Abstract
Objectives. We evaluated the impact of influenza vaccine text message reminders in a low-income obstetric population.
Methods. We conducted a randomized controlled trial that enrolled 1187 obstetric patients from 5 community-based clinics in New York City. The intervention group received 5 weekly text messages regarding influenza vaccination starting mid-September 2011 and 2 text message appointment reminders. Both groups received standard automated telephone appointment reminders. The prespecified endpoints were receipt of either pre- or postpartum influenza vaccination calculated cumulatively at the end of each month (September–December 2011).
Results. After adjusting for gestational age and number of clinic visits, women who received the intervention were 30% more likely to be vaccinated as of December 2011 (adjusted odds ratio [AOR] = 1.30; 95% confidence interval [CI] = 1.003, 1.69 end of September: AOR = 1.34; 95% CI = 0.98, 1.85; October: AOR = 1.35; 95% CI = 1.05, 1.75; November: AOR = 1.27; 95% CI = 0.98, 1.65). The subgroup of women early in the third trimester at randomization showed the greatest intervention effect (December 31: 61.9% intervention vs 49.0% control; AOR = 1.88; 95% CI = 1.12, 3.15).
Conclusions. In this low-income obstetric population, text messaging was associated with increased influenza vaccination, especially in those who received messages early in their third trimester.
There are an estimated 226 000 hospitalizations annually in the United States caused by influenza, with 3000 to 49 000 deaths annually over the past 3 decades.1,2 Pregnant women are at increased risk for influenza morbidity and mortality.3 Although pregnant women represent only 1% of the US population, in the 2009 H1N1 pandemic, they had a disproportionately higher mortality risk.4,5
Infants are also more likely than other age groups to experience influenza-related morbidity and mortality, but infants younger than 6 months of age are too young to be vaccinated. Vaccination during pregnancy helps to protect newborns both through passive transfer of immunity6 and by “cocooning” the newborn from influenza exposure by vaccinating those in close proximity.7 It is, therefore, strongly recommended that women receive influenza vaccination during pregnancy; those who are not vaccinated during pregnancy should be vaccinated in the postpartum period.2,3 Women should be vaccinated early in the fall, as soon as the vaccine becomes available, to achieve protection before influenza begins circulating in the community.3 Despite these recommendations, only 47% of pregnant women in the United States received the influenza vaccine in the 2011–2012 season.8 Protection against influenza is especially important in low-income communities where the risk of influenza transmission is higher.9
Text messaging has been successfully used to increase vaccination coverage in general pediatric and adolescent populations, as well as in an adult travel clinic.10–14 Although pregnant women have demonstrated interest in text messages,15,16 vaccine text message reminder-recalls have been limited in this population. Text messages can be used to remind women to be vaccinated against influenza, remind those who remain unvaccinated, and provide educational information regarding influenza and the vaccine.15,17–19 Most adults in the United States have a cell phone,20 and cell phone use is higher in lower income populations.21
We assessed the impact of influenza vaccine-related text messages in low-income urban obstetric patients. We hypothesized that text messages would be more efficacious compared with usual care.
