Abstract
Mosquito larvae often exhibit different behaviors depending on the aspects of the aquatic environment, including the presence of different physical factors and detrital food sources. Regardless of these physical differences, different genera also devote different amounts of time to different behaviors. To determine if differences existed among four focal mosquito species (Aedes albopictus (Singh), Aedes triseriatus (Say), Culex quinquefasciatus (Say), Culex coronator Dyar & Knab), we recorded behaviors under different food environments (animal detritus, leaf detritus, and inoculum + inert material) and depths (shallow and deep). Based on past work, we predicted that larval mosquitoes in the genus Culex would spend more time filtering or resting at the surface of containers, whereas Aedes mosquitoes would spend more time browsing on surfaces. Behaviors were recorded for 30 min and were used to generate instantaneous scan census of behavior (thrashing, browsing, and resting or filtering) and locations (top, middle, bottom, wall, and detritus) of each larva every minute. There were significant differences in behaviors among the three detritus types and the four species (Culex generally different than Aedes), as well as a significant interaction between depth and detritus type. Consistent with predictions, Culex species spent more time filtering or resting, whereas Aedes larvae spent more time browsing on detritus. However, all four species changed their behavior similarly among the different environments, and Cx. coronator exhibited some similar behaviors as the two Aedes species. These behavioral differences may aid in explaining performance differences between different species and outcomes of interspecific encounters, which in turn can affect adult emergence and patterns of disease.
Keywords: Aedes, behavior, Culex, detritus type, physical factor
Mosquito larvae are found in numerous aquatic habitat types, including ponds, brackish water, pools, flooded ditches, as well as small containers. Of these, containers can be either natural (e.g., tree holes, pitcher plants) or artificial (e.g., tires, cemetery vases), and both represent significant sources of disease vectors (Kitching 2000, Vezzani 2007, Yee 2008). Mosquitoes that inhabit these environments often feed on heterotrophic microorganisms and detritus (Merritt et al. 1992) as these systems normally lack primary productivity. Numerous control strategies have been developed for adult mosquito populations, such as the use of chemicals, predators, parasites, pathogens, and habitat manipulations (Becker et al. 2010); however, the ecology and biology (e.g., feeding behavior, larval nutrition) of the aquatic larvae is an important but often overlooked factor that can inform vector control. Moreover, as feeding is the principle way by which larvae acquire resources before pupation, and pupal size is often correlated to adult size and fecundity, understanding feeding behavior may ultimately inform us on population dynamics of vectors.
Feeding behavior is defined as the way an organism gathers food, and these behaviors can change based on location or movement (Merritt et al. 1992). Mosquitoes feed with their mouthparts by capturing detrital particles or microorganisms that are suspended in the water column or on surfaces (Dahl et al. 1988). Microorganisms, in turn, subsist on nutrients provided by detrital breakdown (Walker et al. 1991). Categories of larval feeding behavior generally have been based on two criteria, particle size range and the general location of the food item (Merritt et al. 1992). Based on these categories, the following four functional groups have been recognized in mosquito larvae: collecting (separated further into collector-filtering and collector-gathering), scraping, shredding, and predators (Merritt et al. 1992, Clements 1999). Collector-filterers (e.g., Culex, Anopheles, Culiseta) remove particles that are suspended in the water column or floating on the water surface (Merritt et al. 1992, Clements 1999), whereas collector-gatherers (e.g., Aedes, Wyeomyia) feed by removing particles deposited on or loosely connected to rocks, vegetation, and other submerged surfaces (Merritt et al. 1992, Clements 1999). All species included in this experiment fall in the collector group. When analyzing the feeding behavior of a mosquito larva, three things are measured: 1) the type of behavior the larva is performing (i.e., activity), 2) the specific location within the water column the behavior is taking place, and 3) the length of time the larva exhibit the behavior (Martin and Bateson 1986, Juliano and Gravel 2002). Although feeding behavior has been compared for pairs of species (e.g., Yee et al. 2004a,b; O’Donnell and Armbruster 2007), few studies have focused on quantifying the feeding behavior of multiple co-occurring species in containers.
Although categorization of mosquito larval feeding modes often suggests that feeding behaviors are rigid, some species can adjust their behavior depending on the food source. For example, Aedes sierrensis (Lud-low) larvae within tree holes are effective at collecting-filtering and collecting-gathering, a term called preyswitching (Eisenberg et al. 2000). In laboratory experiments, Aedes albopictus (Singh), Aedes. triseriatus (Say), and Culex pipiens L. larvae fed predominately by collecting-gathering if leaf detritus was present; however, they predominately fed by collecting-filtering in systems where only microorganisms in the water column were available (Yee et al. 2004b). In an experiment with Ae. albopictus and Ae. triseriatus, both species spent more time feeding on animal versus leaf detritus, although Ae. albopictus larvae spent more time feeding on either detritus type compared with Ae. triseriatus (Kesavaraju et al. 2007). Feeding behavior also appears to be flexible within species across life history stages. For instance. Ae. sierrensis fourth-instar larvae spent more time filtering than second-instars, and predation on protozoans by way of collecting-filtering decreased when a food source that could be acquired by collecting-gathering was present (Eisenberg et al. 2000). Thus, mosquito larvae can exhibit multiple feeding behaviors depending on the food source even if the larvae have a primary feeding behavior.
