Abstract
Background and Purpose
Obesity is an increasing epidemic worldwide; however little is known regarding effects of obesity produced by high-fat diet (HFD) on the cerebral circulation. The purpose of this study was to examine the functional and temporal effects of a HFD on carotid and cerebral vascular function and to identify mechanisms that contribute to such functional alterations.
Methods
Responses of cerebral arterioles (in vivo) and carotid arteries (in vitro) were examined in C57Bl/6 (wild-type) and Nox2-deficient (Nox2−/−) mice fed a control (10%) or a HFD (45 or 60% kcal from fat) for either 8, 12, 30, or 36 wks.
RESULTS
In wild-type mice HFD produced obesity and endothelial dysfunction by 12 and 36 wks in cerebral arterioles and carotid artery, respectively, in wild-type mice. Endothelial function could be significantly improved with Tempol (a superoxide scavenger) treatment in wild-type mice fed a HFD. Despite producing a similar degree of obesity in both wild-type and Nox2−/− mice, endothelial dysfunction was only observed in wild-type, but not in Nox2−/−, mice fed a HFD.
Conclusions
Endothelial dysfunction produced by a HFD occurs in a temporal manner and appears much earlier in cerebral arterioles than in carotid artery. Genetic studies revealed that Nox2-derived superoxide plays a major role in endothelial dysfunction produced by a HFD. Such functional changes may serve to predispose blood vessels to reduced vasodilator responses and thus may contribute to alterations in cerebral blood flow associated with obesity.
Keywords: brain, high-fat diet, type II diabetes, oxidative stress
INTRODUCTION
Obesity, currently a leading public health concern, is associated with an increased risk of vascular disease and cardiovascular events; including carotid artery disease and stroke.1-3 A diet high in caloric fat content is a major contributor to the increasing global incidence of obesity. Previous studies have shown that obesity is associated with endothelial dysfunction in peripheral and cerebral blood vessels.4-12 While much of the information regarding the effects of obesity on the cerebral circulation has come from genetic models of obesity (such as leptin or leptin-receptor deficiency),10-12 very little is known regarding the effects of obesity produced by a high fat diet (HFD) on cerebral blood vessels.
In obese humans, there is a positive correlation between visceral adiposity and markers of oxidative stress.5,9,13,14 Increases in oxidative stress, particularly superoxide, serve to limit nitric oxide bioavailability and in turn endothelial function.15 An important source of vascular superoxide is derived from the enzymatic activity of NADPH oxidase.16-19 While expression of NADPH oxidase appears to be increased in adipose and blood vessels in obesity20,21, the specific contribution of Nox2 (an important catalytic component of HADPH oxidase) to either the impairment of endothelial function in diet-induced obesity has not been defined.
Considering that the incidence of obesity is increasing worldwide and that obesity has been independently linked to cognitive decline,21-24 the first goal of the present study was to examine the temporal and functional effects of HFD on endothelial responses in the carotid artery and cerebral microcirculation. The second goal was to examine the specific contribution of Nox2 to the development of obesity and alterations in cerebral vascular function produced by a HFD.
METHODS
Experimental Animals
Male C57BL/6 (wild-type; #000664) and homozygous Nox2 (Nox2−/−)-deficient (B6.129S6-Cybbtm1Din/J; #002365)25 mice were obtained from Jackson Laboratories (Bar Harbor, ME) at 4-8 wks of age. All protocols conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Experimental Protocols
Wild-type mice were fed either a control diet (10% kcal from fat, #D12450B from Research Diets, New Brunswick, NJ) or a HFD (45% or 60% kcal, #D12451 and #D12492, respectively) for either 8, 12, 30 or 36 wks. Nox2−/− mice were fed either a control or 45% HFD for 12 (cerebral arteriole studies), or a control, 45%, or 60% HFD for 36 (carotid studies) wks.
Studies of Cerebral Arteriolar and Carotid Artery Function
Methods employed to examine responses of cerebral arterioles and carotid arteries in the present study have been described previously by our laboratory and are described in additional detail in the Online Supplement.26-29
Statistical Analysis
All data are expressed as Mean±SE. Cerebral responses are presented as a percent change in diameter compared to baseline and statistical analyses were performed using paired or unpaired t tests. Responses of carotid artery are expressed as a percent relaxation to U46619-induced contraction. Comparisons of relaxation and contraction were made using analysis of variance followed by Bonferroni's multiple comparisons test. Statistical significance was accepted with p<0.05.
