Introduction
Elbow and forearm wounds range from those caused by congenital anomalies, contracture release, tumor excision, and burns, to autoimmune disease, trauma, infection, and exposed prostheses (1). Regardless of the cause, wounds about the elbow and forearm have potentially disabling effects (2), influencing both the ability to work and quality of life. An appreciation for the etiology and socioeconomic implications of upper extremity wounds is imperative for a successful outcome.
Elbow and forearm wounds require a stable and durable solution. Important differences remain, however, regarding the reconstructive demands intrinsic to each anatomic area. Forearm wounds can often be closed directly or covered with skin grafts. Only large wounds or those with exposed neurovascular structures, tendon, or bone typically require flap closure. Conversely, elbow wounds have limited simple solutions for coverage as underlying structures and prostheses are easily exposed (3). Elbow wounds also have the unique requirement of a pliable, yet well-padded reconstruction. These characteristics promote early mobilization to prevent contracture and stiffness (4). As local tissues are frequently involved in the zone of injury or lack characteristics necessary to achieve an optimal outcome, the surgeon often must choose from the myriad of available flap options.
Principles of Reconstruction
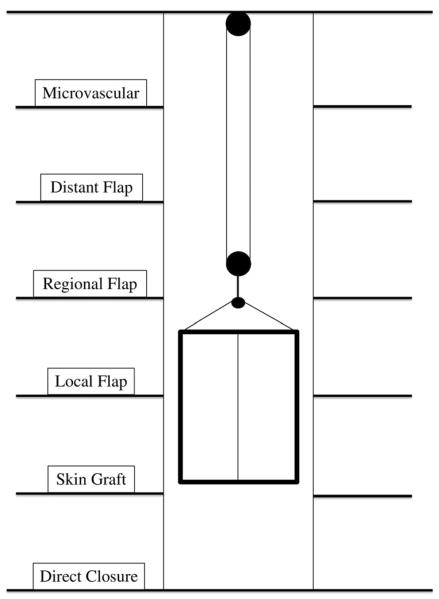
The reconstructive ladder (5) is an important concept in wound management. Large, complex wounds of the forearm and many elbow wounds may require initial management with local, regional, distant, or free flap coverage (reconstructive elevator) (Figure 1) (1, 6). Wound characteristics including etiology, size, and orientation, as well as exposure of or injury to bone and neurovascular structures are important. Patient comorbidities, donor site availability, and clinical status also directly impact the options for flap closure. Previous trauma and surgery to the affected extremity are particularly relevant when considering local and regional flap coverage. Careful identification of the ulnar nerve should be performed during any soft tissue reconstruction of the posterior or medial elbow. As both debridement and flap elevation in this unique area puts the nerve at risk, the surgeon should consider concomitant ulnar nerve transposition (7).
Figure 1.
Reconstructive Elevator Adapted from Bennett N, Choudhary S. Why climb a ladder when you can take the elevator? Plast Reconstr Surg. 2000 May;105(6):2266; with permission.
Timing of definitive reconstruction can affect the ultimate outcome. Reconstruction must be delayed until contamination has been cleared, but should be early enough to facilitate therapy and maintenance of range of motion. Early soft tissue coverage also decreases edema and pain (8). Lister and Scheker recommend immediate coverage (9), although this approach mandates aggressive debridement of all potentially non-viable tissue. Common indications for flap coverage of the elbow and forearm are listed in Box 1.
Fasciocutaneous flaps
The versatility and availability of axial fasciocutaneous flaps have made them a popular option for reconstructing wounds of the upper extremity. These flaps provide adequate soft tissue coverage, good contour, multiple possible orientations for flap inset, and a potential gliding surface for tendons (10, 11, 12, 13). In addition, fasciocutaneous flaps leave virtually no functional deficit (12, 13) and secondary contouring is technically straightforward (14).
Clinical Scenarios
1) Radial forearm pedicle flap
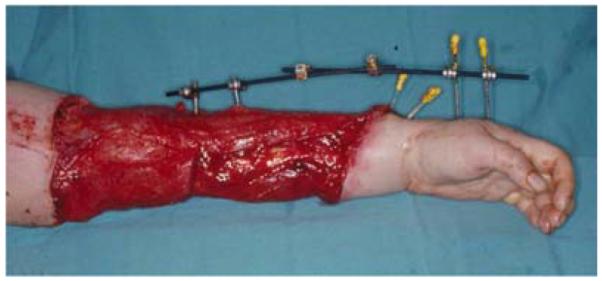
A 19-year-old right-hand dominant male presented to the emergency department after falling 15 feet from a ladder, landing directly on his left elbow. A left supracondylar humerus and distal radius fracture were noted as well as the inability to extend the wrist and fingers. He underwent open reduction and internal fixation (ORIF) of the humerus and distal radius fractures and a completely transected radial nerve was subsequently reconstructed with interposition nerve grafts. Provisional wrist extension was achieved with a simultaneous pronator teres to extensor carpi radialis brevis tendon transfer. Two months post-operatively, the humerus hardware became exposed, requiring washout, wound debridement, and hardware removal. This resulted in a large, complex, elbow wound (Figure 2a). The wound was successfully closed with a proximally-based radial forearm flap (Figures 2b, 2c, 2d).
Figure 2.
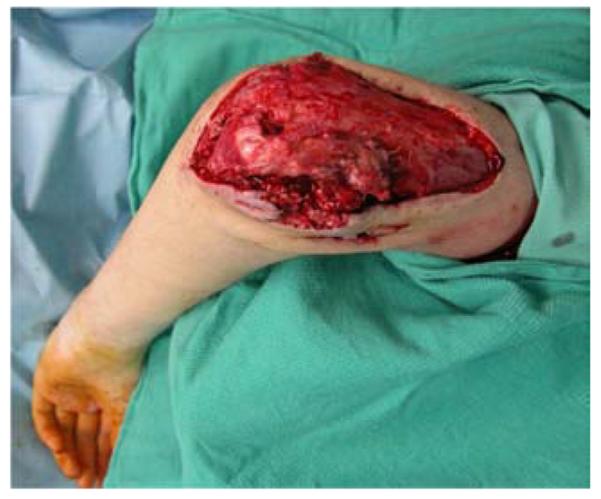
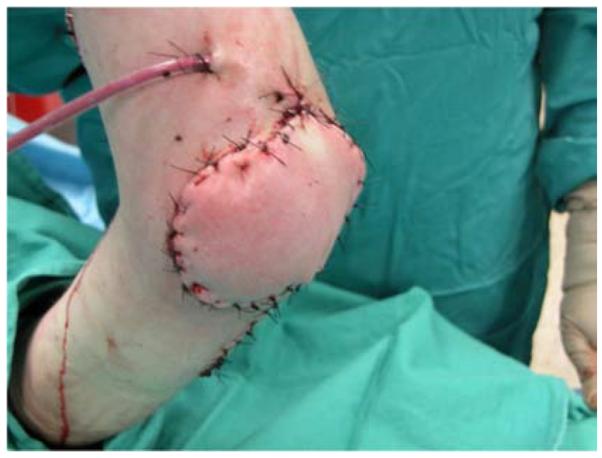
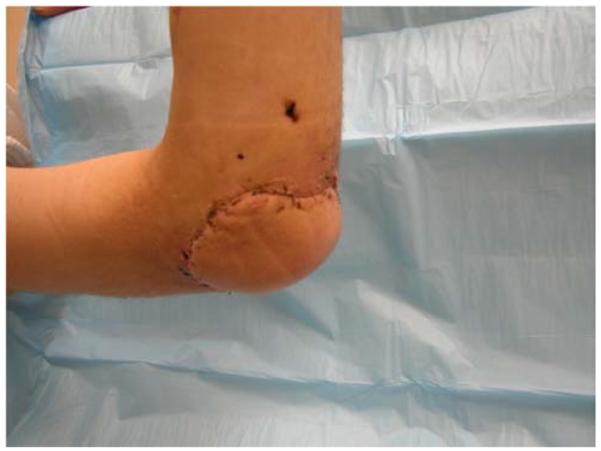
Radial Forearm Pedicle Flap (A) elbow wound after debridement (B) proximally-based radial forearm flap (C) flap inset into wound. (D) post-operative result
Discussion
The radial forearm flap was first described in 1978 by Yang et al. (15) and introduced to the West by Song et al. as the “Chinese Flap” in 1982 (16). This flap is considered to be one of the most reliable and versatile flaps for both pedicle and free flap reconstruction (17). Owing to its long pedicle, pliable soft tissue (18), and ease of dissection, the radial forearm flap is a primary option in upper extremity reconstruction. The radial forearm can be harvested as either a fasciocutaneous or fascia-only flap and can be used for small- and medium-sized defects of the dorsal elbow, antecubital fossa, and in selected proximal forearm wounds. Alternative flaps should be strongly considered, however, in patients with traumatic injury to the volar forearm or an incomplete superficial palmar arch as noted by a pre-operative Allen's test.
