Abstract
Cross-desensitization between G protein-coupled receptors (GPCRs) can play an important role in the regulation of the immune response. Recent research shows that the receptor for nociceptin/orphanin FQ (N/OFQ), designated the orphan opioid receptor-like 1 (ORL1) exerts a significant effect on adaptive immunity. We carried out experiments to determine the capacity of ORL1 to desensitize the major HIV co-receptor CXCR4. Our results show that ORL1 induced significant desensitization of CXCR4 in both CD4-positive T cells and CD14-positive monocytes, as well as the CD4-positive Jurkat T cell, and U937 monocyte-like cell lines. In addition, the cross-desensitization of CXCR4 by ORL1 did not result in detectable internalization of CXCR4 in either primary cells or the hematopoietic cell lines. Finally, results show that the heterologous-desensitization of CXCR4 was associated with reduced susceptibility to HIV-1 infection. Given the relative resistance of CXCR4 to cross-desensitization, our studies suggest that ORL1 possesses a high level of regulatory activity.
Introduction
The function of G protein-coupled receptors (GPCRs) can be regulated on several levels. Of particular interest is the process of desensitization that takes place between GPCRs, which can be the result of either homologous or heterologous desensitization. The former is a rapid event that occurs when a receptor becomes desensitized upon binding of its cognate ligand. The latter is desensitization of a receptor by a separate, unrelated receptor, and does not require agonist stimulation of the desensitized receptor. Further, this desensitization can also, but not always, result in internalization of the receptor.
Many immunologically relevant GPCRs and their ligands have been shown to participate in receptor regulation at the level of cross-desensitization (Steele et al. 2002). It is apparent that the Gi protein-linked chemoattractant receptors exhibit a hierarchy in initiating cross-desensitization, and this is inversely correlated with their susceptibility to desensitization (Steele et al. 2002). For example, there is a hierarchy in the cross-desensitization between GPCRs, where the susceptibility to cross-desensitization varies greatly (Grimm et al 1998; Szabo et al. 2003). Moreover, based on studies with a number of GPCRs, it appears that CXCR4 is relatively resistant as a target for cross-desensitization (Steele et al. 2002).
The Opioid Receptor-Like 1 (ORL1) is expressed abundantly in both the CNS and among cells of the immune system (Peluso et al. 1998). Moreover, a number of laboratories have shown that ORL1 can modulate inflammatory responses, and both innate and adaptive immune responses (Finley et al. 2008; Anton et al. 2010). Because ORL1 appears to be highly expressed by a number of leukocyte populations, it has been suggested that this receptor may potentially be more universally immunomodulatory than the opioids (Finley et al. 2008).
Because ORL1 is expressed by both primary T cells and T cell lines, we wished to determine whether ORL1 might exert a regulatory influence on the function of the chemokine receptor CXCR4. While this receptor is expressed on a wide variety of cell types, it serves as the major HIV co-receptor for T cell tropic HIV-1 strains. Our studies reported here show that ORL1 exhibits the ability to cross-desensitize CXCR4 in both primary leukocytes and hematopoetic cell lines. These results are consistent with studies in a number of experimental systems which suggests that ORL1 can exert substantial immunoregulatory activity.
Materials and methods
Drugs
Both N/OFQ and the ORL-1 antagonist UFP-101 were obtained from Tocris Bioscience (Ellisville, MO).
Cells
U937 cells and Jurkat T cells were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated low-endotoxin fetal calf serum. Freshly isolated CD-14 positive monocytes and CD4-positive T cells were obtained from whole blood of normal HIV-negative donors as previously described using magnetic bead purification (Kaminsky and Rogers 2008), and a protocol and informed consent approved by the Temple University Institutional Review Board.
