Abstract
BACKGROUND
Thrombopoietin mimetic agents are a novel therapeutic option for patients with chronic immune thrombocytopenic purpura (ITP). We report on the use of romiplostim, a thrombopoietin mimetic, throughout pregnancy.
CASE
A 28 year old primigravida with chronic ITP initiated a planned pregnancy on romiplostim. The second and third trimesters were marked by a cyclic pattern of thrombocytopenia requiring supplemental corticosteroids or intravenous immunoglobulin and resultant thrombocytosis. Increased romiplostim doses and daily corticosteroids stabilized the platelet count prior to induction of labor. The delivery was uneventful, though the newborn manifested anemia and intraventricular hemorrhage.
CONCLUSION
The decreased efficacy of romiplostim monotherapy is attributed to increased target-mediated drug disposition and the physiologic changes of pregnancy. Safety concerns still exist for the developmental effects of romiplostim on the fetus.
INTRODUCTION
Immune thrombocytopenic purpura (ITP) is a disorder characterized by antiplatelet autoantibody mediated destruction of existing platelets and decreased production of new platelets. While maternal and fetal morbidity is uncommon in chronic ITP complicating pregnancy, it occurs more frequently in patients with severe thrombocytopenia. Maternal platelet count <20×109/L has been associated with risk of spontaneous bleeding, postpartum hemorrhage, and placental abruption. (1) Fetal effects are also encountered: 5% of newborns will be born with platelets <20×109/L and <1% will have bleeding complications. (1)
The goal of treatment for ITP is to maintain platelets in a range that will prevent episodes of major bleeding (30×109/L - 50×109/L). Individuals who do not respond to conventional therapy may receive second line treatments such as splenectomy or use of alternate agents. Thrombopoietin (TPO) mimetic agents are a novel class of drugs approved by the US Food and Drug Administration for treatment of chronic ITP refractory to first-line therapy. Studies have shown that patients treated with TPO mimetics are able to achieve a durable platelet response (2) and improved health-related quality of life. (3) We report the case of a woman with chronic ITP who initiated a planned pregnancy after achieving a durable platelet response on romiplostim, a TPO mimetic agent. We review challenges to the use of romiplostim for the management of chronic ITP during her pregnancy and the associated neonatal outcome.
CASE
A 28 year-old nulliparous woman diagnosed with ITP refractory to IVIg, prednisone, dexamethasone, azathioprine, vincristine, mycophenolate, and rituximab was seen for a preconception consultation. Initiation of romiplostim (Nplate®, Amgen Inc., Thousand Oaks, CA) five months earlier resulted in a dramatic improvement in her symptoms, with durable platelet response reached at a dose of 3 mcg/kg SQ weekly. At the time of consultation, she expressed a desire to become pregnant while continuing to use romiplostim. Review of the product literature and a search of PubMed using keywords “romiplostim”, “chronic ITP”, and “pregnancy” did not reveal any prior accounts of human reproductive outcome following use of romiplostim in pregnancy. Counseling was provided regarding the risks of pregnancy with chronic ITP and the lack of published data on the safety of romiplostim in pregnancy.
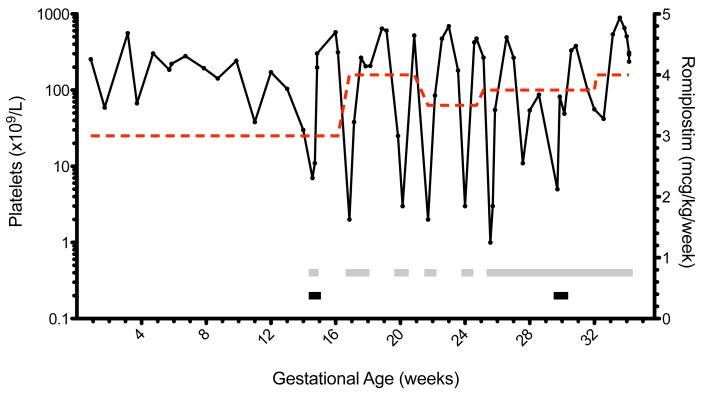
Despite counseling, the patient subsequently initiated prenatal care at 8 weeks gestation. Genetic screening of the fetus was unremarkable; no evidence of aneuploidy was present on first trimester screen. During the first trimester, her platelets were stable on 3 mcg/kg/week of romiplostim. During a routine visit to her hematologist at 14 weeks, she described mild epistaxis and testing revealed a platelet count of 7×109/L. She was subsequently treated with IVIg (0.4 mg/kg x 3 days) and dexamethasone (40 mg IV x 2 days). Dexamethasone was chosen due to a consistent and rapid rise in platelet count, whereas the patient was intolerant of the side effects of prednisone. Courses of corticosteroids and IVIg were used to supplement romiplostim in response to thrombocytopenic episodes (Table). The remainder of the pregnancy was marked by alternating periods of profound thrombocytopenia and thrombocytosis (Figure).