The axis of the flap is from the central antecubital fossa to the radial styloid (8). The proximally based flap used in elbow reconstruction relies on the distal cluster of clinically relevant perforators arising from the radial artery (19). After skin paddle design, a paratenon-sparing dissection proceeds towards the interval between the flexor carpi radialis (FCR) and the brachioradialis (BR) tendons. Prior to distal radial artery ligation, it is prudent to confirm flow through the superficial palmar arch via the ulnar artery (20). Donor site closure frequently requires skin grafting, particularly if the flap is larger than 4cm (21). The muscle of the flexor digitorum superficialis can be used to cover exposed FCR tendon if paratenon is insufficient to support a skin graft (1). Alternatively, a supra-fascial dissection can be performed. This modification preserves the forearm fascia and leaves a well-vascularized barrier of protection over the underlying tendons (22).
In this case, a large and complex wound of the posterior elbow resulted from debridement and hardware removal. The radial forearm flap is an excellent option to close large wounds in this area. As a fasciocutaneous flap, it allows for early range of motion with thin, pliable coverage. It does, however, result in the division of a major artery to the hand and the comorbidity of a skin-grafted donor site. The reverse lateral arm flap may have been considered, though the posterior radial collateral artery was likely compromised by the humerus fracture and fixation. Further, the extent of previous dissection and undermining likely resulted in compromise of the main pedicle, the radial recurrent artery. Free tissue transfer is a reasonable option, but regional fasciocutaneous flaps are preferred when they can provide an adequate closure while also avoiding the use of microsurgical techniques.
2) Reverse lateral arm pedicle flap
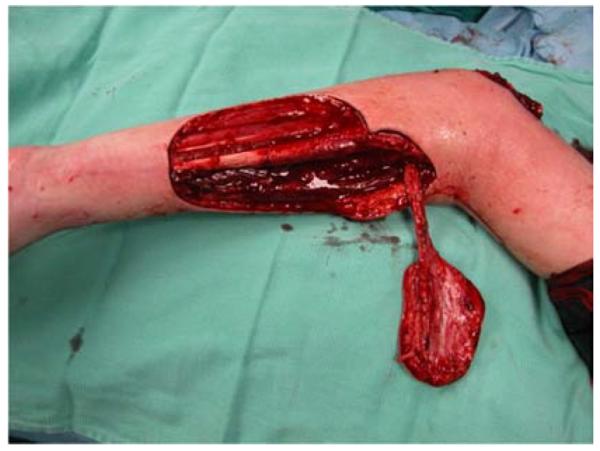
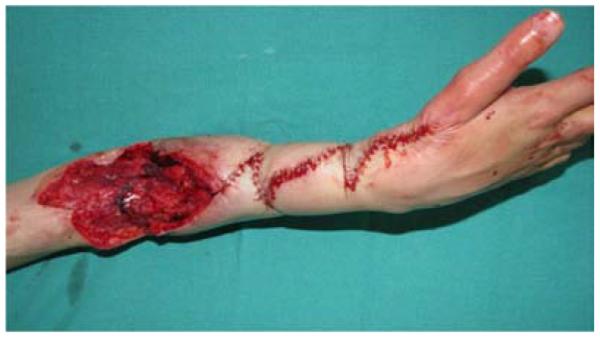
A 65-year-old right-hand dominant female presented to the clinic with a 6-month history of a draining bursa on the right posterior elbow (Figure 3a). Her medical history was notable for rheumatoid arthritis treated with methotrexate and prednisone. A computed tomography scan of the elbow indicated no osteomyelitis. Despite treatment with antibiotics and hyperbaric oxygen, the wound did not improve. At an outside hospital, the wound was debrided and closed primarily. The patient noted wound separation with minimal trauma and requested a second opinion for wound closure. The wound was subsequently debrided and closed with a reverse lateral arm flap (Figures 3b, 3c, 3d).
Figure 3.
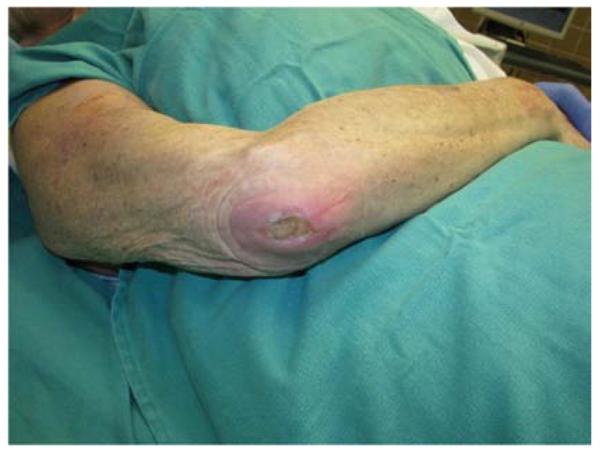
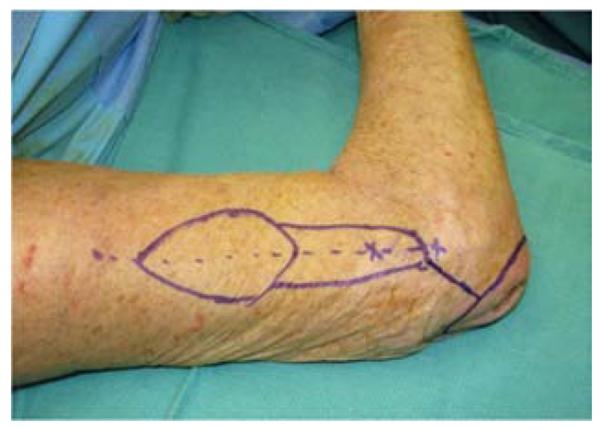
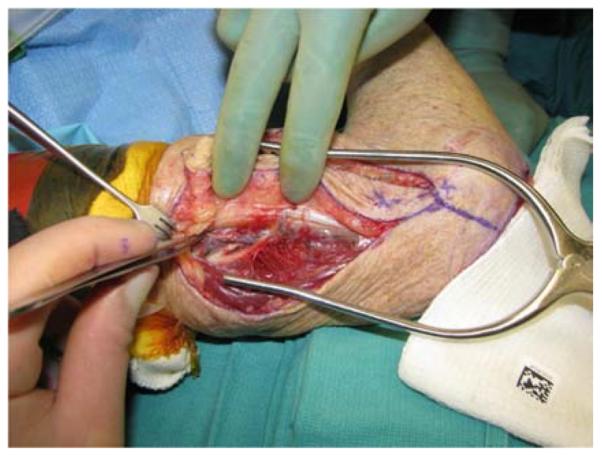
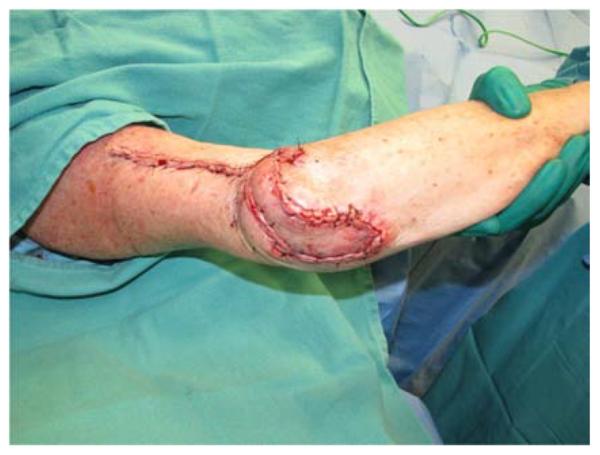
Reverse Lateral Arm Pedicle Flap (A) pre-operative image of elbow wound (B) flap markings (C) flap dissection (D) flap inset into wound.
