Abstract
Chronic rejection predominantly manifested as bronchiolitis obliterans syndrome (BOS), still remains a major problem affecting long-term outcomes in human lung transplantation (LTx). Donor specific antibodies (DSA) and infiltration of neutrophils in the graft have been associated with the development of BOS. This study determines the role of defensins, produced by neutrophils, and its interaction with α-1-antitrypsin (AAT) towards induction of airway inflammation and fibrosis which are characteristic hallmarks of BOS. Bronchoalveolar lavage (BAL) and serum from LTx recipients, BOS+ (n=28), BOS-(n=26) and normal healthy controls (n=24) were analyzed. Our results show that BOS+ LTx recipients had higher α-defensins (HNP1-3) and β-defensin2 HBD2 concentration in BAL and serum compared to BOS-DSA-recipients and normal controls (p=0.03). BOS+ patients had significantly lower serum AAT along with higher circulating concentration of HNP-AAT complexes in BAL (p=0.05). Stimulation of primary small airway epithelial cells (SAECs) with HNPs induced expression of HBD2, adhesion molecules (ICAM and VCAM), cytokines (IL-6, IL-1β, IL-13, IL-8 and MCP-1) and growth-factor (VEGF and EGF). In contrast, anti-inflammatory cytokine, IL-10 expression decreased 2 fold (p=0.002). HNPs mediated SAEC activation was completely abrogated by AAT. In conclusion, our results demonstrates that neutrophil secretory product, α-defensins, stimulate β-defensin production by SAECs causing upregulation of pro-inflammatory and pro-fibrotic signaling molecules. Hence, chronic stimulation of airway epithelial cells by defensins can lead to inflammation and fibrosis the central events in the development of BOS following LTx.
Keywords: HNP- Human Neutrophil Peptide, BOS-Bronchiolitis Obliterans Syndrome, BAL-Bronchoalveolar Lavage, HBD2-Human Beta Defensin 2, SAEC- Small Airway Epithelial Cells
1. Introduction
Long-term survival following human lung transplantation (LTx) is hampered due to chronic allograft rejection, clinically manifested as bronchiolitis obliterans syndrome (BOS) affecting 50–60° of LTx recipients in 5 years [1, 2]. Initial Inflammatory damage such as neutrophilia and their associated products, IL-8, and myeloperoxidase, leading to development of fibrosis around small airways is considered the central pathogenesis of BOS [3, 4]. Although multiple risk factors have been proposed to play major role in the development of BOS, recent evidence strongly suggests towards a role of allo- and auto-immune mediated injury as a significant risk factor [5]. In addition recent studies from our laboratory using a murine model of obliterative airway disease which is considered to have similarity to BOS, we have demonstrated that neutrophil infiltration following administration of antibodies specific to MHC and damage is an important initiating factor towards development of OAD [6]. Bronchoalveolar lavage (BAL) from LTx recipients have shown to contain increased neutrophils and their inflammatory products including human neutrophil peptides (HNP1, 2, 3/α–defensins)[7]. These peptides have been reported to exert chemotactic and cytotoxic activity in BAL and participate in host defense and inflammation [1, 7]. Activation of neutrophils leads to release of HNP1-3. Although normal plasma shows low levels of HNPs, in many inflammatory conditions including sepsis the levels of HNP is elevated > 170μg/ml [8]. Human β-defensins, produced by epithelial cells, also act as chemokines for activation of immature dendritic cells and T cells and provide a link between innate and adaptive immunity[9].
Alpha-1-antitrypsin (AAT), a serine protease inhibitor (serpin), has been shown to form complexes with HNP1-3 and inhibit its inflammatory activity [10]. One of its functions is to inactivate neutrophil elastase and other neutrophil-derived proteinases, and prevent the destruction of pulmonary extracellular matrix. Deficiency of AAT can cause emphysema due to degradation of interstitial elastin [11, 12]. Complex interactions between AAT and defensin are thought to have a role in regulating inflammation [13]. Hence, we propose that an increase in the HNP levels may result in low levels of AAT and this reduction in AAT with concomitant increase in defensins may favor non-alloimmune airway injury with neutrophil degranulation and reduced antiprotease defense.
In this study, we demonstrate that α-defensins stimulate airway epithelial cells to produce β-defensin2 and increased expression of inflammatory cytokines (IL-13, IL-8, IL-1β, IL-6 and MCP-1) that augment the adaptive immune responses. This leads to increased expression of pro-fibrotic growth factors including VEGF and EGF, which can lead to fibrosis, the hallmark of BOS following human LTx.
2. Materials and Methods
2.1 Human subjects
LTx recipients at Washington University Medical Center/Barnes-Jewish Hospital (2000-2007) were enrolled after informed consent according to protocol approved by institutional review board. BOS+ (n = 28) and BOS− (n = 26) patients were matched for gender and time following LTx (Table 1). The BOS+ samples were selected at the time of the initial diagnosis of BOS as determined by pulmonary function tests. The mean follow-up post-LTx I BOS+ cohort was 4.1±2.3 years, while that in the BOS− cohort was 3.6±2.7 years. In addition, serum samples from normal individuals (n = 24) were also analyzed for comparisons. Serum and BAL samples were collected and stored at −70°C until used. All patients included in the study were negative for antibodies to HLA self-antigens. The antibodies to HLA were determined using the LABScreen single antigen assay (One Lambda Inc, CA). Because of fluctuations in mean fluorescence intensity (MFI) between positive controls, a “positive” DSA was defined to be a ratio of sample to positive-control MFI of 0.2 or higher [14]. The antibodies to self-antigens (Collagen-V (Col-V) and Kα1 Tubulin (Kα1T)) by enzyme linked immunosorbent assay (ELISA) as detailed earlier [15, 16]. In brief, 1μg/mL of Col-I, Col-V and Kα1T suspended in phosphate-buffered saline were coated onto an ELISA plate (Col-I and Col-V were obtained from Chemicon/ Millipore, Billerica, MA, and recombinant Kα1T was expressed in our laboratory) and incubated overnight at 4°C. Diluted patient and normal sera were then added to these plates. Detection was done using anti-human IgG-HRP (1:10,000), developed using TMB substrate and read at 450 nm. A sample was considered positive if values were greater than the mean + 2 standard deviations (218±31 ng/mL for Kα1T, 125±23 ng/mL for Col-I and 160±28 ng/mL for Col-V) from normal age-matched sera (n=33, age 47.8±12.4, male=19 and female=14) [17]. Antibody concentration was calculated using a standard curve from known concentrations of Kα1T, Col-I or Col-V antibodies (Santa Cruz Biotechnology, CA). BOS was diagnosed according to International Society for Heart and Lung Transplantation (ISHLT) criteria [18].
