Abstract
The malaria circumsporozoite protein (CS), thrombospondin (TSP), and several other proteins including the terminal complement proteins and the neural adhesion molecules F-spondin and Unc-5, share a cell adhesive sequence. In CS this sequence is designated as region II-plus (EWSPCSVTCGNGIQVRIK) and in TSP it is found in the type I repeats. Previous studies aimed at fine mapping the amino acid residues required for cell adhesion have yielded discrepant results. Here we show in three different cell lines that the downstream basic residues are required for cell adhesion whereas the CSVTCG sequence is not. Using mutant Chinese hamster ovary cells selected for deficiencies in proteoglycan synthesis, we show that in wild type cells, heparan sulfate proteoglycans are the binding sites for this motif. This finding is supported by additional experiments with two other cell lines demonstrating that treatment with heparitinase but not chondroitinase abolishes cell adhesion to peptides representing this motif. Using Chinese hamster ovary cell mutants deficient in heparan sulfate proteoglycans but possessing chondroitin sulfate proteoglycans, we show that cell surface chondroitin sulfate proteoglycans can also mediate binding to this motif although higher concentrations of peptides are required for adhesion. Chondroitinase, but not heparitinase, treatment of these cells destroys cell surface-binding sites. Taken together, these results indicate that cell adhesion to this motif involves an interaction between the downstream positively-charged residues and the negatively charged glycosaminoglycan chains of heparan sulfate, or in some cases chondroitin sulfate, proteoglycans on the cell surface.
The major surface protein of malaria sporozoites, the circumsporozoite protein (CS)1 (reviewed in Ref. 1), contains a cell adhesive sequence (2) called region II-plus (3, 4). This sequence is highly conserved in all species of malaria parasites and is required for CS binding to hepatocytes (5), the target cell of malaria sporozoites. Thrombospondin (TSP), a 420-kDa glycoprotein that functions in the attachment, migration, and proliferation of many different cell types, has several cell adhesive domains (reviewed in Refs. 6–8). One of these lies within the type I repeats and has strong sequence similarity with region II-plus of CS (Fig. 1A). Another sporozoite surface protein, TRAP/SSP2 (9–11), the terminal complement components (12), the complement protein properdin (12), and the neural adhesion molecules F-spondin (13) and Unc-5 (14) also contain similar sequences (Fig. 1A). The importance of this sequence has been demonstrated in several systems. In the life cycle of the malaria parasite, it is responsible for the rapid and specific homing of sporozoites to hepatocytes where the first stage of malaria infection is initiated (15, 16). In thrombospondin this sequence mediates cell adhesion (17), and peptides representing this sequence have anti-angiogenic activity (18) and inhibit platelet aggregation (19) and melanoma metastasis (19).
Fig. 1. Sequences of the cell-adhesive motif in the malaria CS and the type I thrombospondin repeats as well as homologous sequences from other proteins.
Panel A, boxes highlight different portions of this motif: an upstream tryptophan-containing region, a cysteine-containing region and a downstream region containing basic and hydrophobic residues in which the basic residues are shown in bold. Shown are sequences of CS proteins from two human malarias Plasmodium falciparum (51) and Plasmodium vivax (52), the three type I thrombospondin repeats (17), another malaria surface protein TRAP/SSP2 (9–11), the complement protein properdin (12), and the neural adhesion molecules Unc-5 (14) and F-spondin (13). Panel B, peptides used in this study include the full-length region II-plus peptide from P. falciparum CS with a 5-amino acid extension, PGSAN. Truncated and substituted peptides are shown below. Bold letters indicate the changed amino acids and asterisks indicate cysteine residues blocked with acetamide.
The shared adhesive motif includes two cysteines at the amino-terminal and a series of positively charged amino acids at the COOH-terminal (Fig. 1A). Studies aimed at the identification of the amino acids required for cell adhesion have yielded discrepant results. Several groups of investigators, using thrombospondin-derived sequences, have reached the conclusion that the CSVTCG segment of the motif is responsible for cell adhesive activity (2, 19). In contrast, our work in malaria with region II-plus peptides has demonstrated the importance of the downstream positively-charged amino acids (4). We have shown that these residues are required for CS binding to hepatocytes and that they interact with the negatively-charged glycosaminoglycan chains (GAGs) of proteoglycans on the cell membrane.
One important discrepancy between the two series of studies lies in the methodology used to detect the interaction between target cells, and peptides representing various stretches of the adhesion motif. The standard assay used by most investigators measures the binding of live cells to immobilized peptides, while in our previous studies, peptides were in solution and we measured their binding to fixed and immobilized target cells. Although similar peptide ligands were used in all of these studies, cell adhesion is a multivalent process and may involve a different set of receptors than a protein-ligand interaction such as CS binding to hepatocytes. In addition, different cell lines were used in these studies. Our experiments were performed with a hepatoma cell line, HepG2, whereas others used a variety of cell lines none of which was derived from hepatocytes.
In an attempt to resolve these discrepancies, we used a variety of cell lines in a standard cell adhesion assay to analyze the interaction between live cells and peptides representing this cell-adhesive motif from CS (PCSVTCGNGIQVRIK). We tested modifications of both the peptide ligand and the cell surface-binding sites to determine the role of the downstream basic residues and the CSVTCG segment of this motif in cell adhesion.
MATERIALS AND METHODS
Peptides
Peptides were synthesized by Boc chemistry using the multiple peptide synthesis method described by Houghten (20). Cleavage from the resin was performed with hydrofluoric acid in two steps. A low concentration of hydrofluoric acid, used to remove protecting groups from the amino acid side chains, was followed by a high concentration of hydrofluoric acid which removed the peptides from the resin. Purity was verified by high performance liquid chromatography and amino acid analysis. CSVTCG peptides were synthesized in two ways, with blocked cysteines using acetamide groups and also with free sulfhydryls. These peptides were further purified on an FPLC C18 column using an acetonitrile gradient of 1 to 20% with 0.05% trifluoroacetic acid. CSVTCG without blocked cysteines was reduced with 50 mm dithiothreitol for 1 h before purification on the FPLC C18 column. The presence or absence of free sulfhydryl groups in CSVTCG peptides was confirmed with the Ellman reaction (21) before each experiment. The full-length region II-plus peptides used as soluble inhibitors in cell binding assays were partially oxidized and contained tetramers, trimers, dimers, and monomers by gel filtration chromatography and mass spectrometry (4).
