Abstract
Sertoli cells (SCs) regulate testicular fate in the differentiating gonad and are the main regulators of spermatogenesis in the adult testis; however, their role during the intervening period of testis development, in particular during adult Leydig cell (ALC) differentiation and function, remains largely unknown. To examine SC function during fetal and prepubertal development we generated two transgenic mouse models that permit controlled, cell-specific ablation of SCs in pre- and postnatal life. Results show that SCs are required: (1) to maintain the differentiated phenotype of peritubular myoid cells (PTMCs) in prepubertal life; (2) to maintain the ALC progenitor population in the postnatal testis; and (3) for development of normal ALC numbers. Furthermore, our data show that fetal LCs function independently from SC, germ cell or PTMC support in the prepubertal testis. Together, these findings reveal that SCs remain essential regulators of testis development long after the period of sex determination. These findings have significant implications for our understanding of male reproductive disorders and wider androgen-related conditions affecting male health.
Keywords: Diphtheria, Leydig, Male fertility, Peritubular myoid, Sertoli, Testis, Mouse
INTRODUCTION
Sertoli cells (SCs) are essential regulators of testicular fate in the differentiating gonad (reviewed by Cool et al., 2012; Quinn and Koopman, 2012; Sekido and Lovell-Badge, 2013; Warr and Greenfield, 2012), although the role that these cells play in the fetal and prepubertal testis remains largely unknown. Initially, the SCs act as organising centres, enclosing germ cells (GCs) to form testicular cords and secreting factors such as DHH and PDGF, which are essential for development of the fetal population of Leydig cells (FLCs) (Brennan et al., 2003; Yao et al., 2002). Thereafter, the SCs go through a period of intense proliferation during fetal and early neonatal life (Sharpe et al., 2003), which is regulated by a number of factors including androgens (Auharek et al., 2012; Sharpe et al., 2003), FSH (Orth, 1984), activin A (Boitani et al., 1995) and the insulin/IGF1 pathway (Pitetti et al., 2013). The proliferative phase stops shortly before puberty [approximately postnatal day (pnd) 15 in mice] and the SCs undergo a fundamental change and overall increase in activity as they reach adult numbers (O'Shaughnessy et al., 2007; Tarulli et al., 2012). Towards the end of the proliferative phase, a second, adult population of Leydig cells (ALCs) starts to develop from peritubular and perivascular precursor cells (Baker et al., 1999; Nef et al., 2000; O'Shaughnessy et al., 2008b) and this forms the stable population of LCs associated with androgen production throughout adulthood. The peritubular myoid cells (PTMCs), which enclose the seminiferous epithelium, proliferate markedly after birth (Nurmio et al., 2012) and interact closely with SCs to support seminiferous tubule development and function, including deposition of the basement membrane (BM) of the tunica propria (Ailenberg et al., 1990; Skinner et al., 1989; Tung and Fritz, 1987; Tung et al., 1984). GCs also undergo a period of intense proliferation after birth (Nurmio et al., 2012; Vergouwen et al., 1993, 1991) leading up to the onset of meiosis as the animals enter puberty. Currently, we know little about the cellular interactions that drive prepubertal testis development, and whether the SCs retain a pivotal role after initial gonad differentiation or, indeed, whether they play a major role in adult testis function, beyond spermatogenesis, remains unclear.
Controlled cell ablation is a powerful technique that can reveal fundamental information about organ development and function. Studies of testis biology have a well-recognised history of cell ablation studies, mostly using cytotoxins such as busulfan (for GC ablation) (Brinster et al., 2003; Yoshida, 2010) and ethane dimethane sulphonate (EDS) (for LC ablation in rats) (Sharpe et al., 1990). Similar studies to examine the effects of SC ablation on testis function have not been possible, however, as specific cytotoxins that target the SC are not available. To achieve controlled SC ablation, which will allow a systematic review of SC function, it is therefore necessary to take a different approach. Diphtheria toxin (DTX) is an extremely potent cytotoxic protein in some mammalian species. It is made up of two linked A and B peptide subunits with the B subunit able to bind to the HBEGF receptor [also known as diphtheria toxin receptor (DTR)]. Internalisation of the receptor-toxin complex and release of the A subunit (DTA) leads rapidly to apoptotic cell death.
In this study we use two separate transgenic approaches (Brockschnieder et al., 2004; Buch et al., 2005) to target DTR or DTA to the SCs, allowing controlled ablation of the cells in the fetus or at any age after birth. Results show that, far from simply proliferating, the SCs function in the prepubertal testis to actively support postnatal testis development, including the development of the ALC population and retention of the PTMC phenotype. Together, these findings describe a new model of testis development, which encompasses SC-mediated control of ALC development and function. These findings have significant implications for our understanding of both male reproductive disorders and wider androgen-related conditions affecting male health.
RESULTS
SC-specific ablation in Amh-Cre;DTA/iDTR mice
To determine the role of SCs in testis development, two different mouse models were generated which targeted the expression of DTA or DTR to SCs using Amh-Cre. For the first model we bred Amh-Cre+/− males (Lécureuil et al., 2002) to mice carrying a Cre recombinase-inducible DTA fragment (Rosa26-DTA) (Brockschnieder et al., 2004) (Fig. 1A). Activation of Cre recombinase at embryonic day (E) 15 results in expression of DTA, which induces SC death from this time point. Examination of gross testis morphology in adulthood has indicated two divergent phenotypes, with a 60% reduction in testis size in one group (95% of testes examined) and a 90% reduction in the other (5% of testes examined), which could be attributed to whether SC ablation was partial or complete (Fig. 1B; supplementary material Figs S1 and S2).
Fig. 1.
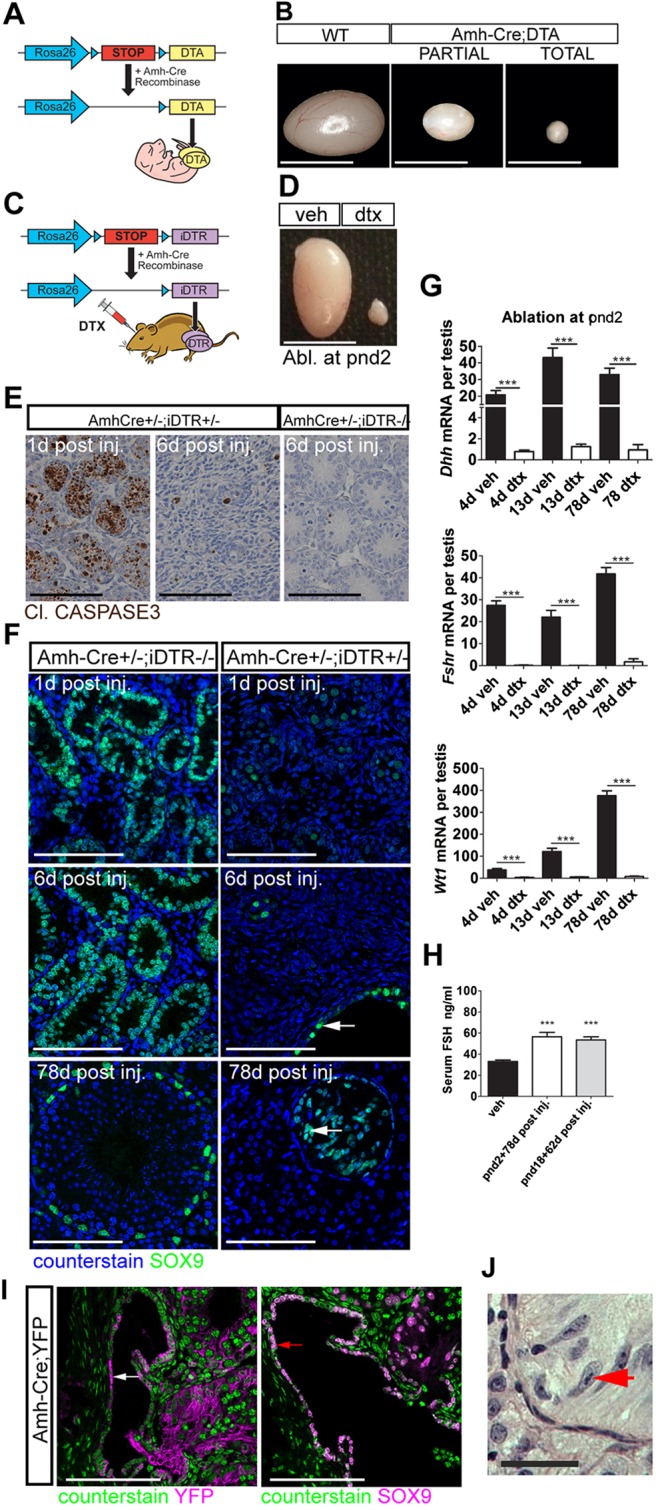
SC-specific ablation. (A) Mouse model one: Amh-Cre:DTA. (B) DTA induced testicular atrophy with different degrees of severity. (C) Mouse model two: Amh-Cre:iDTR. (D) DTX induced testicular atrophy in adulthood following injection at pnd2. (E) Apoptosis is restricted to SCs and is resolved 6 d post DTX injection. (F) SC ablation is mirrored by decreased SC SOX9 expression. Note that the expression of SOX9 is retained in the rete testis epithelium (arrows). Scale bar: 100 µm. (G) Relative expression of the SC-specific markers Dhh, Fshr and Wt1 (one-way ANOVA, n=7-9; ***P<0.001). (H) Circulating FSH concentrations at pnd80 (one-way ANOVA, n=7-9; ***P<0.001). (I) Immunolocalisation of YFP (white arrow), and SOX9 (red arrow) in Amh-Cre:YFP testis (d17). (J) Rete testis epithelium with tripartite nucleolus consistent with an SC origin for these cells (red arrow). WT, wild type; Veh, vehicle control. Scale bars: 500 µm in B,D; 100 µm in E,I; 50 µm in F,J.