Chemotaxis
Analysis of chemotactic activity was carried out as described previously (Szabo et al. 2003) using a 48-well micro chemotaxis chamber, and a semi-permeable polycarbonate PVPF membrane (5 μm pore for U937 cells, monocytes, primary T cells; 3 μm pore for Jurkat T cells). Loaded chambers were incubated at 37°C for 60 min (Jurkat T cells and monocytes) or 90 min (U937 cells and primary T cells). The membranes were fixed and stained for 5 min in each of 3 solutions of the Hema 3 Protocol Fix and Stain Solutions (Fisher Diagnostics, Pittsburgh, PA). The responses were quantified by counting 4 areas of each well under 40× magnification. The 4 counted areas were totaled for each well, averaged across the replicates, and expressed as cells per high powered field (HPF)..
Flow Cytometry
Flow cytometric analysis for the expression of CD4 (Clone S3.5; Invitrogen) and CXCR4 (Clone 12G5; Becton-Dickinson) was carried out as described previously (Szabo et al. 2003) using Q-Dot 605- and PE-conjugated antibodies, respectively.
HIV Susceptibility
The susceptibility to infection with HIV-1 X4 strain MN was carried out by measuring the transcription of the HIV-1 5′ strong-stop (ssHIV) quantitative PCR according to a modification of a method described previously (Szabo et al. 2003; Steele et al. 2003). The ssHIV method allows for a determination of a very early event post-infection and gives a measure of co-receptor function. DNA was isolated from infected cells, and a buffer was created using Roche FastStart DNA Master Hybridization Probe Buffer (Roche Applied Science, Indianapolis, IN), supplemented with 3.5 mM MgCl2, 0.5 μM each of sense and antisense primers (5′-CTTCCCTGATTGGCAGAACT-3′, and 5′-AGCACCATCCAAAGGTCAGT-3′, respectively), 0.2 μM of each hydrolysis probe (Probe 1: 5′-AGTGTGTGCCCGTCTGTTATGTGA[Fluo]-3′; Probe 2: [Red640]CTGGTAGCTAGAGATCCCTCAGATCCTTT[Phosphate]), and 500 ng of DNA. Reaction conditions were: amplification at 95° for 10 min, 35 amplification cycles of denaturation at 95° C for 10s, primer annealing at 55° C for 10s, extension at 72° C for 7s, followed by a cooling step at 40° C for 30 s. The Roche hybridization probe for β-globin was employed as a standard for quantification of the ssHIV product. Real-time PCR was also performed on β-actin in order to normalize the ssHIV products within each sample as described above using actin primers (sense: 5′-CGGGAAATCGTGCGTGACAT-3′; antisense: 5′-GAACTTTGGGGGATGCTCGC-3′), under the same amplifications conditions as described above except for primer annealing at 60° C for 3 s. Melt curve analysis was carried out to assess the homogeneity of each amplicon. The concentrations of ssHIV and actin were determined using LightCycler 4.05 software for each sample. The ssHIV data were normalized to β-actin within each sample, and normalized to the control (no treatment) samples and expressed as mean (± SEM).
Calcium flux
Jurkat cells were incubated 5 μM Fura-2 AM (Invitrogen, Molecular Probes, Eugene, OR) for 30 min at room temperature. Calcium flux was measured with a Fluomax-3 fluorescent spectrophotometer (Horiba Jobin Yvon, Inc.). The ratio of fluorescence at 340 and 380nm was calculated using the DataMax 2.20 software (Horiba Jobin Yvon, Inc.). Cells were first incubated with 10 nM N/OFQ for 60 min before addition of CXCL12. Data are representative of results from 4 independent experiments.
Statistical analysis
Statistical analysis of the difference between groups was assessed using either the Student’s t-test or analysis of variance (ANOVA) followed by Tukey’s test. P<0.05 was taken as the significant level of difference.