RESULTS
Phenotypic Assessment of Obesity Development
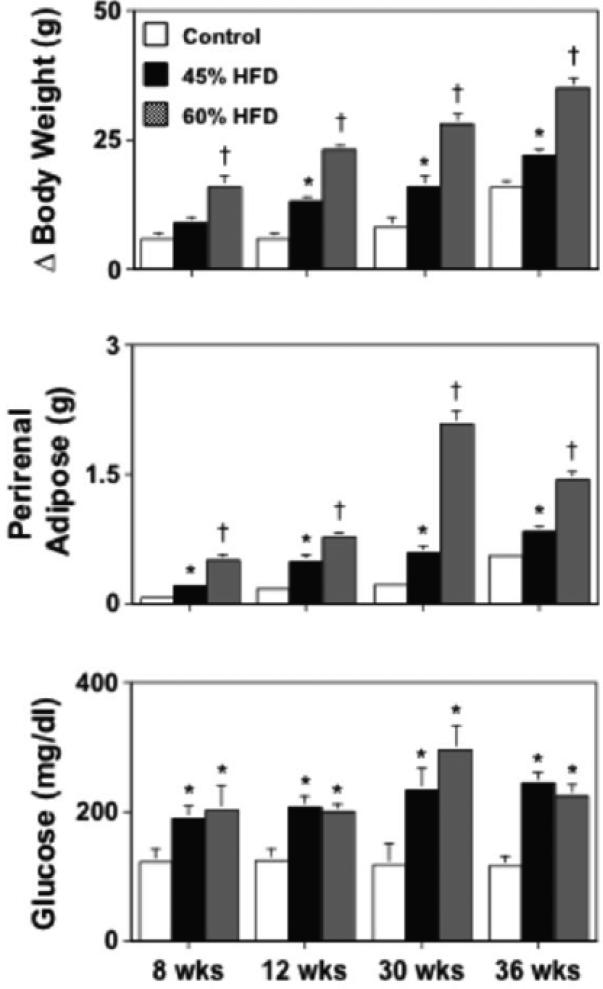
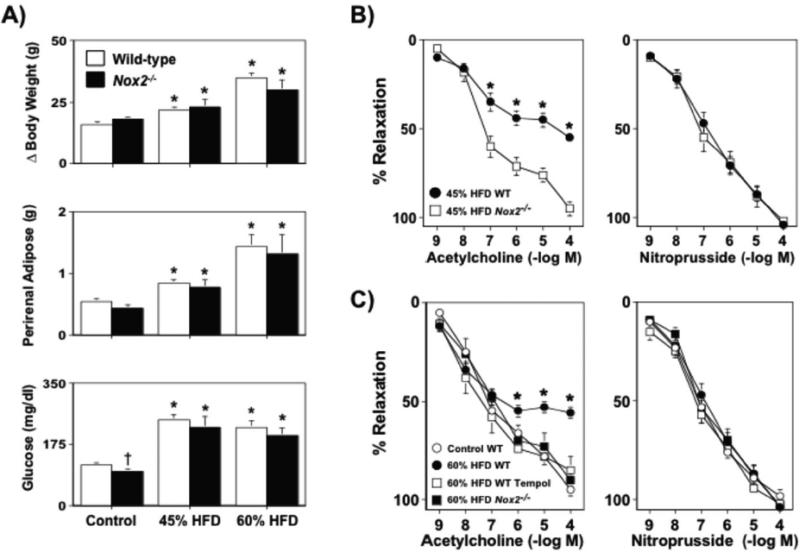
In wild-type mice fed a 45% HFD for 8-36 wks, body weight and perirenal fat mass were significantly greater (P<0.05) than that in wild-type mice fed a control diet (Figure 1). These same parameters were also greater, but to a significantly larger extent (P<0.05), in wild-type mice fed a 60% HFD as compared to either control or 45% HFD fed wild-type mice (Figure 1). Both HFDs produced hyperglycemia in wild-type mice, however the degree of hyperglycemia produced by the two diets was similar (P>0.05; Figure 1).
Figure 1.
Body weight gain, perirenal adipose mass, and fasting blood glucose levels in wild-type mice fed either a control (n=36), 45% (n=47) or 60% (n=18) HFD for 8-36 wks. A 45% HFD was associated with marked increases in body weight gain, perirenal adiposity, and fasting glucose levels, with the exception of fasting glucose levels. A 60% HFD also produced increases in body weight and perirenal adiposity as compared to wild-type mice fed either a control or 45% HFD over the same length of time, however the increase was significantly greater in mice fed a 60% HFD. Mean±SE. *P<0.05 vs. control and †P<0.05 vs. 45% HFD.
Effect of a HFD on Responses of Cerebral Arterioles
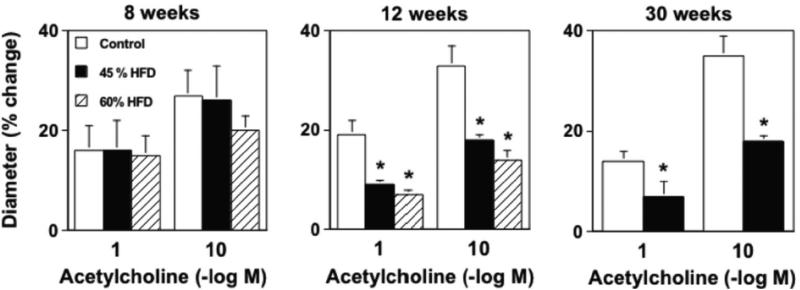
Acetylcholine produced dilatation of cerebral arterioles in wild-type mice fed a control diet and this response was not affected (P>0.05) by the length of time (8, 12, or 30 wks) on the diet (Figure 2). Following 8 wks of either a 45% or 60% HFD responses to acetylcholine were similar to that observed in wild-type mice fed a control diet for 8 wks (Figure 2). In contrast, responses of cerebral arterioles to acetylcholine were markedly reduced in mice fed a 45% HFD for 12 and 30 wks (Figure 2). We anticipated that a 60% HFD might impair responses to acetylcholine at earlier time points and/or to a greater extent, however the degree of dysfunction produced by a 60% HFD was similar to that produced by a 45% HFD at 12 wks. Because a 60% HFD did not produce any additional degree of impairment, we elected not to extend studies with the 60% HFD to 30 wks. Endothelium-independent responses to nitroprusside in cerebral arterioles were not altered by diet or time on diet (Supplemental Figure I).
Figure 2.
