], in which unimpaired interaction between ER and mitochondria is essential for correct cell functioning [39]. For instance, low OXPHOS capacity could lead to a defective Ca2+ homoeostasis and the release of Ca2+ from InsP3-sensitive stores upon activation of Group I mGluR (metabotropic glutamate receptor) [40,41] in anterior cingulate cortex [42] (a key region of brain cognitive and executive functions) could be hampered as well as other Ca2+-mediated processes required for normal neuron function from plasmalemmal excitability to synaptic plasticity [43].
The expression levels of mitochondrial proteins were generally decreased in FXTAS, consistent with the general decrease in protein synthesis mediated by the higher eIF2α phosphorylation observed in these cells. However, not all of the proteins tested were affected to a similar extent. It has been reported that intracellular levels of proteins with relatively rapid degradation or turnover could be critically affected by translation inhibition/attenuation as has been shown for Mcl-1 (myeloid cell leukaemia 1) [44]. By analogy, it could be proposed that CCOIV is a protein with a relatively fast turnover (when compared with ATPB and MnSOD), whose steady-state concentrations would be profoundly affected by the dysregulation of the translation machinery. Thus proteins that have a faster degradation (or turnover) might act as convergence points of several diverse apoptotic stimuli, coupling directly global translation to cell survival or apoptosis.
It is important to note that the mitochondrial changes observed in FXTAS were also present, although to a lesser extent, in premutation carriers without FXTAS, suggesting that these events are early and their onset at the molecular level is probably earlier than the onset of clinical symptoms. A hallmark of FXTAS pathology is the presence of intranuclear inclusions throughout the brain, and in other tissues as well. The accumulation of oxidatively modified proteins, even at early stages when no inclusions are observed, might activate the UPR via PERK and ATF6 (activating transcription factor 6). PERK activation should result in the inhibition of protein translation via phosphorylation of eIF2α.
The present study suggests that the occurrence of nitrated proteins, and perhaps intranuclear inclusions as markers for oxidative/nitrative stress, is secondary to, or follows by a significant period (perhaps years), the onset of MD. It is also possible that the intranuclear inclusions found in FXTAS patients might result from the accumulation over time of oxidatively modified proteins that aggregate and are not processed by the proteasome [45]. The observations of the present study, taken together, indicate that MD and subsequent oxidative/nitrative stress are likely to precede both in age and in CGG repeat number the appearance of overt clinical involvement. This last proposal is of critical importance to the detection and management of FXTAS, since it suggests that early detection of MD may afford early intervention. We speculate that a therapeutic strategy that induces the UPR might prevent neuronal death induced by ER stress and ultimately by low ATP from OXPHOS.
Interestingly, some premutation carriers (15–40%) exhibit signs of autistic behaviour [46,47], attributed to the co-segregation of 15 loci for FMRP cargo (mRNAs) in FXTAS and autism [48]. We have found recently that typical autistic children (without expanded FMR1 alleles) shared many aspects of MD with the defects reported in this study (C. Giulivi, Y.-F. Zhang, A. Omanska-Klusek, C. M. Ross-Inta, S. Wong, I. Hertz-Picciotto, F. Tassone and I. N. Pessah, unpublished work). Thus polymorphisms in some of the FMR1 RNA cargos may contribute to both FXTAS and autism spectrum disorders.
Finally, the results of the present study provide a possible functional basis for the recent observation that the frequencies of small CGG repeat expansions, in the grey zone (40–54 CGG repeats) and low–mid premutation range (<100 CGG repeats) are much higher in individuals with iPD (idiopathic Parkinson’s disease) and other forms of parkinsonism than in the general population [49]. Loesch et al. [49] found that premutation and grey zone alleles were at least 5-fold higher in a screened population of 228 males with iPD (87% of cases) or other forms of parkinsonism. Indeed, all four premutation alleles (56, 58, 83 and 87 CGG repeats) and nine out of ten grey zone alleles were initially diagnosed with iPD. The results of Loesch et al. [49], taken together with those of the present study, raise two important issues. (i) Mitochondrial dysregulation (e.g. decreased Complex I function) in carriers of small CGG-repeat expansions may predispose such individuals to other disorders (e.g. Parkinson’s disease/parkinsonism) that are likely to involve MD, even when the allele size is not sufficient to produce FXTAS. (ii) Subclinical MD may also predispose such carriers to environmental stressors, which may in turn contribute to both the penetrance and the severity of clinical involvement in FXTAS. A better understanding of this latter issue might help us to define which carriers are most likely to develop FXTAS and to design targeted preventative therapies.