Results
ORL1 mediates cross-talk with CXCR4 in human T cells and monocytes
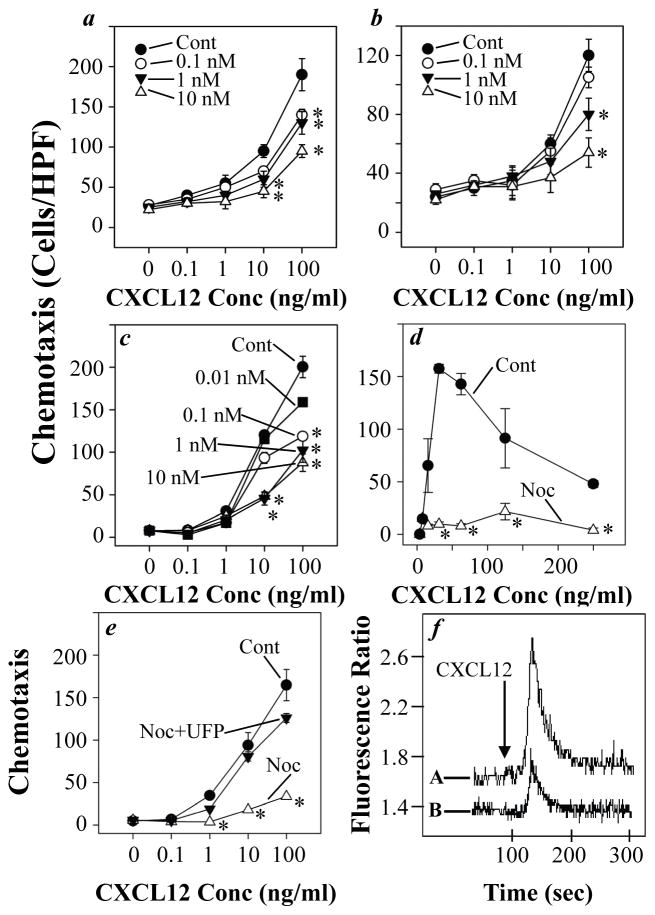
In an effort to determine the capacity of ORL1 to cross-talk with CXCR4, we carried out experiments to examine the effect of N/OFQ on the chemotactic response of CD4-positive T cells and CD14-positive monocytes to CXCL12. The results (Fig. 1a & b) show that the response of primary peripheral leukocytes through CXCR4 is inhibited in a dose-dependent manner following ORL1 activation. The inhibition of the CD4-positive T cell response to CXCL12 was significantly inhibited by pre-treatment with as little as 0.1 nM N/OFQ, while the response of CD14-positive monocytes was apparent with as little as 1 nM N/OFQ.
Fig. 1.
Pre-treatment with N/OFQ inhibits CXCR4-mediated chemotactic responses. Human CD4-positive T cells (a), CD14-positive monocytes (b), Jurkat T cells (c and e), and U937 monocytes (d) were pre-treated with the designated doses of N/OFQ for 60 min, and the chemotactic responses to CXCL12 were determined as described in Materials and methods. Jurkat T cells were also pre-treated with the ORL1 antagonist UFP-101 (100 nM) prior to the treatment with 10 nM N/OFQ (e), and the response to CXCL12 was determined in the same manner. Results are expressed as the number of migrated cells per high-powered field (± SEM) of triplicate samples, and are representative of at least three independent experiments. *, P < 0.05. Jurkat cells were pre-treated with PBS (A) or 10 nM N/OFQ (B), and the calcium response to 50 ng/ml CXCL12 was determined after 60 min (f).