Table 1.
Clinical and demographic characteristics of lung transplant patients
| Clinical and demographic characteristics | BOS+ (n =28) | BOS− (n =26) |
|---|---|---|
|
Age (years)
Mean Follow uppost-LTx (years) |
56.62 ± 7.34 4.1 ± 2.3 |
54.22 ± 8.43 3.6 ± 3.7 |
|
Gender
Female Male |
17 16 |
11 10 |
|
Race
Caucasian African American |
24 4 |
24 2 |
|
Original Disease
Chronic obstructive pulmonary disease Primary pulmonary hypertension Interstitial pulmonary fibrosis Alpha-1 antitrypsin deficiency emphysema |
18 4 4 2 |
15 6 5 0 |
|
Type of Transplant
Bilateral Single |
24 4 |
24 2 |
2.2 Cell lines
SAEC (American Type Culture Collection, Manassas, VA) were cultured in SAGM (Small Airway Epithelial Cell Medium, Cambrex BioScience, Charles City, IA). HAEC (Human Aortic Endothelial Cells, American Type Culture Collection, Manassas, VA) were cultured in EGM (Endothelial Growth Medium, Cambrex BioScience, Charles City, IA).
2.3 ELISA for HNP(1-3), HBD2 and AAT
HNP1-3 (MyBioSource, Inc., San Diego, CA), HBD2 (Phoenix Pharmaceuticals, Burlingame, CA) and human AAT (US Biological, Swampscott, MA) quantification ELISA were performed using kits and the reagents acquired from the respective manufacturer and according to manufacturer’s instructions.
2.4 Measurement of HNP- AAT complexes in BAL
BAL was incubated in microtiter wells coated with HNP1-3 antibodies (Abs). HRP-conjugated AAT Ab was used for detection. Non-complexed HNP in BAL diluted at 1:100 was measured by incubating BAL in microtiter wells coated with AAT Abs. The AAT-HNP complexes were removed. Unbound complexes were transferred to wells coated with AAT Abs following which ELISA for free HNPs and AAT were carried out as mentioned earlier.
2.5 Treatment of SAEC or HAEC cells with HNPs
SAEC/HAEC (25×103), serum starved for 16hrs, were cultured with 5, 10 or 20μg/ml of HNP1 or HNP2 (Bachem, Torrance, CA) with or without equimolar AAT. After 24 hrs, changes in morphology of the cells were observed. Supernatants from the treated cells were tested for HBD2 by ELISA. Epithelial or endothelial cells used as controls were also treated similarly.
2.6 MTT assay
Effect of HNP treatment on viability of epithelial cells was assessed by measuring mitochondrial activity using MTT (4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) assay. After stimulation of SAECs in 96 well plate for 24 hrs with various concentrations of HNPs (5, 10 or 20 μg/ml) in serum free basal medium, the cells were incubated with 5mg/ml MTT in PBS for 2 hrs, lysed with DMSO and read at 562 nm. Viability was calculated as percentage compared to untreated cells.
2.7 Detection of cell surface adhesion molecules (ICAM, VCAM) by Flow cytometry
SAEC (1×106), serum starved for 16hrs, were treated with 1 μg/ml of HNP1 or HNP2 (Bachem, Torrance, CA) for 24 hrs. The cells were collected and tested for the expression of ICAM and VCAM using FITC-labeled Anti-ICAM or Anti-VCAM Abs (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) by Flow cytometry.
2.8 Cytokine and chemokines quantitation by Luminex assay
Cytokines and chemokines (EGF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-8, IL-6, IL-10, IL-12p40/p70, IL-13, IP-10, KC, MCP-1, MIG, MIP-1α, TNF-α and VEGF) in supernatants diluted at 1:1000 obtained from cells cultured with HNPs were analyzed using Luminex Multiplex Bead immunoassays (Invitrogen, Carlsbad, CA) as described by Carson et.al. previously [19].
2.9 Statistical analysis
Five independent experiments (n=5) were performed and data presented as mean±SEM. Statistical differences between means were analyzed using a paired or unpaired Student’s t test, or subjected to analysis of variance and post hoc test. A value of P less than 0.05 was considered significant.