Mosquito larvae may change their foraging activity and feeding behavior during suspension feeding when confronted with structural changes in the environment. In particular, water depth plays an important role in regulating population dynamics of some mosquitoes (Lester and Pike 2003), and changes in depth in aquatic habitats could potentially control for breeding sites in vector species (i.e., Anopheles; Mutero et al. 2000). Water depth also affects larval survival, development time, and accumulation of reserves (e.g., mass) in mosquito larvae (Timmermann and Briegel 1993, Juliano and Stoffregen 1994, Briegel 2002). Some Culex larvae are often found in larger tires that are deeper with higher volumes compared with many Aedes, suggesting that Culex may benefit more than Aedes from deeper environments. Briegel (2002) showed that several species of mosquitoes (e.g., Ae. aegyptii and Cx. pipiens) had lower survival in deeper containers than in shallower containers, whereas Juliano and Stoffregen (1994) showed that individual Ae. triseriatus larvae developed faster and had lower mass in shallower compared with deeper microcosms. Thus, depth and other structural features may play important ecological roles in the performance of larval mosquitoes.
We investigated the behavior of four common container species of mosquito that occur in the southeastern United States: Ae. albopictus, Ae. triseriatus, Culex quinquefasciatus Say, and Culex coronator Dyar & Knab (Yee et al. 2012). Ae. albopictus, the Asian tiger mosquito, is a container specialist endemic to tropical Asia, but the species was introduced to the United States in 1985 via the international tire trade (Hawley 1988), and is a worldwide vector for the dengue virus (Paupy et al. 2009) among other arboviruses. Ae. triseriatus, the eastern tree hole mosquito, is a container specialist endemic to the eastern United States (Livdahl and Willey 1991), and the females are a major vector of the LaCrosse encephalitis virus (Watts et al. 1973). Cx. quinquefasciatus, the southern house mosquito, is a habitat generalist that was first discovered in the United States, but is believed to have been introduced from Africa before its discovery (Vinogradova 2000). This species is the main vector of bancroftian filariasis (Subra 1980) and a major vector of St. Louis encephalitis virus (Gillett 1972). Finally, Cx. coronator females prefer to lay their eggs in pools and ponds, and its endemic range extends from Argentina to the southwestern United States (Goddard et al. 2006). Since 2002, Cx. coronator has extended its range from Oklahoma to North Carolina (Bradley 2004, Debboun et al. 2005, Varnado et al. 2005, Smith et al. 2006, McNelly et al. 2007, Gray et al. 2008, Moulis et al. 2008) and has been identified to co-occur with other species in tires in our study area (Yee et al. 2012). This species is of unknown medical importance in its new range, although females are vectors of Venezuelan equine encephalitis (Sudia and Newhouse 1975) and St. Louis encephalitis (Beadle et al. 1957) in its native range.
In this study, our objectives were to determine if there were differences in behavior among the four species of container mosquitoes. We tested the hypothesis that differences in feeding behavior of larval mosquitoes between genera were greater than differences in feeding behavior within genera. Based on our current knowledge, we predicted that variation in behavior would largely manifest in location and feeding mode differences, with Culex larvae spending more time filter-feeding at the surface of the container, and Aedes larvae spending more time browsing on surfaces (Merritt et al. 1992, Yee et al. 2004b). Because both food resources and depths have shown to affect mosquito performance, we also tested if larvae changed their behaviors in different depths and when presented with different detritus types.
Materials and Methods
Larvae of Ae. albopictus (F1), Ae. triseriatus (F1), Cx. quinquefasciatus (F2 or F3), and Cx. coronator (F4 or F5), came from eggs produced from colonies originally collected from tire piles or tree holes in Mississippi. Specifically, larval Ae. albopictus were collected from Oloh (31.21 °N 89.51 °W), Jackson (32.18 °N 90.11 °W), Gulfport (30.25 °N 89.40 °W), and Natchez (31.33 °N 91.23 °W); Cx. quinquefasciatus and Ae. triseriatus were collected from Hattiesburg (31.19 °N 89.18 °W); and Cx. coronator was collected from Lamar county, MS. Larvae used in behavioral trials were reared to either third or fourth instars (Ae. albopictus, Cx. quinquefasciatus, and Ae. triseriatus), whereas Cx. coronator larvae were second, third, or fourth instars (12% of the larvae used were second instars). Early instars were used for this species due to low numbers of later instars available at the start of the behavioral observations.