Discussion
The lateral arm flap was described by Song et al. in 1982 (23). Though initially utilized as a free flap for head and neck reconstruction, the reverse pedicle modification has been successfully applied in soft tissue reconstruction of the elbow (8). The lateral arm flap is supplied by the posterior radial collateral artery and has collateral flow through the radial recurrent artery in the proximal forearm (24). The radial recurrent vessels provide inflow to the reverse lateral arm flap, with a lesser contribution from a recurrent branch of the posterior interosseous artery (1). This flap may be contraindicated in the presence of trauma to the humerus or in obese patients (25).
Flaps up to 8cm × 15cm have been described, though primary skin closure can be difficult if width exceeds 6cm (26). Elevation of the reverse lateral arm flap begins by marking the longitudinal axis between the deltoid and the lateral epicondyle (27). Centering the flap on this line ensures that the vessels running in the lateral intermuscular septum are captured during elevation. The flap is first dissected posteriorly, then anteriorly along the septum proceeding towards the humerus with careful preservation of the radial nerve. Further dissection elevates the flap from the humerus in a proximal to distal direction after ligation of the proximal posterior radial collateral artery if used as a reverse pedicle flap for elbow coverage.
The reverse lateral arm flap is an excellent option for addressing small- to medium-sized posterior or lateral elbow wounds. It avoids the sacrifice of a major artery (see radial forearm flap) and provides adequate soft tissue for coverage of bone and neurovascular structures. Muscle flaps are not optimal in this location as they can adhere to underlying structures. Of the available options, the reverse lateral arm flap remains the simplest and also avoids the use of microsurgical free tissue transfer.
3) Free anterolateral thigh free flap
A 53-year-old left-hand dominant male presented to the emergency department after sustaining a crush injury to his right forearm and wrist while clearing an auger used to dispense salt from a truck. Exam revealed an open dislocation of his distal radioulnar and radiocarpal joints, a distal radius fracture, and extensive soft tissue injuries to his forearm. He was taken to the operating room for initial debridement and external fixation of distal radioulnar and radiocarpal dislocations. After further debridement (Figure 4a), ORIF of the distal radius fracture and free anterolateral thigh (ALT) flap coverage of the forearm wound were performed (Figures 4b, 4c, 4d).
Figure 4.
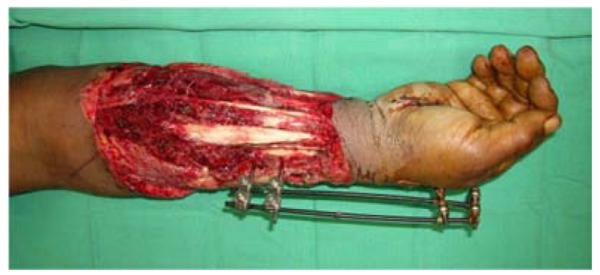
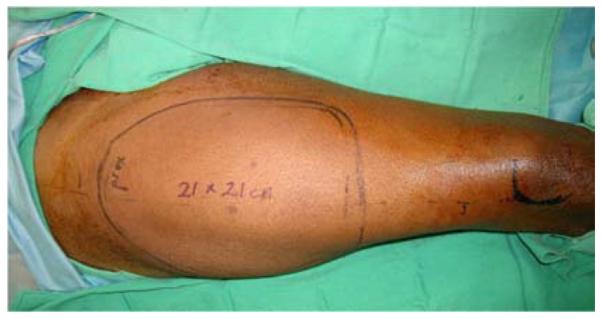
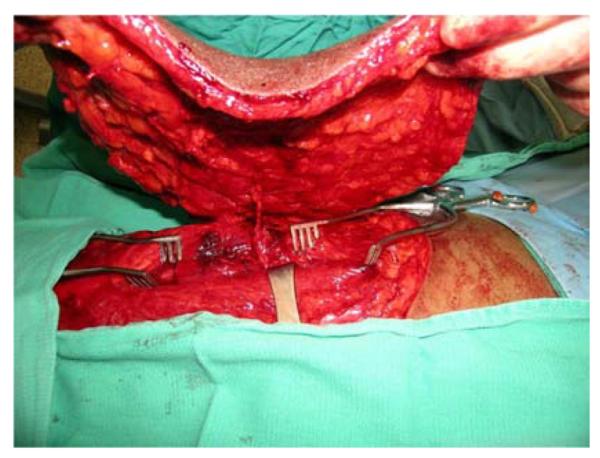
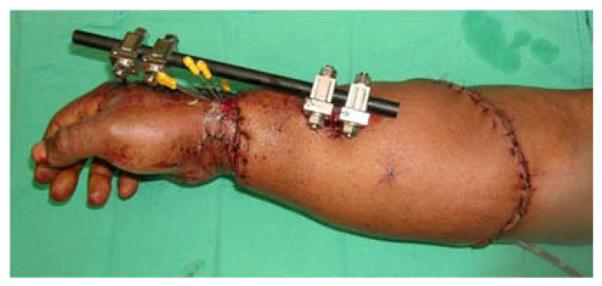
Anterolateral Thigh Free Flap (A) extensive forearm and hand wound after debridement (B) flap markings (C) flap elevation prior to transfer (D) flap inset into wound.
Discussion
The ALT free flap was first reported by Song et al. in 1984 (28). Since that time, it has seen broad application in extremity, trunk, abdomen, as well as head and neck reconstruction (29). The ALT flap is most frequently supplied by musculocutaneous perforators (80%) arising from the lateral circumflex femoral artery (30, 31). Variable pedicle anatomy (32), however, can make dissection difficult. Recent studies have shown that the anteromedial thigh flap may be a useful alternative in patients with inadequate ALT flap perforators discovered intra-operatively (33).
Perforators typically arise within 3cm of the midpoint between the anterior superior iliac spine and the lateral patella. If the perforator is septocutaneous, dissection proceeds quickly in the interval between the rectus femoris and vastus lateralus. The dissection necessarily proceeds more slowly if the perforator is musculocutaneous. In this frequent situation, meticulous technique is required to trace the pedicle through the vastus lateralus (29, 34). The donor site can generally be closed primarily.
Massive wounds of the forearm frequently require free tissue transfer, as no local options are available to provide appropriate coverage of exposed tendons and neurovascular structures. The ALT flap offers ideal characteristics to address the wound described in this case. Muscle or myocutaneous flaps (e.g. latissimus dorsi flap) also would have achieved the goal of wound coverage. This option, however, would have compromised motion and resulted in difficulty with flap elevation for secondary procedures. Pedicle two-stage options (e.g. thoracoabdominal flaps) for extensive forearm wounds result in a large donor site, often requiring skin graft closure. This can lead to a substantial aesthetic deformity and joint stiffness from prolonged immobilization.