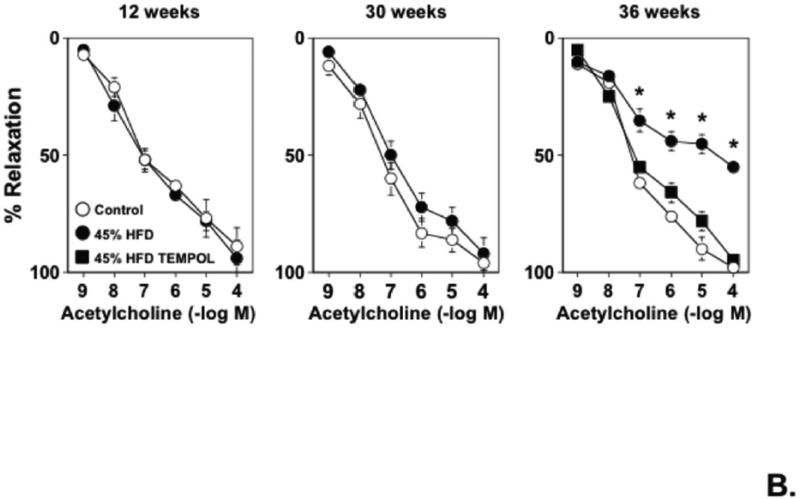
A) Dilatation of cerebral arterioles to acetylcholine were markedly impaired in wild-type mice fed a 45% HFD for 12 and 30, but not 8, wks as compared to those in wild-type mice fed a control diet. A 60% HFD produced a similar degree of impairment of cerebral arteriolar function in wild-type mice, which also occurred in a temporal manner. B) A 45% HFD for 36, but not 12 or 30 wks, was associated with a marked impairment of acetylcholine-induced relaxation in carotid artery as compared to wild-type mice fed a control diet. Tempol restored endothelial responses in wild-type mice fed a 45% HFD for 36 wks, suggesting that the impairment of endothelial function was mediated by superoxide. Mean±SE. *P<0.05 vs. control.
Effect of a HFD on Responses of Carotid Arteries
Responses to acetylcholine were similar in carotid arteries from mice fed a 45% HFD for 8, 12, or 30 wks on a 45% HFD as compared to mice fed a control diet (Supplemental Figure II and Figure 2B). In contrast, responses of carotid arteries to acetylcholine were markedly impaired in mice fed a 45% HFD for 36 wks as compared to mice fed a control diet for the same length of time (Figure 2). Endothelium-independent responses to nitroprusside in carotid artery were unaffected by either HFD or length of time on each diet (Supplemental Figure III).
Endothelial Dysfunction Produced by a HFD is Mediated by Superoxide
Because both a 45% and 60% HFD produced similar degrees of impairment in the cerebral circulation, we elected to examine mechanisms in response to a 45% HFD for 12 weeks (wks) in the cerebral circulation and for 36 wks in carotid arteries in subsequent studies. In order to address a role for superoxide, vascular responses in carotid artery or cerebral arterioles were examined in the presence or absence of Tempol, a superoxide scavenger. While Tempol had no effect on baseline cerebral arteriolar diameter (Supplemental Figure IV), acetylcholine-induced vasodilation was significantly improved in Tempol-treated vessels (Supplemental V). Similarly, responses of carotid arteries to acetylcholine in 36 wk HFD mice were improved towards that observed in wild-type mice fed a control diet following Tempol-treatment (Figure 2). In separate experiments, vascular superoxide levels (as measured using electron spin resonance) in aortic homogenates were significantly higher in wild-type mice fed a HFD compared to wild-type mice fed a control diet (Supplemental Figure VI).
Development of Obesity Produced by a HFD is Independent of Nox2
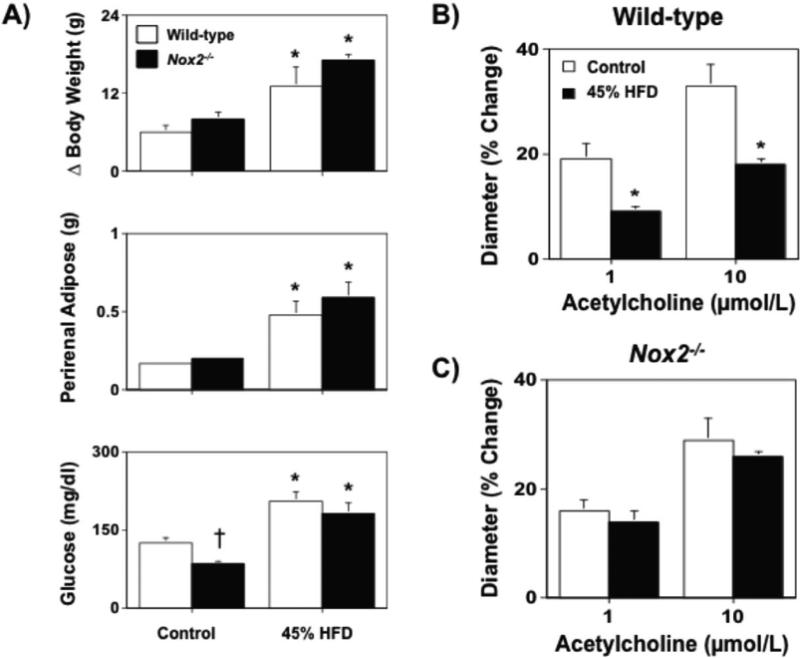
Body weight and visceral adiposity were found to be similar to that in wild-type mice fed a control diet for either 12 or 36 wks, however glucose levels were found to be lower in Nox2−/− fed a control diet as compared to wild-type mice (Figures 3A and 4A). Both 45% and 60% HFDs produced significant, but similar, increases in body weight, visceral adiposity and hyperglycemia in wild-type and Nox2−/− mice (Figure 3A and 4A).
Figure 3.
A) Body weight gain, perirenal adipose mass, and fasting blood glucose levels in wild-type and Nox2−/− mice fed either a control or 45% high fat diet for 12 wks. B and C) While a 45% HFD was associated with marked impairment of acetylcholine-induced dilatation in wild-type mice, cerebral arteriolar response in Nox2−/− were similar to that in wild-type mice fed a control diet and were not affected by a 45% HFD. Mean±SE. *P<0.05 vs. respective control. †P<0.05 vs. wild-type; n=6-10.