Experimental microcosms consisted of 50-ml plastic beakers filled with room temperature water and microorganism inoculum (hereafter, inoculum) created in the laboratory. The inoculum was created 72 h before the start of the experiment by filling a container with 4 cm of reverse osmosis water at room temperature, adding senescent leaves, dried vinegar flies, and spoonfuls of 50/50 Lactalbumin-yeast mixture. To understand if different depths elicited changes in feeding behavior among species, water levels were set at two depths: 50 ml (44 mm, hereafter deep) or 25 ml (23 mm, hereafter shallow). These depths fall within the range of depths found in tires in our study area (D.A.Y., unpublished data). In both cases, water consisted of reverse osmosis water and 0.1 ml of inoculum.
Microcosms held one of three detritus types known to elicit different behaviors among container larvae (Yee et al. 2004b, Kesavaraju et al. 2007). Detritus consisted of either senescent live oak leaves (Quercus virginiana Miller) collected from the Lake Thoreau Environmental Center (31.19 °N 89.18 °W; hereafter leaves), dried vinegar flies (Drosophila melanogaster Meigen; hereafter animal detritus), or inoculum and an inert material (strips of thin plastic). The strips of thin plastic were not measured, but the strips extended a few millimeters above the height of the cup. The inert material was used so that all behavior locations (i.e., detritus) existed in all treatment combinations (depth, species, etc.). Detritus was soaked in 50 ml of water at room temperature 48 h before the start of the experiment. The detrital containers were covered with black screen mesh to reduce the amount of light reaching the detritus. Larvae were starved 24 h at room temperature before the start of the experiment to standardize hunger. Each species (4), detritus (3), and depth (2) combination was replicated 19 times for a total of 456 experimental units. Recordings of replicates were split between 2 d (i.e., 10 replicates on Days 1 and 9 on Day 2).
Behaviors were recorded using a Sony HD 40 GB Handycam. During each run of the behavioral recordings, eight microcosms were recorded undisturbed in an empty room for 30 min. No individual larva was used more than once for recording. All treatment levels and each species were present during each recording. Recordings were viewed by the first author to generate instantaneous scan census of the mosquito’s activities and location of larvae every minute.
Activities recorded included browsing (movement along a surface using its mouthparts), filtering or resting (movement within the water column using its mouthparts or larva not moving), and thrashing (movement through the water column by energetic lateral flexations of the body) (Juliano and Reminger 1992). Locations included the surface (the larvae’s siphon in contact with the water–air interface), middle (larvae located >1 mm from the wall, the water surface, and any detrital surface), wall (larvae located <1 mm from the wall of the container), bottom (larvae located on the bottom of the container and not touching the detrital surface), and detritus (larvae in contact with the detrital surface or inert material) (Juliano and Reminger 1992).
Statistical Analysis
Proportion of time spent performing each behavior was transformed using an arc-sine transformation given that proportional data often fail to meet assumptions of statistical tests. Because some behaviors are correlated with one another, or some behaviors only exist at certain locations, a principal components analysis (PCA) was performed on the transformed data to extract uncorrelated axes of behaviors (Juliano and Gravel 2002). Principal components (PCs) with eigenvalues ≥1.0 were retained for further analysis, whereas those with values <1.0 were ignored (Hatcher and Stepansky 1994). The PC axes were then used as dependent variables in a multivariate analysis of variance (MANOVA) with water depth (50 ml or 25 ml), detritus type (leaves, animal detritus, and inoculum + inert material), species (Ae. albopictus, Ae. triseriatus, Cx. quinquefasciatus, and Cx. coronator) and their interactions as independent factors. Standardized canonical coefficients were used to identify the dependent variable(s) responsible for significant MANOVA effects (Scheiner 2001). Tukey’s honest significant difference post hoc analysis was used to determine if there are differences in feeding behavior as measured by PC axes.
Results
PCA yielded three important axes that summarized 82.9% of variation in behaviors (PC 1 =41.80%, PC 2 = 27.48%, PC 3 = 13.53%). PC 1 separated mosquitoes who were browsing at the detritus (positive scores) from those who were resting or filtering at the surface (negative scores; Table 1). PC 2 separated mosquitoes who were thrashing, in the middle, or at the wall (positive scores) from those who were resting or filtering (negative scores; Table 1). Finally, PC 3 separated mosquitoes who were thrashing or in the middle (positive scores) from those who were at the surface (negative scores; Table 1).
Table 1.