Cells
Mutant and wild-type Chinese hamster ovary (CHO) cells were grown in Ham's F-12 (Life Technologies, Inc.) supplemented with 7.5% fetal calf serum (FCS) and 1 mm glutamine. The growth, isolation of subclones, and cryogenic storage of cells is described in Ref. 22. The mutants used in this study had the following characteristics: mutant pgsA-745 lacks xylosyltransferase activity and produces less than 2% of wild-type levels of glycosaminoglycans (23). pgsD-677 does not produce any heparan sulfate chains and makes greater than normal amounts of chondroitin sulfate due to a deficiency in the chain polymerization activities involved in heparan sulfate biosynthesis (24). pgsE-606 has diminished GlcNAc N-sulfotransferase activity, which results in the formation of heparan sulfate chains with less overall sulfation (25–27). pgsF-17 produces heparan sulfate lacking a specific subset of sulfate groups, namely the 2-O-sulfate groups of iduronic acid and glucuronic acid residues (28). Human cell lines were obtained from the American Type Culture Collection (Rockville, MD). MG63 cells (CRL 1427) are a human osteosarcoma line and were grown in Dulbecco's modified Eagle's medium (Life Technologies, Inc.) with 10% FCS and 1 mm glutamine. K562 cells (CCL 243), a myeloid cell line, were maintained in RPMI 1640 (Life Technologies, Inc.), 10% FCS, and 1 mm glutamine.
Cell Adhesion Assay
96-Well plates (Falcon Pro-Bind, Becton Dickinson, Lincoln Park, NJ) were coated with peptide at the indicated concentrations in phosphate-buffered saline (PBS) overnight at room temperature or for 2 h at 37 °C. Plates were washed twice with PBS and blocked for 1–2 h at 37 °C with PBS, 0.5% bovine serum albumin (product A-7888, Sigma). Log-phase cells were washed twice in PBS and detached with 5 mm EDTA/PBS at 37 °C. FCS was then added to the cells to a final concentration of 5%. The CHO cells required longer incubations in EDTA for detachment and so a different buffer was used since incubation in PBS for 10 min was found to be toxic to these cells. Both CHO mutants and wild-type cells were washed once in 10 mm Hepes, 25 mm sodium bicarbonate, 5 mm EDTA (pH 7.3) and then incubated in this same buffer for 15 min at 37 °C. By trypan blue exclusion 95% of the detached cells were viable after this treatment. After the cells were detached FCS was added as above. Cells were then washed 3 times with their normal growth medium without FCS (incomplete medium) and resuspended at a density of 106/ml in incomplete medium. 105 cells were plated in each well and incubated at 37 °C with 5% CO2 for 1 h. The plates were then centrifuged upside down at 100 × g for 5 min, and the cells remaining in the wells were fixed with 4% paraformaldehyde (Eastman Kodak Co.) in PBS for 10 min followed by 20% methanol for 10 min. The cells were stained with 0.5% crystal violet (Sigma) in 50% methanol for 5 min, washed 3 times with tap water, the stain was eluted from the cells with 0.1 m citrate buffer (pH 4.2) in 50% ethanol and absorbance of the eluant was measured at 540 nm in an Emax microplate reader (Molecular Devices Corp., Menlo Park, CA). Experiments performed to assess the efficiency of cell binding demonstrated that when wells were coated with 10 μg/ml full-length peptide, over 95% of the cells added, bound to the wells (data not shown). In addition, control experiments performed with fibronectin demonstrated that all of the wild-type cell lines bound with the same efficiency to 10 μg/ml fibronectin as they did to region II-plus peptides (data not shown).
Peptide Inhibition
Cells were prepared as outlined above and after 3 washes in incomplete medium were resuspended in incomplete medium with the indicated concentration of peptide. Incubations with peptide were for 30 min at 37 °C on an orbital rotator in sterile Eppendorf tubes that had been blocked with PBS, 5% bovine serum albumin for 2 h at 37 °C to avoid peptide and cell adhesion to the sides of the tubes. The cells were then plated without being washed, in wells that had been coated with 1.25 μg/ml region II-plus peptide and blocked as above.
Glycosaminoglycan Inhibition
Cells were prepared as above and after 3 washes in incomplete medium they were incubated for 10 min on ice with the indicated amounts of heparin (Sigma) or chondroitin sulfate A, B, or C (Sigma) before being plated with the inhibitor in wells that had been coated with 1.25 μg/ml region II-plus peptide and blocked as above. In experiments with CHO mutant pgsD, the wells were coated with 4 μg/ml peptide because this was the lowest concentration of peptide that resulted in maximal binding of the cells.
Enzyme Digestion
Cells were prepared as outlined above and after 3 washes in incomplete medium, were incubated with heparitinase (heparinase III; ICN, Costa Mesa CA) or chondroitinase ABC (ICN) in incomplete media supplemented with 0.5% bovine serum albumin and 20 mm Hepes pH 7.4 for 3 h on an orbital rotator at 37 °C. They were then plated, in the presence of the enzyme, in wells coated with 1.25 μg/ml region II-plus peptide, and blocked with PBS, 0.5% bovine serum albumin. When binding of enzyme-treated CHO mutant pgsD was investigated, plates were coated with 4 μg/ml region II-plus peptide for reasons stated above.
35 Sulfate Labeling of Cells
Subconfluent cells were trypsinized and resuspended in complete medium and 2 × 105 cells/well were plated in 6-well plates (Falcon 3064, Becton Dickinson, Lincoln Park, NJ). The cells were allowed to grow for 18–24 h, the medium was removed and 2 ml of sulfate labeling medium (2.5% dialyzed FCS and 50 μCi of 35SO4/ml in Ham's F-12) was added per well. 24 h later the cells were washed once with PBS and incubated with 100 μg of l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin (Sigma) in 1 ml of PBS for 5 min at room temperature. 50 μg of soybean trypsin inhibitor (Sigma) was then added to each well and the cells, which were now detached, were spun at 70 × g for 5 min at 4 °C and the supernatants were collected and stored at –70 °C for later GAG analysis.