Table.
Treatment of chronic ITP in pregnancy
| Gestational age (week) |
Platelets (min-max) |
Romiplostim dose (mcg/kg/wk) | Corticosteroid | IVIg |
|---|---|---|---|---|
| 14 -15 | 7-303 | 3 | + | + |
| 16 | 2-575 | 4 | + | − |
| 17 | 38-206 | 4 | + | − |
| 18 | 208 | 4 | − | − |
| 19 | 25-604 | 4 | + | − |
| 20 | 187-521 | 4 | − | − |
| 21 | 2 | 3.5 | + | − |
| 22 | 85-474 | 3.5 | − | − |
| 23 | 181-609 | 3.5 | − | − |
| 24 | 2-475 | 3.5 | + | − |
| 25 | 1-267 | 3.75 | + | − |
| 26 | 55-492 | 3.75 | + | − |
| 27 | 11-266 | 3.75 | + | − |
| 28 | 54-87 | 3.75 | + | − |
| 29 | 5-40 | 3.75 | + | − |
| 30 | 300-331 | 3.75 | + | + |
| 31 | 101 | 3.75 | − | − |
| 32 | 42-56 | 4 | − | − |
| 33 | 539-653 | 4 | + | − |
| 34 | 237-508 | 4 | + | − |
Legend: min=minimum; max=maximum; wk=week; IVIg=intravenous immunoglobulin
Course of refractory ITP treated with romiplostim in pregnancy. Solid line - platelet count; dashed line - romiplostim dose; gray bar - dexamethasone administered; black bar - IVIg administered.
At 33 weeks and 6 days, labor was induced for increasing episodes of thrombocytopenia. Induction proceeded with misoprostol and foley bulb, with addition of pitocin for augmentation of labor. The patient received 8 mg of dexamethasone daily and stress dose steroids in active labor (50 mg IV hydrocortisone every 6 hours). Platelets remained between 200×109/L -300×109/L, facilitating epidural placement upon entry into active labor. Labor induction lasted approximately 2 days and resulted in spontaneous vaginal delivery of a 1910 gram male infant with APGAR scores of 8, 9. Estimated blood loss at delivery was 300 mL and no prophylaxis for postpartum hemorrhage was provided. Placental pathology was notable for multiple small infarcts (1-3 cm) in the peripheral parenchyma. After delivery, the patient was restarted on romiplostim (4 mcg/kg) and daily dexamethasone (8 mg). Platelets remained above 200×109/L during the postpartum period.
The infant’s platelets were 70×109/L at birth, but fell to 33×109/L within eight hours. Treatment with IVIg (1 g/kg × 2 days) was initiated. A grade III intraventricular hemorrhage (IVH) was discovered, and the infant was subsequently transfused a unit of platelets. The platelets rose appropriately to 116×109/L and stabilized. The infant received antibiotics for presumed sepsis, a red blood cell transfusion for anemia, and phototherapy for hyperbilirubinemia. Additionally, the infant was noted to have adrenal insufficiency and phimosis.
COMMENT
Romiplostim is approved for the treatment of chronic ITP in adults, though there is no published literature on its use throughout pregnancy. Although a pregnancy registry had been established to collect information on outcomes from women exposed to romiplostim, only one other patient was enrolled at the time of this case. Reproductive studies in rodents suggested an association with fetal thrombocytosis, decreased maternal body weight, post-implantation loss, and increased perinatal pup mortality. Despite these precautions, the patient chose to proceed with pregnancy and accepted the risks of continued use of romiplostim.
The patient maintained stable platelet counts in the first trimester, but developed worsening thrombocytopenia in the early second trimester. Rebound thrombocytopenia, a phenomenon that occurs in patients after withdrawal of TPO receptor agonist treatment, has been associated with highly fluctuating platelet counts. (4) Rebound thrombocytopenia has been hypothesized to originate from increased clearance of endogenous TPO or negative feedback due to a marked increase in platelets and megakaryocytes induced by TPO receptor agonists. (4) The frequency of the observed episodes of rebound thrombocytopenia suggests the efficacy of romiplostim was altered during pregnancy.
Romiplostim pharmacokinetics follows a target-mediated drug disposition (TMDD) model. (5) TPO receptor agonists bind to c-Mpl, a cytokine receptor on the surface of megakaryocytes and platelets, activating transcriptional pathways which lead to an increase in platelet count. The c-Mpl receptors also control the distribution and elimination of the drug. Elimination of romiplostim has been described as non-linear: at low doses the target c-Mpl is the primary elimination pathway, while at high doses c-Mpl becomes saturated and renal elimination plays an increasing role. (5) Normal pregnancy is characterized by low-grade platelet consumption (turnover) compensated by enhanced megakaryopoiesis. A study of platelet production in normal pregnancies found average TPO values twice that of the non-pregnant control group. (6) Increased megakaryopoiesis in a normal pregnancy implicates upregulated c-Mpl, which can alter the pharmacokinetics of romiplostim by enhancing target-mediated elimination.