Rotated factor pattern produced from the PCA from mosquito behaviors
| Behavior or location | PC 1 | PC 2 | PC 3 |
|---|---|---|---|
| Resting or filtering | −78 | −52 | −24 |
| Thrashing | 0 | 51 | 66 |
| Browsing | 94 | 19 | −21 |
| Surface | −69 | −37 | −56 |
| Bottom | −6 | 64 | 38 |
| Wall | 22 | 82 | −17 |
| Middle | −10 | −11 | 90 |
| Detritus | 90 | −34 | −16 |
| Interpretation | Browsing on detritus vs resting or filtering at the surface | Thrashing, at the bottom, or at the wall vs resting or filtering | Thrashing in the middle vs at the surface |
Dependent variables with large loadings (>40) are in bold.
There were significant differences in behaviors among the three detritus types (Pillai’s trace6, 834 = 8.28; P < 0.0001) and the four species (Pillai’s trace9, 1254 = 4.17; P < 0.0001; Fig. 1), as well as a significant interaction between depth and detritus type (Pillai’s trace6, 834 = 2.60; P =0.0166). There was no significant difference in behavior between the two depths (Pillai’s trace3, 416 = 0.04; P = 0.9893), and there was no significant interaction between detritus and species (Pillai’s trace18, 1254 = 1.33; P = 0.1587), species and depth (Pillai’s trace9, 1254 = 0.30; P = 0.9746), nor the interaction among all three factors (Pillai’s trace18, 1254 = 1.34; P = 0.1564).
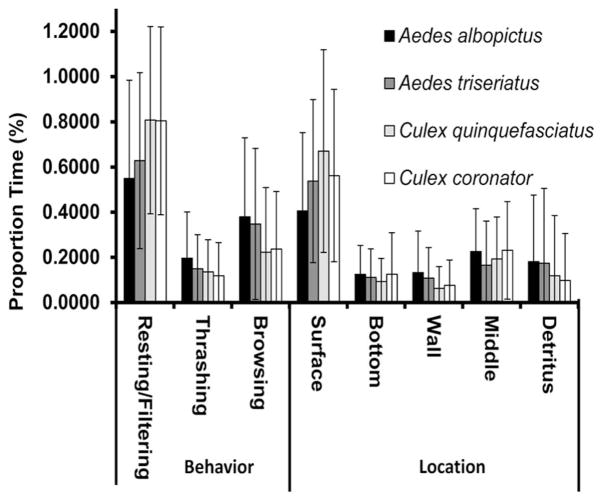
Fig. 1.
Means (±SE) of time spent in different locations and performing different behaviors for each species during behavioral trials. Each mean represents 15–19 replicates (pooled across detrital types and depths).
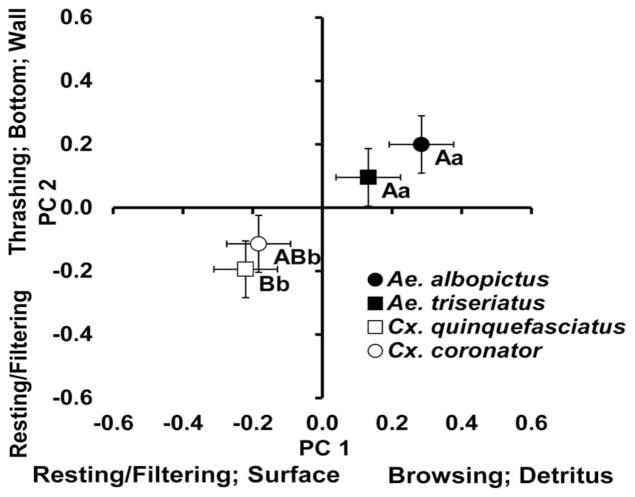
For the significant effect of species, both PC 1 (standardized canonical coefficient [SCC] = 0.8111) and PC 2 (SCC = 0.6330) explained the most variation. Based on mean contrasts for PC 1, there were no differences in behaviors within each genus, but there was a significant difference between Culex and Aedes (Fig. 2). Specifically, Aedes mosquitoes spent more time browsing on detritus, whereas Culex mosquitoes spent more time resting or filtering at the surface (Fig. 2). For PC 2, there were also no differences between species within the same genera, or between Cx. coronator and both Aedes species, whereas there was a significant difference between Cx. quinquefasciatus and both Aedes species (Fig. 2). In general, Ae. albopictus spent more time thrashing, at the wall, or in the middle, whereas Cx. quinquefascitus spent more time resting or filtering, with Ae. triseriatus and Cx. coronator displaying intermediate behaviors (Fig. 2).
Fig. 2.
Bivariate means (±SE) for PC 1 and PC 2 that made the greatest contributions of the effect of species. Activities and positions most closely associated with large positive or negative factor scores are indicated parallel to each axis. Differences in means between species for PC 1 are represented by lowercase letters, while differences in means between species for PC 2 are represented by uppercase letters.