Muscle and Myocutaneous Flaps
Muscle flaps can offer the advantage of dead space obliteration and significant soft tissue coverage with the ability to conform to irregular contours. There is conflicting evidence regarding the advantage of muscle flaps as compared to fasciocutaneous flaps in the setting of open fractures or contamination and osteomyelitis (35, 36). Experimental evidence suggests improved osseous healing and resistance to infection with muscle flaps (35, 37, 38), although outcomes studies in humans show no statistical difference (39, 40). Further, muscle flap elevation for secondary surgery can be difficult. When harvested as a pure muscle flap, a skin graft is mandatory and results in an additional donor site. Despite these disadvantages, multiple reliable muscle or myocutaneous flap options have been used successfully for both elbow and forearm wounds.
Clinical Scenarios
1) Flexor carpi ulnaris pedicle flap
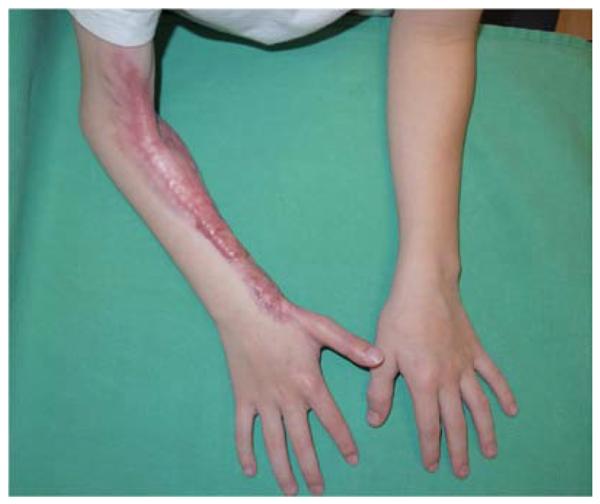
A 26-year-old male was seen in the plastic surgery office in follow-up after sustaining 50% partial- and full-thickness burns to the torso and upper extremities in a house fire. On exam, a fixed left elbow flexion deformity was noted (Figure 5a) associated with heterotopic ossification. The patient was taken to the operating room for excision of the burn scar, removal of heterotopic ossification at the medial epicondyle, ulnar nerve transposition, flexor carpi ulnaris (FCU) pedicle flap, and skin graft coverage (Figures 5b, 5c, 5d, 5e).
Figure 5.
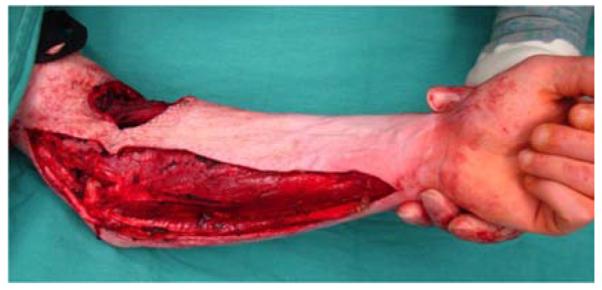
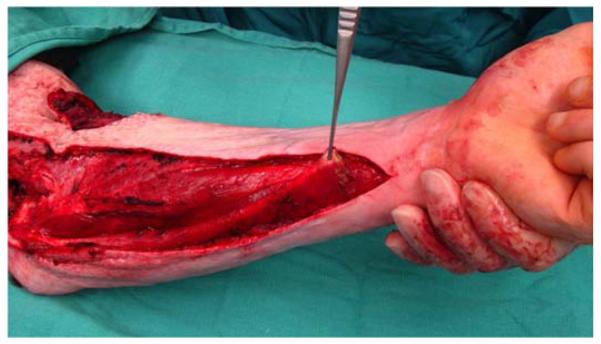
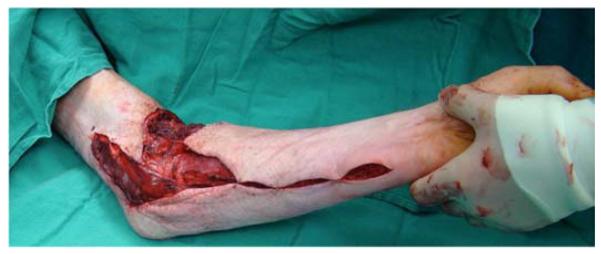
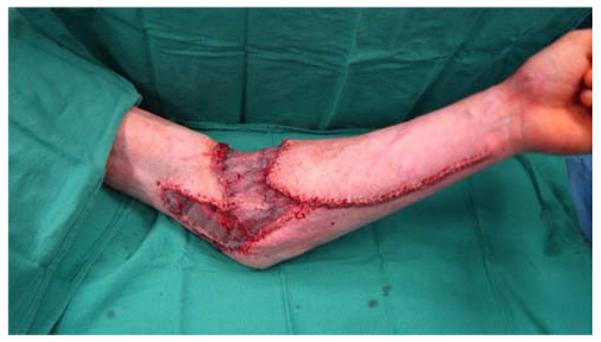
Flexor Carpi Ulnaris Pedicle Flap (A) elbow flexion contracture (B) elbow and forearm wounds after burn contracture release (C) FCU muscle insertion divided and held in forceps (D) FCU muscle reflected proximally for medial epicondyle coverage (E) flap inset and skin graft placed.
Discussion
The FCU muscle is located superficially in the forearm, making dissection straightforward. The dominant blood supply arises from the ulnar artery with a smaller contribution from the posterior ulnar recurrent artery (41). The FCU is innervated by the ulnar nerve and acts to flex and ulnarly-deviate the wrist (3). This flap is useful for small- to medium-sized defects of the elbow.
The axis of the flap is centered on a line from the medial epicondyle and the pisiform (8). With a pivot point approximately 6–8cm from the elbow flexion crease, the flap can be rapidly dissected after division at the musculotendinous junction (1). The FCU can also be split into its two heads, retaining one for wrist flexion and ulnar deviation (42). This may prove useful in patients anticipated to return to normal function after surgery.
In dealing with previously reconstructed burn wounds of the upper extremity, it is important to consider that local fasciocutaneous flaps (i.e. radial forearm, reverse lateral arm) may have been compromised during tangential excision and skin graft closure. Both the FCU and BR muscle flaps will provide efficient coverage of small- to medium-sized wounds of the antecubital fossa or posterior elbow in this situation. A pedicle or free latissimus dorsi (LD) muscle flap would have provided more soft tissue than necessary to close the wound, while also requiring more difficult intra-operative patient positioning. Free fasciocutaneous flaps (e.g. ALT) may be employed for closure but require microsurgical expertise and equipment.
2) Brachioradialis pedicle flap
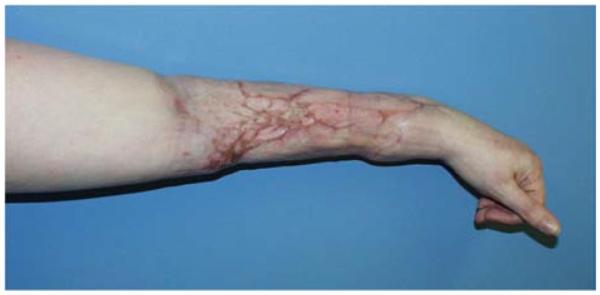
A 71-year-old right-hand dominant male was referred to the plastic surgery clinic with a non-healing wound of the left posterior elbow (Figure 6a). The elbow had been debrided 1 year prior to evaluation for an infected bursa requiring prolonged use of intravenous antibiotics. The wound had been managed with a VAC in the interim. The patient was taken to the operating room for wound excision and a pedicle left BR muscle flap closure (Figures 6b, 6c). The skin was closed directly over the muscle with a stable long-term outcome (Figure 6d).