Figure 4.
A) Body weight gain, perirenal adipose mass, and fasting blood glucose levels in wild-type and Nox2−/− mice fed either a control, 45%, or 60% HFD for 36 wks. B and C) Both a 45% and 60% HFD was associated with marked impairment of acetylcholine-induced dilatation in wild-type mice, whereas responses to acetylcholine in Nox2−/− fed either a 45% or a 60% HFD were similar to that observed in wild-type mice fed a control diet, suggesting a major role for Nox2-derived superoxide in the impairment of endothelial function produced by a HFD. Responses to nitroprusside were not affected by diet or genotype. Mean±SE. *P<0.05 vs. respective control. †P<0.05 vs. wild-type, n=6-9.
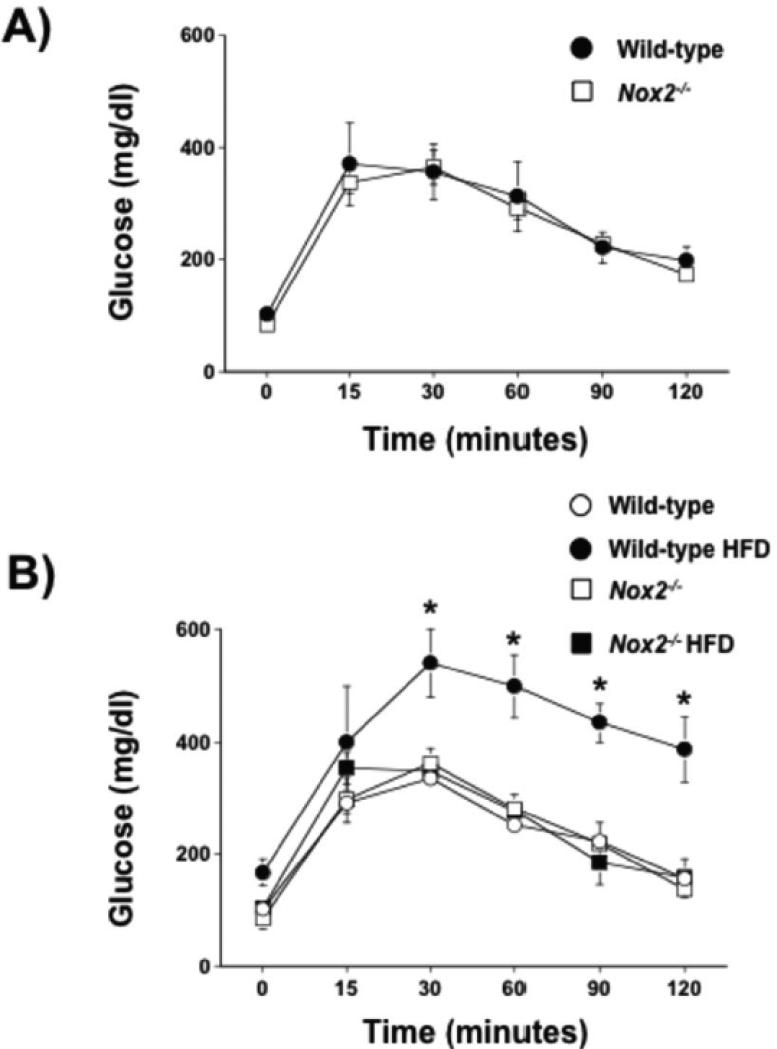
Because a HFD may also promote insulin resistance and because measures of plasma glucose alone may not be reflective of insulin resistance per se, we assessed insulin sensitivity by performing glucose tolerance tests in wild- type and Nox2−/− mice under baseline conditions (prior to the start of the experimental diets) and in wild-type and Nox2−/− mice fed a control or HFD for 12 wks. Under baseline conditions, wild-type and Nox2−/− mice were able to clear a glucose bolus (1 mg/kg i.p.) within 120 min, suggesting that deficiency of Nox2 is not associated with alterations in insulin sensitivity. In contrast, 12 wks of a HFD was associated with insulin a marked impairment of the ability of wild-type mice to clear the same glucose load in wild-type mice fed a control diet. Perhaps more importantly, deficiency of Nox2 prevented the impairment of insulin sensitivity as Nox2−/− were able to clear the glucose bolus at a similar rate as wild-type mice fed a control diet (Figure 5). Taken together, these data suggest that while the development of obesity per se is not affected by Nox2 deficiency, it appears that Nox2-derived superoxide contributes to the development of insulin resistance.
Figure 5.
A) Blood glucose concentrations in wild-type and Nox2−/− mice on a control diet before (baseline; 0 min) and after an intraperitoneal glucose (1 mg/kg) bolus. Mean±SE; n=6/group; P>0.05. B) Blood glucose concentrations in wild-type and Nox2−/− mice fed a control diet or HFD for 12 wks before (baseline; 0 min) and after an intraperitoneal glucose (1 g/kg) bolus. Mean±SE; n=3/group;*P<0.05 vs. wild-type.