GAG Analysis of 35Sulfate-labeled Supernatants
Each supernatant was treated with one-sixth volume of a Pronase solution (Pronase, 1 mg/ml (Boehringer Mannheim), 0.24 m sodium acetate (pH 6.5), and 1.92 m sodium chloride). 1 mg of chondroitin sulfate was added to each solution as a carrier. After overnight incubation, the reaction mixture was diluted 5-fold with water to reduce the salt concentration to ~0.1 m. The solution was applied to a 0.5-ml DEAE-Sephacel column prepared in a disposable polypropylene pipette tip plugged with glass wool. The column was washed with 20 mm sodium acetate buffer (pH 6.0) containing 250 mm sodium chloride. Bound GAGs were eluted with 1 m sodium chloride in 20 mm sodium acetate (pH 6.0) and precipitated with 4 volumes of ethanol at 4 °C for 2 h. The precipitate was dissolved in 1 ml of 0.5 m sodium acetate (pH 5.5) and reprecipitated with ethanol. GAG chains were treated with 1 m sodium borohydride in 0.5 m sodium hydroxide for 24 h at 4 °C to β-eliminate the chains. The samples were diluted with water and purified by another round of DEAE chromatography. The heparan sulfate chains were prepared by treating the samples with 20 milliunits of chondroitinase ABC (Seikagaku, Japan) in 20 mm Tris-HCl (pH 7.4) overnight at 37 °C to depolymerize [35S]chondroitin sulfate. Another round of DEAE-Sephacel chromatography separated the intact HS chains from the degraded chondroitin sulfate. The amount of [35S]heparan sulfate was measured by scintillation counting and the relative amount of heparan sulfate and chondroitin sulfate was calculated.
RESULTS
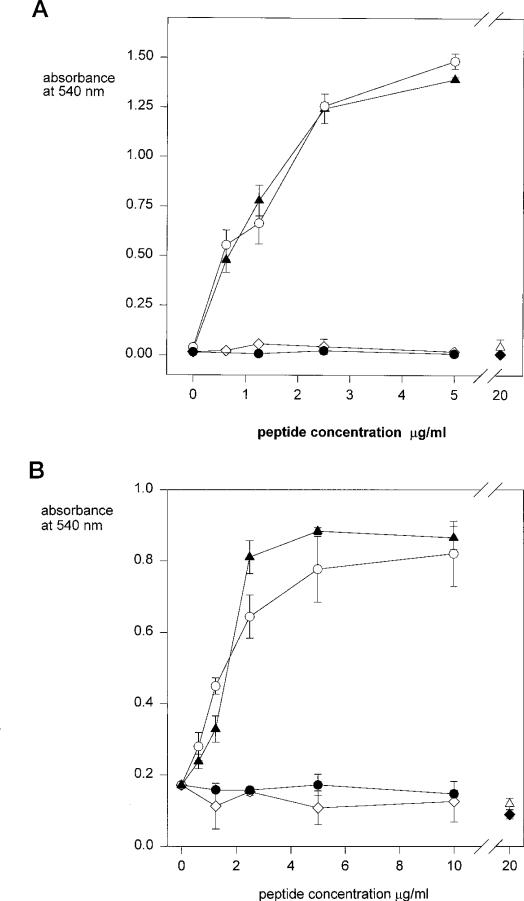
In initial experiments we measured the adhesion of live cells to region II-plus peptides (PCSVTCGNGIQVRIKPGSAN) immobilized onto wells of microtiter plates. For these studies we used two cell lines, one of which, K562 cells, had been previously shown to adhere to the CSVTCG portion of this motif (2). The other cell line, MG63, is a standard cell line used in adhesion studies although the fine specificity of its interaction with this motif had not been previously studied. The full-length region II-plus peptide used in this study (Fig. 1B) contains a 5-amino acid extension, PGSAN, present for reasons extraneous to the current study. We have previously shown that these additional amino acids do not interfere with or enhance binding activity of the peptide (4). As shown in Fig. 2, both MG63 and K562 cells adhere to region II-plus peptides in a dose-dependent manner. To determine which portion of this motif is required for cell adhesion, we tested the truncated and substituted peptides shown in Fig. 1B for cell adhesive activity. When the NH2-terminal portion was truncated but the downstream portion with the basic and hydrophobic residues was intact (CGNGIQVRIKPGSAN), the cells bound to the peptide-coated wells with identical efficiency (Fig. 2). However, neither cell line adhered to peptides in which the downstream basic residues had been replaced by either negatively charged or neutral amino acids (PCSVTCGNGIQVEIEPGSAN and PCSVTCGNGIQVNIN), even though the entire NH2-terminal CSVTCG sequence was present (Fig. 2).
Fig. 2. Adhesion of MG63 and K562 cells to region II-plus peptides.
MG63 cells (panel A) or K562 cells (panel B) were added to the peptide-coated wells, PCSVTCGNGIQVRIKPGSAN (closed triangles); CGNGIQVRIKPGSAN (open circles); PCSVTCGNGIQVEIEPGSAN (open diamonds); PCSVTCGNGIQVNIN (closed circles); CSVTCG with free sulfhydryls (open triangles); CSVTCG with blocked cysteines (closed diamonds). Cells were allowed to adhere for 1 h at 37 °C and bound cells were quantified with crystal violet. Each point was performed in triplicate and shown is the mean with standard deviations.