To complement this model, and to permit selective ablation at key stages of postnatal life, we also bred the Amh-Cre+/− line to mice carrying a Cre-inducible simian HBEGF (iDTR) (Buch et al., 2005) (Fig. 1C). Initial studies showed that injection of 100 ng DTX has no impact on the testis from wild-type, Amh-Cre+/− or iDTR+/− animals, but that it induces specific and complete ablation of SCs in Amh-Cre+/−;iDTR+/− animals, with no evidence of increased apoptosis in other organs (data not shown). On this basis, and to minimise animal numbers, Amh-Cre+/+;iDTR+/+ mice treated with 100 ng DTX or vehicle were used for the remainder of the study (supplementary material Fig. S1).
Injection of DTX induced a reduction in testis size at both postnatal ages tested [pnd2 (Fig. 1D) and pnd18], which was associated with SC apoptosis (Fig. 1E). Following induction of SC apoptosis, macrophage numbers were unchanged 1 day (d) post-ablation (supplementary material Fig. S3). However, an apparent increase in macrophage numbers was observed 4 days post-ablation, consistent with macrophage clearance of cellular debris. Macrophage numbers declined to control levels by 13 d post-ablation. One week after injection of DTX, there was no remaining evidence of SC apoptosis in the testis (Fig. 1E). Confirmation of SC ablation was obtained by immunohistochemical localisation of the SC marker SOX9 (Fig. 1F; supplementary material Fig. S2) and qRT-PCR analysis of the SC-specific transcripts Dhh, Wt1 and Fshr (Fig. 1G; supplementary material Fig. S2). Furthermore, circulating FSH concentrations were significantly increased at all ages [consistent with removal of the SC-dependent hypothalamic-pituitary-gonadal axis negative-feedback loop (Fig. 1H)]. Together, these data confirmed successful ablation of the SC population from the testis in the iDTR model.
Surprisingly, however, the epithelial cells of the rete testis (which express SOX9 and have been hypothesized to be modified SCs) were retained following DTX treatment. To examine this in more detail we bred Amh-Cre+/− mice to a Cre-inducible YFP reporter line (Srinivas et al., 2001), and confirmed that the Amh-Cre transgene is expressed only in a proportion of epithelial cells of the rete testis (Fig. 1I). This mosaic Cre expression explained why a proportion of the SOX9-expressing cells were retained in the rete testis of DTX-treated mice. Furthermore, Amh-Cre-induced YFP labelling of a subset of the rete epithelium suggested that these cells do indeed have an SC origin/phenotype [which is supported by the presence of an archetypal SC tripartite nucleolus in some of the cells (Fig. 1J)]. These observations serendipitously provided a valuable internal control for our subsequent studies.
Ablation of SCs at any age induces loss of all GC types, but GC loss does not impact on testicular architecture
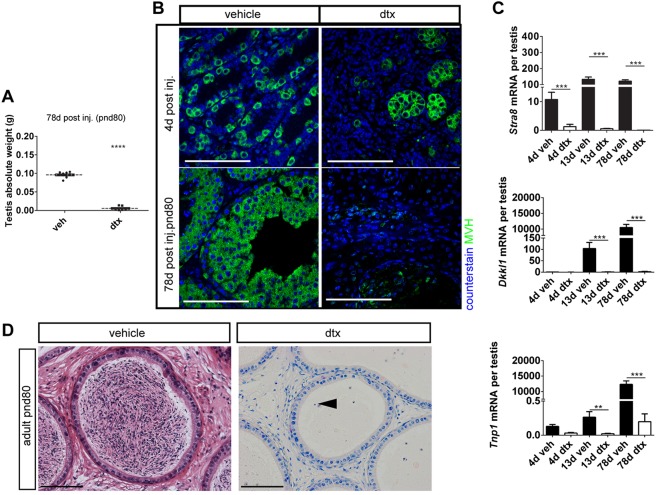
Consistent with their close functional and physical association, ablation of SCs was associated with a significant reduction in testis weight at all time points due to extensive loss of GCs (which normally constitute >80% of testis weight in the adult) (Fig. 2A; supplementary material Fig. S4). Loss of GCs at all stages of development following SC ablation (Fig. 2B; supplementary material Fig. S4) was confirmed by qRT-PCR analysis of gene transcripts associated with specific GC stages (Stra8, spermatogonia; Dkkl1, spermatocyte; and Tnp1, spermatid) (Fig. 2C; supplementary material Fig. S4). Consistent with loss of GCs, spermatozoa were absent from the epididymides of all SC-ablated animals when examined in adulthood (Fig. 2D; supplementary material Fig. S4).
Fig. 2.
Ontogeny of GC loss. (A) Testis weight was significantly reduced in adulthood following SC ablation at pnd2 (t-test, n=9-13; ****P<0.0001). (B) Immunolocalisation of MVH (DDX4) protein (green) identified GC loss following SC ablation. (C) Expression levels of the GC-specific markers Stra8 (spermatogonia), Dkkl1 (spermatocytes) and Tnp1 (spermatids) were all significantly reduced following SC ablation at pnd2 (one-way ANOVA, n=7-9; **P<0.01, ***P<0.001). (D) Consistent with GC loss, spermatozoa were absent and cellular debris remained (arrowhead) in cauda epididymides when examined in adulthood. Scale bars: 100 µm.
To exclude the possibility that GC loss would complicate our study of the somatic cell populations, and also to control for impacts arising from macrophage activation following induction of widespread cellular apoptosis, we generated Stra8-Cre+/−;iDTR+/− mice, which target iDTR expression specifically to the GC lineage (from pnd3) (Sadate-Ngatchou et al., 2008). Injection of 100 ng DTX at pnd10 induced specific ablation of GCs. Consistent with mouse models of busulfan-mediated GC ablation (O'Shaughnessy et al., 2008a,b), we observed no gross somatic cell impact arising from GC loss (supplementary material Fig. S5). Furthermore, compound Amh-Cre+/−;Stra8-Cre+/−;iDTR+/− mice displayed an identical testicular histology to Amh-Cre+/−;iDTR+/− mice following treatment with DTX (supplementary material Fig. S6).
SCs maintain the differentiated PTMC phenotype in prepubertal life
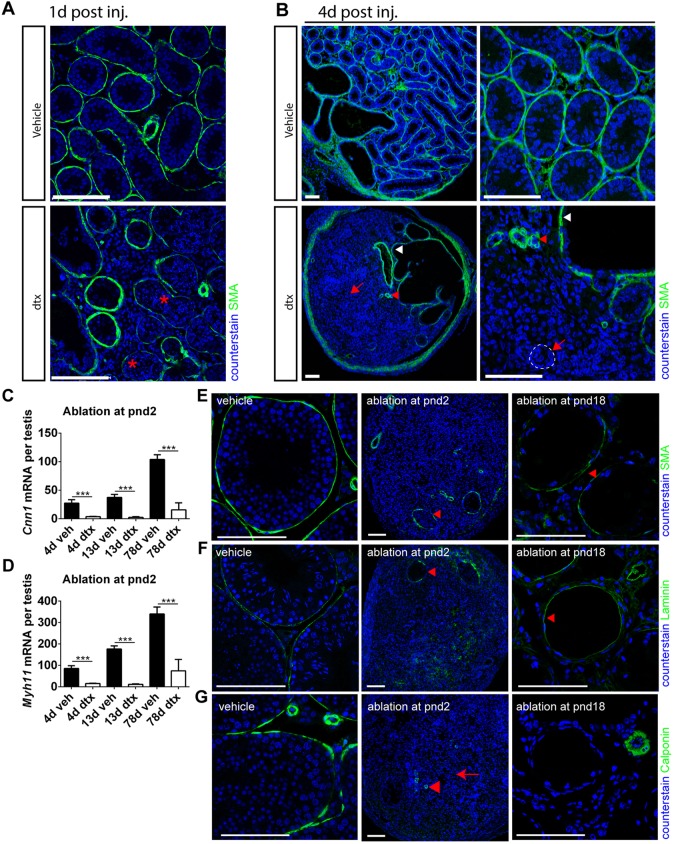
With the specificity of the model validated, we examined the impact of SC ablation on the architecture of the developing testis. SC ablation in fetal life profoundly altered the architecture of the testis in adulthood, with no evidence of recognisable seminiferous tubules and no expression of PTMC marker proteins. Ablation of SCs at pnd2 by DTX injection led to a similar disruption of tubular architecture, with no intact seminiferous tubules present in the testis 4 days after a single injection of DTX on pnd2, although the rete testis remained (Fig. 3A). Collapsed tubule remnants could be seen within the testis parenchyma, with the PTMCs forming concentric rings and occasionally surrounding a surviving spermatogonial stem cell (Fig. 3A). These changes were associated with the loss of normal PTMC markers 1 d or 6 d after injection, although there was no evidence of PTMC apoptosis at these times [by cleaved caspase 3 expression (Fig. 1E)].
Fig. 3.