Supplementary Material
ACKNOWLEDGEMENTS
We express our gratitude to the patients and families that participated in the study.
FUNDING
This study was supported by funds provided by Autism Speaks Foundation [grant number 58739 (to C.G.)], the National Institute on Aging [grant number AG024488 (to P. J.H.)], and by the National Institutes of Health Interdisciplinary Research Consortium (IRC) [grant numbers RL1 AG032119 and UL1 DE19583 (to P. J.H.); and AG032115 (to R. J.H.)].
Abbreviations used
- ATPB
ATPase β-subunit
- BCA
bicinchoninic acid
- CCOIV
cytochrome c oxidase subunit IV
- CNS
central nervous system
- ECL
enhanced chemiluminescence
- eIF2α
eukaryotic translational initiation factor 2α
- ER
endoplasmic reticulum
- FMR1
fragile X mental retardation 1
- FMRP
fragile X mental retardation protein
- FXTAS
fragile X-associated tremor/ataxia syndrome
- iPD
idiopathic Parkinson’s disease
- MD
mitochondrial dysfunction
- MnSOD
manganese superoxide dismutase
- MRCD
mitochondrial respiratory enzyme chain enzyme deficiency
- OXPHOS
oxidative phosphorylation
- PERK
PKR (RNA-dependent protein kinase)-like ER kinase
- POI
primary ovarian insufficiency
- RCR
respiratory control ratio
- RONS
reactive oxygen and nitrogen species
- UPR
unfolded protein response
- VDAC1
voltage-dependent anion channel 1
Footnotes
AUTHOR CONTRIBUTION
Catherine Ross-Inta performed all biochemical assays and Western blots on fibroblast cells, processed data and analysed them statistically. Alicja Omanska-Klusek and Sarah Wong performed genomic DNA extractions, quantifications and protein determinations on samples. Cedrick Barrow performed all biochemical assays and Western blots on brain samples. Dolores Garcia-Arocena cultured the cells and provided them to Cecilia Giulivi’s laboratory; she also performed the genotyping of cells. Christine Iwahashi developed most of the dermal fibroblast lines used in the study (some provided by Elizabeth Berry-Kravis), and provided brain samples to Cecilia Giulivi’s laboratory. Elizabeth Berry-Kravis and Randi Hagerman obtained the samples from patients and provided all clinical information relevant to the study. Cecilia Giulivi, Randi Hagerman and Paul Hagerman discussed the possibility of mitochondrial involvement in FXTAS. Paul Hagerman partially funded the research effort, provided his expertise in FXTAS, discussed experiments and participated in the writing of the paper. Cecilia Giulivi provided her expertise in bioenergetics in physiopathology, designed all experiments, supervised their execution, analysed data, partially funded this project and wrote the paper.