In an effort to extend these findings, we also carried out these studies using the T cell and monocyte-like cell lines, Jurkat and U937, since these cells are more homogeneous. The results from these experiments (Fig. 1c & d) showed that both Jurkat T cells and U937 monocytes failed to exhibit normal CXCR4 activity following pre-treatment with N/OFQ. The response of Jurkat cells to CXCL12 was significantly inhibited following pretreatment with as little as 0.1 nM N/OFQ, and the inhibitory activity of N/OFQ was dose-dependent. Finally, experiments were conducted in which Jurkat T cells were pre-treated with the ORL1 antagonist UFP-101, and then subjected to the analysis of N/OFQ inhibitory activity. The results from this study (Fig. 1e) show that ORL1 antagonist pre-treatment substantially blocked the ability of N/OFQ to inhibit CXCR4 functional activity. Finally, we also tested the effect of N/OFQ treatment on the capacity of Jurkat cells to exhibit a calcium mobilization response to CXCL12. The results (Fig. 1f) show that pretreatment with N/OFQ results in a reduction in the calcium flux response to CXCL12.
The ORL1-induced cross-desensitization of CXCR4 does not require target receptor internalization
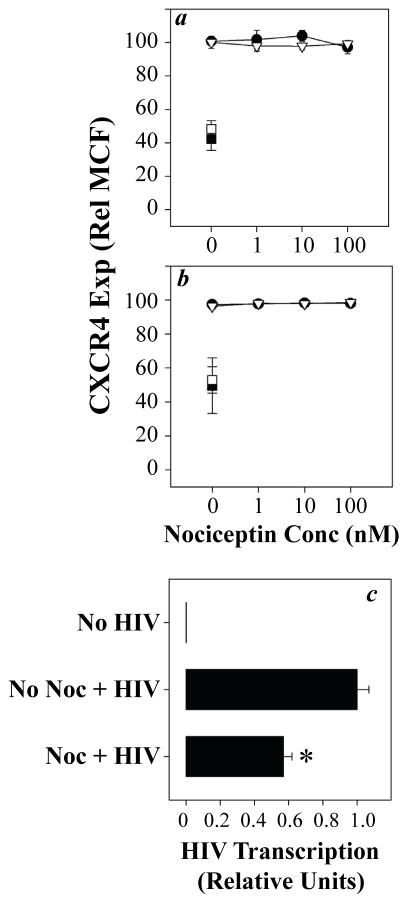
The cross-talk between GPCRs may or may not involve target receptor internalization, and internalization can contribute to the failure of the target receptor to manifest functional activity (Steele et al. 2002). Experiments to determine the effect of N/OFQ pre-treatment on the surface expression of CXCR4 showed (Fig. 2a & b) that N/OFQ pre-treatment failed to alter the expression of CXCR4, and this was apparent at doses of N/OFQ as high as 100 nM. This was in contrast to treatment with CXCL12, which induced a reduction in CXCR4 expression of approximately 55%. These results show that homologous desensitization of CXCR4 with CXCL12 effectively reduces receptor internalization, while cross-desensitization through ORL1 failed to induce detectable expression. These results demonstrate that CXCR4 remains in the outer membrane following the ORL1 activation, but CXCR4 loses functional activity.
Fig. 2.
Pre-treatment with N/OFQ fails to induce internalization of CXCR4, while reducing susceptibility to infection with X4 HIV-1. Human CD4-positive T cells (a, closed symbols), CD14-positive monocytes (a, open symbols), Jurkat T cells (b, closed symbols), and U937 monocytes (b, open symbols) were pre-treated with N/OFQ at the designated concentrations, and the expression of CXCR4 was determined after 60 min by flow cytometry. In each case, cells were also pre-treated with CXCL12 (100 ng/ml) as a positive control for homologous desensitization (square symbols). Results are expressed as a percentage of the CXCR4 expression in control cultures (no pre-treatment with N/OFQ or CXCL12) of triplicate cultures (± SEM), and are representative of 3–5 independent experiments. Assessment of HIV-1 susceptibility was carried out by treating T cells with 1 nM N/OFQ for 60 min, followed by infection with HIV-1 strain MN (MOI 0.1) for 2 hr (c). DNA was isolated from the cells at 2 hr and the reverse transcription of HIV strong-stop cDNA was determined by real time PCR. Results are expressed as the mean (± SEM) of triplicate cultures, and are representative of experiments with 5 independent donors. *, P < 0.05.