3. Results
3.1 Increased concentration of α-defensins (HNP1-3) and β-defensin (HBD2) in the BAL and sera of BOS+ LTx recipients following LTx
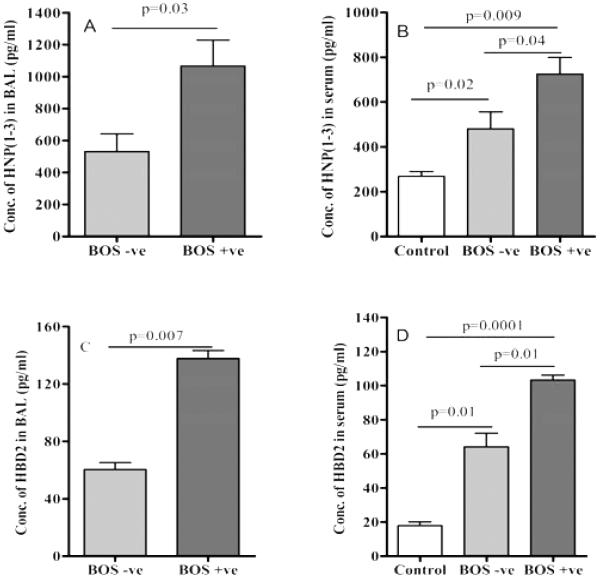
To determine the expression of defensins in pulmonary inflammation following LTx we determined the concentration of HNP1-3 in BAL and sera of BOS+ and BOS− LTx recipients. The sera from age-matched normal individuals were utilized as negative control cohort. As shown in Figure 1A BAL from BOS+ patients demonstrated significantly higher levels of HNP1-3 (1066.4 ± 282.9 vs. 532.6 ± 193.7 pg/ml, p=0.03) when compared to BOS free LTx recipients. The serum HNP1-3 levels were also higher in BOS+ (724.7 ± 129.5 vs. 480 ± 132.1 pg/ml, p=0.04) compared to BOS− LTx recipients and normal sera (268.3 ± 48.2 pg/ml, p=0.009) respectively (Figure 1B). HBD2 concentration was significantly higher (2.28 fold) (Figure 1C) in BAL samples from BOS+ LTx compared to BOS− LTx patients (137.7 ± 8.36 vs. 60.45 ± 9.92 pg/ml, p=0.007). Similarly sera from BOS+ LTx demonstrated elevatedHBD2 concentration (103.2 ± 4.97 pg/ml vs64.0 ± 13.9, p=0.01) compared to BOS− patients (Figure 1D). In addition the serum concentrations of α-defensins (p=0.02) and β-defensin (p=0.01) were also significantly elevated in BOS+LTx recipients compared to normal sera (Figure 1B and 1D). The above findings demonstrate that following LTx there is a significant increase in the expression of of defensins both in lungs and systemic circulation of the patients.
Figure 1. HNP (1-3) in the BAL fluid (A) and serum (B) of BOS+ LTx recipients are higher than BOS− recipients.
HNP (1-3) levels were measured in the BAL fluid and serum (normal control, n= 24) of BOS+ (n= 28) and BOS− (n= 26) by ELISA.
Human β-defensin (HBD2) in the BAL fluid (C) and serum (D) of BOS+ LTx recipients are higher than BOS− recipients. HBD2 levels were measured in the BAL fluid and serum (normal control, n= 24) of BOS+ (n= 28) and BOS− (n= 26) by ELISA.
3.2 BOS+ LTx recipients demonstrate increased concentrations of AAT-HNP complexes in BAL along with decreased unbound AAT in circulation
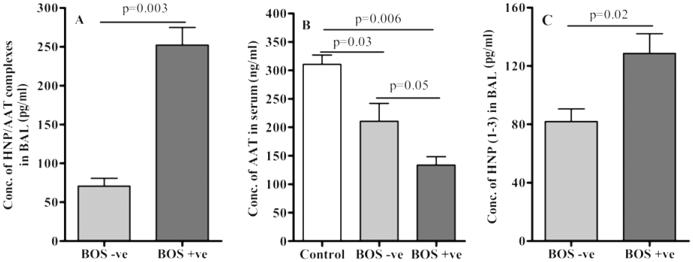
AAT regulates defensins function through formation of complexes [10]. To evaluate the function of AAT in LTx recipients, we determined the concentrations of AAT-HNP complexes in BAL and the levels of free unbound AAT in the sera by ELISA. Concentration of AAT-HNP complexes in BAL samples from BOS+ LTx was 3.6 fold higher (252.4 ± 38.9 vs. 70.8 ± 17.1 pg/ml, p=0.003, n=26, excluding 2 BOS+ patient with AAT deficiency) compared to BOS-LTx (Figure 2A). Further, the corresponding unbound AAT concentrations in the sera of BOS+LTx (133.6 ± 25.6 ng/ml) patients demonstrated markedly less (1.57 fold) concentration compared to BOS− LTx (210.7 ± 54.3 ng/ml, p=0.05) and normal sera (310.6 ± 28.2 ng/ml, 0.0006) respectively (Figure 2B). Further the sera concentrations of AAT in BOS− patients were also significantly less (1.47 fold, p=0.03) compared to normal sera. These results demonstrate that AAT forms complexes with defensins both in BOS+ and BOS− LTx recipients which can be detected in BAL.
Figure 2. High concentrations of HNP-AAT complexes are found in BAL fluid from BOS+ than BOS− patients.
BAL samples from BOS+ (n= 26, excluding 2 BOS+ patient with AAT deficiency) patients were compared to BOS− (n= 26) patients for HNP-AAT complex by ELISA (A). AAT levels in the serum samples from the above BOS+ and BOS− patients and normal sera (n= 24) were also analyzed for the presence of non-complexed AAT and HNP (n=10) using ELISA (B and C).
To determine the non-AAT complexed free HNP1-3 in BAL we incubated the BAL samples thrice (2hr at 37°C) with excess amount of polyclonal AAT Abs to remove AAT-HNP complexes. After incubations and spinning (10,000 rpm) the unbound supernatant fractions was tested to confirm complete removal of AAT using ELISA. These AAT depleted BAL samples were then utilized to measure the concentration of remaining HNP1-3. As shown in Figure 2C, BAL from BOS+ LTx contained higher HNP1-3 levels than BAL from BOS− LTx (128.66 ± 23.15 vs. 82.0 ± 14.79 pg/ml, p=0.02) demonstrating that substantial free HNP1-3 exists in the BAL of BOS+ patients even after forming complex with AAT. We propose that the free HNPs may play a role in persistent activation of airway cells following LTx.