Endothelial Dysfunction Produced by a HFD is Mediated by Nox2
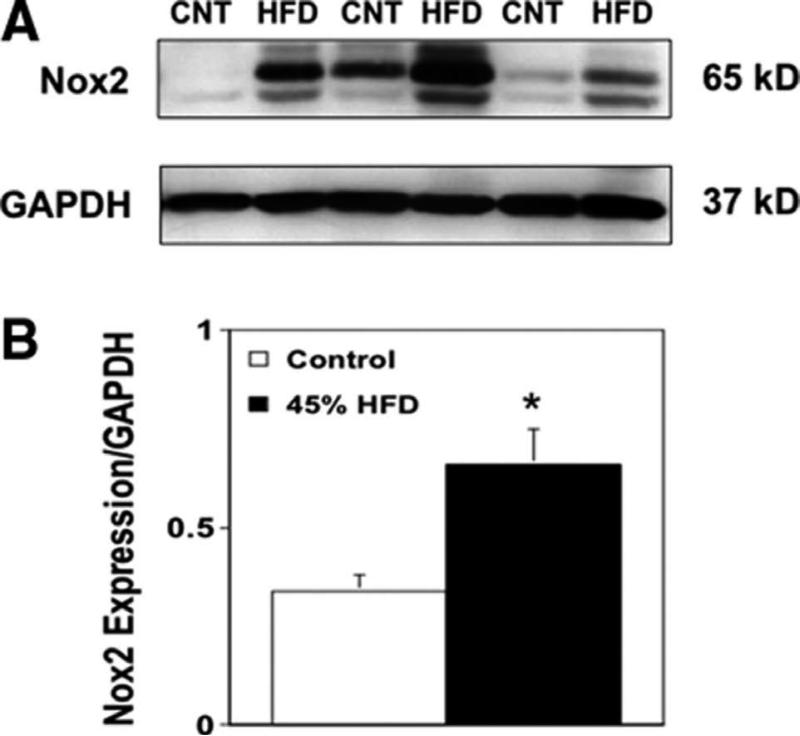
In an attempt to identify the enzymatic source of superoxide responsible for the impairment of endothelial function, we next performed experiments to determine the role of NAD(P)H oxidase in the impairment of vascular function in our model. Vascular Nox2 expression was higher in aorta from wild-type mice fed a 45% HFD compared with their control diet counterparts (Figure 6), consistent with our measurements of vascular superoxide (Supplemental Figure VI).
Figure 6.
A) Western blot for Nox2 expression in vascular tissue (aortic homogenates) from wild-type mice fed a control or a 45% HFD. GAPDH expression was used as a loading control and for standardization of Nox2 expression. B) A HFD was associated with approximately 2-fold higher levels of Nox2 expression in vascular tissue from mice fed a HFD compared to mice fed a control diet. Changes in protein expression were quantified by densitometry. Mean±SE; n=3/group; *P<0,05 vs. control (CNT)
In separate experiments, we also sought to determine a potential mechanism that might contribute to the impairment of endothelial function in cerebral arterioles, but not carotid artery, following a 12 wk HFD. Thus, we elected to examine expression of eNOS and CD36 as endothelium-dependent response in cerebral arterioles have be shown to be mediated in large part by nitric oxide and eNOS and as CD36 (a major scavenger receptor responsible for the uptake of oxidized LDL) has been shown previously to be associated with endothelial dysfunction. Expression of eNOS and CD36 was found to be similar in brains from wild-type mice fed a control or HFD (Supplemental Figure IX). Taken together, our molecular data demonstrate that a HFD is associated with increased vascular expression of Nox2 and independent of alterations in eNOS and CD36 expression. While these molecular findings provide additional supporting evidence that the impairment of endothelial function in obesity is mediated, in large part, by increases in Nox2-derived superoxide, we cannot completely rule out the possibility that translational and/or post-translational alterations in eNOS and/or CD36 may also play a role. For example, CD36 expression has been shown to be modulated at the level of translation under conditions of hyperglycemia.30
Endothelial responses were normal in Nox2−/− mice fed a control diet. In contrast, a 45% and 60% HFD produced marked impairment of endothelial responses in wild-type mice, whereas a HFD did not produce alterations in endothelial responses in either cerebral arterioles or carotid arteries of Nox2−/−mice (Figures 3B and 4B). Endothelium-independent responses to nitroprusside were not altered by Nox2 deficiency alone or Nox2 deficiency in combination with a HFD in either or cerebral arterioles (data not shown) or carotid arteries (Figure 4B and 4C).
DISCUSSSION
There are several major new findings of the present study. First, a HFD produced endothelial dysfunction, which occurred in a temporal manner and appeared much earlier in cerebral arterioles than that in carotid arteries. Second, endothelial dysfunction produced by high fat was mediated by superoxide as a superoxide scavenger was very effective at improving endothelial function in wild-type mice fed a HFD. Third, endothelial dysfunction in both carotid artery as well as the cerebral circulation was absent in Nox2 deficient mice fed a HFD. Taken together, these findings provide the first direct evidence that Nox2-derived superoxide contributes to carotid and cerebral endothelial dysfunction in a model of diet-induced obesity.