Based on the inhibition of CXCR4 functional activity, we wished to determine whether the ORL1-induced cross-talk with CXCR4 might be associated with an alteration in HIV-1 co-receptor activity. Experiments in which we pre-treated CD4-positive T cells with N/OFQ, and then subjected these cells to infection with the X4 HIV-1 strain MN, showed (Fig. 2c) that N/OFQ pre-treatment induced a significant inhibition of HIV-1 LTR strong-stop expression by approximately 45%. The magnitude of the inhibition was consistent with the inhibition of the chemotactic activity of CXCR4 observed for CD4-positive primary T cells (Fig. 1a). Finally, additional studies have shown that the treatment with N/OFQ does not result in a change in expression of CD4 (data not shown).
Discussion
Several laboratories have shown that leukocytes express ORL1 (Finley et al. 2008), and leukocytes are a source of N/OFQ (Miller and Fulford 2007). Moreover, N/OFQ is stored in neutrophil granules, and this mediator is released upon degranulation of the neutrophils (Fiset et al. 2003), suggesting that N/OFQ is likely to be upregulated at sites of inflammation. Indeed, there is evidence that N/OFQ can directly modulate components of the inflammatory response (Goldfarb et al. 2006; Kaminsky and Rogers 2008).
The cross-desensitization of CXCR4 by ORL1 would be expected to occur in conditions where an elevation in the levels of N/OFQ occur, and CXCR4 expression by inflammatory cells is upregulated in certain inflammatory states (Buckley et al. 2000). The process of ORL-1 mediated cross-desensitization is likely to contribute to inflammatory cell “traffic-control” at sites of inflammation. The migration of inflammatory cells is dependent on the ability to decipher a complex mixture of chemotactic signals and “prioritize” among the chemoattractants and ignore undesirable chemoattractants. Several mechanisms have been identified which account for this selective cellular guidance, including heterologous desensitization (Steele et al. 2002). It is apparent that chemoattractant receptors exhibit a hierarchy in which there are strong, intermediate, and weak receptors for initiating cross-desensitization, and this is inversely correlated with their susceptibility to desensitization. For example, the formyl peptide receptor (FPR) is a strong desensitizer, and FPR is relatively resistant as a target for cross-desensitization (Shen et al. 2000). Previous studies suggest that CXCR4 is a strong desensitizer, and is relatively resistant to cross-desensitization (Steele et al. 2002). However, while CXCR4 is relatively resistant, it can be successfully cross-desensitized by certain CCR5 agonists (Hecht et al. 2003). Given the relative resistance of CXCR4 to cross-talk, it was somewhat surprising that ORL1 would successfully desensitize this chemokine receptor. However, previous studies have shown that ORL1 mediates cross-desensitization of the μ-opioid receptor (Mandyam et al. 2002). It is likely that the failure of ORL1 to more completely cross-desensitize CXCR4 may reflect the apparent resistance of this receptor to this cross-talk.
Finally, an additional consequence of the ORL1-mediated inhibition of CXCR4 is apparent from the results showing that HIV-1 coreceptor function is inhibited. This result was not entirely unexpected, given the previous findings which show that cross-desensitization of either CXCR4 or CCR5 is associated with a reduction in coreceptor activity (Steele et al. 2002). For example, the cross-desensitization of CXCR4 by FPR is associated with significantly reduced CXCR4 co-receptor function (Shen et al. 2000). The biochemical basis for the reduced co-receptor function associated with cross-desensitization remains uncertain.
Acknowledgments
Support: The authors wish to acknowledge the support from the National Institutes of Health for the following grant support: DA14230 (TJR), DA16544 (TJR), PO1DA23828 (TJR), P30DA13429 (TJR), DA06650 (TJR), T32DA07237 (DEK)
We would like to acknowledge the expert assistance of the Temple University Flow Cytometry Facility.