REFERENCES
- 1.Berry-Kravis E, Goetz CG, Leehey MA, Hagerman RJ, Zhang L, Li L, Nguyen D, Hall DA, Tartaglia N, Cogswell J, et al. Neuropathic features in fragile X premutation carriers. Am. J. Med. Genet. A. 2007;143:19–26. doi: 10.1002/ajmg.a.31559. [DOI] [PubMed] [Google Scholar]
- 2.Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 3.Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am. J. Hum. Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA, J. Am. Med. Assoc. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 5.Amiri K, Hagerman RJ, Hagerman PJ. Fragile X-associated tremor/ataxia syndrome: an aging face of the fragile X gene. Arch. Neurol. 2008;65:19–25. doi: 10.1001/archneurol.2007.30. [DOI] [PubMed] [Google Scholar]
- 6.Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 7.Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) . Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum. Mol. Genet. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study - preliminary data. Am. J. Med. Genet. 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz CE, Dean J, Howard-Peebles PN, Bugge M, Mikkelsen M, Tommerup N, Hull C, Hagerman R, Holden JJ, Stevenson RE. Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am. J. Med. Genet. 1994;51:400–402. doi: 10.1002/ajmg.1320510419. [DOI] [PubMed] [Google Scholar]
- 11.Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 12.Beckel-Mitchener A, Greenough WT. Correlates across the structural, functional, and molecular phenotypes of fragile X syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:53–59. doi: 10.1002/mrdd.20009. [DOI] [PubMed] [Google Scholar]
- 13.El-Osta A. FMR1 silencing and the signals to chromatin: a unified model of transcriptional regulation. Biochem. Biophys. Res. Commun. 2002;295:575–581. doi: 10.1016/s0006-291x(02)00682-4. [DOI] [PubMed] [Google Scholar]
- 14.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Arocena D, Yang JE, Brouwer JR, Tassone F, Iwahashi C, Berry-Kravis EM, Goetz CG, Sumis AM, Zhou L, Nguyen DV, et al. Fibroblast phenotype in male carriers of FMR1 premutation alleles. Hum. Mol. Genet. 2010;19:299–312. doi: 10.1093/hmg/ddp497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross-Inta CM, Zhang YF, Almendares A, Giulivi C. Threonine-deficient diets induced changes in hepatic bioenergetics. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1130–G1139. doi: 10.1152/ajpgi.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solien J, Haynes V, Giulivi C. Differential requirements of calcium for oxoglutarate dehydrogenase and mitochondrial nitric-oxide synthase under hypoxia: impact on the regulation of mitochondrial oxygen consumption. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005;142:111–117. doi: 10.1016/j.cbpb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Traaseth N, Elfering S, Solien J, Haynes V, Giulivi C. Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle. Biochim. Biophys. Acta. 2004;1658:64–71. doi: 10.1016/j.bbabio.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Elfering SL, Haynes VL, Traaseth NJ, Ettl A, Giulivi C. Aspects, mechanism, and biological relevance of mitochondrial protein nitration sustained by mitochondrial nitric oxide synthase. Am. J. Physiol. Heart. Circ. Physiol. 2004;286:H22–H29. doi: 10.1152/ajpheart.00766.2003. [DOI] [PubMed] [Google Scholar]
- 20.Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- 21.Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J. Neurol. Sci. 1992;113:91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- 22.Boffoli D, Scacco SC, Vergari R, Solarino G, Santacroce G, Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim. Biophys. Acta. 1994;1226:73–82. doi: 10.1016/0925-4439(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 23.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 24.Brand MD, Hafner RP, Brown GC. Control of respiration in non-phosphorylating mitochondria is shared between the proton leak and the respiratory chain. Biochem. J. 1988;255:535–539. [PMC free article] [PubMed] [Google Scholar]
- 25.Estabrook RW. Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. Methods Enzymol. 1967;10:41–47. [Google Scholar]
- 26.Sherratt HS, Watmough NJ, Johnson MA, Turnbull DM. Methods for study of normal and abnormal skeletal muscle mitochondria. Methods Biochem. Anal. 1988;33:243–335. doi: 10.1002/9780470110546.ch6. [DOI] [PubMed] [Google Scholar]
- 27.Chance B, Williams GR. A simple and rapid assay of oxidative phosphorylation. Nature. 1955;175:1120–1121. doi: 10.1038/1751120a0. [DOI] [PubMed] [Google Scholar]