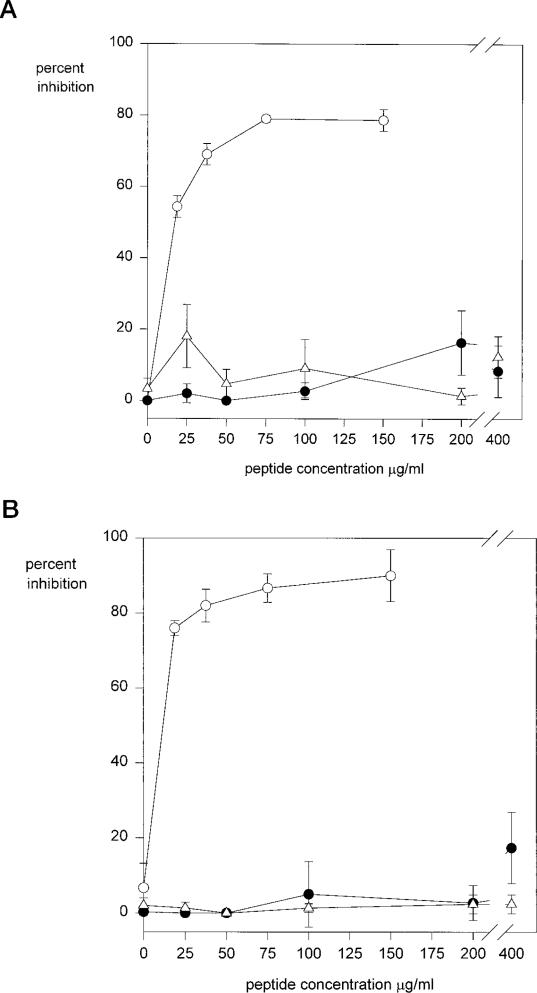
The apparent lack of activity of CSVTCG is in contradiction with the results of Tuszynski et al. (19). In their studies, however, the short CSVTCG peptide was the immobilized target, rather than the longer constructs described above. We therefore synthesized CSVTCG peptides to use in adhesion assays with MG63 and K562 cells. We synthesized two CSVTCG peptides, one in which the cysteines were blocked with acetamide groups and another with free sulfhydryl groups (Fig. 1B). As shown in Fig. 2, however, neither cell line adhered to the wells coated with these peptides. To investigate whether these short peptides had indeed bound to the plastic, we performed experiments with a slightly longer and more hydrophobic, 125I-labeled YCSVTCG peptide. We found that, as is frequently the case for short peptides, YCSVTCG bound very poorly to plastic (data not shown) and therefore could not be reliably used as a substrate for cell adhesion. For this reason, we tested CSVTCG as a soluble inhibitor of cell adhesion to immobilized PCSVTCGNGIQVRIKPGSAN.
As shown in Fig. 3, adhesion of both cell lines is inhibited by soluble full-length peptide, whereas CSVTCG peptide, at high concentrations has no inhibitory activity. Because the oxidation state of the cysteines in CSVTCG could influence the cell adhesive activity of this sequence, we used CSVTCG peptides whose cysteines were blocked and peptides whose cysteines contained free sulfhydryls. Neither peptide inhibited cell adhesion to the full-length sequence.
Fig. 3. Peptide inhibition of cell adhesion to region II-plus peptides.
MG63 cells (panel A) or K562 cells (panel B) were preincubated with inhibitor peptides PCSVTCGNGIQVRIKPGSAN (open circles); CSVTCG, cysteines blocked (open triangles); CSVTCG, cysteines reduced (closed circles); for 30 min at 37 °C and then plated in wells coated with 1.25 μg/ml region II-plus peptide (PCSVTCGNGIQVRIKPGSAN) for 1 h. Bound cells were quantified with crystal violet. Each point was performed in triplicate, and shown is the percent inhibition of binding compared with controls with no inhibitor.
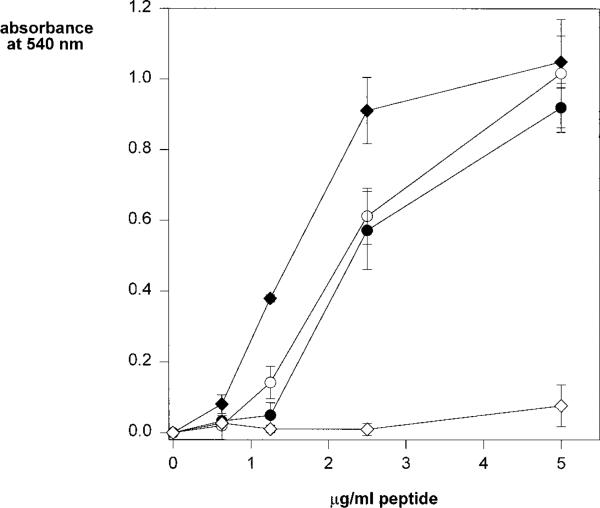
The requirement of the downstream basic residues for cell adhesive activity suggested that an ionic interaction between this sequence and negatively-charged cell surface molecules was involved. Others have shown that this motif binds to sulfated glycoconjugates (17, 29–33) and our previous studies using fixed immobilized cells demonstrated that soluble region II-plus peptides bound to the heparan sulfate (HS) GAGs of cell surface HSPGs (4, 34). To investigate which sulfated glycoconjugate on the cell surface bound to this motif in live-cell adhesion assays, we used mutant CHO cells selected for deficiencies in GAG synthesis. As shown in Fig. 4, wild-type CHO cells, similarly to MG63 and K562 cells, adhere to the full-length region II-plus peptide (PCSVTCGNGIQVRIKPGSAN). Mutant CHO cell line pgsA, lacking proteoglycan GAGs, does not bind to the peptide. When GAGs are partially undersulfated, as in mutant cell lines pgsE and pgsF, there is decreased cell binding when compared with wild-type cells (Fig. 4). These results suggest that the GAGs of cell surface proteoglycans mediate binding to region II-plus peptides and that this is due, at least in part, to an ionic interaction between the basic residues of this cell adhesive motif and the negatively charged sulfate moieties of cell surface GAGs.
Fig. 4. Wild-type and mutant CHO cell adherence to region II-plus peptides.
CHO wild-type (closed diamonds); pgsE-606, under-sulfated GAGs (closed circles); pgsF-17, undersulfated GAGs (open circles); pgsA-745, 6% normal GAGs (open diamonds) were allowed to adhere to wells coated with region II-plus peptide (PCSVTCGNGIQVRIKPGSAN), for 1 h and bound cells were quantified with crystal violet. Each point was performed in triplicate and shown are the means with standard deviations.