For the depth by food interaction, again both PC 1 (SCC =−0.6270) and PC 2 (SCC =0.7493) were large compared with PC 3 (SCC = 0.4319). Based on PC 1, larvae spent more time browsing on detritus in shallow leaf environments, but larvae exhibited combinations of behaviors in the other environments (Fig. 3). Larvae also spent more time at resting or filtering in shallow leaf environments, compared with more time thrashing, in the middle, or at the bottom in shallow animal environments, and exhibited a combination of behaviors in the deep or shallow microorganism only environments and deep leaf or animal environments (Fig. 3).
Fig. 3.
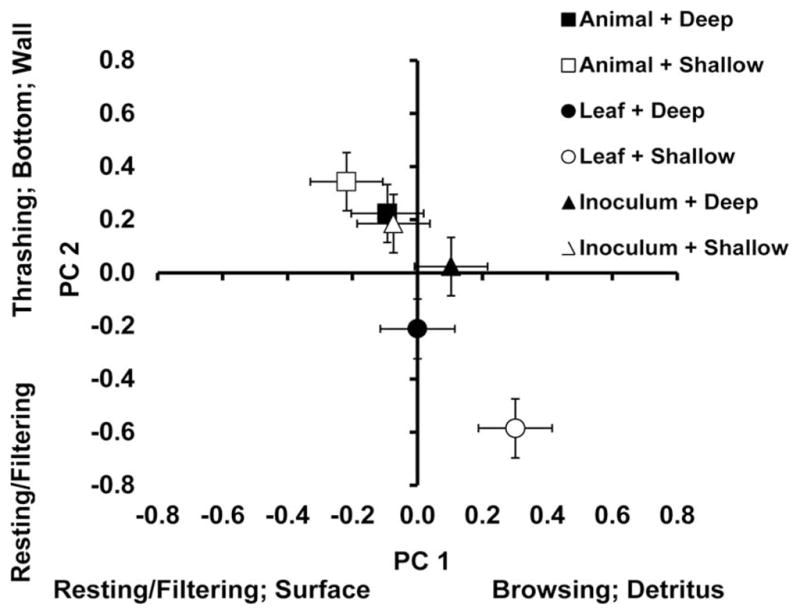
Bivariate means (±SE) for PC 1 and PC 2 that made the greatest contributions to the interaction between depth and detritus type. Activities and positions most closely associated with large positive or negative factor scores are indicated parallel to each axis.
Discussion
Our hypothesis of differences among the four species of mosquito larvae was partially supported. Specifically, differences in behavior between Aedes and Culex mosquitoes were significantly greater than differences between individual Culex or individual Aedes. Our prediction that Culex species would often spend more time filtering or resting, whereas Aedes larvae would often spend more time browsing on detritus was supported (Fig. 2). These findings were consistent with other studies that have investigated larval behavior between these same genera (e.g., Yee et al. 2004b). Aedes larvae may spend more time browsing as they may be more sensitive to certain phagostimulants that allow the larvae to locate microorganisms (Merritt et al. 1992, Clements 1999, Yee et al. 2004b). In Culex larvae, phagostimulants in the water cause larvae to spend more time beating their mouthparts (i.e., filtering; Dadd 1970, Merritt et al. 1992). In our experiment, we observed some Culex larvae spending time filtering near the detritus, suggesting that they may have picked up some phagostimulant cues in the water column.
Although there were strong differences in some behaviors between the genera, these differences were not rigid across different food environments. Specifically, all larvae changed their behavior equally across the different food and depth environments, a result consistent with the findings of Yee et al. (2004a,b). These authors also found that their species changed their behavior from filtering to browsing when given a leaf surface (Yee et al. 2004b), a result consistent with our findings. However, in our study larvae did not browse on detrital surfaces at the same rate in animal only environments compared with leaf only environments. One possible reason for animal environments not eliciting as much browsing is that the vinegar flies did not sink in most of the containers, perhaps leading to larvae filtering more near the surface. Workman and Walton (2003) observed that Culex larvae filtered more at the surface in high quality environments than in low quality environments, perhaps as this gave these animals the advantage of also maintaining access to the atmosphere for breathing. Animal detritus is a higher quality food source than leaf detritus (Yee and Juliano 2006, Yee et al. 2007), and thus larvae may have only needed to filter-feed in animal environments to acquire the same nutritional resources as browsing in leaf environments.
For nonfeeding behaviors and locations (PC 2, Fig. 2), Cx. coronator was not significantly different in terms of behaviors than either of the Aedes species. One plausible explanation is that mosquitoes of different genera have adapted similar responses to movement within the container. Another plausible explanation is that Cx. coronator and Cx. quinquefasciatus are noncontainer species that use containers (Dyar and Knab 1906, Arnett 1950, Subra 1980), and thus the larvae may exhibit more general behaviors compared with other Culex in these nontypical environments. Due to a lack of sufficient later instars, a small percentage (12%) of the Cx. coronator larvae were early instars, and thus similarities to both Aedes and Cx. quinquefasciatus could be because of behavioral switching of different developmental stages (Juliano et al. 1993, Eisenberg et al. 2000). However, it is unlikely that inclusion of these early instars would have influenced the overall results, given that the variation in behaviors around the mean was similar compared with other species (Fig. 2). Thus, it appears that Cx. coronator does exhibit feeding behaviors that are intermediate compared with other container species. As the basic ecology, including foraging, is relatively unknown for this species, future work should focus on other interactions with other container species across their new geographic range.