Obesity is Associated with Endothelial Dysfunction in Cerebral Circulation
Endothelium-dependent responses in cerebral blood vessels are impaired in a number of genetic models of obesity, mostly models of leptin and leptin-receptor deficiency.11,12,27,28 While such genetic models are informative, deficiency of either leptin or the leptin-receptor is extremely rare in humans and not always associated with obesity.31 In contrast, high fat feeding is a well-established model of diet-induced obesity that recapitulates key features of obesity in humans, e.g., mice fed a HFD primarily gain weight in the form of visceral adipose (intra-abdominal fat) similar to that in obese humans.32-34 In the present study, a HFD produced a marked degree of obesity as evidenced by increased visceral adiposity in wild-type mice. In addition, the HFD produced hyperglycemia, as fasting glucose levels were significantly higher in high fat fed wild-type mice. These findings are consistent with previous studies using the same (or similar) HFD to produce obesity and hyperglycemia in C57Bl/6 mice.32-36
While obesity is associated with endothelial dysfunction in a number of peripheral blood vessels, very little is known regarding the effect of diet-induced obesity on endothelial responses in cerebral blood vessels and even less is known regarding temporal effects.4-12 In the present study, we examined the temporal effects of a HFD on responses in carotid artery and cerebral blood vessels. We found that a HFD was associated with temporal increases in body weight and adiposity in wild-type mice. However, despite marked increases in adiposity early on, endothelium-dependent responses to acetylcholine were similar in cerebral arterioles of wild-type mice fed a HFD for 8 wks as compared to wild-type mice fed a control diet. By 12 wks, cerebral arteriolar responses to acetylcholine were impaired (~50% less than that observed in wild-type mice fed a control diet) in wild-type fed a HFD. Arteriolar responses to acetylcholine were impaired to a similar extent in wild-type mice fed a 45% HFD for 30 wks as that observed at 12 wks. These data suggest that a HFD impairs endothelial function in cerebral arterioles by at least 12 wks and that a HFD is not associated with additional reductions in endothelial responses in cerebral arterioles with longer time points of high fat feeding, at least up to 30 wks.
In contrast, responses to acetylcholine were normal in carotid arteries from wild-type mice fed either a control or 45% HFD for 8-30 wks. Endothelial dysfunction eventually became evident in carotid artery arteries in wild-type mice, but only after 36 wks of high fat feeding. These data suggest that unlike cerebral arterioles, where endothelial dysfunction is apparent as early as 12 wks, a much longer exposure to a HFD is required to produce similar dysfunction in carotid arteries. These data suggest that cerebral arterioles appear to be more sensitive than carotid artery to the effects of a HFD. While the specific mechanism(s) that account for the earlier impairment of endothelial function produced by a HFD are unclear, it is possible that these effects may be reflective of the fact that cerebral blood vessels have be shown to have a higher capacity to generate superoxide. Indeed, it has been shown previously that NADPH oxidase activity and function is greater in cerebral versus that in systemic arteries.37 The fact that Tempol could improve endothelial function in both cerebral arterioles and carotid arteries in wild-type mice fed a HFD and that Nox2-deficiency served to protect endothelial function would also be consistent with this concept.
In addition to the temporal effects of a HFD on vascular responses, very little is known regarding the effect of varying the percentage of dietary fat on vascular responses. Thus, we compared and contrasted the effects of a 45% HFD with that of a 60% HFD. We predicted that the degree of impairment would be directly proportional to the caloric fat content present in the two diets. However, while a 60% HFD produced a greater degree of obesity and visceral adiposity, it was not associated with a greater degree of endothelial dysfunction in either cerebral arterioles or carotid arteries than that produced by a 45% HFD. While the reason for this difference is not clear, we speculate that the increase in vascular Nox2 expression produced by a HFD is maximal with a 45% HFD and that increasing the fat content to 60% is simply not additive at the time points examined in the present study. The fact that Nox2-deficiency was sufficient to protect against the negative effects of a HFD on endothelial function is supportive in this possibility.
The effects of a HFD appeared to be selective for endothelium, as responses to nitroprusside in both cerebral arterioles and carotid artery were similar in high fat fed (both 45% and 60%) mice as compared to that observed in control mice. Thus, the present findings not only demonstrate that endothelial dysfunction occurs in a temporal- and vessel-dependent manner and that the maximal degree of endothelial dysfunction produced by a HFD appears to occur with a 45% HFD. Taken together, these findings also established time points that then allowed the design of studies to test mechanisms associated with impairment of endothelial function.
Endothelium-dependent responses in cerebral arterioles and carotid artery in the mouse are mediated in large part by nitric oxide synthase and nitric oxide.38-41 It is fairly well established that increased superoxide contributes to endothelial dysfunction via superoxide-mediated inactivation of nitric oxide bioavailability.15 In the present study, a superoxide scavenger effectively restored cerebral arteriolar and carotid artery responses in wild-type mice fed a 45% or 60% HFD towards normal. In addition, vascular superoxide levels were found to be greater in wild-type mice fed a 60% HFD for 36 wks compared to controls. Thus, our pharmacological data combined with measurements of superoxide strongly implicate a role for superoxide in the impairment of endothelial function in carotid and cerebral vessels in obesity.
Nox2 Deficiency Limits Endothelial Dysfunction in the Cerebral Circulation Associated with a HFD
While our data with Tempol implicate superoxide in the impairment of endothelial function in the cerebral circulation produced by a HFD, it does not provide information regarding the enzymatic source of superoxide. Previous studies have implicated a number of source(s) of superoxide, such as xanthine oxidase, uncoupled eNOS, or NADPH oxidase that contribute to endothelial dysfunction in a number of diseases that affect the vasculature such as diabetes and hypertension.15 Thus, to determine the enzymatic source of superoxide in our model, we examined obesity development and vascular function following a HFD in mice genetically deficient in Nox2, a major catalytic isoform of NADPH oxidase.
Body weight, perirenal adipose mass, as well as plasma glucose levels were similar in wild-type and Nox2−/− mice fed a control diet. A HFD produced similar increases in body weight and perirenal adipose mass, as well as plasma glucose levels in wild-type and Nox2−/− mice. Taken together, these data suggest that Nox2 deficiency does not alter metabolic phenotypes under baseline conditions or in response to a HFD. In terms of vascular responses, Nox2 deficiency was not associated with alterations in endothelial function as responses to acetylcholine were similar in wild-type and Nox2−/− mice fed a control diet. These data are consistent with previous findings and suggest that normally superoxide levels are very low and that under normal conditions Nox2 expression and/or activity is not sufficient to limit endothelial function.41-43 While Nox2 deficiency did not affect obesity development, endothelial responses in both cerebral arterioles and carotid artery to acetylcholine were normal in Nox2−/−mice fed a HFD.