METHODS
This randomized controlled trial included women who initiated prenatal care at 1 of 5 multispecialty community-based clinics affiliated with an academic medical center in New York City, New York, during the 2011–2012 influenza season. The 5 sites are part of a centrally administered ambulatory care network, are located within 2 miles of the medical center, and use a common electronic health record (EHR). Four sites are staffed by 1 obstetric group practice, and the fifth site is staffed by family medicine providers. These sites routinely provide influenza vaccination to pregnant women. The clinics primarily serve a publicly insured, Latina population. Uninsured pregnant women seen at these sites are routinely enrolled in Medicaid, which covers vaccination.19
The study used a 2-step inclusion process. Women were eligible for a screening text message if they (1) had a first trimester obstetric visit between February 1 and August 15, 2011, at 1 of the 5 clinical sites; (2) had an estimated date of delivery after August 31, 2011; and (3) had a cell phone number recorded in the institution’s registration system. Vaccination was not offered for the 2011–2012 season before August 15, 2011. All women meeting these criteria were sent an introductory text message that informed them that they might receive pregnancy-related health messages. Women were instructed that they could stop receiving messages either by sending a text reply or by calling a phone number. Messages were sent in English or Spanish based on the patient’s language preference as specified in the registration system. Messages provided an option to have the message resent in the other language. Messages that received an automated bounce response were resent 1 week later. Text messages were sent using a customized text-messaging platform integrated with the institution’s immunization information system, EzVac. EzVac automatically collects outpatient and inpatient vaccine administration data from the common EHR used at the study sites and the hospital.10
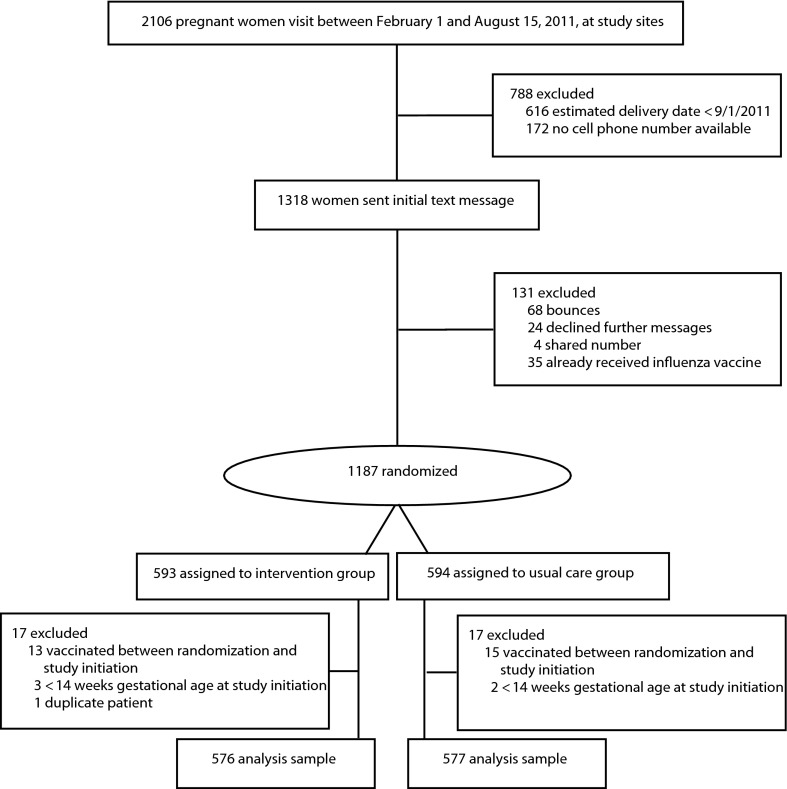
Women were eligible for the trial if they had a cell phone able to receive text messages, defined as no automated bounce response on the 2 attempts. Exclusion criteria included (1) request to stop messages, (2) having the same phone number listed in the database as another person meeting eligibility criteria, and (3) previous receipt of an influenza vaccination in the 2011–2012 season (Figure 1).
FIGURE 1—
Study flow diagram: Influenza Vaccine Text Message Reminders Study, New York, NY, 2011.
Eligible women were individually randomized to the text messaging intervention or to usual care using 1:1 allocation stratified by clinic site, using the random sample algorithm in SPSS version 19.0 (SPSS Inc., Chicago, IL) with a randomly generated start point. The study analyst was blinded to which group received the intervention. To detect an absolute difference of 9% or greater between groups in influenza vaccination, as observed from a pilot study in 294 pregnant women (unpublished data), we needed a minimum of 458 participants in each group that allowed for 5% type I error, 80% power, and equal allocation.
Intervention
Women in the intervention group received a sequence of 5 weekly, automated text message influenza vaccine reminders that were developed based on focus groups.15 The first message was introductory to let women know they were due for an influenza vaccine. Three other messages provided educational information, including (1) that pregnant women and their newborns are at increased risk for influenza-related illness, (2) vaccine safety, and (3) that doctors recommend the influenza vaccine. Some messages suggested that women discuss the vaccine at their next prenatal visit. The fifth message was interactive; women could select to receive more information regarding influenza risk, common misperceptions regarding the influenza vaccines, side effects, and need for yearly influenza vaccination (data available as a supplement to the online version of this article at http://www.ajph.org). A final message was sent to assess satisfaction with the text messaging service to which participants were asked to reply whether they “really liked” the messages, thought they were “ok,” or “did not like” them. The messages were written at a fourth grade level per the Flesch-Kincaid readability statistic.