Figure 6.
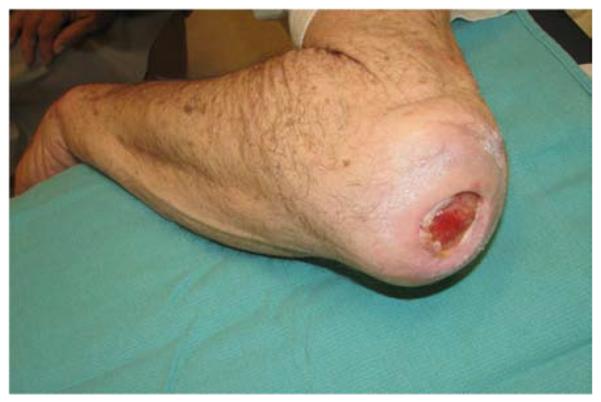
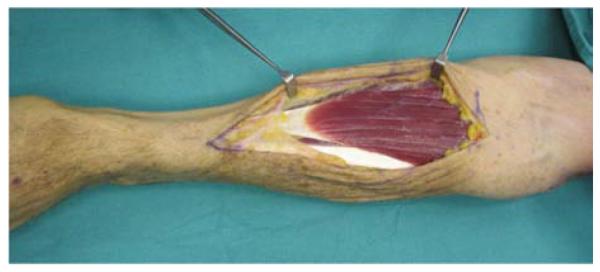
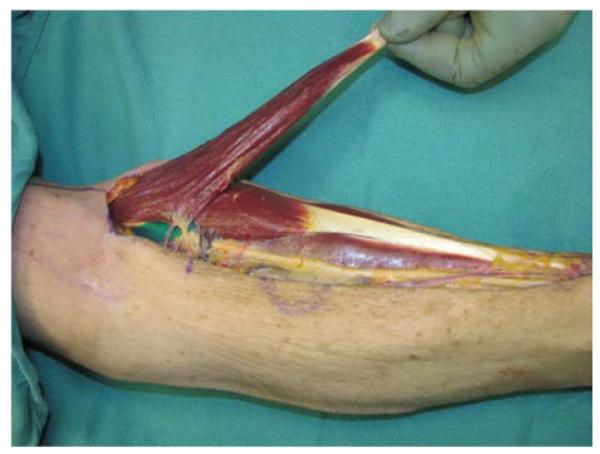
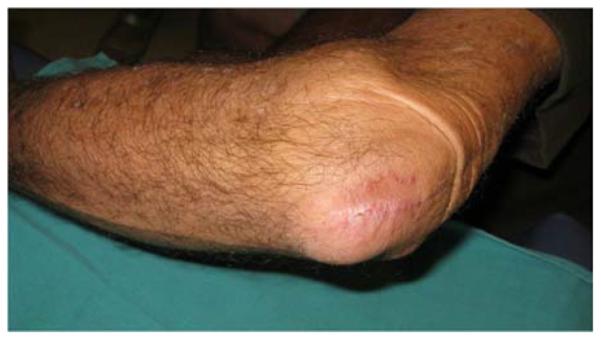
Brachioradialis Pedicle Flap (A) pre-operative image of elbow wound (B) flap dissection, sparing the dorsal radial sensory nerve (C) flap elevated (D) long-term result after primary closure over the FCU muscle flap.
Discussion
The BR is another workhorse flap for small- to medium-sized soft tissue reconstruction at the elbow and can be elevated with minimal morbidity. It is the most superficial muscle on the radial aspect of the forearm originating proximally on the humerus between the brachialis and triceps (3). At its origin, the BR is between 7–8cm wide, and tapers distally providing approximately 15cm of muscle (43). The blood supply arises from the radial artery, the radial recurrent artery, or both (44).
The muscle is exposed through a longitudinal incision over the proximal forearm. The musculotendinous junction is then divided and the muscle reflected proximally revealing the pedicle approximately 10cm distal to the origin. Extreme care is taken to preserve the dorsal sensory branch of the radial nerve (7). Muscle transposition to the wound is technically easier if the origin is elevated off of the humerus (1). The muscle flap can then be inset and covered with a split thickness skin graft, if necessary.
Multiple local and regional options are available to address wounds in this location. Because no appreciable skin deficit resulted from wound excision, a regional muscle flap was sufficient to provide durable, well-vascularized soft tissue under the skin closure. The BR is an excellent option in this situation as a result of the minimal functional deficit incurred after elevation. The FCU muscle flap is an alternative option and a reverse lateral arm flap is useful when a skin deficit exists.
3) Latissimus Dorsi free flap
A 32-year-old right-hand dominant female presented to the emergency department after a motor vehicle collision. On examination of the left upper extremity, the forearm skin was degloved from the elbow to distal forearm and the hand was well-perfused. A distal radius fracture was noted on x-ray. The patient was taken to the operating room for debridement of the wound and external fixation of a comminuted and intra-articular distal radius fracture (Figure 7a). At the time of debridement, it was discovered that the FCU and flexor digitorum profundus (FDP) tendons were avulsed at their musculotendinous junctions. As such, the extensor carpi radialis longus tendon was transferred to the FDP to maintain finger flexion. The wound was subsequently closed with an LD muscle free flap and split-thickness skin grafts (Figures 7b, 7c, 7d).
Figure 7.
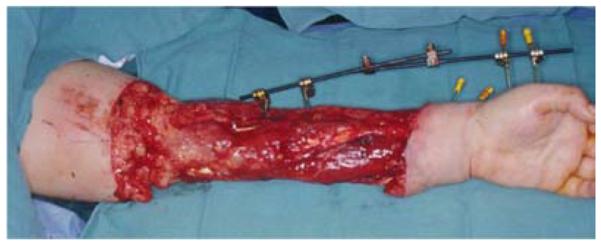
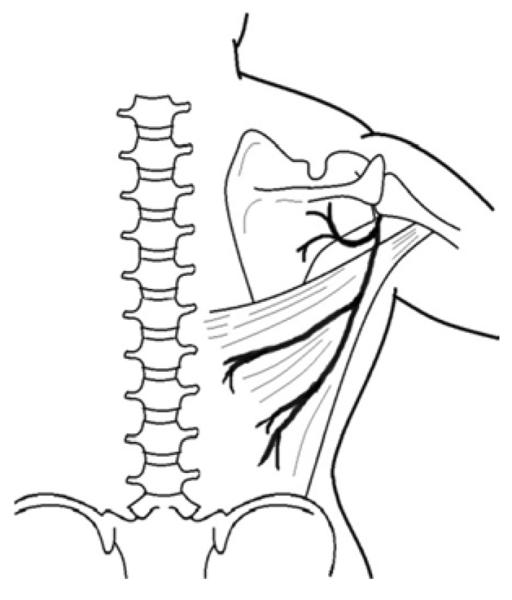
Latissimus Dorsi Free Flap (A) forearm wound after debridement (B) arterial anatomy of flap (C) free latissimus dorsi muscle flap after inset (D) long-term result.
Discussion
The LD muscle originates from the lower six thoracic vertebrae, thoracolumbar fascia, and the iliac crest, converging to insert on the bicipital groove of the humerus (45). The dominant vascular pedicle arises from the thoracodorsal artery with segmental perfusion from multiple lumbar perforators (4). With dimensions averaging 25cm × 35cm (46), this flap is a versatile, reliable, and technically straightforward option for pedicle coverage of the elbow and proximal forearm (47) or free flap coverage of massive forearm defects (48). The flap may also be used as a functional muscle transfer to restore elbow flexion or extension (49, 50). An alternative flap should be chosen in paraplegic or crutch-dependent patients as muscle harvest would lead to functional disability (3).