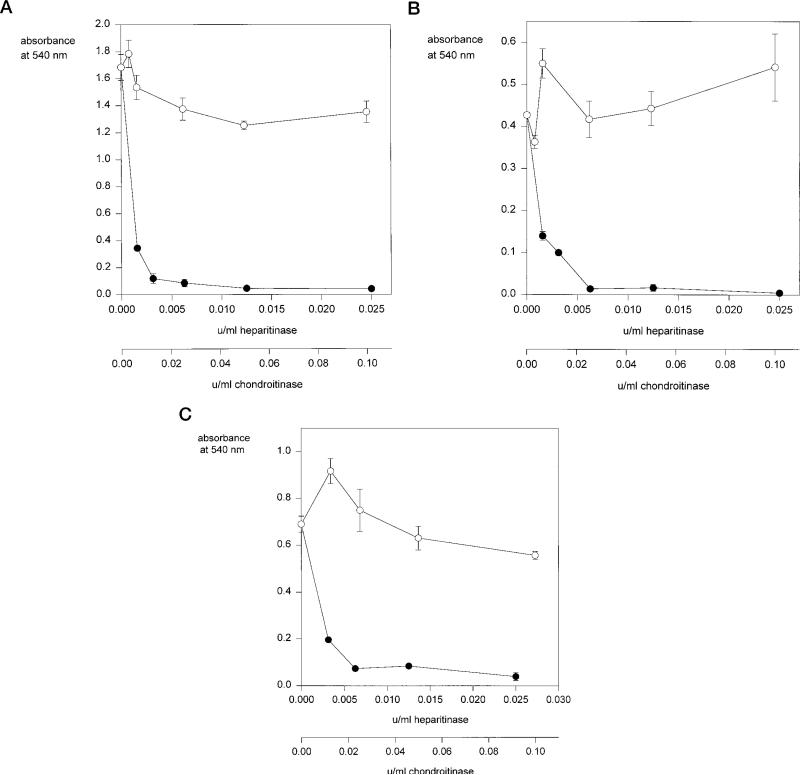
We then used GAG lyases to investigate the identity of the cell surface GAGs which mediate adhesion to this motif since these cell lines have chondroitin sulfate proteoglycans as well as HSPGs (Table I). As shown in Fig. 5, heparitinase treatment abolishes adhesion of MG63, K562, and wild-type CHO cells to this sequence whereas chondroitinase treatment does not significantly decrease cell binding, suggesting that HS and not chondroitin sulfate GAGs mediate binding to this motif. To confirm this finding, we performed studies with the CHO mutant pgsD, which has very low amounts of cell surface HS (<2% of wild-type cells) but increased amounts of chondroitin sulfate GAGs (24). To our surprise, we found that these cells bound to region II-plus peptides although with lower efficiency when compared with wild-type CHO cells (Fig. 6A). The role of chondroitin sulfate GAGs in binding of the CHO pgsD cells to region II-plus peptides was confirmed with the demonstration that heparitinase treatment of these cells had little effect on cell adhesion whereas chondroitinase treatment completely abolished binding to region II-plus peptides (Fig. 6B). These results suggest that both HSPGs and chondroitin sulfate proteoglycans can mediate binding to this motif. However, HSPG-deficient pgsD cells require higher concentrations of peptide for binding when compared with wild-type cells, suggesting that HSPGs bind with higher avidity to this motif than chondroitin sulfate.
Table I.
Relative amounts of cell surface GAGs on cells used in this study
| Cell type | Heparan sulfate | Chondroitin sulfatea |
|---|---|---|
| % | ||
| CHO K1 | 63 | 37 |
| CHO pgsF | 50 | 50 |
| CHO pgsE | 49 | 51 |
| CHO pgsD | <2b | >98b |
| MG63 | 50 | 50 |
| K562 | 25 | 75 |
Calculated from [35S]GAG-[35S]HS.
Ref. 24.
Fig. 5. Adhesion of cells to region II-plus peptides after treatment with GAG lyases.
MG63 cells (panel A), K562 cells (panel B), or CHO wild-type cells (panel C) were preincubated with the indicated concentrations (units/ml) of chondroitinase ABC (open circles) or heparitinase (closed circles) for 3 h at 37 °C, then added to wells coated with 1.25 μg/ml region II-plus peptide (PCSVTCGNGIQVRIKPGSAN) and allowed to adhere for 1 h at 37 °C. Bound cells were quantified with crystal violet. Each point was performed in triplicate and shown are the means with standard deviations.
Fig. 6. Adhesion of mutant CHO pgsD to region II-plus peptides before and after treatment with GAG lyases.
Panel A, CHO wild-type cells (closed diamonds) or CHO pgsD-677 cells (open circles) were allowed to adhere to wells coated with region II-plus peptide (PCSVTCGNGIQVRIKPGSAN), for 1 h and bound cells were quantified with crystal violet. Each point was performed in triplicate and shown are the means with standard deviations. Panel B, CHO pgsD-677 cells were preincubated with either chondroitinase ABC (open circles) or heparitinase (closed circles) for 3 h at 37 °C, then added to wells coated with 4.0 μg/ml region II-plus peptide (PCSVTCGNGIQVRIKPGSAN) and allowed to adhere for 1 h at 37 °C. Bound cells were quantified with crystal violet. Each point was performed in triplicate and shown are the means with standard deviations.
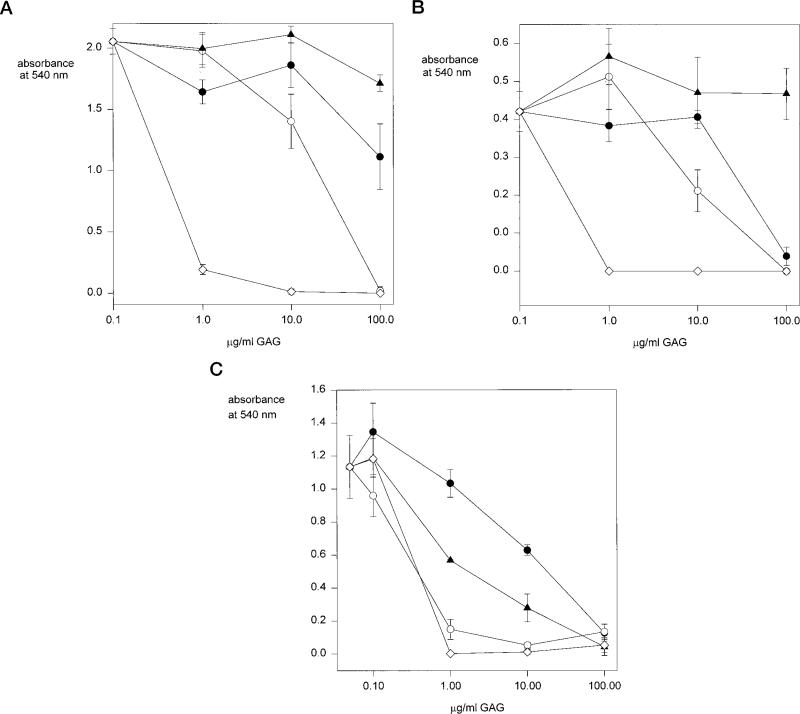
We then used soluble GAGs as competitive inhibitors of cell adhesion to compare the avidity of binding of chondroitin sulfate and heparan sulfate to this motif (Fig. 7). Binding of MG63 cells, K562 cells (data not shown), and wild-type CHO cells is completely inhibited in the presence of 1 μg/ml heparin. Higher concentrations of chondroitin sulfate B (10–100 μg/ml) can inhibit binding of these cells to region II-plus peptides, however, chondroitin sulfates A and C do not significantly inhibit cell adhesion. Binding of CHO mutant pgsD to region II-plus peptides is inhibited in the presence of 1 μg/ml heparin, similar to the wild-type cell line. However, 10-fold less chondroitin sulfate B is required to inhibit adhesion of these cells when compared with wild-type cells and chondroitin sulfates A and C also inhibit at lower concentrations. In these experiments, cell surface GAGs are competing with soluble GAGs for binding to immobilized region II-plus peptides. These results therefore demonstrate that higher concentrations of soluble chondroitin sulfate are required to compete with cell surface HS than with cell surface chondroitin sulfate for binding to this motif.