Water depth in particular plays an important role in regulating population dynamics of some mosquitoes (Lester and Pike 2003), and water depth can also affect larval survival, development time, and accumulation of reserves (e.g., mass) in mosquito larvae (Timmermann and Briegel 1993, Juliano and Stoffregen 1994, Briegel 2002). Water depth can also play a role in larval behavior. For example, shallower environments may prevent Culex larvae from filter feeding, while Aedes larvae in deeper environments may spend significant energy browsing at the bottom and then returning to the surface to breathe. Yet, there was no significant difference between the two depths in our study. Larvae performed similar behaviors in deep and shallow animal environments, as well as deep and shallow inoculum only environments (Fig. 3). One possible explanation is that the difference in depth between 50 ml and 25 ml may not have been significant enough to elicit differences in larval behavior. Thus, extreme container depths could be chosen instead of those that are average or median to elicit larvae to change their behavior.
Our results differed from those of Walker and Merritt (1991), who observed that Ae. triseriatus spent more time browsing at or near the surface, whereas we observed both Aedes species spent more time browsing on detritus. Walker and Merritt (1991) observed multiple larvae in one container, which could have led to larvae being forced to browse at the surface to avoid competition or for space considerations. Our results also differed from those of Kesavaraju et al. (2007) who observed that Ae. triseriatus and Ae. albopictus spent more time browsing at an animal patch when given a choice between leaf and animal detritus. In this experiment, both Aedes species spent more time browsing on a leaf compared with animal detritus, although these types of detritus were not offered in a choice experiment, and Kesavaraju et al. (2007) measured behavior in only one depth. Increased browsing on leaves over animal carcasses could be explained by the fact that leaves were much larger than vinegar fly carcasses, and could have provided a stronger phagostimulant for browsing during the limited time the larvae were in the containers. Our experiment showed that different Aedes species did not differ in their overall behavior, while differences between different Aedes species have been found in other experiments under different experimental protocols (Yee et al. 2004b, O’Donnell and Armbruster 2007). These experimental differences suggest that larval behavior is flexible even between species in the same genus.
Besides differences between these experimental outcomes and other studies, we limited our behavior categories to resting or filtering, browsing, and thrashing, whereas Walker and Merritt (1991) observed and recorded autogrooming as well. This behavior is defined as when a larva flexes to clean its siphon or body with its mouthparts. However, larvae also may be inadvertently feeding on particles that are trapped on the siphon or hairs on the body. We observed larvae performing this behavior, but we included it in the resting or filtering category. Categorization of behaviors is challenging, especially as larvae likely exhibit a range of behaviors between the rigid categories often used by observers.
Our experiment has shown that there are significant differences in behaviors between Aedes and Culex mosquitoes. Because defining and classifying different behaviors is difficult (Merritt et al. 1992), certain sets of larval behaviors should not be assigned to one genus or another (Walker and Merritt 1991). This is evidenced by the fact that Cx. coronator exhibited some behaviors similar to Aedes species, and that all four species changed their behavior equally among the different food and depth environments. Differences in behavior and flexibility in feeding behavior may lead different species to either be competitively dominant over other species (Yee et al. 2004a), better at avoiding predators (Sih 1986, Grill and Juliano 1996, Kesavaraju and Juliano 2004), or have increased survival in general (Kesavaraju et al. 2007). More studies on feeding behavior are needed to fully understand the differences among mosquito species, to determine the mechanisms for why certain species behave one way over another, and why behaviors change across different environments.
Acknowledgments
We thank W. C. Glasgow, S. Schelble, A. Reeves, N. Ezeakacha, and C. Bofill for assistance in the laboratory. This work was supported by the Department of Biological Sciences at the University of Southern Mississippi and by a grant to D. A. Yee from the National Institutes of Health (R15 [AI]-92404-01A1).