The first message was sent during the third week of September, and the last in the series was sent during the third week in October; messages were discontinued for a woman once she was vaccinated. The satisfaction message was sent to all at the beginning of November. Unvaccinated women in the intervention group also received 2 text message appointment reminders, which included a reminder to ask for the influenza vaccine at their next prenatal visit. The first was sent for the first appointment between September 21 and November 2, 2011, and the second was sent for the first appointment between November 3 and December 15, 2011. Women in both groups received routine automated telephone pre- and postnatal appointment reminders provided directly from the clinic network.
Statistical Analyses
The prespecified trial endpoints were the receipt of vaccination calculated cumulatively at the end of each month during the intervention and an observation period (i.e., September 30, October 31, November 30, and December 31, 2011). These endpoints were selected to better address protection during variable times of onset of influenza activity in a community.22 Vaccination data were collected from the hospital immunization information system, EzVac. Vaccinations were administered (or not administered) as part of the routine care by the clinic sites. We derived baseline characteristics of age, insurance, language, and clinic site from the institution’s registration system.
We evaluated differences in proportions receiving influenza vaccine at each endpoint between randomized groups by using the χ2 test and reported asymptotic 95% confidence intervals (CIs). We used multivariable logistic regression analyses to adjust for baseline differences between the groups. Results were also adjusted for the number of visits between the start of the intervention on September 19, 2011, and the end of the observation period on December 31, 2011, for those with visits, to account for more potential opportunities for vaccination. Results were also stratified by gestational age at start of the intervention: less than 14 weeks, 14 to 27 weeks, 28 to 33 weeks, and 34 weeks or more (including postpartum). These gestational age groups were selected a priori based on gestational age–specific differences in visit frequency and content. Analyses were conducted with SPSS 19.0.
RESULTS
Of the 2106 women who met the visit eligibility criteria, 1187 met all criteria and were randomized (Figure 1). Five women at less than 14 weeks gestational age were removed from further analysis, as were 28 women who were vaccinated after randomization but before the intervention, and 1 duplicate patient. The remaining 1153 women constituted the analytical group; intervention and usual care groups were similar with regard to baseline demographic characteristics, but differed in gestational age at the start of the intervention (Table 1). The mean number of clinic visits during the intervention period for those with visits did not differ (intervention group [4.57 ±2.3] vs usual care group [4.74 ±2.3]; P = .25). The majority of vaccines administered were given prepartum (84.1% intervention; 82.4% usual care; P = .62).
TABLE 1—
Baseline Characteristics of Pregnant Women Randomized to Text Message Reminders vs Usual Care: New York, NY, 2011
| Characteristic | Intervention (n = 576), No. (%) | Usual Care (n = 577), No. (%) | P |
| Age, y | .14 | ||
| < 20 | 41 (7.1) | 50 (8.7) | |
| 20–29 | 338 (58.7) | 308 (53.4) | |
| 30–39 | 179 (31.1) | 189 (32.8) | |
| ≥ 40 | 18 (3.1) | 30 (5.2) | |
| GA at text message initiation | .004 | ||
| 14–27 wk | 194 (33.7) | 241 (41.8) | |
| 28–33 wk | 139 (24.1) | 143 (24.8) | |
| ≥ 34 wk | 243 (42.2) | 193 (33.4) | |
| Previous pregnancies | .24 | ||
| First pregnancy | 153 (26.6) | 136 (23.6) | |
| Previously pregnant | 423 (73.4) | 441 (76.4) | |
| Insurance at start of pregnancy | .22 | ||
| Uninsureda | 179 (31.1) | 181 (31.4) | |
| Medicaid/SCHIP | 394 (68.4) | 387 (67.1) | |
| Private | 3 (0.5) | 9 (1.6) | |
| Language | .95 | ||
| English | 193 (33.5) | 188 (32.6) | |
| Spanish | 377 (65.5) | 383 (66.4) | |
| Other | 6 (1.0) | 6 (1.0) |
Note. GA = gestational age; SCHIP = State Children's Health Insurance Program.
Of note, these uninsured women would be covered by Medicaid for their pregnancy.