3.3 Treatment of SAEC with increasing concentrations of HNP affects cell viability
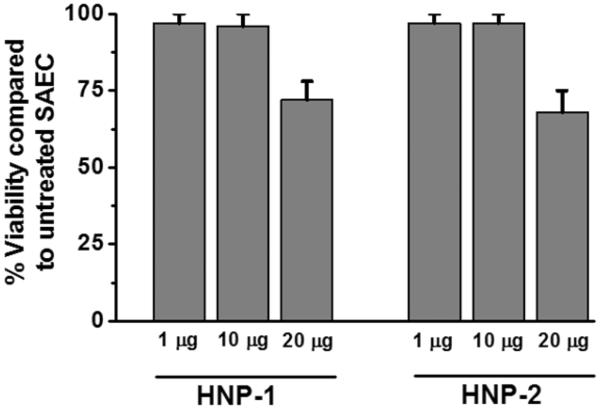
Lung epithelium is the immediate target for the host immune response following LTx. To determine the activation potential of HNPs on airway cells a dose dependent effect of HNP1 or HNP2 treatment on epithelial cell viability was analyzed using MTT assay. Twenty-four hours treatment with >20 μg/ml of HNP1/HNP2 resulted in cytotoxicity to SAECs reducing cell survival to less than 76° (Figure 3). However, a physiological concentration, i.e., 1μg/ml and a 10 fold increased levels did not result in cytotoxicity and the viability remained greater than 95°.
Figure 3. SAEC treatment with HNPs leads to cell death.
SAEC grown in 24 well plates were treated with 1, 10 and 20 μg/ml HNP1 or HNP2 respectively for MTT assay. Five independent experiments (n=5) were performed and data presented as mean±SEM; significance (p<0.05) was determined by student t-test.
3.4 HNP activation of SAEC induced expression of inflammatory adhesion molecules and β-defensins
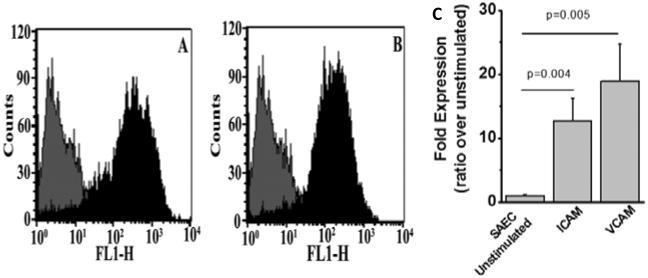
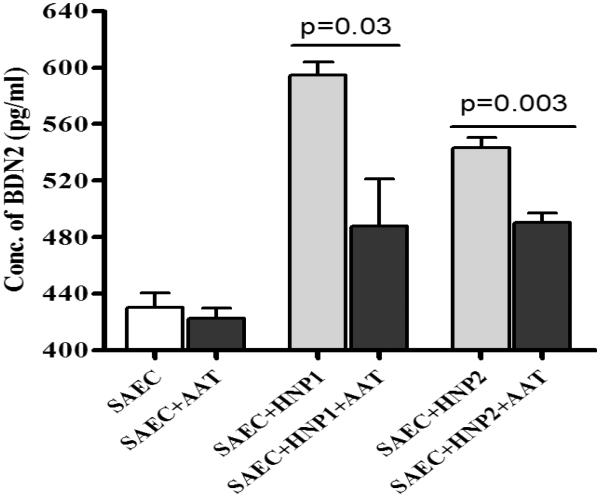
To evaluate the role of HNPs towards upregulation of inflammatory adhesion molecules, we incubated SAEC with HNP 1 (0.5μg/ml)/ HNP2 (0.5μg/ml) for 72h and analyzed the expression of cell surface adhesion molecules, ICAM and VCAM by FACS. To specifically determine the stimulatory effect of HNP on SAEC, we have preferred to employ purified HNP instead of BAL from BOS+ or BOS− patients. As demonstrated in Figure 4A and 4B, expression of both ICAM (12 fold, p=0.004) and VCAM (18 fold, p=0.005) were up-regulated on SAEC upon HNP1 treatment (Figure 4C). Similar results were also observed upon treatment with HNP 2 (data not shown).Further, culture supernatants from SAEC treated with HNP 1 (0.5μg/ml)/HNP2 (0.5μg/ml) with or without AAT for 48 hrs were analyzed for HBD2 (β-defensins) by ELISA. Results presented in Figure 5 shows significant increases in HBD2 levels in response to HNP1 or HNP2 treatment (p < 0.001). More importantly this is inhibited in the presence of AAT. Hence, higher production of α-defensins by neutrophils can lead to elevated levels of β-defensin inflammatory adhesion molecule production from the epithelial cells.
Figure 4. Treatment of SAEC with HNP1 or HNP2 results in ICAM and VCAM expression.
ICAM and VCAM were analyzed on the surface of SAEC treated with HNP1 (0.5 μg/ml) and HNP2 (0.5μg/ml) by using FITC conjugated Anti-ICAM (A) and Anti-VCAM (B) Abs in Flow cytometric detection and the fold increase in expression is represented in figure 4C. (A-B) light color represented unstimulated cells and dark color represented HNP stimulated SAECs. Five independent experiments (n=5) were performed and data presented as mean±SEM; significance (p<0.05) was determined by student t-test.
Figure 5. Treatment of SAEC with HNP1 or 2 results in more HBD2 production from the cells, which is inhibited in the presence of AAT.