References Cited
- Arnett RH. Notes on the distribution, habits, and habitats of some panama culicines (Diptera: Culicidae), (Continued) JNY Entomol Soc. 1950;58:99–115. [Google Scholar]
- Beadle LD, Menzies GC, Hayes GR, Von Zuben FJ, Eads RB. Vector evaluation and control: St. Louis encephalitis in Hidalgo County. Texas Public Health Rep. 1957;72:531–536. [PMC free article] [PubMed] [Google Scholar]
- Becker N, Petrić D, Zgomba M, Boase C, Madon M, Dahl C, Kaiser A. Mosquitoes and their control. 2. Springer; Berlin, Germany: 2010. [Google Scholar]
- Bradley KK. Oklahoma State Report: Twenty- Fifth Biennial State Public Health Vector Control Conference. 2004 ( http://www.cdc.gov/ncidod/dvbid/westnile/conf/25thbiennialVectorControl/pdf/OklahomaReport_Bradley.pdf)
- Briegel H. Physiological bases of mosquito ecology. J Vector Ecol. 2002;28:1–11. [PubMed] [Google Scholar]
- Clements AN. The biology of mosquitoes, vol. 2. Sensory reception and behavior. CAB International; Wallingford, United Kingdom: 1999. [Google Scholar]
- Dadd RH. Relationship between filtering activity and ingestion of solids by larvae of the mosquito Culex pipiens: a method for assessing phagostimulant factors. J Med Entomol. 1970;7:708–712. doi: 10.1093/jmedent/7.6.708. [DOI] [PubMed] [Google Scholar]
- Dahl C, Widahl L, Nilsson C. Functional analysis of the suspension feeding system in mosquitoes (Diptera: Culicidae) Ann Entomol Soc Am. 1988;81:105–127. [Google Scholar]
- Debboun M, Kuhr DD, Rueda LM, Pecor JE. First record of Culex (Culex) coronator in Louisiana, U.S.A. J Am Mosq Control Assoc. 2005;21:455–457. doi: 10.2987/8756-971X(2006)21[455:FROCCC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dyar HG, Knab F. The larvae of Culicidae classified as independent organisms. JNY Entomol Soc. 1906;14:169–230. 242. [Google Scholar]
- Eisenberg JNS, Washburn JO, Schreiber SJ. Generalist feeding behaviors of Aedes sierrensis larvae and their effects on protozoan populations. Ecology. 2000;81:921–935. [Google Scholar]
- Gillett JD. The mosquito: Its life, activities, and impact on human affairs. Doubleday; Garden City, NY: 1972. [Google Scholar]
- Goddard J, Varnado WC, Harrison BA. Notes on the ecology of Culex coronator Dyar and Knab, in Mississippi. J Am Mosq Control Assoc. 2006;22:622–625. doi: 10.2987/8756-971X(2006)22[622:NOTEOC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gray KM, Burkett-Cadena ND, Eubanks MD. Distribution expansion of Culex coronator in Alabama. J Am Mosq Control Assoc. 2008;24:585–587. doi: 10.2987/08-5778.1. [DOI] [PubMed] [Google Scholar]
- Grill CP, Juliano SA. Predicting species interactions based on behavior: predation and competition in container-dwelling mosquitoes. J Am Ecol. 1996;65:63–76. [Google Scholar]
- Hatcher L, Stepansky EJ. A step by step approach to using SAS system for univariate and multivariate analyses. SAS Institute; Cary, NC: 1994. [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;4:1–39. [PubMed] [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behavior: an experiment with tree hole mosquitoes. Behav Ecol. 2002;13:301–311. [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behavior of larval treehole mosquitoes: geographic and ontogenetic differences. Oikos. 1992;63:465–476. [Google Scholar]
- Juliano SA, Stoffregen TL. Effects of habitat drying on size at and time to metamorphosis in the tree hole mosquito Aedes triseriatus. Oceologia. 1994;97:369–376. doi: 10.1007/BF00317327. [DOI] [PubMed] [Google Scholar]
- Juliano SA, Hechtel LJ, Waters JR. Behavior and risk of predation in larval tree hole mosquitoes: effects of hunger and population history of predation. Oikos. 1993;68:229–241. [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Yee DA, Juliano SA. Interspecific and intraspecific differences foraging preferences of container-dwelling mosquitoes. J Med Entomol. 2007;44:215–221. doi: 10.1603/0022-2585(2007)44[215:iaidif]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitching RL. Food webs and container habitats: the natural history and ecology of phytotelmata. Cambridge University Press; Cambridge, United Kingdom: 2000. [Google Scholar]
- Lester PJ, Pike AJ. Container surface area and water depth influence the population dynamics of the mosquito Cx. pervigilans (Diptera: Culicidae) and its associated predators in New Zealand. J Vector Ecol. 2003;28:267–274. [PubMed] [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Martin P, Bateson P. Measuring behavior: an introductory guide. Cambridge University Press; Cambridge, United Kingdom: 1986. [Google Scholar]
