Abstract
Vaccine development for the blood stages of malaria has focused on the induction of antibodies to parasite surface antigens, most of which are highly polymorphic. An alternate strategy has evolved from observations that low-density infections can induce antibody-independent immunity to different strains. To test this strategy, we treated parasitized red blood cells from the rodent parasite Plasmodium chabaudi with seco-cyclopropyl pyrrolo indole analogs. These drugs irreversibly alkylate parasite DNA, blocking their ability to replicate. After administration in mice, DNA from the vaccine could be detected in the blood for over 110 days and a single vaccination induced profound immunity to different malaria parasite species. Immunity was mediated by CD4+ T cells and was dependent on the red blood cell membrane remaining intact. The human parasite, Plasmodium falciparum, could also be attenuated by treatment with seco-cyclopropyl pyrrolo indole analogs. These data demonstrate that vaccination with chemically attenuated parasites induces protective immunity and provide a compelling rationale for testing a blood-stage parasite-based vaccine targeting human Plasmodium species.
Introduction
The development of a vaccine for malaria is seen as the best hope to significantly reduce the burden of disease (1–3). Vaccine development for the blood stages has focused on individual antigens expressed on the parasite or infected rbc surface primarily because antibodies induced by recombinant forms of these antigens can induce immunity in model systems. However, despite initial promise with one vaccine candidate, MSP2 (4), none of these subunit vaccine approaches have translated to success in late-stage clinical trials (e.g., refs. 5, 6), although there has been no follow-up with MSP2. Evidence suggests that this is in large part due to the extensive polymorphisms that occur in these proteins (7) and to the fact that very high titer antibody responses are required for efficacy (8, 9). Virtually all vaccine trials have focused on Plasmodium falciparum, even though the other species that infect humans, particularly Plasmodium vivax, also cause significant morbidity and mortality (10).
Over the last 10 years, evidence has accumulated that low-density blood-stage infections that are curtailed by antimalaria drug therapy (and in which parasitemia is below the threshold detectable by microscopy) can induce immunity in both human and rodent systems (11, 12). Although further work is required to validate results from human volunteers (11), due to concerns with the half-life of the drug previously used (13), in rodent models, immunity was shown to be species- and strain-transcendent (12). Unlike immunization with a subunit vaccine, exposure to whole organisms has the advantage of inducing immune responses to multiple plasmodial antigens, many of which are likely to be highly conserved between strains or species (14). An additional difference between subunit vaccination and immunity induced by exposure to a low-density infection is that immunity in the latter is antibody independent and T cell dependent (11, 12). While high parasite load is a critical determinant driving T cells to undergo apoptosis during normal infection, exposure to parasites at low density leads to T cell activation (11, 15). In humans, this immunity involves both CD4+ and CD8+ T cells as well as induction of nitric oxide synthase and the absence of any detectable antibody response to defined merozoite surface proteins or to antigens expressed on the surface of infected rbcs (11). Subsequent experiments demonstrated that low doses of Plasmodium chabaudi parasites killed by freeze/thawing, when combined with the Th1 T cell–activating adjuvant CpG, could also induce antibody-independent immunity to various strains and species of Plasmodium (16). The adjuvant was critical for vaccine efficacy. Others have recently shown protection following immunization with low doses of nucleoside transporter-1 genetically attenuated Plasmodium yoelii blood-stage parasites which induced a limited microscopic infection during the immunization period (17). However, these approaches are marred by difficulties in identifying a suitable human-compatible adjuvant capable of replicating the immunity induced in mice by killed parasites (16) and concerns that a parasite genetically modified in only one or a few regions of its genome might revert to a virulent phenotype (18).
Given that exposure to live parasites at low density (without an adjuvant) induces immunity, we asked whether low doses of live blood-stage parasites attenuated by a chemical treatment that irreversibly alkylates the parasite’s DNA in multiple sites (19) could also induce immunity. While chemically attenuated intact sporozoite stage parasites (the stage inoculated by mosquitoes and which travel to the liver to continue their life cycle) can induce immunity (20, 21), DNA chemical attenuation of blood stages has never been pursued as a vaccine strategy.
Results
Attenuated parasites persist and induce cross-species protective immunity.