SAEC grown in 6 well plates were treated with HNP1 or HNP2 with or without equimolar AAT as mentioned in methods for 24 hrs. HBD2 production was measured by ELISA in the culture supernatant. Five independent experiments (n=5) were performed and data presented as mean±SEM; significance (p<0.05) was determined by student t-test.
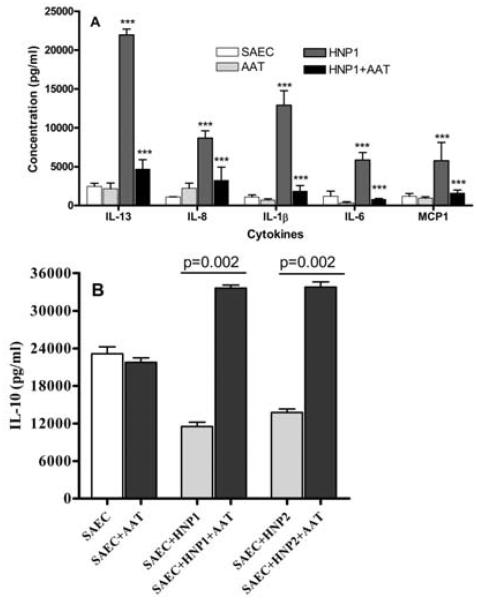
3.5 Increase in secretion of cytokines IL-13, IL-8, IL-1β, IL-6 and MCP-1 by SAEC following addition of HNP1 or HNP2
To determine the role of HNP towards induction of pro-inflammatory cytokines by airway epithelial cells, culture supernatants from SAEC treated with HNP 1 (0.5μg/ml)/HNP2 (0.5μg/ml), were analyzed for various cytokines by Luminex assay. The effect of AAT on cytokine production induced by HNP was also determined. Expression and secretion of pro-inflammatory cytokines (Figure 6A) in the culture supernatant were increased following incubation with HNP1/2 (IL-13 (9 folds), IL-8 (8 folds), IL-1β (11 folds), IL-6 (5 fold) and MCP-1 (5 fold) over unstimulated cells. A similar increase was also noted following incubation with HNP2 (data not shown). These cytokine stimulatory effects of both HNP1/2 were completely abrogated in the presence of AAT. It is also of interest to note that incubation of SAEC with HNP1/ 2 also resulted in down regulation of anti-inflammatory cytokine, IL-10 (2 fold, p<0.03). However, AAT reversed the effects of defensins resulting in significant increase in IL-10 levels (1.7 fold, p=0.002) (Figure 6B).
Figure 6. (A) Treatment of SAEC with HNP1 results in production of cytokines, which is inhibited in the presence of AAT.
SAEC grown in 6 well plates were treated with 1μg/ml HNP1 with or without equimolar AAT as mentioned in methods for 24 hrs. Cytokines production was measured by Luminex method in the culture supernatant. (B) Treatment of SAEC with HNP1 or 2 results in less IL-10 production from the cells. SAEC grown in 6 well plates were treated with 10μg/ml HNP1 or HNP2 as mentioned in methods for 24 hrs. IL-10 production was measured by Luminex method in the culture supernatant. Five independent experiments (n=5) were performed and data presented as mean±SEM; significance (p<0.05) was determined by student t-test.
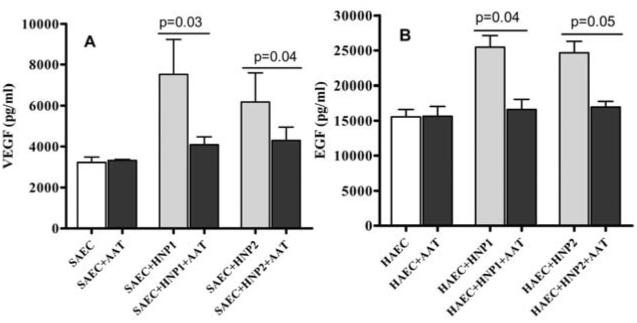
3.6 Increased pro-fibrotic growth factor expression by SAEC following stimulation with HNP1/2
Epithelial proliferation, migration and differentiation are predominantly mediated by growth factors and their receptors. Hence, culture supernatants from SAEC treated with 1μg/ml HNP1 or HNP2, with or without AAT for 24 hrs was collected and tested for the presence of growth factors by Luminex assay. Increased concentration of VEGF (6100 vs 3700 pg/ml, p<0.05) and EGF (25,500 vs 15,500 pg/ml, p<0.05) following incubation of SAEC with HNPs are noted which were completely abrogated by AAT (Figure 7). These confirm the notion that HNPs induce pro-fibrotic growth factors which play a crucial role in the development of fibrotic changes seen in BOS.
Figure 7. Growth factors are produced in response to HNP treatment. (A) Treatment of SAEC with HNP1 or HNP2 results in VEGF production from the cells, which is inhibited in the presence of AAT.
SAEC grown in 6 well plates were treated with 10μg/ml HNP1 or HNP2 with or without equimolar AAT as mentioned in methods for 24 hrs. Growth factor production was measured by Luminex method in the culture supernatant. (B) Treatment of HAEC with HNP1 or 2 results in EGF production from the cells, which is inhibited in the presence of AAT. HAEC grown in 6 well plates were cultured with 10μg/ml HNP1 or HNP2 with or without equimolar AAT as mentioned in methods for 24 hrs. Growth factor production was measured by Luminex method in the culture supernatant. Five independent experiments (n=5) were performed and data presented as mean±SEM; significance (p<0.05) was determined by student t-test.