Fig. 7. Heparin and chondroitin sulfate inhibition of cell adhesion to region II-plus peptides.
MG63 cells (panel A), CHO wild-type cells (panel B), or CHO pgsD-677 cells increased chondroitin sulfate, decreased HS (panel C) were preincubated with chondroitin sulfate A (closed circles), chondroitin sulfate B (open circles), chondroitin sulfate C (closed triangles), or heparin (open diamond) for 10 min on ice and then added to wells coated with region II-plus peptide (PCSVTCGNGIQVRIKPGSAN) and allowed to adhere for 1 h at 37 °C. Bound cells were quantified by crystal violet. Each point was performed in triplicate and shown are the means with standard deviations.
DISCUSSION
These studies confirm the findings of Prater et al. (17) demonstrating the cell adhesive properties of the conserved motif found within the type I repeats of TSP. Our fine mapping of the residues required for cell adhesion, however, are not in agreement with previous studies (2, 19). The present findings demonstrate that the downstream basic residues are required for cell adhesion whereas the CSVTCG sequence is not. If CSVTCG is present without the downstream basic residues, cell adhesive activity is lost whereas truncated peptides beginning with the second cysteine and including the downstream residues support cell adhesion with an efficiency equal to that of full-length peptide. Unlike other investigators, we could not demonstrate cell adhesive activity using the short CSVTCG peptide either when it was used to coat microtiter plate wells or when used as a soluble inhibitor.
More studies are required to explain the role of the CSVTCG portion of the motif. One possibility is that its role is structural. We have previously shown that only multimers of the downstream basic residues bind to cell surface HSPGs (4). Consistent with this finding is the fact that most proteins containing this motif have two to six copies of this sequence (6, 12–14) so that folding of the protein could bring multiple GAG-binding sequences together. Of all the proteins containing this motif, only the malaria CS and TRAP/SSP2 proteins do not contain multiple copies of this sequence. In the case of CS, however, the requirement for aggregation is met by the high density of CS molecules, as well as the presence of disulfide-linked aggregates (4), on the surface of the parasite. CSVTCG may therefore contribute to the formation of a structure that aligns the downstream basic residues of these motifs from different regions of the same protein or, in the case of CS, from different CS monomers. This may be accomplished either via disulfide bond formation between cysteines, or via hydrophobic interactions between the side chains of the intervening amino acids, from different region II-plus sequences or type I TSP repeats. The importance of hydrophobic residues in the formation of structures in which the basic residues are aligned with one another has been demonstrated for other heparin-binding proteins whose crystal structures have been resolved or inferred (35, 36). In this study, the requirement for aggregation was met by immobilization of the peptides in microtiter wells. In this way, the peptides are within sufficient proximity of one another such that their heparin binding activity is intact. In the experiments where region II-plus peptides are used as soluble inhibitors they had been oxidized and contained tetramers, trimers, and dimers as well as monomers (4).
Alternately the CSVTCG portion of the motif may bind to a cell surface receptor after the downstream basic residues bind to proteoglycans. TSP is known to bind to CD36 (37–40) and some investigators have suggested that this binding is CSVTCG-dependent (41–43). However, in some of these studies the peptides used contained the downstream basic residues as well as CSVTCG so it is not clear which portion of the motif binds to CD36.
We provide additional evidence for the involvement of the downstream basic residues in cell adhesion with the demonstration that negatively-charged GAGs of cell surface proteoglycans serve as binding sites for this motif. Mutant cells lacking GAGs and cells whose GAGs have been digested with enzymes do not bind to region II-plus peptides. In addition, experiments with mutant cell lines pgsE and pgsF show that altered sulfation of HS decreases cell adhesion to region II-plus peptides. These two mutant cell lines have different sulfotransferase deficiencies (25, 28). pgsE cells lack the enzyme responsible for removal of N-acetyl groups and the addition of N-sulfate groups to a subset of N-acetylglucosamine residues in HS. This enzyme acts first during polymer modification reactions and creates regions of modified residues interspersed with regions of unmodified residues. After N-deacetylation/N-sulfation, the adjacent glucuronic acid residues epimerize to iduronic acid, and sulfate groups are added to the hydroxyl groups at C2 of the uronic acids and C6 of the glucosamine residues. The modifications occur in blocks along the chains much like variably sized beads on a string. The defect in pgsE cells depresses N-sulfotransferase activity by about a factor of 2 to 3, causing a decrease in the frequency of modified blocks along the chain (25). Interestingly, the overall degree of modification within the blocks does not change significantly (26). The structure of HS from pgsF cells differs significantly from that of pgsE cells. These cells lack the 2-O-sulfotransferase that acts on the uronic acid residues within the blocks (28). A side effect of this mutation is that the chains are actually more highly N-sulfated, suggesting that the blocks of modified residues are somewhat longer or more frequent. Nevertheless, the lack of 2-O-sulfotransferase activity diminishes the degree of sulfation within the blocks. Both strains attach to region II-plus peptides to a lesser extent than wild-type cells, suggesting that both the degree of sulfation (pgsE) and the pattern of sulfation (pgsF) are important.
Here we demonstrate that in addition to HS, cell surface chondroitin sulfate can also serve as adhesion receptors for this motif. The mutant CHO cell line pgsD lacks the enzymes required for HS GAG chain polymerization and makes ~3-fold more chondroitin sulfate than wild-type cells so that the total amount of GAGs remains the same. This mutant binds to region II-plus peptides and binding is abolished with chondroitinase but not heparitinase treatment of the cells. These cells, however, require higher concentrations of peptide for adhesion and binding is more easily inhibited with sulfated compounds when compared with wild-type cells, suggesting that there is a lower avidity interaction between chondroitin sulfate and this cell adhesive motif.