We initially treated P. chabaudi parasitized rbcs (prbcs), 98% of which were at the early “ring” stage, with the seco-cyclopropyl pyrrolo indole analogs AS-I-145 (centanamycin), TH-III-149 (tafuramycin A), and AS-VIII-104 for 40 minutes in vitro. The prbcs were washed 3 times to remove excess drug, and then 106 treated cells were administered i.v. to immunodeficient SCID mice to ascertain attenuation. Mice were followed for 6 weeks for the appearance of any “breakthrough,” parasites but none were detected microscopically. We also analyzed the blood from SCID and normal mice receiving centanamycin-attenuated and WT parasites using a quantitative PCR (qPCR) assay based on the 18S rRNA gene of Plasmodium (Figure 1A). Based on this assay, we showed that most attenuated (and WT) parasites were removed from the blood within 5 minutes of administration. For up to 24 hours after administration of either attenuated or WT parasites to normal mice, DNA levels were similar. After that time, DNA levels then increased rapidly in mice given WT parasites as the infection progressed. However, DNA from the attenuated parasites persisted at low but fluctuating levels in the blood of SCID and normal mice for over 110 days. In a repeat experiment, we used 15 mice and divided them into 3 groups of 5 so that more blood could be taken from each mouse at each time point up to day 51 (collecting 50 μl instead of 20 μl of blood to increase the sensitivity of the assay) (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI66634DS1). As reported in Figure 1, DNA was detectable at low but fluctuating levels and all mice survived. It was not clear whether these low but fluctuating levels of DNA represented viable parasites or simply DNA from nonviable parasites. To explore further whether or not viable parasites were present in the blood of vaccinated mice, xenodiagnosis was undertaken. Blood from vaccinated mice was transferred to naive BALB/c mice (100 μl per mouse). None of the 20 mice that received the blood developed a patent parasitemia, suggesting that the parasites were not viable in the donors at the time of transfer.
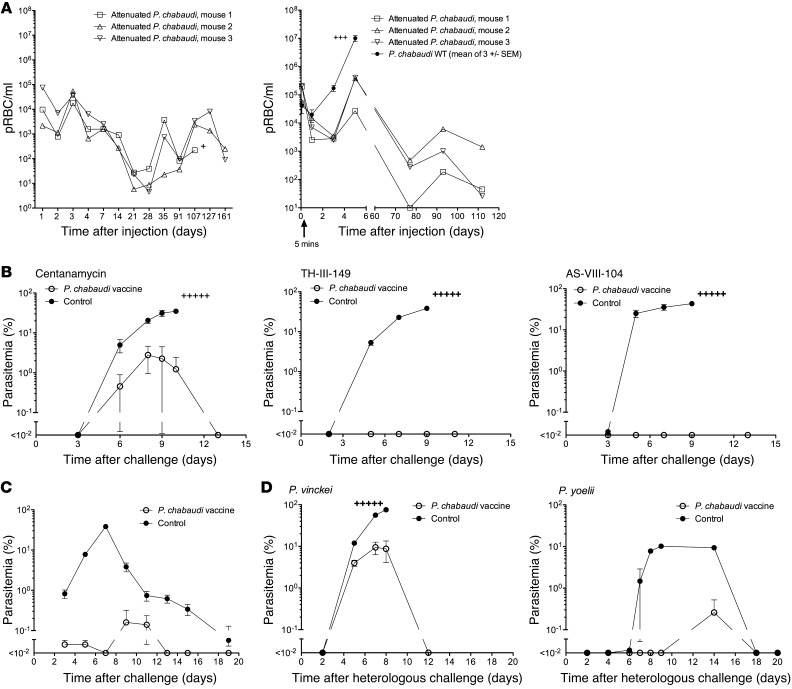
Figure 1. Immunization with attenuated parasites.
(A) Immunodeficient SCID mice (left panel) or immunocompetent A/J mice (right panel) were administered 106 (left panel) or 107 (right panel) P. chabaudi prbcs attenuated with 2 μM centanamycin or 107 WT parasites (right panel). qPCR was performed to estimate parasite density in the blood at various time points. y axes show the estimated parasite density. (B) To assess immunity, A/J mice (5 per group) were immunized with a single dose of 106P. chabaudi prbcs attenuated with centanamycin, TH-III-149, or AS-VIII-104, as indicated, or left untreated. All were challenged 5 weeks later with 105P. chabaudi prbcs i.v. (C) To assess immunity in a different mouse strain, C57BL/6 mice were either immunized with a single dose of 106P. chabaudi prbcs attenuated with centanamycin or given a saline injection (control) and challenged 9 weeks later with 105P. chabaudi prbcs i.v. (D) TH-III-149 (for P. vinckei challenge) or centanamycin (for P. yoelii challenge) were used to attenuate P. chabaudi, and mice were immunized with a single dose of 106 prbcs and challenged 4 weeks later with either 104P. vinckei or 104P. yoelii prbcs i.v. Plus signs indicate that mice succumbed to the infection.