4. Discussion
Long term outcomes following LTx are significantly affected due to development of chronic rejection (BOS). Several immunological as well as non-immune factors are postulated in its pathogenesis. Amongst these, increased neutrophil infiltration in the lungs has been shown to be associated with the development of BOS following LTx [20, 21]. Based on this we hypothesized that initial inflammatory injury and activation of airway epithelial cells as a major initiating factor towards development of BOS. Further it has been demonstrated that direct activation of endothelial cells by antibodies to HLA can induce pro-inflammatory and pro-fibrotic signaling cascade [22, 23]. To avoid possible co-variate bias, in this study we selected LTx patients who were negative for donor specific HLA antibodies (DSA) and auto-antibodies (anti-collagenV and anti-kα1tubulin). Our results clearly demonstrate that BAL and sera from LTx recipients who developed BOS has increased concentration of HNP1-3 (α-defensins) (Figure 1). Defensins are small molecular peptides that play an important role in cellular infiltration and effector functions at the site of infection or injury [24]. Though defensins have been found to be increased following lung injury, including transplantation, the mechanisms by which defensins contribute to the development of chronic rejection remains undefined. We demonstrate that levels of defensins are significantly elevated in the serum as well as BAL samples of LTx recipients diagnosed with BOS. Further, we present data that defensins o induce the secretion of cytokines, chemokines and fibrogenic growth factors by airway epithelial cells which we propose to play a significant role in the pathogenesis of BOS. A detailed correlative study with respect to the entire cell profile, microbial analyses, and BOS phenotypes (NRAD, RAS etc) would be warranted in our future studies.
A complex interaction between innate and adaptive immunity contribute to the propagation of airway inflammation in BOS. Defensins, a part of the innate immune system [25], are small molecules released at the site of injury that promotes inflammation and resistance to infections. They have also been shown to affect immune functions and mediate wound repair processes [13]. Analysis of the levels of α-defensins and β-defensins in the serum of LTx recipients with and without BOS (Figure 1) has demonstrated a significant increase in defensins in BOS+ LTx recipients compared to the BOS− patients. Similarly, defensin levels in BAL from the LTx recipients were significantly higher in the BOS+ when compared to BOS− patients. These results are in agreement with reports of increased levels of defensins in LTx recipients with BOS [7, 26].
AAT, a serine proteinase inhibitor that forms complexes with α-defensins, can inhibit the functions of defensins in addition to decreasing its elastase-inhibitory activity [10, 27]. Elastase is vital to phogocytosis of debris in the lungs resulting as by-product of infection or inhaled pollutants. Following neutrophil elastase activity, AAT inactivates elastase and prevents further damage to lung tissue. We demonstrate in this report that AAT levels are lower in patients with increased defensin due to increased complex formation between the two (Figure 2). These results suggest that measurement of HNP1-3; HBD2 and AAT levels in the serum of LTx patients may offer a simple, non-invasive diagnostic adjunct for the diagnosis of chronic lung allograft rejection.
Inflammation and inflammation induced diapedesis in the lungs and airways is regulated [28] by integrin mediated cell-cell interactions [29] between the epithelial cells. In order to determine the effect of defensins on epithelial cells, we incubated SAEC with varying concentrations of defensins and analyzed its effect expression of pro-inflammatory and pro-fibrotic signaling molecules. Exposure of epithelial cells to defensins as seen in Figure 3, demonstrated morphological changes and clustering. There was also an increase in adhesion molecules ICAM and VCAM on the surface of epithelial cells after treatment with HNP1 or HNP2. Although the secretory inflammatory cytokines are expressed by 24 hrs following HNP stimulation, the membrane targeting proteins ICAM and VCAM were detected 48 to 72 hrs following the HNP stimulation of SAECs. Exposure of epithelial cells to neutrophil defensins have been shown to result in chemotaxis of inflammatory cells and proliferation of epithelial cells and fibroblasts [30]. This may in part be due to the ability of α-defensins to lyse lung epithelial cells [31] and/or induction of IL-8 production in these cells [20]. Neutrophil defensins have been shown to cause proliferation of epithelial cells, to enhance lung epithelial airway remodeling and mucin gene expression [32, 33] whereas β-defensins promote differentiation of keratinocytes [34]. This is in agreement with our hypothesis that α-defensins can activate SAEC to produce β-defensins which in turn act as chemoattractants for neutrophils and macrophages, further increasing defensin production (HNP1-3) by the infiltrating neutrophils as well as HBD2 from the epithelial cells.
The airway remodeling process comprises of epithelial proliferation, migration and differentiation that are regulated by growth factors and their receptors [35]. Our results presented in Figure 7A and B also show upregulation of growth factors (VEGF, EGF) on SAECs in response to α-defensins. Growth factor VEGF has been shown to be involved in LTx rejection [36]. The VEGF concentration in BAL is particularly decreased at early time points following LTx, which gradually increase in the absence of rejection or infection. Further, VEGF becomes lower during active rejection or CMV pneumonia[36]. Increased VEGF seen in our study (Figure 6A) may contribute towards fibro proliferative changes seen in BOS following human LTx.
It has been shown that HNPs link innate and adaptive immunity through activation of co-stimulatory molecules in lung epithelial cells and CD4+ lymphocytes [37]. HBD-1 and HBD-2 bind to a chemokine receptor CCR-6 [38]. Several studies have shown an important contribution for pro-inflammatory cytokines in the development of BOS [39, 40]. HBD-2 bind toll-like receptors (TLR) in the epithelium and activate NFkB pathway and cytokine gene upregulation. In our study, several cytokines (IL-1β, IL-13, IL-6 and IL-8) and chemokines (MCP-1) were upregulated upon incubation of SAECs with HNP1 or 2. We also demonstrate that IL-6 and IL-1β, major pro-inflammatory cytokines, were upregulated upon stimulation of epithelial cells by HNPs. Chemokine MCP-1 is also produced by epithelial cells on treatment with HNPs (Figure 6A). This can also increase monocytes infiltration into the lung leading to inflammation since monocyte-chemotactic activity of defensins has been reported earlier [41, 42]. We propose that the inflammatory milieu along with epithelial proliferation and remodeling can expose self antigens including Kα1 Tubulin and Collagen V to the immune system giving rise to autoimmune responses which are considered to be important in the pathogenesis of BOS [16, 43].