Monotherapy with romiplostim was insufficient to maintain stable platelet counts beyond the first trimester. Use of romiplostim in combination with corticosteroids and IVIg was necessary as the patient became increasingly refractory to treatment. These treatments may work synergistically by decreasing platelet destruction (corticosteroids/IVIg) and increasing platelet production (TPO receptor agonist). During the postpartum period, romiplostim and maintenance corticosteroids were able to stabilize the platelet count without further refractory episodes. Notably, a report was recently published of a patient who received a brief course of romiplostim in the 3rd trimester for secondary ITP associated with systemic lupus erythematosus. (7) A similar fluctuation in the platelet count was observed, but due to the short course of treatment no trend could be discerned.
Infants born to mothers with ITP are estimated to have a 10% risk of having a platelet count <50×109/L at the time of delivery. (8) A drop in platelet count may occur in the days following birth as antibody-sensitized platelets are destroyed by the infant’s spleen. Neonatal intracerebral hemorrhage is estimated to occur in <1% of newborns with ITP. (8) In the case above, it was presumed that neonatal thrombocytopenia and intracerebral hemorrhage were a result of maternal anti-platelet antibodies as well as rebound thrombocytopenia associated with clearance of romiplostim from the newborn. Stable platelet counts after transfusion were reassuring for a lack of lingering romiplostim effect and resolution of maternal anti-platelet antibodies. The newborn’s adrenal insufficiency, associated with increased antenatal glucocorticoid use, resolved after birth. However, mild delay of motor skills and benign external hydrocephalus were noted at a 5 month follow-up visit. While delayed motor skills are likely related to prematurity, the finding of hydrocephalus cannot be excluded as a complication of romiplostim or high-dose corticosteroid exposure in a newborn with grade III IVH.
As improved quality of life is achieved for patients with refractory ITP, women of childbearing age will consider their reproductive options. This case relates our experience with the use of a TPO mimetic for refractory ITP in pregnancy. In this case, romiplostim was inadequate as monotherapy beyond the first trimester. Co-administration of corticosteroids and IVIg was necessary to resolve episodes of severe thrombocytopenia in an increasingly refractory patient. The decreased efficacy of romiplostim may be related to physiologic changes of pregnancy, suggesting that further pharmacokinetic studies are needed to determine an optimal dosing regimen. Safety concerns still exist for the developmental effects of TPO mimetics on the fetus, which may be clarified through further post-marketing surveillance.
Acknowledgments
ASP received support from T32 GM 86330-1 A1 (NIH-NIGMS) for his work in clinical pharmacology.
Footnotes
DISCLOSURE: The authors report no conflict of interest.
REFERENCES
- 1.Kelton JG. Idiopathic thrombocytopenic purpura complicating pregnancy. Blood Reviews. 2002;16:43–46. doi: 10.1054/blre.2001.0181. [DOI] [PubMed] [Google Scholar]
- 2.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 3.George JN, Mathias SD, Go RS, Guo M, Henry DH, Lyons R, et al. Improved quality of life for romiplostim-treated patients with chronic immune thrombocytopenic purpura: results from two randomized, placebo-controlled trials. Br J Haematol. 2009;144:409–15. doi: 10.1111/j.1365-2141.2008.07464.x. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara K, Kambara N. Highly Fluctuating Thrombocytopenia Developing in a Patient with Immune Thrombocytopenia (ITP) while Administering Romiplostim. Intern Med. 2012;51:1399–401. doi: 10.2169/internalmedicine.51.7106. [DOI] [PubMed] [Google Scholar]
- 5.Wang YM, Sloey B, Wong T, Khandelwal P, Melara R, Sun YN. Investigation of the pharmacokinetics of romiplostim in rodents with a focus on the clearance mechanism. Pharm Res. 2011;28:1931–8. doi: 10.1007/s11095-011-0420-y. [DOI] [PubMed] [Google Scholar]
- 6.Frölich MA, Datta S, Corn SB. Thrombopoietin in normal pregnancy and preeclampsia. Am J Obstet Gynecol. 1998;179:100–4. doi: 10.1016/s0002-9378(98)70257-1. [DOI] [PubMed] [Google Scholar]
- 7.Alkaabi J, Alkindi S, Riyami NA, Zia F, Balla L, Balla S. Successful treatment of severe thrombocytopenia with romiplostim in a pregnant patient with systemic lupus erythematosus. Lupus. 2012;21:1571–4. doi: 10.1177/0961203312463621. [DOI] [PubMed] [Google Scholar]
- 8.Webert KE, Mittal R, Sigouin C, Heddle NM, Kelton JG. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood. 2003;102:4306. doi: 10.1182/blood-2002-10-3317. [DOI] [PubMed] [Google Scholar]