Parasites attenuated with each of the seco-cyclopropyl pyrrolo indole analogs were next administered i.v. to immunocompetent A/J mice. The infection is lethal in this mouse strain. Five weeks after a single immunizing dose of 106 attenuated P. chabaudi parasites, the mice were challenged with 105 homologous WT parasites and were strongly protected (Figure 1B). Clinical scores were recorded for all mice and none of the vaccinated mice demonstrated any adverse clinical effects as a result of the vaccination or the challenge infection. Although P. chabaudi is not lethal in C57BL/6 mice, we observed that the centanamycin-attenuated vaccine could also induce strong antiparasitic immunity in this mouse strain (Figure 1C). In various experiments, control A/J mice immunized with uninfected rbcs treated with centanamycin, injected with saline, or receiving no treatment all developed a rapid infection following challenge and succumbed (data not shown). In other experiments, we observed protection following immunization with 104 attenuated parasites (data not shown) and also observed that 3 immunizations did not lead to enhanced protection over a single immunization (Supplemental Figure 2).
We next asked whether immunity was long lived and observed that A/J mice challenged 6 months after a single immunization with attenuated prbcs from a different parasite, P. yoelii, were strongly protected against homologous challenge (Supplemental Figure 3). To ask whether immunity resulted in cross-species protection, we vaccinated mice with centanamycin-attenuated parasites prepared from P. chabaudi strain AS, challenged them with the heterologous species, Plasmodium vinckei and P. yoelii, and also observed strong protection (Figure 1D). Mice were also vaccinated with centanamycin-attenuated parasites prepared from P. chabaudi strain AS and challenged initially with the homologous strain, then 3 months later with the heterologous strain, P. chabaudi AJ. We observed strong protection against the original homologous parasite and the subsequent heterologous challenge (Supplemental Figure 4).
Attenuated parasites induce a cellular immune response.
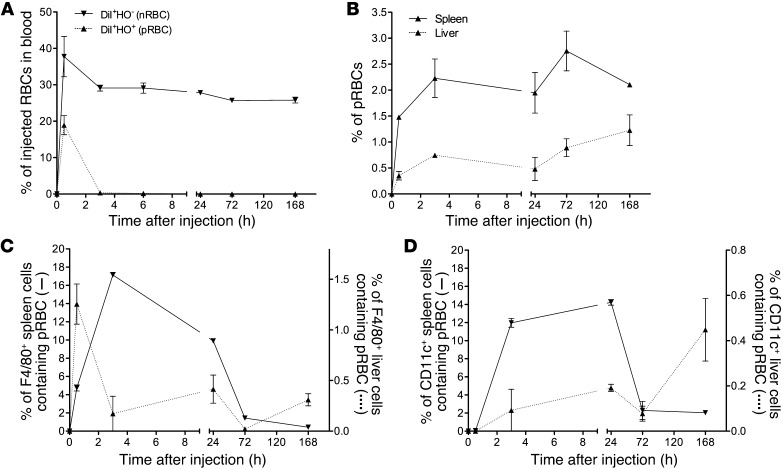
To examine the nature of immunity, we initially determined the fate of attenuated P. chabaudi prbcs in vivo. We confirmed the rapid diminution of attenuated parasite numbers in the blood shown by qPCR (Figure 1) using an assay in which we tracked parasitized and normal rbcs by labeling the rbc membranes with DiI (22) and parasite DNA with Hoechst (HO) stain 34580. We demonstrated that chemically attenuated prbcs were removed from the circulation far more rapidly than normal rbcs (nrbcs) (Figure 2A). Attenuated parasites accumulated in the spleen and liver as determined by flow cytometry (Figure 2B) and were shown to be inside F4/80+ cells (macrophages) and CD11c+ dendritic cells (Figure 2, C and D). Confocal imaging confirmed that HO+DiI+ cells could be seen within F4/80+ cells in the spleen (Supplemental Figure 5B). These data suggested that attenuated parasites might rapidly activate the immune system.