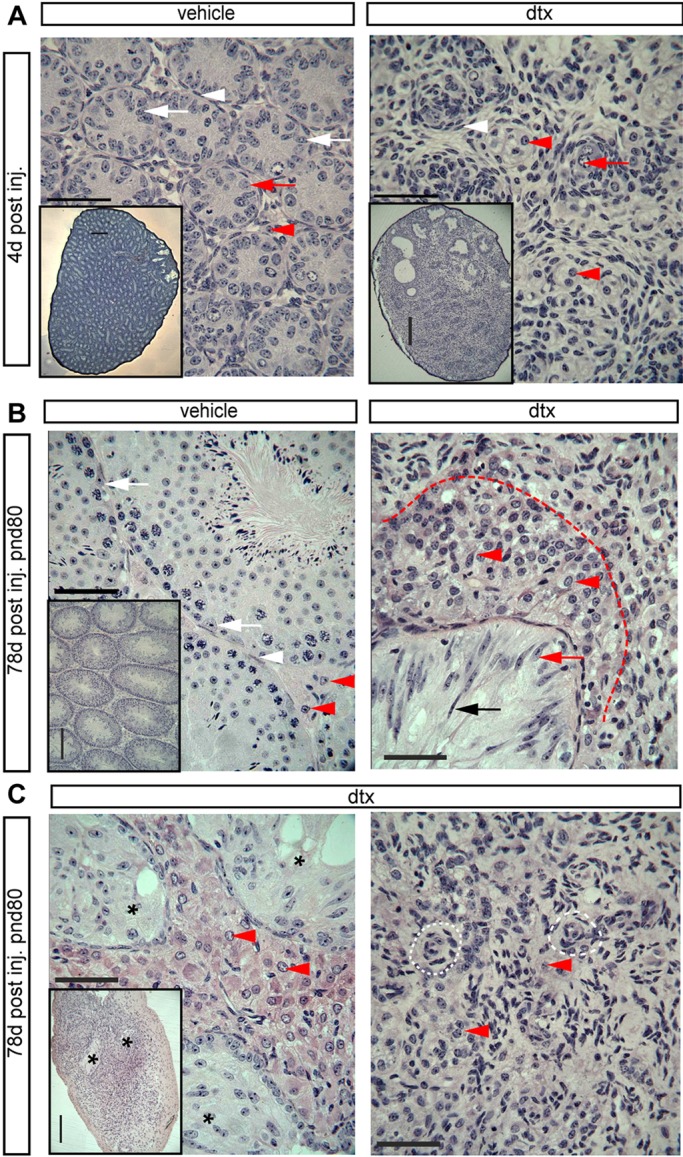
Testicular histology following SC ablation at pnd2. (A) Testicular histology of testes 4 d after ablation at pnd2. In DTX-treated animals no intact seminiferous tubules are present, although the rete testis remains present. In control testes, primordial germ cells (red arrows) and SCs (white arrows) are present inside the tubules and are surrounded by an intact PTMC layer (white arrowheads). FLCs (red arrowheads) are apparent in the interstitial tissue. In DTX-treated animals the tubules appear to have collapsed with the PTMCs forming concentric rings (white arrowheads), occasionally surrounding a surviving spermatogonial stem cell (red arrow). LCs remain present in the tissue between the collapsed tubules (red arrowheads). The insets, which are at lower magnification, show the overall structure of the seminiferous tubules in vehicle- or DTX-treated testes. (B) Testicular histology of adult testes (80 d) after ablation at pnd2. Representative SCs (white arrows), PTMCs (white arrowheads) and LCs (red arrowheads) are highlighted in vehicle-treated testis. In DTX-treated animals there was no tubular structure, although the rete testis remained intact (black arrow). The epithelium of the rete testis either had an SC-like appearance or had a highly elongated, pseudostratified appearance (black and red arrows). Abundant LCs were present in the vicinity of the rete testis (red arrowheads and delineated by the red dashed line), but there was a sharp reduction in LC numbers further from the rete testis. (C) Some variation between animals was seen in the size of the LC population surrounding the rete testis (black asterisks). In the parenchyma of the testis, concentric circles of cells were seen that might represent collapsed tubules seen at pnd6 (white dashed line). LCs were present but scarce in this region (red arrowheads). Scale bars: 50 µm in A-C; 250 µm in insets.
Smooth muscle actin (SMA) is expressed by the PTMC and usually surrounds the developing seminiferous tubules, but it appeared discontinuous 1 d after DTX treatment on pnd2, as the tubular architecture was being lost (Fig. 4A). By 4 d post-ablation, SMA expression in DTX-treated animals was restricted to the testicular vasculature, the rete testis and tunica albuginea (Fig. 4B), consistent with disruption to seminiferous tubule morphology (Fig. 3A). Similarly, expression of smooth muscle myosin heavy chain (smMHC; a specific marker of smooth muscle cells) and calponin (a functional marker of PTMC contractility) was disrupted in the PTMC 4 d after DTX injection on pnd2 (supplementary material Fig. S7). In addition, the seminiferous tubule BM, as characterised by laminin immunolocalisation, also appeared disrupted and diffuse at this time (supplementary material Fig. S7). These observations were mirrored by transcript levels of the PTMC markers Cnn1 and Myh11, both of which showed significantly reduced expression 4 d, 13 d and 78 d after pnd2 SC ablation (Fig. 4C,D). It is well established that loss of SMA, smMHC and calponin expression represents a marker of smooth muscle cell dedifferentiation (reviewed by Owens et al., 2004) and, considering also the absence of apoptosis, strongly suggests that PTMCs undergo dedifferentiation after neonatal SC ablation. Together, these data suggest that SCs act during the fetal and perinatal period both to promote PTMC specification and to maintain continued PTMC fate in cells immediately abutting the SCs.
Fig. 4.
SCs maintain the PTMC differentiated phenotype in prepubertal life. (A) Disruption of PTMCs (as indicated by loss of SMA, asterisks) 1 d post DTX injection at pnd2. (B) Four days after injection, loss of SMA expression is consistent with a collapse of the tubular architecture (arrows and dashed line). Note that SMA expression is also restricted to blood vessels (red arrowhead) and rete testis (white arrowhead). (C,D) Expression of myoid cell markers (C) Cnn1 and (D) Myh11 following SC ablation at pnd2 (one-way ANOVA, n=7-9, ***P<0.001). (E) SMA expression is retained if SC ablation occurs at pnd18 (arrowhead). (F) Disruption to tubular basement membrane (BM) (laminin) at pnd2, whereas rete testis BM remains intact (arrowhead), whereas there is retention of gross tubule morphology from pnd18 (arrowhead). (G) Loss of calponin expression, a functional marker of PTMCs, at both pnd2 [arrow; whereas blood vessels retain it (arrowhead)], consistent with a collapse of the tubular architecture, and at pnd18 albeit with retention of gross tubule morphology. Scale bars: 100 µm.
To confirm this relationship, we isolated testes at pnd8, made single-cell suspensions and xenografted the cellular milieu under the skin of nude mice. Complete disruption of the testicular architecture provided a further opportunity to test whether PTMCs require the proximity of SCs to retain their differentiated state. Examination 28 d later demonstrated that PTMCs only retained a smooth muscle identity when abutting the SCs of the reformed seminiferous tubules (supplementary material Fig. S8), thus confirming the close functional relationship between these cell types at this age.
SC control of PTMC fate is temporally restricted to prepubertal life
To establish whether control of PTMC fate was temporally restricted, we examined testes collected at pnd80 from animals that had undergone SC ablation at d2 or d18. At d80, following SC ablation on pnd2, the tunica of the testis was clearly thickened (Fig. 3C) and the rete testis remained intact but no other tubular structures were apparent (Fig. 3C). In the parenchyma of the testis some concentric circles of cells were seen, which might represent PTMCs from previously collapsed tubules seen at pnd6 (Fig. 3A,C). No PTMC markers could be seen, however, by immunohistochemistry, apart from around blood vessels, and the expression of Cnn1 and Myh11 was markedly reduced (Fig. 4C,D). By contrast, in adult (pnd80) mice treated with DTX at pnd18, the tubular structure of the testis remained intact (Fig. 5), although the tubules were marked by the presence of calcium salt deposits in the lumen (Fig. 5B). PTMCs were present around the tubules (Fig. 5B, white arrowhead), forming multilayers in places. The lumen of some of the tubules also contained a number of unidentified cells (Fig. 5B). PTMC SMA expression was retained in these animals (Fig. 4E).
Fig. 5.
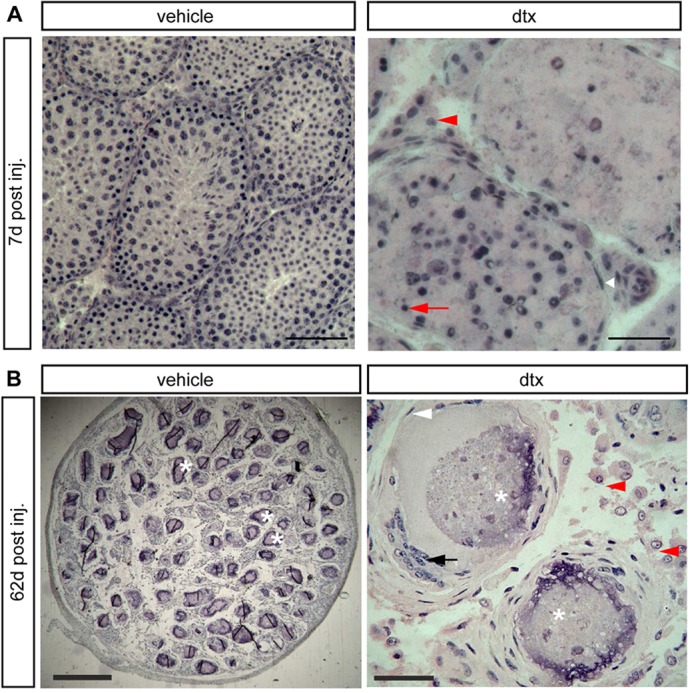
Testicular histology following SC ablation at pnd18. (A) Testicular histology of mice 7 d after ablation at pnd18. Testes retained tubular architecture with representative PTMCs (white arrowhead) surrounding tubules and LCs in the interstitium (red arrowhead). Seminiferous tubules exhibited altered spermatogenesis and apoptotic GCs were clearly visible (red arrow). Scale bars: 100 µm (left), 50 µm (right). (B) In adult mice (pnd80) injected at pnd18, the tubular structure of the testis remained intact, although the tubules were marked by calcium salt deposits in the lumen (asterisks). PTMCs were present around the tubules (white arrowhead), forming multilayers in places. The lumen of some of the tubules contained unidentified cells (black arrow). LCs (B, red arrowheads) were present between the tubules. Scale bars: 400 µm (left), 50 µm (right).