In conclusion, results presented in this communication demonstrate that α-defensins produced by neutrophils, infiltrate into the transplanted lung stimulate β-defensin production by epithelial cells. This leads to increase in cellular activation resulting in increased production of inflammatory cytokines in the local area. Chronic stimulation of epithelial cells by the resulting defensins can also result in increased expression of cell adhesion molecules, pro-inflammatory cytokines, and pro-fibrotic growth factor production resulting in epithelial cell proliferation and remodeling that contribute towards the development of BOS. We propose that the growth factors produced not only by epithelial cells but also endothelial cells upon chronic stimulation by defensins can lead to the fibrotic changes seen in BOS. Therefore, defensins interactions with epithelial and endothelial cells of the transplanted lung serves as a primary link between the innate and adaptive immunity following human LTx and play an important role in the pathogenesis of BOS.
Acknowledgements
The authors thank Ms. Billie Glasscock for her assistance in preparing and submitting this manuscript. This work was supported by NIH HL056643 and HL092514 to TM. DS is the recipient of an ISHLT Research Fellowship.
Abbreviations
- AAT
α-1-antitrypsin
- BAL
bronchoalveolar lavage
- BOS
bronchiolitis obliterans syndrome
- Col-V
Collagen-V
- DSA
donor specific antibodies
- ELISA
enzyme-linked immunosorbent assay
- EGF
epidermal growth factor
- HAEC
human aortic endothelial cells
- HBD2
human β-defensin2
- HNP
human neutrophil peptide
- IL
interleukin
- ICAM
intercellular adhesion molecule
- Kα1T
Kα1 Tubulin
- LTx
lung transplantation
- MFI
mean fluorescence intensity
- MCP-1
monocyte chemotactic protein-1
- SAEC
small airway epithelial cells
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sundaresan S, Trulock EP, Mohanakumar T, Cooper JD, Patterson GA. Prevalence and outcome of bronchiolitis obliterans syndrome after lung transplantation. Washington University Lung Transplant Group. Ann Thorac Surg. 1995;60(5):1341. doi: 10.1016/0003-4975(95)00751-6. [DOI] [PubMed] [Google Scholar]
- 2.Valentine VG, Robbins RC, Berry GJ, Patel HR, Reichenspurner H, Reitz BA, Theodore J. Actuarial survival of heart-lung and bilateral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant. 1996;15(4):371. [PubMed] [Google Scholar]
- 3.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166(4):440. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 4.Yousem SA, Suncan SR, Ohori NP, Sonmez-Alpan E. Architectural remodeling of lung allografts in acute and chronic rejection. Arch Pathol Lab Med. 1992;116(11):1175. [PubMed] [Google Scholar]
- 5.Tiriveedhi V, Sarma N, Mohanakumar T. An important role for autoimmunity in the immunopathogenesis of chronic allograft rejection. Int J Immunogenet. 2012;39(5):373. doi: 10.1111/j.1744-313X.2012.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiriveedhi V, Takenaka M, Sarma NJ, Gelman AG, Mohanakumar T. Anti-major histocompatibility complex-induced obliterative airway disease: selective role for CD4 and CD8 T cells in inducing immune responses to self-antigens. J Heart Lung Transplant. 2013;32(7):714. doi: 10.1016/j.healun.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson RL, Hiemstra PS, Ward C, Forrest IA, Murphy D, Proud D, Lordan J, Corris PA, Fisher AJ. Antimicrobial peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Eur Respir J. 2008;32(3):670. doi: 10.1183/09031936.00110807. [DOI] [PubMed] [Google Scholar]
- 8.Panyutich AV, Panyutich EA, Krapivin VA, Baturevich EA, Ganz T. Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J Lab Clin Med. 1993;122(2):202. [PubMed] [Google Scholar]
- 9.Schutte BC, McCray PB., Jr. [beta]-defensins in lung host defense. Annu Rev Physiol. 2002;64:709. doi: 10.1146/annurev.physiol.64.081501.134340. [DOI] [PubMed] [Google Scholar]
- 10.Panyutich AV, Hiemstra PS, van Wetering S, Ganz T. Human neutrophil defensin and serpins form complexes and inactivate each other. Am J Respir Cell Mol Biol. 1995;12(3):351. doi: 10.1165/ajrcmb.12.3.7873202. [DOI] [PubMed] [Google Scholar]
- 11.Hiemstra PS, van Wetering S, Stolk J. Neutrophil serine proteinases and defensins in chronic obstructive pulmonary disease: effects on pulmonary epithelium. Eur Respir J. 1998;12(5):1200. doi: 10.1183/09031936.98.12051200. [DOI] [PubMed] [Google Scholar]
- 12.Van Wetering S, Mannesse-Lazeroms SP, Dijkman JH, Hiemstra PS. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J Leukoc Biol. 1997;62(2):217. doi: 10.1002/jlb.62.2.217. [DOI] [PubMed] [Google Scholar]
- 13.van Wetering S, Sterk PJ, Rabe KF, Hiemstra PS. Defensins: key players or bystanders in infection, injury, and repair in the lung? J Allergy Clin Immunol. 1999;104(6):1131. doi: 10.1016/s0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- 14.Morris GP, Phelan DL, Jendrisak MD, Mohanakumar T. Virtual crossmatch by identification of donor-specific anti-human leukocyte antigen antibodies by solid-phase immunoassay: a 30-month analysis in living donor kidney transplantation. Hum Immunol. 2010;71(3):268. doi: 10.1016/j.humimm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiriveedhi V, Gautam B, Sarma NJ, Askar M, Budev M, Aloush A, Hachem R, Trulock E, Myers B, Patterson AG, Mohanakumar T. Pre-transplant antibodies to Kalpha1 tubulin and collagen-V in lung transplantation: clinical correlations. J Heart Lung Transplant. 2013;32(8):807. doi: 10.1016/j.healun.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26(8):782. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Carson RT, Vignali AA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods. 1999;227(1-2):41. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 20.Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272(5 Pt 1):L888. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 21.Riise GC, Williams A, Kjellstrom C, Schersten H, Andersson BA, Kelly FJ. Bronchiolitis obliterans syndrome in lung transplant recipients is associated with increased neutrophil activity and decreased antioxidant status in the lung. Eur Respir J. 1998;12(1):82. doi: 10.1183/09031936.98.12010082. [DOI] [PubMed] [Google Scholar]