With the patient in the lateral decubitus position, the incision is centered between the posterior axillary fold and the midpoint of the iliac crest. The skin flaps are raised exposing the muscle that is then elevated off of the fascia and rhomboid muscles (8). If used for pedicle soft tissue coverage, the insertion is left intact to prevent excess traction on the thoracodorsal artery during transposition and inset (1). The donor site is closed primarily over a drain unless a skin paddle exceeding 10cm has been harvested with the muscle necessitating a skin graft for donor site closure (45).
In this case, a massive forearm soft tissue defect was closed with an LD muscle free flap and skin graft. This flap is useful for muscle or myocutaneous coverage of large upper extremity defects, and effectively fills dead spaces. While this achieved stable wound coverage, secondary contouring and tendon reconstruction is made simpler with fasciocutaneous flaps (e.g. ALT). Despite this, the flap size requirement of a circumferential arm wound precludes the use of any standard fascial flap. Harvest of a flap with the dimensions necessary in this case would cause considerable donor site morbidity and also extend beyond the angiosome of the fascial flap pedicle. In situations requiring coverage of circumferential wounds with large dead spaces, flat muscle flaps, such as the LD or rectus abdominis, are good options. A thoracoabdominal pedicle flap may also be a reasonable option, but would result in a substantial donor site defect requiring a skin graft. This option also mandates multiple operations, while also leading to sequelae of immobilization.
Distant two-stage pedicle flaps
Distant two-stage pedicle flaps have a role when local and regional flaps are inappropriate or when recipient vessels for free tissue transfer are in the zone of injury or absent (51). Further, distant pedicle flaps do not require microsurgical expertise and are a practical option when patient-related factors preclude the prolonged procedure times typical of microsurgical free tissue transfer (3, 52, 53, 54, 55). These flaps can provide a large amount of skin and soft tissue for coverage of the forearm (up to 22cm in length) (3). Distant flaps are used infrequently owing to the immobilization required after flap inset, the need for several secondary operations, and prolonged length of hospitalization as compared to free tissue transfer (4, 56).
Clinical Scenario
1) Intercostal artery perforator pedicle flap
A 7-year-old right-hand dominant male presented with his parents to the hand surgery clinic nine months after a right upper extremity crush injury sustained in an ATV accident. At the time of his injury, he underwent brachial artery reconstruction with a reversed saphenous vein graft at an outside hospital. His median nerve was transected, but not reconstructed, and his wounds were closed with a combination of skin grafts and secondary intention. His parents desired evaluation of an elbow flexion contracture and an insensate thumb, index, and middle finger (Figure 8a). He was taken to the operating room for excision of scar contracture and partial z-plasty closure (Figure 8b), median nerve reconstruction with sural nerve grafts, biceps tendon lengthening, and intercostal artery perforator pedicle flap closure of the antecubital wound (Figures 8c, 8d). He returned to the operating room 4 weeks later for flap division with a stable long-term outcome (Figure 8e).
Figure 8.
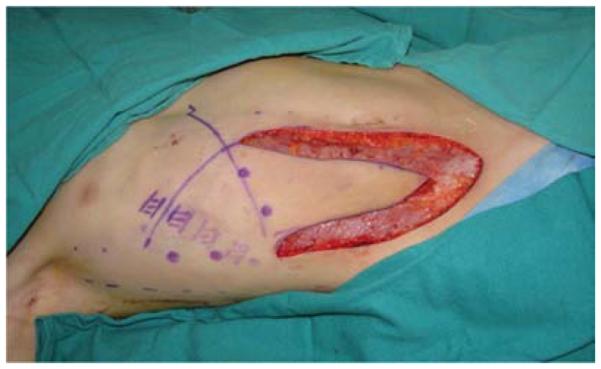
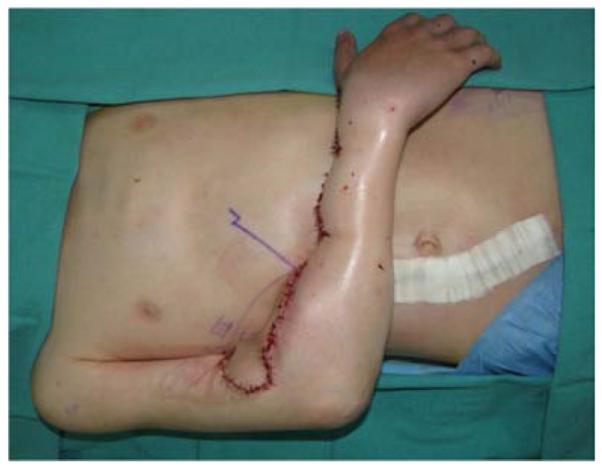
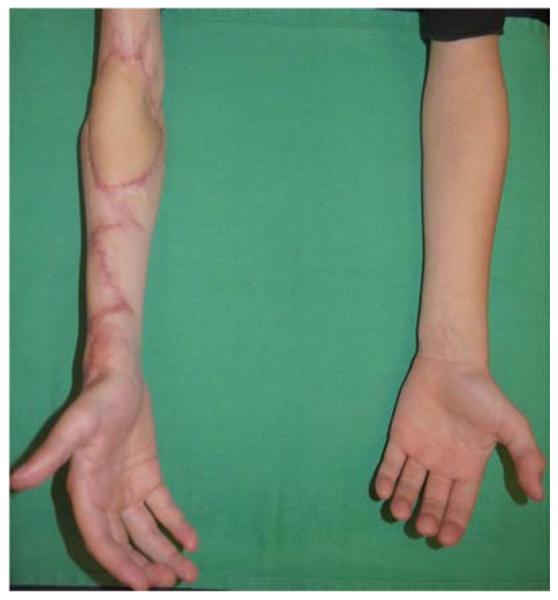
Intercostal Artery Perforator Pedicle Flap (A) elbow flexion and forearm scar contractures (B) elbow and forearm wound after debridement and adjacent z-plasty (C) flap design and elevation (D) flap inset (E) long-term result.
Discussion
Pedicle thoracoabdominal flaps were first described in the 1940's (57, 58) The vast network of perforators arising from the superior epigastric, inferior epigastric, deep circumflex iliac, intercostal, and subcostal arteries (59) provide a multitude of options for flap design and inset. Depending on size and orientation, thoracoabdominal flaps may have a random component (60), particularly at the margins of larger flaps. These flaps are indicated in larger wounds of the forearm when microsurgical options are unavailable or contraindicated.
Free fasciocutaneous flap coverage of the wound seen in Figure 8b would be an optimal choice. This was relatively contraindicated in the setting of a previous brachial artery reconstruction. No local options were available that would offer sufficient and durable coverage over the exposed vital structures of the antecubital fossa. An LD muscle flap would have provided ample soft tissue. Secondary surgery would prove difficult after this flap, as muscle flaps frequently adhere to underlying structures.
Conclusions
Wounds about the elbow and forearm present unique anatomical and functional challenges for the reconstructive surgeon. The ultimate success of reconstruction relies not only upon stable wound coverage, but also early motion of adjacent muscles, tendons, and joints. As such, management requires a thorough understanding of the advantages and limitations of local, regional, distant, and free flap options. Patient-related and surgeon-dependent variables play an important role in determining the most appropriate flap choice for a given wound. Soft tissue coverage of the elbow and forearm can be successfully performed using the techniques presented.
Synopsis.