The overall maintenance of tubular structure in mice treated with DTX after pnd18 suggests that the BM laid down by SCs and PTMCs is sufficient by this age to provide structural support to the seminiferous tubules. Consistent with this hypothesis, immunolocalisation studies in adult (pnd80) testes from mice treated with DTX at pnd18 indicated normal laminin distribution (Fig. 4F); however, PTMC function might be impacted as calponin expression is lost from PTMCs, yet retained in blood vessels (Fig. 4G). In testes from adult animals treated with DTX at pnd2, both laminin and calponin expression localised only to the blood vessels and to the rete testis (Fig. 4F). Together, these data suggest that SC maintenance of the differentiated PTMC phenotype is restricted to prepubertal life.
FLCs function independently in the neonatal testis
Differentiation of the FLC population is critically dependent on SCs in fetal life, but the role of SCs in supporting FLCs beyond this point is unknown. Following SC ablation at E15, a population of LCs is present in adulthood (Fig. 6A), masculinisation of the male fetus is unaffected, and circulating testosterone is normal in the adult (Fig. 6B), indicating that functional LCs remain after SC ablation in fetal life. To examine the relationship between FLCs and SCs in more detail, LC number and function were examined 4 d after SC ablation at pnd2. Morphologically distinct LCs were apparent in the interstitial tissue between the collapsed tubules (Fig. 6A) in these animals and total FLC numbers were unaffected by SC ablation (Fig. 6C and Fig. 7A). In addition, cell function was similar to that of the control, with expression of Star and Cyp11a1 unchanged and relatively small (but significant) changes in expression of Cyp17a1, Hsd3b1 and Lhcgr despite the complete loss of SCs, GCs and PTMCs (Fig. 6C). Conversely, Hsd17b3 expression was markedly reduced by neonatal SC ablation, consistent with the expression of this gene in the fetal/neonatal SC and the described role of the SCs in testosterone production prior to puberty (O'Shaughnessy et al., 2000; Shima et al., 2013). We conclude that, following initial specification, the FLC population is retained and apparently functions relatively normally, independent of SC, GC and PTMC input in fetal and neonatal life.
Fig. 6.
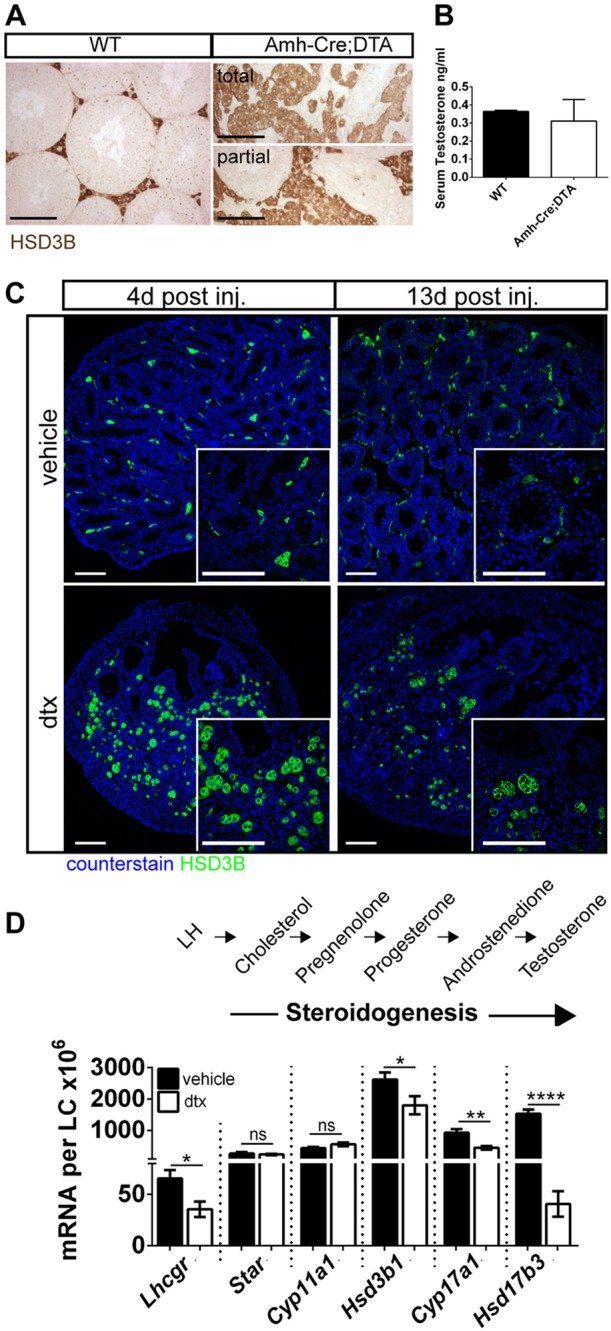
FLCs are retained and function independently in perinatal life. (A) Immunolocalisation of LCs (HSD3B) and (B) circulating testosterone in adult (pnd60) wild-type and Amh-Cre:DTA testes (t-test, n=3-6). (C) FLCs (HSD3B) in Amh-Cre:iDTR animals 4 d and 13 d following SC ablation at pnd2. Note retention of the FLC population despite SC, GC and PTMC loss. (D) Comparative testicular expression of steroidogenic transcripts 4 d following SC ablation at pnd2, when only FLCs are present (t-tests, n=7-9; *P<0.05, **P<0.01, ****P<0.0001; ns, not significant). Scale bars: 100 µm.
Fig. 7.
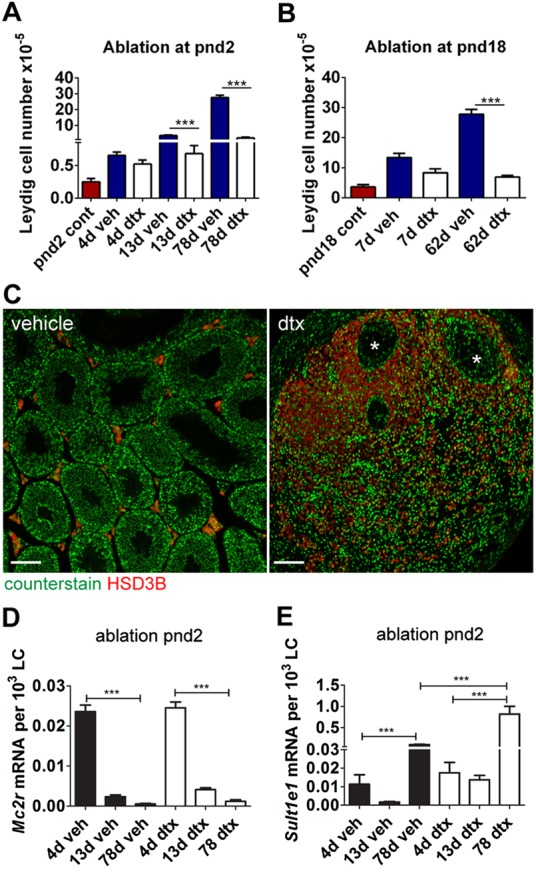
SC ablation reduces final ALC number in adulthood. (A,B) Total LC number per testis at key time points following SC ablation at pnd2 (A) or pnd18 (B) (one-way ANOVA, n=4-12). (C) LCs (HSD3B) are localised around the rete testis (asterisks) in adulthood following SC ablation at pnd2. (D,E) Markers of FLC (Mc2r) (D) and ALC (Sult1e1) (E) show similar gene expression profiles in vehicle-treated and pnd2 SC ablation groups (one-way ANOVA, n=7-9; ***P<0.001).
Neonatal or prepubertal ablation of SCs markedly reduces final LC number in adulthood
LC numbers in the mouse increase markedly at puberty as the ALC population develops (Baker and O'Shaughnessy, 2001; Vergouwen et al., 1993). To determine whether this is directed by SCs, total LC number was counted following SC ablation in neonatal life (pnd2) or during the window of LC proliferation at pnd18 (Fig. 7A,B). In control animals, LC number increased ∼30-fold from pnd2 to pnd80. In adult animals, following SC ablation at pnd2, abundant LCs were present surrounding the rete testis (Fig. 3C and Fig. 7C) but there was a sharp reduction in LC numbers more distant from the rete testis (Fig. 7C). When SCs were ablated at pnd18, LCs in the adult were present in the interstitium between the tubules (Fig. 5A). In both groups (SC ablation at pnd2 or pnd18), ablation of SCs resulted in a significant reduction in the numbers of LCs in adulthood (supplementary material Table S1), so that by pnd80 LC numbers in SC-ablated testes were only 7% (pnd2 ablation, Fig. 7A) or 24% (pnd18 ablation, Fig. 7B) of control levels. This demonstrates that SCs are fundamentally required in peripubertal life to achieve normal numbers of LCs in adulthood. Clustering of the LCs around the rete testis following SC ablation at pnd2 is also consistent with the interpretation that SCs are required for LC development in postnatal life, since this is the only area in which SC-like cells remain.
The LCs that develop following SC ablation are adult-type LCs
The LCs that develop postnatally in the mouse comprise a distinct adult population that arises from peritubular and, possibly, perivascular progenitor cells starting around pnd10 (Baker et al., 1999; Landreh et al., 2013; Nef et al., 2000; O'Shaughnessy et al., 2008b). To determine which type of LC develops following SC ablation in the neonatal testis, we measured key molecular markers associated with FLCs and ALCs in the residual population of LCs present during puberty and in adulthood (Fig. 7D,E). Mc2r expression is restricted to FLCs (O'Shaughnessy et al., 2003) and, consistent with this, Mc2r was detected in both control and DTX-treated mice 4 d after treatment on pnd2. Examination at 13 d and 78 d post-ablation demonstrated a significant reduction in Mc2r transcript levels compared with 4 d post-ablation, which mirrored observations in control animals (Fig. 7D). Conversely, expression of the ALC marker Sult1e1 (Song et al., 1997) demonstrated the opposite profile (Fig. 7E), with a significant increase in expression at pnd80 in both control and SC-ablated testes, together confirming that LCs developing after SC ablation are of the ALC type. Similar ALC development was also confirmed following SC ablation in fetal life. Compared with the control, expression of these markers (per LC) was significantly greater at pnd80 following SC ablation at pnd2 (Fig. 7E), suggesting increased activity per LC, as discussed below.