The cumulative vaccination rates by December 31, 2011, were 49.3% in the intervention group versus 46.6% in the usual care group (difference 2.7%; 95% CI = −3.2%, 8.6%; Table 2). After adjusting for gestational age and number of clinic visits, women who received intervention were more likely to receive an influenza vaccination (adjusted odds ratio [AOR] = 1.30; 95% CI = 1.003, 1.69). Tests for interactions between clinic site and intervention were nonsignificant. Differences accrued early in the fall and then were sustained (Table 2; data available as a supplement to the online version of this article at http://www.ajph.org). The greatest effects were seen early in the intervention (October 31: AOR = 1.35; 95% CI = 1.05, 1.75). The median number of messages sent before a woman in the intervention group was vaccinated was 3 (interquartile range = 3), which represents 3 weeks.
TABLE 2—
Cumulative Influenza Vaccination Coverage by Month of Assessment for Pregnant Women Randomized to Text Message Reminders Vs Usual Care: New York, NY, 2011
| Month | Intervention (n = 576), No. (%) | Usual Care (n = 577), No. (%) | Absolute Difference, % (95% CI) | Relative Rate (95% CI) | AORa (95% CI) | AORb (95% CI) |
| By September 30 | 111 (19.3) | 88 (15.3) | 4.0 (−0.51, 8.60) | 1.26 (0.98, 1.63) | 1.34 (0.98, 1.83) | 1.34 (0.98, 1.85) |
| By October 31 | 252 (43.8) | 228 (39.5) | 4.2 (−1.6, 10.1) | 1.11 (0.97, 1.27) | 1.29 (1.01, 1.64) | 1.35 (1.05, 1.75) |
| By November 30 | 273 (47.4) | 259 (44.9) | 2.5 (−3.4, 8.4) | 1.06 (0.93, 1.20) | 1.22 (0.96, 1.54) | 1.27 (0.98, 1.65) |
| By December 31 | 284 (49.3) | 269 (46.6) | 2.7 (−3.2, 8.6) | 1.06 (0.94, 1.19) | 1.24 (0.97, 1.57) | 1.30 (1.003, 1.69) |
Note. AOR = adjusted odds ratio; CI = confidence interval.
Odds ratio adjusted for gestational age at start of intervention.
Odds ratio adjusted for gestational age at start of intervention and number of visits between start of intervention and end of review period.
The greatest effect of text messaging was seen among women who were in their early third trimester (28–33 weeks gestation) at the start of the intervention. Among these women, there was up to a 15% absolute difference in vaccination between those in the intervention group and those in the usual care group (Table 3). The difference between groups was sustained through December 31 (61.9% intervention vs 49.0% usual care; P = .029). These differences also persisted after adjusting for number of clinic visits (September 30: AOR = 1.80; 95% CI = 1.01, 3.20; October 31: AOR = 2.07; 95% CI = 1.24, 3.44; November 30: AOR = 1.86; 95% CI = 1.10, 3.11; December 31: AOR = 1.88; 95% CI = 1.12, 3.15).