Although there are a number of co-morbidities associated with a HFD that may ultimately serve to promote increases in vascular superoxide and endothelial dysfunction, it is often difficult to establish cause and effect relationships. While hypercholesterolemia might be considered to be an important factor in this regard, previous studies have shown that a HFD is associated with only minimal to modest increases in plasma cholesterol in wild-type (C57Bl/6) mice.35,36,43,44 This is in contrast to the marked increase in plasma cholesterol levels observed in atherosclerotic mouse models, such as the apolipoprotein E (ApoE) or the low density lipoprotein receptor (LDLR) fed a normal (500-800 mg/dl range) or Western (high cholesterol) (>2,000 mg/dl) diet.45-48 More importantly, endothelial function appears to be normal in wild-type and ApoE and LDLR mice in which plasma cholesterol levels are elevated as high as 500 mg/dl while on a normal diet.46 In contrast, a high cholesterol diet appears to impair endothelial function in ApoE and LDLR-deficient, but not wild-type, mice.45
Similarly, a number of studies have shown that a HFD is associated with minimal to modest alterations in plasma LDL, HDL, and triglyceride levels.35,36,44 Based on such previous studies, we would predict that the HFD would have minimal effects on plasma lipids in the wild-type (C57Bl/6) mice used in the present study. Based on recent report, we would also predict that Nox2-deficiency per se or in combination with a HFD would have little to no effect on plasma lipids.44 Thus, while it may be difficult to assign a specific role for any one factor in promoting the endothelial dysfunction observed with a HFD, our data in Nox2-derived superoxide in the impairment of endothelial function in carotid artery and cerebral microvessels is most likely related to the effects of a HFD independent of alterations in plasma lipid profiles.
Conclusions
These findings provide genetic evidence for a major role of Nox2-derived superoxide in impairment of endothelial function in carotid artery and cerebral microvessels in response to a HFD. Our data also demonstrate that Nox2- deficiency is not associated with alterations in the development of obesity produced by high fat, suggesting that the protective effect of Nox2 deficiency on endothelial function may reflect loss of Nox2 in the vessel wall. While results derived from the present study cannot differentiate between the effects of obesity and/or hyperglycemia per se on endothelial function, we would propose that impairment of vascular function in carotid and cerebral microvessels could conceivably contribute, in part, to the cognitive decline and dementia associated with obesity and type II diabetes. This idea is consistent with emerging data that suggest that obesity with a high degree of visceral adipose accumulation, is associated with an increased risk of dementia.49
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This study was supported by National Institutes of Health grants HL-089884 and HL-107632 (to S.P.D.), NS-24621 and HL-62984 (to F.M.F), HL-089067 (to J.F.) NS-054688 (to A.E.), American Heart Association grant (EIA-0740002N to A.E.), and Veterans Administration Merit grant (BX000347 to A.E.). This study was also supported in part by a Synergy Award from the Georgia Health Sciences University's Diabetes and Obesity Discovery Institute (to A.E., J.F. and S.P.D).
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.De Michele M, Panico S, Iannuzzi A, Celentano E, Ciardullo AV, Galasso R, et al. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke. 2002;33:2923–2928. doi: 10.1161/01.str.0000038989.90931.be. [DOI] [PubMed] [Google Scholar]
- 2.Winter Y, Rohrmann S, Linseisen J, Lanczik O, Ringleb PA, Hebebrand J, et al. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke. 2008;39:3145–3151. doi: 10.1161/STROKEAHA.108.523001. [DOI] [PubMed] [Google Scholar]
- 3.Bodenant M, Kuulasmaa K, Wagner A, Kee F, Palmieri L, Ferrario MM, et al. Measures of abdominal adiposity and the risk of stroke: The MOnica Risk, Genetics, Archiving and Monograph (MORGRAM) Study. Stroke. 2011;42:2872–2877. doi: 10.1161/STROKEAHA.111.614099. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin. Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campia U, Tesauro M, Carrillo C. Human obesity and endothelium-dependent responsiveness. Br J Pharmacology. 2012;165:561–573. doi: 10.1111/j.1476-5381.2011.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stapleton PA, James ME, Goodwill AG, Frisbee JC. Obesity and endothelial dysfunction. Pathophysiology. 2008;15:79–89. doi: 10.1016/j.pathophys.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas SR, Chen K, Keaney JF., Jr Oxidative stress and endothelial nitric oxide bioactivity. Antioxid Redo Signal. 2003;5:181–194. doi: 10.1089/152308603764816541. [DOI] [PubMed] [Google Scholar]
- 9.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in The Framingham Study. Arterioscler. Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 10.Katakam PV, Domoki F, Snipes JA, Busija AR, Jarajapu YP, Busija DW. Impaired mitochondria-dependent vasodilation in cerebral arteries of Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R289–R298. doi: 10.1152/ajpregu.90656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erdös B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 2004;53:1352–1359. doi: 10.2337/diabetes.53.5.1352. [DOI] [PubMed] [Google Scholar]
- 12.Phillips SA, Sylvester FA, Frisbee JC. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R522–R530. doi: 10.1152/ajpregu.00655.2004. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross- sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 15.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 16.Sun H, Zheng H, Molacek E, Fang Q, Patel KP, Mayhan WG. Role of NAD(P)H oxidase in alcohol-induced impairment of endothelial nitric oxide synthase-dependent dilation of cerebral arterioles. Stroke. 2006;37:495–500. doi: 10.1161/01.STR.0000199033.06678.c3. [DOI] [PubMed] [Google Scholar]