SC ablation reduces the number of ALC progenitor cells
To establish why ALC numbers are reduced in adulthood following neonatal SC ablation, we initially examined the prepubertal testis for evidence of increased cell apoptosis (cleaved caspase 3 immunolocalisation) or decreased proliferation (Ki67 immunolocalisation) in the interstitium, but found no evidence of either in developing ALCs (data not shown). We then determined whether the ALC progenitor cells were affected by SC ablation using the established progenitor cell marker nestin (Davidoff et al., 2004; Ge et al., 2006; Landreh et al., 2013). Results showed that ablation of SCs at pnd2 (Fig. 8A) and pnd18 (Fig. 8B) was associated with loss of ALC progenitor cells, which would explain the reduction in ALC numbers observed in adulthood. However, ALC progenitor cells were retained around the rete testis, consistent with the observation that ALC development is restricted to this region following SC ablation (Fig. 8A).
Fig. 8.
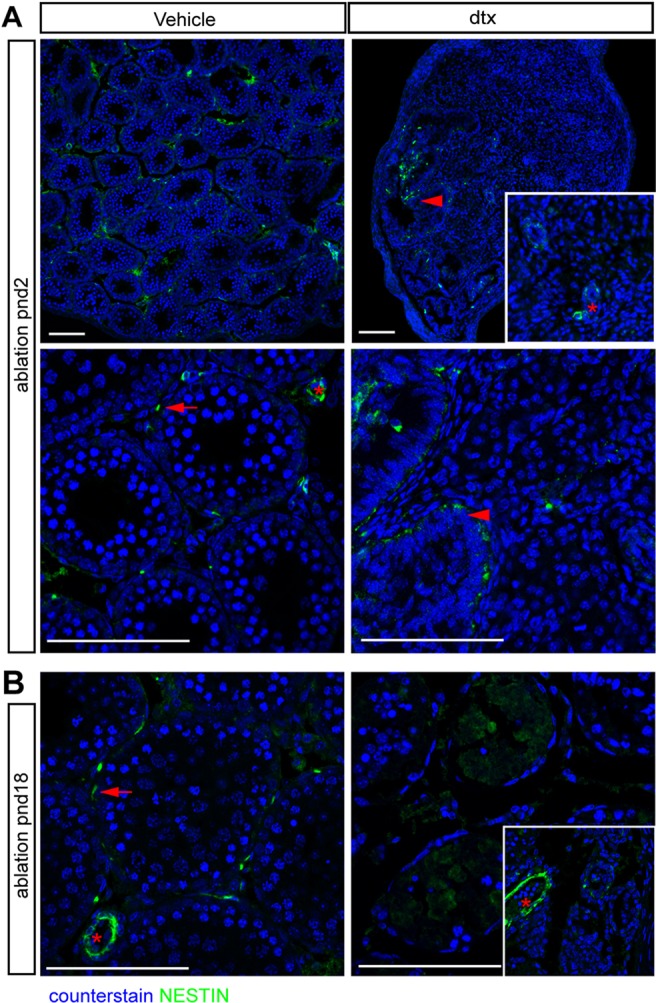
ALC progenitor cells are restricted to the rete testis following SC ablation. LC progenitor cells (as marked by nestin) localise to peritubular (arrows) and perivascular (asterisk) regions in vehicle-treated testis (A,B). By contrast, LC progenitor cells are absent from the testicular parenchyma 13 d after SC ablation at pnd2 (A) and 7 d after SC ablation at pnd18 (B), but remain adjacent to the rete testis (A, arrowheads). Scale bars: 100 µm.
LC function compensates to maintain circulating testosterone
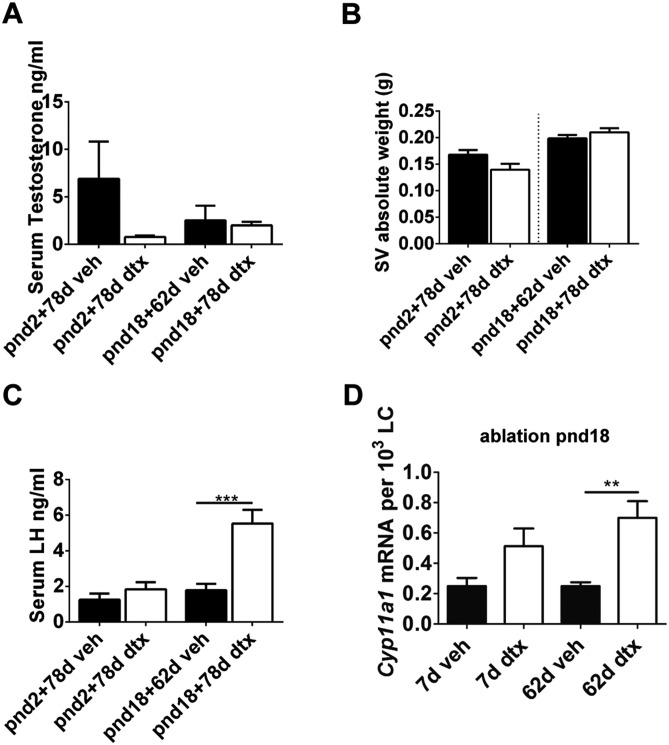
Despite the significant reduction in LC number, circulating testosterone concentrations in adulthood were not significantly different from those of control animals following SC ablation at any age (Fig. 6B and Fig. 9A), and seminal vesicle weights (Fig. 9B) (a biomarker of circulating testosterone) did not differ. Mean LH levels were significantly higher following ablation at pnd18, but did not differ at other time points (Fig. 9C). Examination of ALC function showed that transcript levels of the key steroidogenic enzyme Cyp11a1 were increased in the LCs remaining following SC ablation, which suggests that these cells are functionally compensating for the 70% reduction in LC numbers (Fig. 9D). Together, these data indicate that, following SC ablation, a compensatory response occurs in the remaining LCs to increase steroid output and thus maintain circulating testosterone concentrations.
Fig. 9.
Functional compensation of ALCs after SC ablation. (A) Serum testosterone (one-way ANOVA, n=9-12), (B) seminal vesicle weight (t-test, n=9-12) and (C) LH concentrations in adulthood following SC ablation at pnd2 and pnd18 (ANOVA, n=9-12). (D) Expression of Cyp11a1 following SC ablation at pnd18 (ANOVA, n=7-9; **P<0.01, ***P<0.001).
DISCUSSION
Since their initial description (Sertoli, 1865), SCs have been linked to most aspects of testicular development and function. Their fundamental role in sex determination and spermatogenesis is well established, but their role in other aspects of testis development and function has attracted considerable speculation. The results described here now outline the extent of SC involvement in testis development and show that they are required (1) to maintain the differentiated PTMC phenotype in fetal and neonatal life, (2) to preserve the ALC progenitor cell population and (3) to stimulate ALC development. By contrast, these studies also show, for the first time, that SCs are not required to maintain the fetal population of LCs, which show apparently normal activity in the absence of SCs, PTMCs and GCs.
The PTMCs develop initially during early testis differentiation from interstitial cells (Combes et al., 2009) and, although they clearly provide structure to the developing tubules, they might also play a more fundamental role in testis development given their importance in the adult animal (Welsh et al., 2009). Results from both SC ablation and testicular reconstitution experiments (supplementary material Fig. S8) in this study now show that the maintenance of PTMC fate during fetal and neonatal development is critically dependent on the presence and physical proximity of the SC. The nature of this interaction is unknown but, given the rapidity with which the PTMCs dedifferentiate after SC ablation (3 days before a significant macrophage response to SC ablation), active paracrine signalling appears likely, as suggested by earlier in vitro studies (Ailenberg et al., 1990; Skinner et al., 1989). Previous work has shown that DHH is required for normal development of both PTMCs and ALCs (Clark et al., 2000) and Dhh transcript levels were markedly reduced following SC ablation (Fig. 1; supplementary material Fig. S2), consistent with a role in this process. Alternatively, as both the SC and the PTMC contribute to the formation of the BM (Tung and Fritz, 1987), it is also possible that rapid changes in the nature of the BM contribute to the loss of PTMCs. Previous studies have reported that PTMCs will only retain their normal phenotype in culture on BM derived from the tubules (Tung and Fritz, 1986), which is also consistent with this hypothesis. Embryonic development of the testis cords fails in mice lacking SOX8 and SOX9 (Barrionuevo et al., 2009), but this appears to be due to loss of cell adhesion molecules, whereas extracellular matrix components are apparently normal, and the fate of the PTMC in these animals is not clear. Results from the current study also show that, by pnd18, the nature of the dynamic between the PTMC and SC has changed. The PTMCs no longer require SCs to retain their differentiated state, although their functional activity is reduced. This change in relationship might be a consequence of a terminal differentiation step in the PTMC during late neonatal development or because the BM of the tubules has developed sufficiently to maintain PTMC differentiation.
The relationship between the SC and LC during development has been the subject of considerable speculation as both cell types are crucial for normal testicular function. Results of the SC ablation studies reported here now make the relationship between these cell types clear: presence of the SC is essential for the initial differentiation of both fetal and adult populations of LCs. The FLC population arises soon after initial testis differentiation under the control of SC-derived factors such as DHH and PDGF (Brennan et al., 2003; Yao et al., 2002) as well as factors derived from other cell types in the developing testis (Kitamura et al., 2002). Our SC ablation studies now show, however, that once differentiated the continued function of the FLCs appears to be largely independent of not only the SC, but also of the GC and PTMC. Previous studies have also shown that progressive fetal loss of SCs, caused by deletion of Wt1, is not associated with loss of the FLC (Gao et al., 2006), which is consistent with this hypothesis. Activity of the FLC population in rodents has also been shown to be independent of hormone (LH) stimulation (O'Shaughnessy et al., 1998; Zhang et al., 2001) and so, once formed, these cells appear to act autonomously to secrete androgens. The independence of these cells might be a reflection of their importance in ensuring normal masculinisation of the developing fetus.