Using some of the same mutant CHO cell lines, others have shown that adhesion to the entire TSP molecule is largely dependent upon cell surface HSPGs, although a mutant deficient in HSPGs but possessing chondroitin sulfate proteoglycans (pgsD) also binds to TSP (44, 45). In addition, a more recent study has shown that myoblasts bind to TSP via cell surface chondroitin sulfate and that this interaction is mediated in part by the type I repeats of TSP (46). Our demonstration that the motif of basic and hydrophobic residues found within the type I repeats can bind to chondroitin sulfate, suggests that these residues are involved in the adhesion of mutant CHO cells and myoblasts to TSP.
Interestingly, K562 cells have relatively more chondroitin sulfate than heparan sulfate proteoglycans on their surface and yet heparinase digestion of these cells abolishes binding to region II-plus peptides and, in contrast to the CHO pgsD cells, chondroitinase digestion does not. This may be due to differences in the type and structure of the chondroitin sulfate, to differences in the display of cell surface chondroitin sulfate or in the absolute amount of chondroitin sulfate chains possessed by these two cell lines. It has been previously shown for the syndecans that chondroitin sulfate chains are located near the transmembrane domain of the core protein and HS chains are found more NH2-terminal (47, 48). In the CHO pgsD mutant, chondroitin sulfate chains are substituted for HS chains2 and so at least a portion of them will be located N-terminal and may therefore be more accessible for binding to receptors.
Another region within the type I repeats of TSP has been shown to have heparin binding activity (49, 50). NH2-terminal to the CSVTCG sequence is a conserved tryptophan-containing region, SHWSPWSS. The minimum sequence necessary for heparin binding activity is the pentapeptide WSPWS. However, not all of the proteins shown in Fig. 1 contain this heparin-binding sequence, including the malaria CS proteins. It is possible that WSPWS has a different GAG chain specificity than the downstream cluster of basic amino acids. Alternately, when both heparin-binding motifs are present, as in the case of TSP, the affinity of the cell surface receptor-ligand interaction may increase. In support of this, Guo et al. (49) have shown that when a cluster of positively charged residues was added to the SHWSPWSS sequence, heparin binding activity of the peptide was enhanced 10-fold (49).
In some instances, therefore, the CSVTCG sequence is flanked on both sides by heparin- or GAG-binding domains. We previously discussed the possibility that CSVTCG performs a structural role, aligning the downstream basic residues from different region II-plus motifs, or type I TSP repeats, for multimeric binding to HSPGs. An alternate possibility is that, in some cases, it may be part of a structural motif that permits sequences flanking it on both sides to bind to HSPGs. It is also possible that binding of the downstream basic residues of the region II-plus motif, and/or the WSPWS sequence discussed above, to HSPGs is a prerequisite for the participation of the CSVTCG sequence in a subsequent binding event. Future work will hopefully elucidate the function of the CSVTCG portion of this motif which together with existing data will shed some light on the significance of this conserved sequence.
Acknowledgments
We thank Rocio Farfan for technical assistance, Dr. Laura Spinardi for help with the cell binding assay, and Drs. Victor Nussenzweig and Filippo Giancotti for helpful discussions and review of the manuscript.
Footnotes
This work was supported by National Institutes of Health Physician Scientist Award K11 AI-01175 (to P. S.), Short Term Training Students in Health Professional Schools Grant 5T35-DK-07421 (to S. M. G.), National Institutes of Health Grants PO1 AI35703 (to P. C. and J. D. E.) and RO1 GM33063 (to J. D. E.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: CS, circumsporozoite protein; TSP, thrombospondin; GAG, glycosaminoglycan; CHO, Chinese hamster ovary; FCS, fetal calf serum; PBS, phosphate-buffered saline; HS, heparan sulfate; HSPG, heparan sulfate proteoglycan.
L. Zhang and J. Esko, unpublished data.
REFERENCES
- 1.Nussenzweig V, Nussenzweig RS. In: Advances in Immunology. Dixon FJ, editor. Vol. 45. Academic Press, Inc.; San Diego: 1989. pp. 283–334. [DOI] [PubMed] [Google Scholar]
- 2.Rich KA, George FWI, Law JL, Martin WJ. Science. 1990;249:1574–1577. doi: 10.1126/science.2120774. [DOI] [PubMed] [Google Scholar]
- 3.Dame JB, Williams JL, McCutchan TF, Weber JL, Wirtz RA, Hockmeyer WT, Maloy WL, Haynes JD, Schneider I, Roberts DD, Sanders GS, Reddy EP, Diggs CL, Miller LH. Science. 1984;225:593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- 4.Sinnis P, Clavijo P, Fenyo D, Chait BT, Cerami C, Nussenzweig V. J. Exp. Med. 1994;180:297–306. doi: 10.1084/jem.180.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, Santos MJ, Nussenzweig V. Cell. 1992;70:1021–1035. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- 6.Lawler J, Hynes RO. J. Cell Biol. 1986;103:1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frazier WA. Curr. Opin. Cell Biol. 1991;3:792–799. doi: 10.1016/0955-0674(91)90052-z. [DOI] [PubMed] [Google Scholar]
- 8.Mosher DF. Annu. Rev. Med. 1990;41:85–97. doi: 10.1146/annurev.me.41.020190.000505. [DOI] [PubMed] [Google Scholar]