TABLE 3—
Influenza Vaccination Coverage, by Gestational Age at Enrollment, for Pregnant Women Randomized to Text Message Reminders Vs Usual Care: New York, NY, 2011
| Gestational Age at Start Date Based on EDD/Review Date | Cumulative by September 30 | P | Cumulative by October 31 | P | Cumulative by November 30 | P | Cumulative by December 31 | P |
| 14–27 wk | .73 | .34 | .38 | .47 | ||||
| Intervention, no. (%) | 29 (14.9) | 99 (51.0) | 112 (57.7) | 117 (60.3) | ||||
| Usual care, no. (%) | 39 (16.2) | 112 (46.5) | 129 (53.5) | 137 (56.8) | ||||
| Absolute difference (95% CI) | −1.2% (−8.5, 6.1) | 4.6 (−5.3, 14.5) | 4.2 (−5.6, 14.1) | 3.5 (−6.3, 13.2) | ||||
| RR (95% CI) | 0.92 (0.59, 1.44) | 1.10 (0.91, 1.33) | 1.08 (0.91, 1.28) | 1.06 (0.91, 1.24) | ||||
| 28–33 wk | .049 | .009 | .03 | .029 | ||||
| Intervention, no. (%) | 39 (28.1) | 80 (57.6) | 84 (60.4) | 86 (61.9) | ||||
| Usual care, no. (%) | 26 (18.2) | 60 (42.0) | 68 (47.6) | 70 (49.0) | ||||
| Absolute difference (95% CI) | 9.9 (−0.6, 20.4) | 15.6 (3.4, 27.8) | 12.8 (0.63, 25.1) | 12.9 (0.71, 25.1) | ||||
| RR (95% CI) | 1.54 (0.996, 2.39) | 1.37 (1.08, 1.74) | 1.27 (1.02, 1.58) | 1.26 (1.02, 1.56) | ||||
| ≥ 34 wk | .095 | .82 | .92 | .79 | ||||
| Intervention, no. (%) | 43 (17.7) | 73 (30.0) | 77 (31.7) | 81 (33.3) | ||||
| Usual care, no. (%) | 23 (11.9) | 56 (29.0) | 62 (32.1) | 62 (32.1) | ||||
| Absolute difference (95% CI) | 5.8 (−1.3, −12.9) | 1.0 (−8.1, 10.1) | −0.4 (−9.7, −8.8) | 1.2 (−8.1, −10.5) | ||||
| RR (95% CI) | 1.49 (0.93, 2.38) | 1.04 (0.77, 1.39) | 0.99 (0.75, 1.30) | 1.04 (0.79, 1.36) |
Note. CI = confidence interval; EDD = estimated date of delivery; RR = relative rate.
Sensitivity analyses yielded similar adjusted odds when the 28 women who had been vaccinated before the start of the intervention were included in the analyses. Likewise, similar results were seen when the 225 women who were retrospectively found to have had a pregnancy outcome before the start of the intervention were removed from the analysis. When the 10 women who did not receive any text messages were removed from the analyses (i.e., analyses only including those “on treatment”), the following effects were seen: September 30: AOR = 1.35; 95% CI = 0.98, 1.86; October 31: AOR = 1.39; 95% CI = 1.08, 1.80; November 30: AOR = 1.31; 95% CI = 1.01, 1.69; and December 31: AOR = 1.33; 95% CI = 1.02, 1.72.
Nearly one quarter (23.2%) of the women who were not vaccinated by December 31 had an influenza vaccine order written, but the vaccine was not administered; this occurred primarily for vaccinations ordered in the outpatient setting. The proportion of such women did not differ between intervention and usual care groups. Overall, influenza vaccination coverage for the entire cohort (intervention and usual care combined) remained low, 48.0% by December 31; the small family medicine site had higher coverage of 76.9%, whereas the obstetric sites had coverage rates from 41.5% to 52.2%.
Only 10 women declined further messages during the study. Women who were at less than 14 weeks gestational age at the start of the intervention were more likely to decline further messages. All women in the intervention group were sent a text message to assess their satisfaction with the text message service. Only 12.5% replied; 83.3% of those reported they liked it, and an additional 4.2% reported that it was “ok.”
The allocated time to update the preexisting text messaging system for this project was approximately 60 hours, at an estimated cost of US $2700 for programming time. The ongoing personnel costs were an additional $45 (1 hour) per week for preparation and monitoring of messaging. The cost of the more than 3000 text messages was approximately US $84.