ALC development in the mouse starts at about pnd7 after birth, during the period of SC proliferation (Nef et al., 2000; O'Shaughnessy et al., 2005, 2012; Vergouwen et al., 1993). ALC numbers increase rapidly up to puberty, although steroidogenic activity of the cells is low until about pnd25 (Baker and O'Shaughnessy, 2001; O'Shaughnessy et al., 2002; Vergouwen et al., 1993). There is no doubt that proliferation of the LCs and then activation at around pnd25 is linked to the pubertal rise in LH, since LC numbers and activity remain very low in post-pubertal animals lacking LH or its receptor (Baker and O'Shaughnessy, 2001; Ma et al., 2004; O'Shaughnessy and Baker, 1998; Zhang et al., 2001). Our SC ablation studies show, however, that the differentiation/development of normal LC numbers in the prepubertal/pubertal period is also critically dependent upon the presence of SCs. Current evidence suggests that the ALCs differentiate largely from progenitor cells in a peritubular location (Ariyaratne et al., 2000; Ge et al., 2006; Landreh et al., 2013; O'Shaughnessy et al., 2008b), and the data presented here indicate that it is this progenitor cell population that requires the presence of SCs in the adjacent tubules. Failure of ALC development following neonatal or pubertal SC ablation appears to be a direct result, therefore, of loss of the ALC progenitor cells. It remains to be determined whether the SCs act directly to maintain the ALC stem/progenitor cell niche or whether they act through stimulation/maintenance of the PTMC. In either case, potential intermediaries include DHH and PDGF, which have both been shown to be involved in PTMC and ALC development and, possibly, stem cell maintenance and differentiation (Basciani et al., 2010; Clark et al., 2000; Gnessi et al., 2000; Park et al., 2007; Schmahl et al., 2008). Other SC factors might also, of course, be involved in this process, and a recent study (De Gendt et al., 2014) has identified 508 SC-specific genes in the adult mouse testis, a number of which encode secreted proteins that could be directly involved in the regulation of ALC development.
The presence of normal adult circulating testosterone levels and normal seminal vesicle weights in SC-ablated mice, despite a 70-90% reduction in ALC number, indicates that there must be a marked increase in activity of the remaining ALCs. This is consistent with the increase in Cyp11a1 transcript levels seen after SC ablation, as the cholesterol side-chain cleavage enzyme CYP11A1 catalyses one of the rate-limiting steps in testosterone synthesis (Nolan and Payne, 1990). Circulating testosterone levels are normally part of a homeostatic control mechanism regulating LH secretion and LC activity, but LH levels were not significantly affected following SC ablation on pnd2. This would suggest that increased activity of the remaining ALCs is probably mediated through altered paracrine control mechanisms. The increase in LH following SC ablation at pnd18 shows that the ALCs require increased stimulation in these animals to maintain testosterone output. It is not clear why there is a difference in the requirement for additional LH support between adult animals from the pnd2 and pnd18 ablation groups. The testicular architecture in the two groups differs, however, with tubules and PTMCs retained following ablation at pnd18, which might alter the responsiveness of the ALCs.
Ablation of the SC population at pnd2 caused a predictable invasion of macrophages into the interstitium 4 days later, although it had largely resolved after 13 days. The timing of this invasion makes it unlikely that the increase in macrophage numbers is responsible for the loss of PTMC phenotype, which starts within 1 day of SC ablation. It remains possible, nevertheless, that changes in ALC differentiation may have been affected by altered macrophage numbers. Previous studies have suggested that macrophages generally enhance LC function and development (Chen et al., 2002; Gaytan et al., 1994), although activation of macrophage invasion can have an inhibitory effect (Hales, 2002). In this case, it appears unlikely that the marked reduction in ALC development following SC ablation is caused by the transient increase in macrophage numbers for the following three reasons. (1) ALCs continue to develop around the rete testis following SC ablation at pnd2. Thus, although there might be macrophage effects in the interstitium after SC ablation, this does not affect ALC differentiation or the presence of LC progenitor cells where SC-like cells are present. (2) GC ablation prior to formation of the blood-testis barrier will induce a similar immune response in the testis to SC ablation. Data in supplementary material Fig. S4 show, however, that this has no effect on ALC progenitor cells (as determined by nestin staining), nor indeed on LC development (as determined by HSD3B staining). (3) In rats treated with EDS to ablate the ALC population there is a marked macrophage invasion of the interstitium but this does not prevent the ALC progenitor cells from repopulating the testis (e.g. Teerds and Rijntjes, 2007). Thus, although there may be knock-on effects of the macrophage invasion after SC ablation, it is unlikely that they explain the changes in PTMC or ALC development. Nevertheless, we cannot exclude the possibility that these secondary effects might contribute to the phenotype seen after SC ablation.
Previous studies have shown that the gonad remains capable of sex reversal even in adulthood. Normally, in the female, FOXL2 acts to maintain ovarian phenotype and in its absence granulosa cells will be reprogrammed into SCs and theca cells will develop into LCs (Ottolenghi et al., 2005). It has also been shown that loss of DMRT1 in SCs leads to reprogramming of the testis towards an ovarian phenotype associated with increased FOXL2 expression in the SC (Matson et al., 2011). The DTX-induced loss of SCs in this study did not lead to any increase in expression of ovarian transcript markers and there were no clear morphological changes in the LC population. This might suggest that any change in gonadal phenotype after loss of DMRT1 requires the presence of SCs and is consistent with a crucial role for the SC in regulating testis structure/function.
In the adult animal both ends of the seminiferous tubules open into the rete testis through the connecting transition zone and tubuli recti (Dym, 1974, 1976; Nykänen, 1979). The rete testis is at the interface between structures derived from the embryonic testis cords and the mesonephric tubules and it has a simple cuboidal epithelium in the mouse, which differs from the normal SC epithelium of the tubules. In some species [e.g. Bovini (Wrobel, 2000)] the rete testis clearly has an extratesticular origin but data from this study now strongly suggest that at least some of the cells of the rete epithelium in the mouse are SC-like and express at least some SC markers (SOX9). These SC-like cells also appear to be able to maintain ALC precursor/stem cells and allow ALC differentiation in the immediate vicinity of the rete testis, although it is not clear whether this SC-like activity is normal in these cells or whether it is induced by loss of SCs in other parts of the testis. The elongated phenotype of the rete epithelium after SC ablation in the neonate (Fig. 1J) certainly differs from the morphology of normal cells, which would be consistent with altered function of the cells. Although these rete cells clearly show SC-like activity, it remains unclear whether they also share the same lineage as the SC.
In conclusion, the development and application of these novel transgenic models has, for the first time, permitted acute cell-specific ablation of SCs from the developing testis. Exploitation of the DTX-mediated approach has allowed specific and rapid removal of SCs from the testis, leading to a greater understanding of the function of the SC during testis development. Together, these studies demonstrate that the SCs not only control sex determination and spermatogenesis, but also act to direct testicular development and function throughout fetal, neonatal and prepubertal life. The data also show that the testis can display a high level of adaption to maintain circulating testosterone levels despite a 90% loss of ALCs. This probably means, however, that the cells are functioning at a much higher level of activity, which will reduce the ability of the system to respond to challenges and might lead to premature, age-related loss of ALC activity.
MATERIALS AND METHODS
Generation of mouse models
Mice were housed under standard conditions of care and bred as described in the Results. Amh/Stra8;iDTR mice were injected subcutaneously with a single acute dose (100 ng in 50 µl) of DTX (Sigma-Aldrich) or with 50 µl sterile water (vehicle). Experiments passed local ethical review and were conducted with licensed permission under the UK Animal Scientific Procedures Act (1986), Home Office licence number PPL 60/4200, and the ethical guidelines of the Direction Générale de la Santé of the Canton de Genève.
qRT-PCR
RNA isolation and real-time PCR were carried out as previously described (Hu et al., 2007; O'Shaughnessy and Murphy, 1993). Primers are listed in supplementary material Table S2.
Stereology, histology and immunohistochemistry
Tissues were fixed in Bouin's solution for 6 h, and embedded in paraffin for immunohistochemistry or in Technovit 7100 resin (Heraeus Kulzer, Wehrheim, Germany) for stereology. Stereology was carried out as previously described (O'Shaughnessy et al., 2012). For histology, slides were stained with Hematoxylin and Eosin. For immunolocalisation, slides were treated as previously described (Welsh et al., 2009). Negative controls lacking primary antibody were used throughout. A minimum of three different animals from each group were tested and vehicle and DTX samples were processed simultaneously on the same slide, and each protocol was carried out on a minimum of three separate occasions. Antibodies used are detailed in supplementary material Table S3.
FSH, LH and testosterone
Serum follicle stimulating hormone (FSH), luteinizing hormone (LH) and testosterone levels were measured as described previously (Corker and Davidson, 1978; McNeilly et al., 2000).
Testis dissociation and xenografting
Testes were isolated from pnd8 mice, fragmented and digested to obtain a single-cell suspension, and injected (5-6 grafts per animal) subcutaneously into the back of castrated male CD1 nude mice as previously described (Mitchell et al., 2010). Grafts of Matrigel (BD Biosciences) suspended isolated cells were retrieved 28 d later, weighed and fixed in Bouin's solution for 2 hours.
Statistical analysis
Data were analysed using Prism (version 5, GraphPad Software) by Student's t-test or one-way ANOVA with the appropriate post-hoc tests (Turkey's multiple comparison or Dunnet's test). When required, data were normalised by Box-Cox transformation. Values are expressed as mean±s.e.m.