- McNelly JR, Smith M, Micher-Stevens KM, Harrison BA. First record of Culex coronator from Alabama. J Am Mosq Control Assoc. 2007;23:473–475. doi: 10.2987/5575.1. [DOI] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:379–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Moulis RA, Russell JD, Lewandowski HB, Jr, Thompson PS, Heusel JL. Culex coronator in coastal Georgia and South Carolina. J Am Mosq Control Assoc. 2008;24:588–590. doi: 10.2987/5766.1. [DOI] [PubMed] [Google Scholar]
- Mutero CM, Blank H, Konradsen F, vander Hoek W. Water management for controlling the breeding of Anopheles mosquitoes in rice irrigation schemes in Kenya. Acta Trop. 2000;76:253–263. doi: 10.1016/s0001-706x(00)00109-1. [DOI] [PubMed] [Google Scholar]
- O’Donnell DL, Armbruster P. Comparison of larval foraging behavior of Aedes albopictus and Aedes japonicus (Diptera: Culicidae) J Med Entomol. 2007;44:984–989. doi: 10.1603/0022-2585(2007)44[984:colfbo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Paupy C, Delatte H, Bagny L, Corvel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. MANOVA: Multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. 2. Oxford University Press, Inc; New York, NY: 2001. pp. 99–115. [Google Scholar]
- Sih A. Antipredator responses and the perception of danger by mosquito larvae. Ecology. 1986;67:434–441. [Google Scholar]
- Smith JP, Walsh JD, Cope EH, Tennant RA, Kozak JA, Darsie RF. Culex coronator Dyar and Knab: a new Florida species record. J Am Mosq Control Assoc. 2006;22:330–332. doi: 10.2987/8756-971X(2006)22[330:CCDAKA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Subra R. Biology and control of Culex pipiens quinquefasciatus Say, 1823 (Diptera, Culicidae) with special reference to Africa. Insect Sci Appl. 1980;1:319–338. [Google Scholar]
- Sudia WD, V, Newhouse F. Epidemic Venezuelan equine encephalitis in North America: a summary of virus-vector-host relationships. Am J Epidemiol. 1975;101:1–13. doi: 10.1093/oxfordjournals.aje.a112066. [DOI] [PubMed] [Google Scholar]
- Timmermann SE, Briegel H. Water depth and larval density affect development and accumulation of reserves in laboratory populations of mosquitoes. Bull Soc Vector Ecol. 1993;18:174–187. [Google Scholar]
- Varnado WC, Goddard J, Harrison BA. New state record of Culex coronator Dyar and Knab (Diptera: Culicidae) from Mississippi. Proc Entomol Soc Wash. 2005;107:476–477. [Google Scholar]
- Vezzani D. Review: artificial container-breeding mosquitoes and cemeteries: a perfect match. Trop Med Int Health. 2007;12:299–313. doi: 10.1111/j.1365-3156.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- Vinogradova EB. Culex pipiens pipiens mosquitoes: taxonomy, distribution, ecology, physiology, genetic, applied importance and control. Pensoft Publishers; Sofia, Bulgaria: 2000. [Google Scholar]
- Walker ED, Merritt RW. Behavior of larval Aedes triseriatus (Diptera: Culicidae) J Med Entomol. 1991;28:581–589. doi: 10.1093/jmedent/28.5.581. [DOI] [PubMed] [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991:1529–1546. [Google Scholar]
- Watts DM, Pantuwatana S, DeFoliart GR, Yuill TM, Thompson WH. Transovarial transmission of LaCrosse virus (California encephalitis group) in the mosquito Aedes triseriatus. Science. 1973;182:1140–1141. doi: 10.1126/science.182.4117.1140. [DOI] [PubMed] [Google Scholar]
- Workman PD, Walton WE. Larval behavior of four Culex (Diptera: Culicidae) associated with treatment wetlands in the southwestern United States. J Vector Ecol. 2003;28:213–228. [PubMed] [Google Scholar]
- Yee DA. Tires as habitats for mosquitoes: a review of studies within the eastern United States. J Med Entomol. 2008;45:581–593. doi: 10.1603/0022-2585(2008)45[581:tahfma]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yee DA, Juliano SA. Consequences of detritus type in an aquatic microsystem: effects on water quality, micro-organisms, and performance of the dominant consumer. Freshwater Biol. 2006;51:448–459. doi: 10.1111/j.1365-2427.2005.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Inter-specific differences in feeding behavior and survival under food-limited conditions for larval Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Ann Entomol Soc Am. 2004a;97:720–728. doi: 10.1603/0013-8746(2004)097[0720:IDIFBA]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Larval feeding behavior of three co-occurring species of container mosquitoes. J Vector Ecol. 2004b;29:315–322. [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kaufman MG, Juliano SA. The significance of ratios of detritus types and microorganism productivity to competitive interactions between aquatic insect detritivores. J Anim Ecol. 2007;76:1105–1115. doi: 10.1111/j.1365-2656.2007.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Allgood D, Kneitel JM, Kuehn KA. Constitutive differences between natural and artificial container mosquito habitats: vector communities, resources, microorganisms, and habitat parameters. J Med Entomol. 2012;49:482–491. doi: 10.1603/me11227. [DOI] [PubMed] [Google Scholar]