Elbow and forearm wounds have distinct reconstructive requirements, but both require a durable and pliable solution. Pedicle and free fasciocutaneous and muscle flaps, as well as distant (two-stage) flaps have a role in reconstruction of wounds in these unique areas. We present practical surgical cases as a guide to soft tissue reconstruction of the elbow and forearm.
Key Points
Forearm wounds can generally be closed by recruitment of local tissues. Only those wounds with exposed neurovascular structures, tendon, or bone typically require flap coverage.
Elbow wounds frequently involve bone, joints, tendons, neurovascular structures, and prostheses. These factors, combined with the requirement for early motion, mandate a well-padded and durable flap reconstruction.
There are a multitude of options for reconstructing elbow and forearm wounds. It is imperative to understand the indications, advantages, and disadvantages of each flap.
Box 1 Common Indications for Elbow and Forearm Soft Tissue Reconstruction
Contracture release
Trauma with exposure of bone, joint, tendon, or neurovascular structures
Prosthetic exposure or prevention of prosthetic exposure
Infection-related wounds
Tumor extirpation-related wounds
Autoimmune disease-related wounds
Acknowledgements
Supported in part by grants from the National Institute on Aging and National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR062066) and from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (2R01 AR047328-06) and a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) (to Dr. Kevin C. Chung).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giele H. Soft tissue coverage around the elbow. In: Stanley D, Trail I, editors. Operative Elbow Surgery: Expert Consult. Churchill Livingstone; London, England: 2012. p. 719. [Google Scholar]
- 2.Sharpe F, Stevanovic M, Itamura JM. Soft tissue coverage of the elbow. In: Mirzayan R, Itamura JM, editors. Shoulder and Elbow Trauma. Thieme; New York, NY: 2004. p. 89. [Google Scholar]
- 3.Stevanovic M, Sharpe F. Soft-tissue coverage of the elbow. Plast Reconstr Surg. 2013 Sep;132(3):387e–402e. doi: 10.1097/PRS.0b013e31829ae29f. [DOI] [PubMed] [Google Scholar]
- 4.Jensen M, Moran SL. Soft tissue coverage of the elbow: a reconstructive algorithm. Orthop Clin North Am. 2008 Apr;39(2):251–64. vii. doi: 10.1016/j.ocl.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Levin LS. The reconstructive ladder. An orthoplastic approach. Orthop Clin North Am. 1993 Jul;24:393–409. [PubMed] [Google Scholar]
- 6.Bennett N, Choudhary S. Why climb a ladder when you can take the elevator? Plast Reconstr Surg. 2000 May;105(6):2266. doi: 10.1097/00006534-200005000-00062. [DOI] [PubMed] [Google Scholar]
- 7.Patel KM, Higgins JP. Posterior elbow wounds: soft tissue coverage options and techniques. Orthop Clin North Am. 2013 Jul;44(3):409–17. doi: 10.1016/j.ocl.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Moran SL, Johnson CH. Skin and Soft Tissue: Pedicled Flaps. In: Berger RA, Weiss APC, editors. Hand Surgery. Lippincott Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- 9.Lister G, Scheker L. Emergency free flaps to the upper extremity. J Hand Surg Am. 1988 Jan;13(1):22–8. doi: 10.1016/0363-5023(88)90193-1. [DOI] [PubMed] [Google Scholar]
- 10.Pribaz JJ, Chan RK. Where do perforator flaps fit in our armamentarium? Clin Plast Surg. 2010 Oct;37(4):571–9. doi: 10.1016/j.cps.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Orgill DP, Pribaz JJ, Morris DJ. Local fasciocutaneous flaps for olecranon coverage. Ann Plast Surg. 1994;32(1):27–31. doi: 10.1097/00000637-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Fassio E, Laulan J, Aboumoussa J, et al. Serratus anterior free fascial flap for dorsal hand coverage. Ann Plast Surg. 1999 Jul;43(1):77–82. doi: 10.1097/00000637-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Geddes CR, Morris SF, Neligan PC. Perforator flaps: evolution, classification, and applications. Ann Plast Surg. 2003 Jan;50(1):90–9. doi: 10.1097/00000637-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Davami B, Porkhamene G. Versatility of local fasciocutaneous flaps for coverage of soft tissue defects in upper extremity. J Hand Microsurg. 2011 Dec;3(2):58–62. doi: 10.1007/s12593-011-0040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang GF, Chen PJ, Gao YZ, et al. Forearm free skin flap transplantation. Chin Med J. 1981;61:139–141. doi: 10.1016/s0007-1226(97)91363-1. [DOI] [PubMed] [Google Scholar]
- 16.Song R, Gao Y, Song Y, et al. The forearm flap. Clin Plast Surg. 1982;9:21–26. [PubMed] [Google Scholar]
- 17.Lytle IF, Chung KC. Radial forearm flap. In: Rayan GM, Chung KC, editors. Flap Reconstruction of the Upper Extremity: A Master Skills Publication. American Society for Surgery of the Hand; Rosemont, IL: 2009. pp. 97–105. [Google Scholar]
- 18.Jones NF, Jarrahy R, Kaufman MR. Pedicled and free radial forearm flaps for reconstruction of the elbow, wrist, and hand. Plast Reconstr Surg. 2008 Mar;121(3):887–98. doi: 10.1097/01.prs.0000299924.69019.57. [DOI] [PubMed] [Google Scholar]
- 19.Saint-Cyr M, Mujadzic M, Wong C, et al. The radial artery pedicle perforator flap: vascular analysis and clinical implications. Plast Reconstr Surg. 2010;125:1469–78. doi: 10.1097/PRS.0b013e3181d511e7. [DOI] [PubMed] [Google Scholar]
- 20.Megerle K, Sauerbier M, Germann G. The evolution of the pedicled radial forearm flap. Hand (N Y) 2010 Mar;5(1):37–42. doi: 10.1007/s11552-009-9231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zenn MR, Jones Glyn. Reconstructive Surgery: Anatomy, Technique, and Clinical Applications. Quality Medical Publishing, Inc.; St. Louis, Missouri: 2012. Radial Forearm Flap; p. 855. [Google Scholar]
- 22.Lutz BS, Wei FC, Chang SC, et al. Donor site morbidity after suprafascial elevation of the radial forearm flap: a prospective study in 95 consecutive cases. Plast Reconstr Surg. 1999 Jan;103(1):132–137. doi: 10.1097/00006534-199901000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Song R, Song Y, Yu Y, et al. The upper arm free flap. Clin Plast Surg. 1982;9:27–35. [PubMed] [Google Scholar]
- 24.Prantl L, Schreml S, Schwarze H, et al. A safe and simple technique using the distal pedicled reversed upper arm flap to cover large elbow defects. J Plast Reconstr Aesthet Surg. 2008;61(5):546–51. doi: 10.1016/j.bjps.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Sauerbier M, Geissler G. Lateral Arm Flap for Hand Wrist Coverage. In: Moran SL, Cooney WP, editors. Soft Tissue Surgery: Master Techniques in Orthopedic Surgery. Lippincott Williams and Wilkins; Philadelphia, PA: 2009. pp. 179–180. [Google Scholar]
- 26.Slutsky DJ. Lateral Arm Flap. In: Rayan GM, Chung KC, editors. Flap Reconstruction of the Upper Extremity: A Master Skills Publication. American Society for Surgery of the Hand; Rosemont, IL: 2009. pp. 189–194. [Google Scholar]
- 27.Tung TC, Wang KC, Fang CM, et al. Reverse pedicled lateral arm flap for reconstruction of posterior soft-tissue defects of the elbow. Ann Plast Surg. 1997 Jun;38(6):635–41. doi: 10.1097/00000637-199706000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg. 1984;37:149–159. doi: 10.1016/0007-1226(84)90002-x. [DOI] [PubMed] [Google Scholar]