Supplementary Material
Acknowledgements
We thank Nathan Jeffrey, Mike Millar, Nancy Evans, Forbes Howie, Mike Dodds and Nicolas Veillard for technical support; Richard Sharpe for constructive comments on the manuscript; and Professors Braun and Waisman and the Jackson Laboratory (Maine, USA) for provision of the Stra8-Cre and HBEGF (iDTR) mice.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
Designed the study: D.Re., P.J.O., T.C.F., S.N. and L.B.S. Carried out experiments: D.Re., P.J.O., J.-L.P., A.M., L.O., L.M., Y.T.T., L.C. and L.B.S. Analysed results: D.Re., P.J.O., S.N. and L.B.S. Provided novel research tools: D.Ri., F.G., R.T.M. and R.v.H. Wrote the manuscript: D.Re., P.J.O. and L.B.S.
Funding
This work was funded by a Biotechnology and Biological Sciences Research Council (BBSRC) Project Grant [BB/J015105/1 to L.B.S., P.J.O. and T.C.F.]; a Medical Research Council (MRC) Programme Grant [G1100354/1 to L.B.S.]; a Swiss National Science Foundation grant [#3100A 135227] and by the Département de l'Instruction Publique of the Canton de Genève (to S.N. and J.-L.P.). T.C.F. is supported by a BBSRC grant [BB/JO1446X/1] to The Roslin Institute. Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.107029/-/DC1
References
- Ailenberg M., Tung P. S., Fritz I. B. (1990). Transforming growth factor-beta elicits shape changes and increases contractility of testicular peritubular cells. Biol. Reprod. 42, 499-509 10.1095/biolreprod42.3.499 [DOI] [PubMed] [Google Scholar]
- Ariyaratne H. B. S., Mendis-Handagama S. M. L. C., Hales D. B., Mason J. I. (2000). Studies on the onset of Leydig precursor cell differentiation in the prepubertal rat testis. Biol. Reprod. 63, 165-171 10.1095/biolreprod63.1.165 [DOI] [PubMed] [Google Scholar]
- Auharek S. A., Lara N. L. M., Avelar G. F., Sharpe R. M., Franca L. R. (2012). Effects of inducible nitric oxide synthase (iNOS) deficiency in mice on Sertoli cell proliferation and perinatal testis development. Int. J. Androl. 35, 741-751 10.1111/j.1365-2605.2012.01264.x [DOI] [PubMed] [Google Scholar]
- Baker P. J., O'Shaughnessy P. J. (2001). Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction 122, 227-234 10.1530/rep.0.1220227 [DOI] [PubMed] [Google Scholar]
- Baker P. J., Sha J. A., McBride M. W., Peng L., Payne A. H., O'Shaughnessy P. J. (1999). Expression of 3beta-hydroxysteroid dehydrogenase type I and VI isoforms in the mouse testis during development. Eur. J. Biochem. 260, 911-917 10.1046/j.1432-1327.1999.00245.x [DOI] [PubMed] [Google Scholar]
- Barrionuevo F., Georg I., Scherthan H., Lécureuil C., Guillou F., Wegner M., Scherer G. (2009). Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev. Biol. 327, 301-312 10.1016/j.ydbio.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Basciani S., Mariani S., Spera G., Gnessi L. (2010). Role of platelet-derived growth factors in the testis. Endocr. Rev. 31, 916-939 10.1210/er.2010-0004 [DOI] [PubMed] [Google Scholar]
- Boitani C., Stefanini M., Fragale A., Morena A. R. (1995). Activin stimulates Sertoli cell proliferation in a defined period of rat testis development. Endocrinology 136, 5438-5444 [DOI] [PubMed] [Google Scholar]
- Brennan J., Tilmann C., Capel B. (2003). Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 17, 800-810 10.1101/gad.1052503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster C. J., Ryu B.-Y., Avarbock M. R., Karagenc L., Brinster R. L., Orwig K. E. (2003). Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol. Reprod. 69, 412-420 10.1095/biolreprod.103.016519 [DOI] [PubMed] [Google Scholar]
- Brockschnieder D., Lappe-Siefke C., Goebbels S., Boesl M. R., Nave K.-A., Riethmacher D. (2004). Cell depletion due to diphtheria toxin fragment A after Cre-mediated recombination. Mol. Cell. Biol. 24, 7636-7642 10.1128/MCB.24.17.7636-7642.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch T., Heppner F. L., Tertilt C., Heinen T. J. A. J., Kremer M., Wunderlich F. T., Jung S., Waisman A. (2005). A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419-426 10.1038/nmeth762 [DOI] [PubMed] [Google Scholar]
- Chen J.-J., Lukyanenko Y., Hutson J. C. (2002). 25-hydroxycholesterol is produced by testicular macrophages during the early postnatal period and influences differentiation of Leydig cells in vitro. Biol. Reprod. 66, 1336-1341 10.1095/biolreprod66.5.1336 [DOI] [PubMed] [Google Scholar]
- Clark A. M., Garland K. K., Russell L. D. (2000). Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol. Reprod. 63, 1825-1838 10.1095/biolreprod63.6.1825 [DOI] [PubMed] [Google Scholar]
- Combes A. N., Wilhelm D., Davidson T., Dejana E., Harley V., Sinclair A., Koopman P. (2009). Endothelial cell migration directs testis cord formation. Dev. Biol. 326, 112-120 10.1016/j.ydbio.2008.10.040 [DOI] [PubMed] [Google Scholar]
- Cool J., DeFalco T., Capel B. (2012). Testis formation in the fetal mouse: dynamic and complex de novo tubulogenesis. Wiley Interdiscip. Rev. Dev. Biol. 1, 847-859 10.1002/wdev.62 [DOI] [PubMed] [Google Scholar]
- Corker C. S., Davidson D. W. (1978). A radioimmunoassay for testosterone in various biological fluids without chromatography. J. Steroid. Biochem. 9, 373-374 10.1016/0022-4731(78)90634-9 [DOI] [PubMed] [Google Scholar]
- Davidoff M. S., Middendorff R., Enikolopov G., Riethmacher D., Holstein A. F., Müller D. (2004). Progenitor cells of the testosterone-producing Leydig cells revealed. J. Cell Biol. 167, 935-944 10.1083/jcb.200409107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K., Verhoeven G., Amieux P. S., Wilkinson M. F. (2014). Research resource: genome-eide identification of AR-regulated genes translated in sertoli cells in vivo using the RiboTag approach. Mol. Endocrinol. me.2013-1391 10.1210/me.2013-1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M. (1974). The fine structure of monkey Sertoli cells in the transitional zone at the junction of the seminiferous tubules with the tubuli recti. Am. J. Anat. 140, 1-25 10.1002/aja.1001400102 [DOI] [PubMed] [Google Scholar]
- Dym M. (1976). The mammalian rete testis--a morphological examination. Anat. Rec. 186, 493-523 10.1002/ar.1091860404 [DOI] [PubMed] [Google Scholar]
- Gao F., Maiti S., Alam N., Zhang Z., Deng J. M., Behringer R. R., Lécureuil C., Guillou F., Huff V. (2006). The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc. Natl. Acad. Sci. U.S.A. 103, 11987-11992 10.1073/pnas.0600994103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan F., Bellido C., Aguilar E., van Rooijen N. (1994). Requirement for testicular macrophages in Leydig cell proliferation and differentiation during prepubertal development in rats. J. Reprod. Fertil. 102, 393-399 10.1530/jrf.0.1020393 [DOI] [PubMed] [Google Scholar]
- Ge R. S., Dong Q., Sottas C. M., Papadopoulos V., Zirkin B. R., Hardy M. P. (2006). In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc. Natl. Acad. Sci. U.S.A. 103, 2719-2724 10.1073/pnas.0507692103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnessi L., Basciani S., Mariani S., Arizzi M., Spera G., Wang C., Bondjers C., Karlsson L., Betsholtz C. (2000). Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (PDGF)-A-deficient mice. J. Cell Biol. 149, 1019-1026 10.1083/jcb.149.5.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales D. B. (2002). Testicular macrophage modulation of Leydig cell steroidogenesis. J. Reprod. Immunol. 57, 3-18 10.1016/S0165-0378(02)00020-7 [DOI] [PubMed] [Google Scholar]
- Hu L., Monteiro A., Johnston H., King P., O'Shaughnessy P. J. (2007). Expression of Cyp21a1 and Cyp11b1 in the fetal mouse testis. Reproduction 134, 585-591 10.1530/REP-07-0133 [DOI] [PubMed] [Google Scholar]
- Kitamura K., Yanazawa M., Sugiyama N., Miura H., Iizuka-Kogo A., Kusaka M., Omichi K., Suzuki R., Kato-Fukui Y., Kamiirisa K., et al. (2002). Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat. Genet. 32, 359-369 10.1038/ng1009 [DOI] [PubMed] [Google Scholar]
- Landreh L., Stukenborg J.-B., Söder O., Svechnikov K. (2013). Phenotype and steroidogenic potential of PDGFRalpha-positive rat neonatal peritubular cells. Mol. Cell. Endocrinol. 372, 96-104 10.1016/j.mce.2013.03.019 [DOI] [PubMed] [Google Scholar]
- Lécureuil C., Fontaine I., Crepieux P., Guillou F. (2002). Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 33, 114-118 10.1002/gene.10100 [DOI] [PubMed] [Google Scholar]
- Ma X., Dong Y., Matzuk M. M., Kumar T. R. (2004). Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc. Natl. Acad. Sci. U.S.A. 101, 17294-17299 10.1073/pnas.0404743101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson C. K., Murphy M. W., Sarver A. L., Griswold M. D., Bardwell V. J., Zarkower D. (2011). DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101-104 10.1038/nature10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly J. R., Saunders P. T. K., Taggart M., Cranfield M., Cooke H. J., McNeilly A. S. (2000). Loss of oocytes in Dazl knockout mice results in maintained ovarian steroidogenic function but altered gonadotropin secretion in adult animals. Endocrinology 141, 4284-4294 10.1210/endo.141.11.7764 [DOI] [PubMed] [Google Scholar]
- Mitchell R. T., Saunders P. T. K., Childs A. J., Cassidy-Kojima C., Anderson R. A., Wallace W. H. B., Kelnar C. J. H., Sharpe R. M. (2010). Xenografting of human fetal testis tissue: a new approach to study fetal testis development and germ cell differentiation. Hum. Reprod. 25, 2405-2414 10.1093/humrep/deq183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef S., Shipman T., Parada L. F. (2000). A molecular basis for estrogen-induced cryptorchidism. Dev. Biol. 224, 354-361 10.1006/dbio.2000.9785 [DOI] [PubMed] [Google Scholar]