DISCUSSION
Influenza vaccination is important for the health of both pregnant women and their newborns. This randomized controlled study illustrated the potential of using text messages to encourage influenza vaccination in this population. Vaccination differences between groups were observed after accounting for the number of clinic visits made by each woman, suggesting that the intervention was helpful in ways other than having a woman attend a visit. Although vaccination coverage remained low, influenza vaccine coverage for the intervention group in this study was higher than national coverage for the same period (43.2% by November 2011, the standard review date used by the Centers for Disease Control and Prevention).23 The greatest absolute differences between the intervention and usual care groups were seen early in the fall, the most beneficial time to vaccinate, because women are protected before influenza begins circulating in a community.3 Delay of vaccination reduces both effectiveness and cost effectiveness of influenza vaccination of pregnant women.24
Many other interventions in pregnant women have focused on provider education or system interventions, such as the use of standing orders or educational posters.25–27 Reminder-recalls are not commonly employed. In previous work, our group used text messaging vaccine reminder-recalls to improve influenza vaccination in pediatric and adolescent populations.10 Text messaging is simple and easily scalable, particularly when immunization data are available in an immunization registry or EHR system. Once a system is set up, a large number of text messages can be sent rapidly, simultaneously, and inexpensively. This can substantially lower the cost compared with many other methods of communication. Although modest, the increases in vaccination observed in our study could yield considerable public health benefits if achieved on a national level. National programs like Text4Baby illustrated the interest in and potential reach of text messages for pregnant women. As of September 2013, more than 235 000 pregnant women and more than 384 000 new mothers received messages through Text4Baby.28
Although the intervention was modestly effective for the entire intervention cohort, it had a larger absolute effect for women early in their third trimester of pregnancy. Interestingly, even in the usual care population, women in their early third trimester at the start of the fall had higher vaccination rates than those who were later in their third trimester at that time, suggesting that vaccination practices might differ for women at that gestational age; this finding deserves further study. Because the intervention seemed to result in the largest absolute differences for this group, it might indicate that they could be a particularly good target for interventions. Importantly, pregnant women in the third trimester have been shown to be at higher risk than those in other trimesters to have an influenza-like illness episode.29 These women also accounted for the majority of pregnant women with the severe 2009 H1N1 infection.30 Of note, a recent study in low-income, primarily African American women did not demonstrate an effect of text messages on influenza vaccination in pregnant women, but only included women who were at less than 28 weeks gestational age at enrollment.31
Vaccine safety fears have been shown in pregnant women.27,32 Women earlier in their pregnancy might have had greater concerns regarding safety and fetal development, whereas those closer to the end of their pregnancy might think that they no longer needed the vaccine for themselves. Although we included in the text messages that vaccination of pregnant women has direct beneficial effects on the health of their newborns,6 future interventions could further emphasize that message. Another study showed that when women understood this “two-for-one” benefit of the influenza vaccine, they were more accepting of the vaccine.18
This study took place in a busy clinic setting with a population with many competing priorities. Understanding problems in workflow and streamlining vaccination might be important, such that once a vaccination decision is made, it is implemented effectively and immediately. For example, we observed that nearly one quarter of women who remained unvaccinated by the end of the study had an order written for an influenza vaccine in the EHR. We also noted differences in vaccination patterns among provider types. Pregnant women who were seen by family medicine providers were more likely to be vaccinated against influenza than those seen by obstetrics and gynecology providers. Other studies showed poorer vaccination practices among obstetrics and gynecology providers versus adult primary care providers.33 In addition, the 2011–2012 season was a relatively mild one for influenza, which might have led to lower overall vaccination rates. Finally, although the text messaging intervention was targeted solely at patient factors, multiple factors, including provider and systemic factors, played a role in influenza vaccination.34–36
Several potential limitations should be noted. Vaccination might have been underreported; however, vaccine documentation occurred automatically once an order was recorded as “administered” in the EHR. Although a woman could have been vaccinated at an outside site, nationally most pregnant women received the influenza vaccine at a provider’s office.23 If underreporting occurred, it was likely that intervention and usual care groups were equally affected. Second, we were limited to the socioeconomic and demographic characteristic data in the EHR. Third, although women who responded to the satisfaction question liked the text messages, the response rate was very low. In a previous telephone survey of 175 pregnant women who received text messages in a small pilot study, the response rate was 89%, and the majority of respondents (95.3%) were satisfied to very satisfied with receiving the text messages, and 95.9% were somewhat to very likely to recommend them to other pregnant women. Finally, findings might not be fully generalizable because this study took place in a single primarily low-income urban population. Strengths of our study included the randomized design, the low cost, and the high potential for dissemination of the intervention.
In conclusion, although overall coverage remained low, influenza vaccine text message reminders increased vaccination among pregnant and recently postpartum low-income women, after adjusting for gestational age and number of visits. The largest differences were found in women who started receiving text messages in their early third trimester.
Acknowledgments
This study was supported by grant R40 MC17169 (M. S. S.) from the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The clinicaltrials.gov identifier for the study is NCT01146912.