- 22.Valenzuela NM, Mulder A, Reed EF. HLA class I antibodies trigger increased adherence of monocytes to endothelial cells by eliciting an increase in endothelial P-selectin and, depending on subclass, by engaging FcgammaRs. J Immunol. 2013;190(12):6635. doi: 10.4049/jimmunol.1201434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed EF. Mechanisms of action and effects of antibodies on the cells of the allograft. Hum Immunol. 2012;73(12):1211. doi: 10.1016/j.humimm.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Jaramillo A, Naziruddin B, Zhang L, Reznik SI, Smith MA, Aloush AA, Trulock EP, Patterson GA, Mohanakumar T. Activation of human airway epithelial cells by non-HLA antibodies developed after lung transplantation: a potential etiological factor for bronchiolitis obliterans syndrome. Transplantation. 2001;71(7):966. doi: 10.1097/00007890-200104150-00023. [DOI] [PubMed] [Google Scholar]
- 25.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23(2):327. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 26.Ross DJ, Cole AM, Yoshioka D, Park AK, Belperio JA, Laks H, Strieter RM, Lynch JP, Kubak B, Ardehali A, Ganz T. Increased bronchoalveolar lavage human beta-defensin type 2 in bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004;78(8):1222. doi: 10.1097/01.tp.0000137265.18491.75. [DOI] [PubMed] [Google Scholar]
- 27.Panyutich AV, Hiemstra PS, van Wetering S, Ganz T. Human neutrophil defensin and serpins form complexes and inactivate each other. Am J Respir Cell Mol Biol. 1995;12(3):351. doi: 10.1165/ajrcmb.12.3.7873202. [DOI] [PubMed] [Google Scholar]
- 28.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr., Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133(4):921. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shock A, Laurent GJ. Adhesive interactions between fibroblasts and polymorphonuclear neutrophils in vitro. Eur.J.Cell Biol. 1991;54:211. [PubMed] [Google Scholar]
- 30.van Wetering S, Tjabringa GS, Hiemstra PS. Interactions between neutrophil-derived antimicrobial peptides and airway epithelial cells. J Leukoc Biol. 2005;77(4):444. doi: 10.1189/jlb.0604367. [DOI] [PubMed] [Google Scholar]
- 31.Aarbiou J, Tjabringa GS, Verhoosel RM, Ninaber DK, White SR, Peltenburg LT, Rabe KF, Hiemstra PS. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm Res. 2006;55(3):119. doi: 10.1007/s00011-005-0062-9. [DOI] [PubMed] [Google Scholar]
- 32.Aarbiou J, Ertmann M, van Wetering S, van Noort P, Rook D, Rabe KF, Litvinov SV, van Krieken JH, de Boer WI, Hiemstra PS. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J Leukoc Biol. 2002;72(1):167. [PubMed] [Google Scholar]
- 33.Aarbiou J, Verhoosel RM, Van Wetering S, De Boer WI, Van Krieken JH, Litvinov SV, Rabe KF, Hiemstra PS. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am J Respir Cell Mol Biol. 2004;30(2):193. doi: 10.1165/rcmb.2002-0267OC. [DOI] [PubMed] [Google Scholar]
- 34.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, Ganz T. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002;118(2):275. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 35.Levine SJ. Bronchial epithelial cell-cytokine interactions in airway inflammation. J Investig Med. 1995;43(3):241. [PubMed] [Google Scholar]
- 36.Meyer KC, Cardoni AL, Xiang Z, Cornwell RD, Love RB. Vascular endothelial growth factor in human lung transplantation. Chest. 2001;119(1):137. doi: 10.1378/chest.119.1.137. [DOI] [PubMed] [Google Scholar]
- 37.Vaschetto R, Grinstein J, Del Sorbo L, Khine AA, Voglis S, Tullis E, Slutsky AS, Zhang H. Role of human neutrophil peptides in the initial interaction between lung epithelial cells and CD4+ lymphocytes. J Leukoc Biol. 2007;81(4):1022. doi: 10.1189/jlb.0706435. [DOI] [PubMed] [Google Scholar]
- 38.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286(5441):952. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 39.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, Meyers B, Schuessler R, Trulock EP, Patterson GA, Mohanakumar T. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 40.Rizzo M, Sundaresan S, Lynch J, Trulock EP, Cooper J, Patterson GA, Mohanakumar T. Increased concentration of soluble human leukocyte antigen class I levels in the bronchoalveolar lavage of human pulmonary allografts. J Heart Lung Transplant. 1997;16(11):1135. [PubMed] [Google Scholar]
- 41.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84(6):2017. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271(6):2935. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 43.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6(8):1799. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]