- Nolan C. J., Payne A. H. (1990). Genotype at the P450scc locus determines differences in the amount of P450scc protein and maximal testosterone production in mouse Leydig cells. Mol. Endocrinol. 4, 1459-1464 10.1210/mend-4-10-1459 [DOI] [PubMed] [Google Scholar]
- Nurmio M., Kallio J., Adam M., Mayerhofer A., Toppari J., Jahnukainen K. (2012). Peritubular myoid cells have a role in postnatal testicular growth. Spermatogenesis 2, 79-87 10.4161/spmg.20067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykänen M. (1979). Fine structure of the transitional zone of the rat seminiferous tubule. Cell Tissue Res. 198, 441-454 10.1007/BF00234189 [DOI] [PubMed] [Google Scholar]
- Orth J. M. (1984). The role of follicle-stimulating hormone in controlling Sertoli cell proliferation in testes of fetal rats. Endocrinology 115, 1248-1255 10.1210/endo-115-4-1248 [DOI] [PubMed] [Google Scholar]
- Ottolenghi C., Omari S., Garcia-Ortiz J. E., Uda M., Crisponi L., Forabosco A., Pilia G., Schlessinger D. (2005). Foxl2 is required for commitment to ovary differentiation. Hum. Mol. Genet. 14, 2053-2062 10.1093/hmg/ddi210 [DOI] [PubMed] [Google Scholar]
- Owens G. K., Kumar M. S., Wamhoff B. R. (2004). Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767-801 10.1152/physrev.00041.2003 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Baker P. J. (1998). Role of gonadotrophins in determining Sertoli cell function during fetal development. J. Reprod. Fertil. Abstract Series 21 [Google Scholar]
- O'Shaughnessy P. J., Murphy L. (1993). Cytochrome P-450 17α-hydroxylase protein and mRNA in the testis of the testicular feminized (Tfm) mouse. J. Mol. Endocrinol. 11, 77-82 10.1677/jme.0.0110077 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Baker P., Sohnius U., Haavisto A. M., Charlton H. M., Huhtaniemi I. (1998). Fetal development of Leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology 139, 1141-1146 10.1210/en.139.3.1141 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Baker P. J., Heikkila M., Vainio S., McMahon A. P. (2000). Localization of 17beta-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis--androstenedione is the major androgen secreted by fetal/neonatal leydig cells. Endocrinology 141, 2631-2637 10.1210/en.141.7.2631 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Willerton L., Baker P. J. (2002). Changes in Leydig cell gene expression during development in the mouse. Biol. Reprod. 66, 966-975 10.1095/biolreprod66.4.966 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Fleming L. M., Jackson G., Hochgeschwender U., Reed P., Baker P. J. (2003). Adrenocorticotropic hormone directly stimulates testosterone production by the fetal and neonatal mouse testis. Endocrinology 144, 3279-3284 10.1210/en.2003-0277 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Baker P. J., Johnston H. (2005). Neuroendocrine regulation of leydig cell development. Ann. N. Y. Acad. Sci. 1061, 109-119 10.1196/annals.1336.013 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Abel M., Charlton H. M., Hu B., Johnston H., Baker P. J. (2007). Altered expression of genes involved in regulation of vitamin A metabolism, solute transportation, and cytoskeletal function in the androgen-insensitive tfm mouse testis. Endocrinology 148, 2914-2924 10.1210/en.2006-1412 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Hu L., Baker P. J. (2008a). Effect of germ cell depletion on levels of specific mRNA transcripts in mouse Sertoli cells and Leydig cells. Reproduction 135, 839-850 10.1530/REP-08-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Morris I. D., Baker P. J. (2008b). Leydig cell re-generation and expression of cell signaling molecules in the germ cell-free testis. Reproduction 135, 851-858 10.1530/REP-07-0529 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Monteiro A., Abel M. (2012). Testicular development in mice lacking receptors for follicle stimulating hormone and androgen. PLoS ONE 7, e35136 10.1371/journal.pone.0035136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. Y., Tong M., Jameson J. L. (2007). Distinct roles for Steroidogenic factor 1 and Desert hedgehog pathways in fetal and adult Leydig cell development. Endocrinology 148, 3704-3710 10.1210/en.2006-1731 [DOI] [PubMed] [Google Scholar]
- Pitetti J.-L., Calvel P., Zimmermann C., Conne B., Papaioannou M. D., Aubry F., Cederroth C. R., Urner F., Fumel B., Crausaz M., et al. (2013). An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol. Endocrinol. 27, 814-827 10.1210/me.2012-1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn A., Koopman P. (2012). The molecular genetics of sex determination and sex reversal in mammals. Semin. Reprod. Med. 30, 351-363 10.1055/s-0032-1324718 [DOI] [PubMed] [Google Scholar]
- Sadate-Ngatchou P. I., Payne C. J., Dearth A. T., Braun R. E. (2008). Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 46, 738-742 10.1002/dvg.20437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl J., Rizzolo K., Soriano P. (2008). The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev. 22, 3255-3267 10.1101/gad.1723908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido R., Lovell-Badge R. (2013). Genetic control of testis development. Sex. Dev. 7, 21-32 10.1159/000342221 [DOI] [PubMed] [Google Scholar]
- Sertoli E. (1865). Dell’ esistenza di particolari cellule ramificate nei canalicoli seminiferi del testicolo umano. Morgagni 7, 31-40 [Google Scholar]
- Sharpe R. M., Maddocks S., Kerr J. B. (1990). Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am. J. Anat. 188, 3-20 10.1002/aja.1001880103 [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., McKinnell C., Kivlin C., Fisher J. S. (2003). Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125, 769-784 10.1530/rep.0.1250769 [DOI] [PubMed] [Google Scholar]
- Shima Y., Miyabayashi K., Haraguchi S., Arakawa T., Otake H., Baba T., Matsuzaki S., Shishido Y., Akiyama H., Tachibana T., et al. (2013). Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol. Endocrinol. 27, 63-73 10.1210/me.2012-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K., Takacs K., Coffey R. J. (1989). Transforming growth factor-alpha gene expression and action in the seminiferous tubule: peritubular cell-Sertoli cell interactions. Endocrinology 124, 845-854 10.1210/endo-124-2-845 [DOI] [PubMed] [Google Scholar]
- Song W.-C., Qian Y., Sun X., Negishi M. (1997). Cellular localization and regulation of expression of testicular estrogen sulfotransferase. Endocrinology 138, 5006-5012 10.1210/endo.138.11.5512 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.-S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarulli G. A., Stanton P. G., Meachem S. J. (2012). Is the adult sertoli cell terminally differentiated? Biol. Reprod. 87, 13 10.1095/biolreprod.111.095091 [DOI] [PubMed] [Google Scholar]
- Teerds K. J., Rijntjes E. (2007). Dynamics of Leydig cell regeneration after EDS. A model for postnatal Leydig cell regeneration. In The Leydig Cell in Health and Disease (ed. Anita P., Matthew H.), pp. 91-116 Totowa: Humana [Google Scholar]
- Tung P. S., Fritz I. B. (1986). Cell-substratum and cell-cell interactions promote testicular peritubular myoid cell histotypic expression in vitro. Dev. Biol. 115, 155-170 10.1016/0012-1606(86)90237-X [DOI] [PubMed] [Google Scholar]
- Tung P. S., Fritz I. B. (1987). Morphogenetic restructuring and formation of basement membranes by Sertoli cells and testis peritubular cells in co-culture: inhibition of the morphogenetic cascade by cyclic AMP derivatives and by blocking direct cell contact. Dev. Biol. 120, 139-153 10.1016/0012-1606(87)90112-6 [DOI] [PubMed] [Google Scholar]
- Tung P. S., Skinner M. K., Fritz I. B. (1984). Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann. N. Y. Acad. Sci. 438, 435-446 10.1111/j.1749-6632.1984.tb38304.x [DOI] [PubMed] [Google Scholar]
- Vergouwen R. P.F. A., Jacobs S. G. P. M., Huiskamp R., Davids J. A. G., de Rooij D. G. (1991). Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J. Reprod. Fertil. 93, 233-243 10.1530/jrf.0.0930233 [DOI] [PubMed] [Google Scholar]
- Vergouwen R. P. F. A., Huiskamp R., Bas R. J., Roepers-Gajadien H. L., Davids J. A. G., de Rooij D. G. (1993). Postnatal development of testicular cell populations in mice. J. Reprod. Fertil. 99, 479-485 10.1530/jrf.0.0990479 [DOI] [PubMed] [Google Scholar]
- Warr N., Greenfield A. (2012). The molecular and cellular basis of gonadal sex reversal in mice and humans. Wiley Interdiscip. Rev. Dev. Biol. 1, 559-577 10.1002/wdev.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M., Saunders P. T. K., Atanassova N., Sharpe R. M., Smith L. B. (2009). Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 23, 4218-4230 10.1096/fj.09-138347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel K.-H. (2000). Morphogenesis of the bovine rete testis: the intratesticular rete and its connection to the seminiferous tubules. Anat. Embryol. (Berl.) 202, 475-490 10.1007/s004290000133 [DOI] [PubMed] [Google Scholar]
- Yao H. H.-C., Whoriskey W., Capel B. (2002). Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 16, 1433-1440 10.1101/gad.981202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. (2010). Stem cells in mammalian spermatogenesis. Dev. Growth Differ. 52, 311-317 10.1111/j.1440-169X.2010.01174.x [DOI] [PubMed] [Google Scholar]
- Zhang F.-P., Poutanen M., Wilbertz J., Huhtaniemi I. (2001). Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol. Endocrinol. 15, 172-183 10.1210/mend.15.1.0582 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.