We would like to thank the FluText and EzVac teams, New York-Presbyterian Hospital for its support of the EzVac Immunization Information System, and New York-Presbyterian Hospital Ambulatory Care Network. We would like to thank Rajasekhar Ramakrishnan, ScD, and Stephen Holleran for their statistical assistance. We also thank Steven Shea, MD, MS, for his critical review of the article.
Note. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding agency. The funding agency had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the article.
Human Participant Protection
This study was approved by the Columbia University Medical Center institutional review board.
References
- 1.Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza—United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59(33):1057–1062. [PubMed] [Google Scholar]
- 2.Fiore AE, Uyeki TM, Broder K et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)–United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61(32):613–618. [PubMed] [Google Scholar]
- 4.Siston AM, Rasmussen SA, Honein MA et al. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamieson DJ, Honein MA, Rasmussen SA et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 6.Zaman K, Roy E, Arifeen SE et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. Erratum: N Engl J Med. 2009;360(6):648. [DOI] [PubMed] [Google Scholar]
- 7.Guzman-Cottrill JA, Phillipi CA, Dolan SA, Nyquist AC, Win A, Siegel J. Free vaccine programs to cocoon high-risk infants and children against influenza and pertussis. Am J Infect Control. 2012;40(9):872–876. doi: 10.1016/j.ajic.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Influenza vaccination coverage among pregnant women: 2011-12 influenza season, United States. MMWR Morb Mortal Wkly Rep. 2012;61(38):758–763. [PubMed] [Google Scholar]
- 9.Lee BY, Brown ST, Bailey RR et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011;30(6):1141–1150. doi: 10.1377/hlthaff.2010.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA. 2012;307(16):1702–1708. doi: 10.1001/jama.2012.502. [DOI] [PubMed] [Google Scholar]
- 11.Stockwell MS, Kharbanda EO, Andres R et al. Text4Health: impact of text message reminder-recalls for pediatric and adolescent immunizations. Am J Public Health. 2012;102(2):e15–e21. doi: 10.2105/AJPH.2011.300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI. Text message reminders to promote human papillomavirus vaccination. Vaccine. 2011;29(14):2537–2541. doi: 10.1016/j.vaccine.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 13.Ahlers-Schmidt CR, Chesser AK, Nguyen T et al. Feasibility of a randomized controlled trial to evaluate Text Reminders for Immunization Compliance in Kids (TRICKs) Vaccine. 2012;30(36):5305–5309. doi: 10.1016/j.vaccine.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 14.Vilella A, Bayas JM, Diaz MT et al. The role of mobile phones in improving vaccination rates in travelers. Prev Med. 2004;38(4):503–509. doi: 10.1016/j.ypmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Kharbanda EO, Vargas CY, Castano PM, Lara M, Andres R, Stockwell MS. Exploring pregnant women’s views on influenza vaccination and educational text messages. Prev Med. 2011;52(1):75–77. doi: 10.1016/j.ypmed.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Raine R, Cartwright M, Richens Y, Mahamed Z, Smith D. A qualitative study of women’s experiences of communication in antenatal care: identifying areas for action. Matern Child Health J. 2010;14(4):590–599. doi: 10.1007/s10995-009-0489-7. [DOI] [PubMed] [Google Scholar]
- 17.Lu AB, Halim AA, Dendle C et al. Influenza vaccination uptake amongst pregnant women and maternal care providers is suboptimal. Vaccine. 2012;30(27):4055–4059. doi: 10.1016/j.vaccine.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Meharry PM, Colson ER, Grizas AP, Stiller R, Vazquez M. Reasons why women accept or reject the trivalent inactivated influenza vaccine (TIV) during pregnancy. Matern Child Health J. 2013;17(1):156–164. doi: 10.1007/s10995-012-0957-3. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard-Rohner G, Meier S, Ryser J et al. Acceptability of maternal immunization against influenza: the critical role of obstetricians. J Matern Fetal Neonatal Med. 2012;25(9):1800–1809. doi: 10.3109/14767058.2012.663835. [DOI] [PubMed] [Google Scholar]
- 20.Zichuhr K, Smith A. Digital differences. Pew Internet & American Life Project. Available at: http://www.pewinternet.org/∼/media//Files/Reports/2012/PIP_Digital_differences_041312.pdf. Accessed May 30, 2012. [Google Scholar]
- 21.Lee S, Elkasabi M, Streja L. Increasing cell phone usage among Hispanics: implications for telephone surveys. Am J Public Health. 2012;102(6):e19–e24. doi: 10.2105/AJPH.2012.300681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofstetter A, Natarajan K, Rabinowitz D et al. Timeliness of pediatric influenza vaccination compared to seasonal influenza activity in an urban community, 2004-2008. Am J Public Health. 2013;103(7):e50–e58. doi: 10.2105/AJPH.2013.301351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. Pregnant women and flu shots. Internet Panel Survey, United States. November 2011. Available at: http://www.cdc.gov/flu/professionals/vaccination/pregnant-women.htm. Accessed September 7, 2012.