- 9.Robson KJH, Hall JRS, Jennings MW, Harris TJR, Marsh K, Newbold CI, Tate VE, Weatherall DJ. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- 10.Hedstrom RC, Campbell JR, Leef ML, Charoenvit Y, Carter M, Sedegah M, Beaudoin RL, Hoffman SL. Bull. W. H. O. 1990;68:152–157. [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers WO, Rogers MD, Hedstrom RC, Hoffman SL. Mol. Biochem. Parasitol. 1992;53:45–52. doi: 10.1016/0166-6851(92)90005-5. [DOI] [PubMed] [Google Scholar]
- 12.Goundis D, Reid KBM. Nature. 1988;335:82–85. doi: 10.1038/335082a0. [DOI] [PubMed] [Google Scholar]
- 13.Klar A, Baldassare M, Jessell TM. Cell. 1992;69:95–110. doi: 10.1016/0092-8674(92)90121-r. [DOI] [PubMed] [Google Scholar]
- 14.Leung-Hagestejin C, Spence AM, Stern BD, Zhou Y, Su MW, Hedgecock EM, Culotti JG. Cell. 1992;71:289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- 15.Cerami C, Frevert U, Sinnis P, Takacs B, Nussenzweig V. J. Exp. Med. 1994;179:695–701. doi: 10.1084/jem.179.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinnis P, Willnow TE, Briones MRS, Herz J, Nussenzweig V. J. Exp. Med. 1996;184:945–954. doi: 10.1084/jem.184.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prater CA, Plotkin J, Jaye D, Frazier WA. J. Cell Biol. 1991;112:1031–1040. doi: 10.1083/jcb.112.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. J. Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuszynski GP, Rothman VL, Deutch AH, Hamilton BK, Eyal J. J. Cell Biol. 1992;116:209–217. doi: 10.1083/jcb.116.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houghten RA. Proc. Natl. Acad. Sci. U. S. A. 1985;82:5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellman GL. Arch. Biochem. Biophys. 1958;74:443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- 22.Esko JD. Methods Cell Biol. 1989;32:387–422. doi: 10.1016/s0091-679x(08)61183-8. [DOI] [PubMed] [Google Scholar]
- 23.Esko JD, Stewart TE, Taylor WH. Proc. Natl. Acad. Sci. U. S. A. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lidholt K, Weinke JL, Kiser CS, Lugemwa FN, Bame KJ, Cheifetz S, Massague J, Lindahl U, Esko JD. Proc. Natl. Acad. Sci. U. S. A. 1992;89:2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bame KJ, Esko JD. J. Biol. Chem. 1989;264:8059–8065. [PubMed] [Google Scholar]
- 26.Bame KJ, Lidholt K, Lindahl U, Esko JD. J. Biol. Chem. 1991;266:10287–10293. [PubMed] [Google Scholar]
- 27.Bame KJ, Reddy RV, Esko JD. J. Biol. Chem. 1991;266:12461–12468. [PubMed] [Google Scholar]
- 28.Bai X, Esko JD. J. Biol. Chem. 1996;271:17711–17717. doi: 10.1074/jbc.271.30.17711. [DOI] [PubMed] [Google Scholar]
- 29.Cerami C, Kwakye-Berko F, Nussenzweig V. Mol. Biochem. Parasitol. 1992;54:1–12. doi: 10.1016/0166-6851(92)90089-3. [DOI] [PubMed] [Google Scholar]
- 30.Pancake SJ, Holt GH, Mellouk S, Hoffman SL. J. Cell Biol. 1992;6:1351–1357. doi: 10.1083/jcb.117.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holt GD, Krivan HC, Gasic GJ, Ginsburg V. J. Biol. Chem. 1989;264:12138–12140. [PubMed] [Google Scholar]
- 32.Roberts DD, Haverstick DM, Dixit VM, Frazier WA, Santoro SA, Ginsburg V. J. Biol. Chem. 1985;260:9405–9411. [PubMed] [Google Scholar]
- 33.Roberts DD, Williams SB, Gralnick HR, Ginsburg V. J. Biol. Chem. 1986;261:3306–3309. [PubMed] [Google Scholar]
- 34.Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. J. Exp. Med. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St. Charles R, Walz DA, Edwards BFP. J. Biol. Chem. 1989;264:2092–2099. [PubMed] [Google Scholar]
- 36.Gan Z-R, Li Y, Chen Z, Lewis SD, Shafer JA. J. Biol. Chem. 1994;269:1301–1305. [PubMed] [Google Scholar]
- 37.Asch AS, Barnwell J, Silverstein RL, Nachman RL. J. Clin. Invest. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverstein RL, Baird M, Lo SK, Yesner LM. J. Biol. Chem. 1992;267:16607–16612. [PubMed] [Google Scholar]
- 39.Kieffer N, Nurden AT, Hasitz M, Titeux M, Breton-Gorius J. Biochem. Biophys. Acta. 1988;967:408–415. doi: 10.1016/0304-4165(88)90104-3. [DOI] [PubMed] [Google Scholar]
- 40.McGregor JL, Catimel B, Parmentier S, Clezardin P, Dechavanne M, Leung LLK. J. Biol. Chem. 1989;264:501–506. [PubMed] [Google Scholar]
- 41.Li W-X, Howard RJ, Leung LLK. J. Biol. Chem. 1993;268:16179–16184. [PubMed] [Google Scholar]
- 42.Catimel B, Leung L, Ghissasi H, Mercier N, McGregor J. Biochem. J. 1992;284:231–236. doi: 10.1042/bj2840231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asch AS, Silbiger S, Heimer E, Nachman RL. Biochem. Biophys. Res. Commun. 1992;182:1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- 44.Murphy-Ullrich JE, Westrick LG, Esko JD, Mosher DF. J. Biol. Chem. 1988;263:6400–6406. [PubMed] [Google Scholar]
- 45.Kaesberg PR, Ershler WB, Esko JD, Mosher DF. J. Clin. Invest. 1989;83:994–1001. doi: 10.1172/JCI113986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams JC, Lawler J. Mol. Biol. Cell. 1994;5:423–437. doi: 10.1091/mbc.5.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, David G, Esko JD. J. Biol. Chem. 1995;270:27127–27135. doi: 10.1074/jbc.270.45.27127. [DOI] [PubMed] [Google Scholar]
- 48.Kokenyesi R, Bernfield M. J. Biol. Chem. 1994;269:12304–12309. [PubMed] [Google Scholar]
- 49.Guo NH, Krutzsch HC, Negre E, Vogel T, Blake DA, Roberts DD. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3040–3044. doi: 10.1073/pnas.89.7.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo N, Krutzsch HC, Negre E, Zabrenetzky VS, Roberts DD. J. Biol. Chem. 1992;267:19349–19355. [PubMed] [Google Scholar]
- 51.de la Cruz VF, Lal AA, McCutchan TF. J. Biol. Chem. 1987;262:11935–11940. [PubMed] [Google Scholar]
- 52.McCutchan TF, Lal AA, De La Cruz VF, Miller LH, Maloy WL, Charoenvit Y, Beaudoin RL, Guerry P, Wistar R, Hoffman SL, Hockmeyer WT, Collins WE, Wirth D. Science. 1985;230:1381–1383. doi: 10.1126/science.2416057. [DOI] [PubMed] [Google Scholar]