- 29.Wei FC, Jain V, Celik N, et al. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg. 2002 Jun;109(7):2219–26. doi: 10.1097/00006534-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Luo S, Raffoul W, Luo J, et al. Anterolateral thigh flap: a review of 168 cases. Microsurgery. 1999;19:232–238. doi: 10.1002/(sici)1098-2752(1999)19:5<232::aid-micr5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.Kimata Y, Uchiyama K, Ebihara S, et al. Anatomic variations and technical problems of the anterolateral thigh flap: a report of 74 cases. Plast Reconstr Surg. 1998 Oct;102(5):1517–23. doi: 10.1097/00006534-199810000-00026. [DOI] [PubMed] [Google Scholar]
- 32.Lakhiani C, Lee MR, Saint-Cyr M. Vascular anatomy of the anterolateral thigh flap: a systematic review. Plast Reconstr Surg. 2012 Dec;130(6):1254–68. doi: 10.1097/PRS.0b013e31826d1662. [DOI] [PubMed] [Google Scholar]
- 33.Yu P, Selber J, Liu J. Reciprocal dominance of the anterolateral and anteromedial thigh flap perforator anatomy. Ann Plast Surg. 2013 Jun;70(6):714–6. doi: 10.1097/SAP.0b013e318241446c. [DOI] [PubMed] [Google Scholar]
- 34.Wang HT, Fletcher JW, Erdmann D, et al. Use of the anterolateral thigh free flap for upper-extremity reconstruction. J Hand Surg Am. 2005 Jul;30(4):859–64. doi: 10.1016/j.jhsa.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Chan JK, Harry L, Williams G, et al. Soft-tissue reconstruction of open fractures of the lower limb: muscle versus fasciocutaneous flaps. Plast Reconstr Surg. 2012 Aug;130(2):284e–295e. doi: 10.1097/PRS.0b013e3182589e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tintle SM, Levin LS. Injury. Jan 24, 2013. The reconstructive microsurgery ladder in orthopaedics. [DOI] [PubMed] [Google Scholar]
- 37.Gosain A, Chang N, Mathes S, et al. A study of the relationship between blood flow and bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast Reconstr Surg. 1990 Dec;86(6):1152–62. [PubMed] [Google Scholar]
- 38.Calderon W, Chang N, Mathes SJ. Comparison of the effect of bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast Reconstr Surg. 1986 May;77(5):785–94. doi: 10.1097/00006534-198605000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000 May;48(5):913–7. doi: 10.1097/00005373-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Hallock GG. Relative donor-site morbidity of muscle and fascial flaps. Plast Reconstr Surg. 1993 Jul;92(1):70–6. doi: 10.1097/00006534-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Payne DE, Kaufman AM, Wysocki RW, et al. Vascular perfusion of a flexor carpi ulnaris muscle turnover pedicle flap for posterior elbow soft tissue reconstruction: a cadaveric study. J Hand Surg Am. 2011 Feb;36(2):246–51. doi: 10.1016/j.jhsa.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 42.Wysocki RW, Gray RL, Fernandez JJ, et al. Posterior elbow coverage using whole and split flexor carpi ulnaris flaps: a cadaveric study. J Hand Surg Am. 2008 Dec;33(10):1807–12. doi: 10.1016/j.jhsa.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 43.McGeorge DD, Arnstein PM, Stilwell JH. The distally-based brachioradialis muscle flap. Br J Plast Surg. 1991 Jan;44(1):30–2. doi: 10.1016/0007-1226(91)90173-h. [DOI] [PubMed] [Google Scholar]
- 44.Leversedge FJ, Casey PJ, Payne SH, et al. Vascular anatomy of the brachioradialis rotational musculocutaneous flap. J Hand Surg Am. 2001 Jul;26(4):711–21. doi: 10.1053/jhsu.2001.26200. [DOI] [PubMed] [Google Scholar]
- 45.Pierce TD, Tomaino MM. Use of the pedicled latissimus muscle flap for upper-extremity reconstruction. J Am Acad Orthop Surg. 2000 Sep-Oct;8(5):324–31. doi: 10.5435/00124635-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Chang LD, Goldberg NH, Chang B, et al. Elbow defect coverage with a one-staged, tunneled latissimus dorsi transposition flap. Ann Plast Surg. 1994 May;32(5):496–502. doi: 10.1097/00000637-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Vedder NB, Hanel DP. The Mangled Upper Extremity. In: Green DP, Hotchkiss RN, Pederson WC, editors. Green's Operative Hand Surgery. 6th Ed. Churchill Livingstone; New York, NY: 2011. p. 1634. [Google Scholar]
- 48.Bakri K, Moran SL. Initial assessment and management of complex forearm defects. Hand Clin. 2007 May;23(2):255–68. vii. doi: 10.1016/j.hcl.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Stern PJ, Neale HW, Gregory RO, et al. Latissimus dorsi myocutaneous flap for elbow flexion. J Hand Surg [Am] 1982;7:25–30. doi: 10.1016/s0363-5023(82)80009-9. [DOI] [PubMed] [Google Scholar]
- 50.Hovnanian AP. Latissimus dorsi transplantation for loss of flexion or extension at the elbow. Ann Surg. 1956;143:493. doi: 10.1097/00000658-195604000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rockwell WB, Ley E. Abdominal Flaps. In: Rayan GM, Chung KC, editors. Flap Reconstruction of the Upper Extremity: A Master Skills Publication. American Society for Surgery of the Hand; Rosemont, IL: 2009. pp. 171–177. [Google Scholar]
- 52.Davis WM, McCraw JB, Carraway JH. Use of a direct, transverse, thoracoabdominal flap to close difficult wounds of the thorax and upper extremity. Plast Reconstr Surg. 1977;60:526–533. doi: 10.1097/00006534-197710000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Fisher J. External oblique fasciocutaneous flap for elbow coverage. Plast Reconstr Surg. 1985;75:51–59. doi: 10.1097/00006534-198501000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Chow JA, Bilos ZJ, Hui P, et al. The groin flap in reparative surgery of the hand. Plast Reconstr Surg. 1986 Mar;77(3):421–6. doi: 10.1097/00006534-198603000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Arner M, Möller K. Morbidity of the pedicled groin flap. A retrospective study of 44 cases. Scand J Plast Reconstr Surg Hand Surg. 1994 Jun;28(2):143–6. doi: 10.3109/02844319409071192. [DOI] [PubMed] [Google Scholar]
- 56.Goertz O, Kapalschinski N, Daigeler A, et al. The effectiveness of pedicled groin flaps in the treatment of hand defects: results of 49 patients. J Hand Surg Am. 2012 Oct;37(10):2088–94. doi: 10.1016/j.jhsa.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Brown JB, Cannon B, Graham WC, et al. Direct flap repair of defects of the arm and hand: preparation of gunshot wounds for repair of nerves, bones and tendons. Ann Surg. 1945;122(4):706–15. doi: 10.1097/00000658-194510000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cannon B, Trott AW. Expeditious use of direct flaps in extremity repairs. Plast Reconstr Surg. 1949;4:415–9. doi: 10.1097/00006534-194909000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Manahan M, Silverman RP. Chest and Abdominal Wall Reconstruction. In: Guyuron B, Eriksson E, Persing J, editors. Plastic Surgery: Indications and Practice. Saunders Elsevier; Philadelphia, PA: 2009. [Google Scholar]
- 60.Farber GL, Taylor KF, Smith AC. Pedicled thoracoabdominal flap coverage about the elbow in traumatic war injuries. Hand (NY) 2010 Mar;5(1):43–8. doi: 10.1007/s11552-009-9213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]