- 24.Myers ER, Misurski DA, Swamy GK. Influence of timing of seasonal influenza vaccination on effectiveness and cost-effectiveness in pregnancy. Am J Obstet Gynecol. 2011;204(6, Suppl 1):S128–S140. doi: 10.1016/j.ajog.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Ogburn T, Espey EL, Contreras V, Arroyo P. Impact of clinic interventions on the rate of influenza vaccination in pregnant women. J Reprod Med. 2007;52(9):753–756. [PubMed] [Google Scholar]
- 26.Mouzoon ME, Munoz FM, Greisinger AJ et al. Improving influenza immunization in pregnant women and healthcare workers. Am J Manag Care. 2010;16(3):209–216. [PubMed] [Google Scholar]
- 27.Yudin MH, Salripour M, Sgro MD. Impact of patient education on knowledge of influenza and vaccine recommendations among pregnant women. J Obstet Gynaecol Can. 2010;32(3):232–237. doi: 10.1016/s1701-2163(16)34449-8. [DOI] [PubMed] [Google Scholar]
- 28. Johnson & Johnson. Text4Baby Enrollment Data. Available at: https://text4baby.org/index.php/partner-resources/105-text4baby-enrollment-data. Accessed October 3, 2013.
- 29.Lindsay L, Jackson LA, Savitz DA et al. Community influenza activity and risk of acute influenza-like illness episodes among healthy unvaccinated pregnant and postpartum women. Am J Epidemiol. 2006;163(9):838–848. doi: 10.1093/aje/kwj095. [DOI] [PubMed] [Google Scholar]
- 30.Van Kerkhove MD, Vandemaele KA, Shinde V et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8(7):e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moniz MH, Hasley S, Meyn LA, Beigi RH. Improving influenza vaccination rates in pregnancy through text messaging: a randomized controlled trial. Obstet Gynecol. 2013;121(4):734–740. doi: 10.1097/AOG.0b013e31828642b1. [DOI] [PubMed] [Google Scholar]
- 32.Fisher BM, Scott J, Hart J, Winn VD, Gibbs RS, Lynch AM.Behaviors and perceptions regarding seasonal and H1N1 influenza vaccination during pregnancy. Am J Obstet Gynecol 20112046 suppl 1S107–S111. [DOI] [PubMed] [Google Scholar]
- 33.Tan TQ, Bhattacharya L, Gerbie MV. Awareness, perceptions and knowledge of recommended adult vaccines among a nationwide sample of adult primary care providers. J Reprod Med. 2011;56(7-8):301–307. [PubMed] [Google Scholar]
- 34.Vitek WS, Akers A, Meyn LA, Switzer GE, Lee BY, Beigi RH. Vaccine eligibility and acceptance among ambulatory obstetric and gynecologic patients. Vaccine. 2011;29(11):2024–2028. doi: 10.1016/j.vaccine.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Tong A, Biringer A, Ofner-Agostini M, Upshur R, McGeer A. A cross-sectional study of maternity care providers’ and women’s knowledge, attitudes, and behaviours towards influenza vaccination during pregnancy. J Obstet Gynaecol Can. 2008;30(5):404–410. doi: 10.1016/s1701-2163(16)32825-0. [DOI] [PubMed] [Google Scholar]
- 36.Power ML, Leddy MA, Anderson BL, Gall SA, Gonik B, Schulkin J. Obstetrician-gynecologists’ practices and perceived knowledge regarding immunization. Am J Prev Med. 2009;37(3):231–234. doi: 10.1016/j.amepre.2009.05.019. [DOI] [PubMed] [Google Scholar]