Footnotes
Conflict of Interest Disclosure: The authors have no known conflicts of interest concerning the results reported in this manuscript.
Contributor Information
David E. Kaminsky, Department of Microbiology and Immunology, Temple University School of Medicine, 3400 N. Broad Street Philadelphia, PA 19140, USA
Thomas J. Rogers, Fels Institute for Cancer Research and Molecular Biology, Center for Inflammation, Translational and Clinical Lung Research, Temple University School of Medicine, 3307 N. Broad Street Philadelphia, PA 19140, USA
References
- Anton B, Leff P, Meissler JJ, Calva JC, Acevedo R, Salazar A, Matus M, Flores A, Martinez M, Adler MW, Gaughan JP, Eisenstein TK. Nociceptin/orphanin FQ suppresses adaptive immune responses in vivo and at picomolar levels in vitro. J Neuroimmune Pharmacol. 2010;5:143–154. doi: 10.1007/s11481-010-9190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, Amara A, Curnow SJ, Lord JM, Scheel-Toellner D, Salmon M. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423–3429. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol. 2008;252:146–154. doi: 10.1016/j.cellimm.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset ME, Gilbert C, Poubelle PE, Pouliot M. Human neutrophils as a source of nociceptin: a novel link between pain and inflammation. Biochem. 2003;42:10498–10505. doi: 10.1021/bi0300635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb Y, Reinscheid RK, Kusnecov AW. Orphanin FQ/nociceptin interactions with the immune system in vivo: gene expression changes in lymphoid organs and regulation of the cytokine response to staphylococcal enterotoxin A. J Neuroimmunol. 2006;176:76–85. doi: 10.1016/j.jneuroim.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J Exp Med. 1999;188:317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht I, Cahalon L, Hershkoviz R, Lahat A, Franitza S, Lider O. Heterologous desensitization of T cell functions by CCR5 and CXCR4 ligands: inhibition of cellular signaling, adhesion and chemotaxis. International Immunol. 2003;15:29–38. doi: 10.1093/intimm/dxg002. [DOI] [PubMed] [Google Scholar]
- Kaminsky DE, Rogers TJ. Suppression of CCL2/MCP-1 and CCL5/RANTES expression by nociceptin in human monocytes. J Neuroimm Pharmacol. 2008;3:75–82. doi: 10.1007/s11481-007-9086-y. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Thakker DR, Christensen JL, Standifer KM. Orphanin FQ/nociceptin-mediated desensitization of opioid receptor-like 1 receptor and mu opioid receptors involves protein kinase C: a molecular mechanism for heterologous cross-talk. J Pharmacol Exp Ther. 2002;302:502–509. doi: 10.1124/jpet.102.033159. [DOI] [PubMed] [Google Scholar]
- Miller TR, Fulford AJ. Regulation of nociceptin/orphaninFQ secretion by immune cells and functional modulation of interleukin-2. Peptides. 2007;28:2243–2252. doi: 10.1016/j.peptides.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gaveriaux-Ruff C. Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol. 1998;81:184–192. doi: 10.1016/s0165-5728(97)00178-1. [DOI] [PubMed] [Google Scholar]
- Shen W, Li B, Wetzel MA, Rogers TJ, Henderson EE, Su SB, Gong W, Le Y, Sargeant R, Dimitrov DS, Oppenheim JJ, Wang JM. Down-regulation of the chemokine receptor CCR5 by activation of chemotactic formyl peptide receptor in human monocytes. Blood. 2000;96:2887–2894. [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Steele AD, Szabo I, Bednar F, Rogers TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine & Growth Factor Reviews. 2002;13:209–222. doi: 10.1016/s1359-6101(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, Oppenheim JJ, Rogers TJ. Selective inactivation of CCR5 and decreased infectivity of R5 hiv-1 strains mediated by opioid-induced heterologous desensitization. J Leuk Biol. 2003;74:1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]