- 28.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiMarco T, Giulivi C. Current analytical methods for the detection of dityrosine, a biomarker of oxidative stress, in biological samples. Mass Spectrom. Rev. 2007;26:108–120. doi: 10.1002/mas.20109. [DOI] [PubMed] [Google Scholar]
- 30.Fujisawa Y, Kato K, Giulivi C. Nitration of tyrosine residues 368 and 345 in the β-subunit elicits FoF1-ATPase activity loss. Biochem. J. 2009;423:219–231. doi: 10.1042/BJ20090594. [DOI] [PubMed] [Google Scholar]
- 31.Haynes V, Traaseth NJ, Elfering S, Fujisawa Y, Giulivi C. Nitration of specific tyrosines in FoF1 ATP synthase and activity loss in aging. Am. J. Physiol. Endocrinol. Metab. 2010;298:E978–E987. doi: 10.1152/ajpendo.00739.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soong NW, Hinton DR, Cortopassi G, Arnheim N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat. Genet. 1992;2:318–323. doi: 10.1038/ng1292-318. [DOI] [PubMed] [Google Scholar]
- 33.Robinson BH. Use of fibroblast and lymphoblast cultures for detection of respiratory chain defects. Methods Enzymol. 1996;264:454–464. doi: 10.1016/s0076-6879(96)64041-5. [DOI] [PubMed] [Google Scholar]
- 34.Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129:256–271. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- 35.Prostko CR, Dholakia JN, Brostrom MA, Brostrom CO. Activation of the double-stranded RNA-regulated protein kinase by depletion of endoplasmic reticular calcium stores. J. Biol. Chem. 1995;270:6211–6215. doi: 10.1074/jbc.270.11.6211. [DOI] [PubMed] [Google Scholar]
- 36.Kudo T, Katayama T, Imaizumi K, Yasuda Y, Yatera M, Okochi M, Tohyama M, Takeda M. The unfolded protein response is involved in the pathology of Alzheimer’s disease. Ann. N.Y. Acad. Sci. 2002;977:349–355. doi: 10.1111/j.1749-6632.2002.tb04837.x. [DOI] [PubMed] [Google Scholar]
- 37.Hershey JW. Translational control in mammalian cells. Annu. Rev. Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- 38.Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat. Rec. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- 39.Rutter GA, Rizzuto R. Regulation of mitochondrial metabolism by ER Ca2+ release: an intimate connection. Trends Biochem. Sci. 2000;25:215–221. doi: 10.1016/s0968-0004(00)01585-1. [DOI] [PubMed] [Google Scholar]
- 40.Rae MG, Martin DJ, Collingridge GL, Irving AJ. Role of Ca2+ stores in metabotropic l-glutamate receptor-mediated supralinear Ca2+ signaling in rat hippocampal neurons. J. Neurosci. 2000;20:8628–8636. doi: 10.1523/JNEUROSCI.20-23-08628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinke B, Sandkuhler J. Group I metabotropic glutamate receptor-induced Ca2+-gradients in rat superficial spinal dorsal horn neurons. Neuropharmacology. 2007;52:1015–1023. doi: 10.1016/j.neuropharm.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Fukushima H, Kida S, Zhuo M. Ca2+/calmodulin-dependent protein kinase IV links group I metabotropic glutamate receptors to fragile X mental retardation protein in cingulate cortex. J. Biol. Chem. 2009;284:18953–18962. doi: 10.1074/jbc.M109.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 44.Adams KW, Cooper GM. Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J. Biol. Chem. 2007;282:6192–6200. doi: 10.1074/jbc.M610643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giulivi C, Pacifici RE, Davies KJ. Exposure of hydrophobic moieties promotes the selective degradation of hydrogen peroxide-modified hemoglobin by the multicatalytic proteinase complex, proteasome. Arch. Biochem. Biophys. 1994;311:329–341. doi: 10.1006/abbi.1994.1245. [DOI] [PubMed] [Google Scholar]
- 46.Reiss AL, Freund L. Fragile X syndrome, DSM-III-R, and autism. J. Am. Acad. Child Adolesc. Psychiatry. 1990;29:885–891. doi: 10.1097/00004583-199011000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Feinstein C, Reiss AL. Autism: the point of view from fragile X studies. J. Autism Dev. Disord. 1998;28:393–405. doi: 10.1023/a:1026000404855. [DOI] [PubMed] [Google Scholar]
- 48.Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 49.Loesch DZ, Khaniani MS, Slater HR, Rubio JP, Bui QM, Kotschet K, D’Souza W, Venn A, Kalitsis P, Choo AKH, et al. Small CGG repeat expansion alleles of FMR1 gene are associated with parkinsonism. Clin. Genet. 2009;76:471–476. doi: 10.1111/j.1399-0004.2009.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bourgeois JA, Cogswell JB, Hessl D, Zhang L, Ono MY, Tassone F, Farzin F, Brunberg JA, Grigsby J, Hagerman RJ. Cognitive, anxiety and mood disorders in the fragile X-associated tremor/ataxia syndrome. Gen. Hosp. Psychiatry. 2007;29:349–356. doi: 10.1016/j.genhosppsych.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.