- 17.Didion SP, Hathaway CA, Faraci FM. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am J Physiol Heart Circ Physiol. 2001;281:H1697–H1703. doi: 10.1152/ajpheart.2001.281.4.H1697. [DOI] [PubMed] [Google Scholar]
- 18.Didion SP, Faraci FM. Effects of NADH and NADPH on superoxide levels and cerebral vascular tone. Am J Physiol Heart Circ Physiol. 2002;282:H688–H695. doi: 10.1152/ajpheart.00576.2001. [DOI] [PubMed] [Google Scholar]
- 19.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, et al. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47phox expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- 21.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Kingwell K. Overweight or obesity during midlife is associated with late-life dementia. Nat Rev Neurol. 2011;8:299. doi: 10.1038/nrneurol.2011.74. [DOI] [PubMed] [Google Scholar]
- 23.Xu Wl Atti AR, Gatz M Pederson NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34:222–229. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 26.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2576–2581. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- 27.Didion SP, Lynch CM, Faraci FM. Cerebral vascular dysfunction in TallyHo mice: a new model of Type II diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H1579–1583. doi: 10.1152/ajpheart.00939.2006. [DOI] [PubMed] [Google Scholar]
- 28.Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of rho-kinase in cerebral arterioles in type II diabetes. Stroke. 2005;36:342–347. doi: 10.1161/01.STR.0000152952.42730.92. [DOI] [PubMed] [Google Scholar]
- 29.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 30.Griffin E, Re A, Hamel N, Fu C, Bush H, McCaffrey T, et al. A link between diabetes and atherosclerosis: glucose regulates expression of CD36 at the level of translation. Nature Med. 2001;7:840–846. doi: 10.1038/89969. [DOI] [PubMed] [Google Scholar]
- 31.Farooqi IS, O'Rahilly S. Monogenic human obesity syndromes. Recent Prog Horm Res. 2004;59:409–424. doi: 10.1210/rp.59.1.409. [DOI] [PubMed] [Google Scholar]
- 32.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Brownlow BS, Petro A, Feinglos MN, Surwit RS. The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol Behav. 1996;60:37–41. doi: 10.1016/0031-9384(95)02210-4. [DOI] [PubMed] [Google Scholar]
- 34.Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism. 1993;42:1405–1409. doi: 10.1016/0026-0495(93)90190-y. [DOI] [PubMed] [Google Scholar]
- 35.Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ Res. 2005;96:1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 36.Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis. 1998;136:17–24. doi: 10.1016/s0021-9150(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 37.Miller AA, Drummond GR, Schmidt HW, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- 38.Didion SP, Faraci FM. Ceramide-induced impairment of endothelial function is prevented by CuZn superoxide dismutase overexpression. Arterioscler Thromb Vasc Biol. 2005;25:90–95. doi: 10.1161/01.ATV.0000149868.74075.5d. [DOI] [PubMed] [Google Scholar]
- 39.Faraci FM, Sigmund CD, Shesely EG, Maeda N, Heistad DD. Responses of carotid artery in mice deficient in expression of the gene for endothelial NO synthase. Am J Physiol. 1998;274:H564–H570. doi: 10.1152/ajpheart.1998.274.2.H564. [DOI] [PubMed] [Google Scholar]
- 40.Faraci FM, Lynch C, Lamping KG. Responses of cerebral arterioles to ADP:eNOS-dependent and eNOS-independent mechanisms. Am J Physiol Heart Circ Physiol. 2004;287:H2871–H2876. doi: 10.1152/ajpheart.00392.2004. [DOI] [PubMed] [Google Scholar]
- 41.Meng W, Ma J, Ayata C, Hara H, Huang PL, Fishman MC, et al. ACh dilates pial arterioles in endothelial and neuronal NOS knockout mice by NO- dependent mechanisms. Am J Physiol. 1996;271:H1145–H1150. doi: 10.1152/ajpheart.1996.271.3.H1145. [DOI] [PubMed] [Google Scholar]
- 42.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, et al. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 43.Girouard H, Park L, Anrather J, Zhou J, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through Nox-2-derived radicals. Arterioscler Thromb Vasc. Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 44.Pepping JK, Freeman LR, Gupta S, Keller JN, Bruce-Keller AJ. NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high- fat diet. Am J Physiol Endocrinol Metab. 2013;304:E392–E404. doi: 10.1152/ajpendo.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonthu S, Heistad DD, Chappell DA, Lamping KG, Faraci FM. Atherosclerosis, vascular remodeling, and impairment of endothelium-dependent relaxation in genetically altered hyperlipidemic mice. Atheroscler Thromb Vasc Biol. 1997;17:2333–2340. doi: 10.1161/01.atv.17.11.2333. [DOI] [PubMed] [Google Scholar]
- 46.Lamping KG, Nuno DW, Chappell DA, Faraci FM. Agonist-specific impairment of coronary vascular function in genetically altered, hyperlipdemic mice. Am J Physiol. 1999;276:R1023–R1029. doi: 10.1152/ajpregu.1999.276.4.R1023. [DOI] [PubMed] [Google Scholar]
- 47.D'Uscio LV, Smith LA, Katusic ZS. Hypercholesterolemia impairs endothelium-dependent relaxations in common carotid arteries of apolipoprotein E-deficient mice. Stroke. 2001;32:2658–2664. doi: 10.1161/hs1101.097393. [DOI] [PubMed] [Google Scholar]
- 48.D'Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, et al. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arteroscler Thromb Vasc Biol. 2001;21:1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- 49.Cereda E, Sansone V, Meola G, Malavazos AE. Increased visceral adipose tissue rather than BMI as a risk factor for dementia. Age Ageing. 2007;36:488–491. doi: 10.1093/ageing/afm096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.