Figure 2. Tracking of attenuated parasites stained with DiI and HO. rbcs from a P. chabaudi–infected mouse (parasitemia, 39.8%; Supplemental Figure 5A) were treated with centanamycin, stained with DiI and HO, and then injected i.v. into naive AJ mice (3 mice, 5 × 107 cells per mouse). rbcs from an uninfected mouse were stained with DiI alone and injected into other mice (3 mice, 5 × 107 cells per mouse).
DiI+HO– cells (nrbcs) or DiI+HO+ cells (prbcs) in the peripheral blood of recipient mice were analyzed over time by flow cytometry and the percentage of these cells of the total injected nrbcs or prbcs calculated based on an assumption of the recipient mice each containing a total of 5 × 109 rbcs (A). Spleen and liver cells of recipient mice were prepared by mechanical disruption. rbcs were gated and DiI+HO+ cells identified. The percentages of DiI+HO+ cells of the total rbcs in the spleen and liver were then calculated (B). Free rbcs were then lysed using NH4Cl-Tris buffer to allow estimation of rbc uptake by white cells. Percentages of DiI+HO+F4/80+ or DiI+HO+CD11c+ cells in total F4/80+ (C) or CD11c+ cells (D) in the spleen and liver were then calculated. Two independent experiments were performed (total n = 4–6 mice per time point), and representative graphs are shown. Means ± SEM are shown.
To measure early activation of the immune system, we monitored CD11a and CD49d expression on T cells (23) and observed that within 5 days of administration of centanamycin-attenuated P. chabaudi prbcs, 1.2% of peripheral blood CD4+ T cells showed significant activation above the level of activation in mice that received a saline injection (P < 0.0001) and that the mean number of activated cells rose to 5% by 21 days (Figure 3A and Supplemental Figure 6). There was less activation of CD8+ cells. When tested 2 months after vaccination, spleen cells showed a strong proliferative response to homologous prbcs as well as to prbcs of the heterologous species P. yoelii, but not to uninfected rbcs (Figure 3B). Spleen cells from a naive mouse did not respond to the parasites.
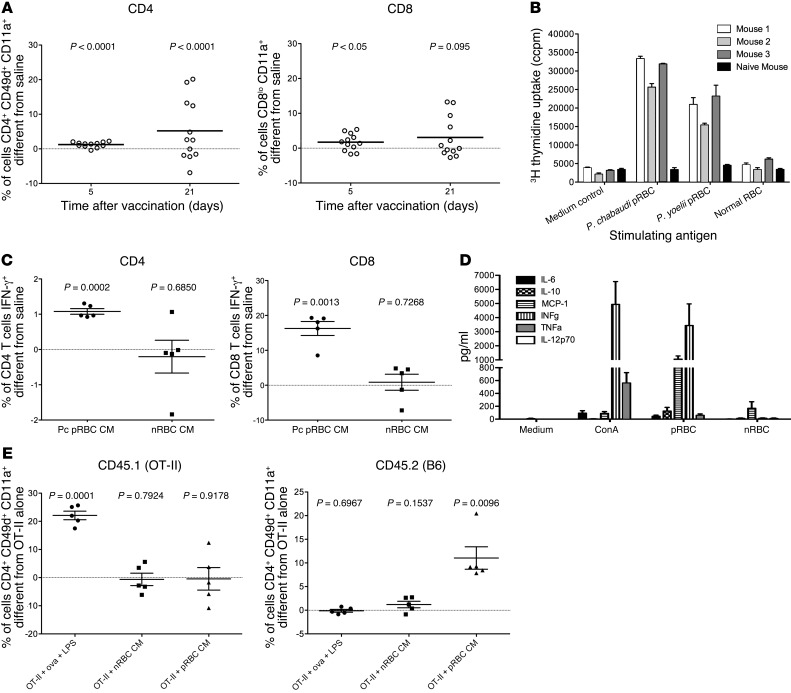
Figure 3. Immune responses induced by chemically attenuated parasites.
(A) C57BL/6 mice (12 per group) were vaccinated with 106P. chabaudi prbcs attenuated with centanamycin or injected with saline. Phenotypes of blood CD4+ and CD8+ cells were assessed on days 5 and 21 after vaccination. Values for means of the saline group were subtracted from values for each vaccinated mouse. Each circle represents 1 mouse. Horizontal bars represent means. One sample t test was performed comparing values to the saline mean of zero. (B) Three A/J mice were immunized with 3 doses of vaccine (each, 106 centanamycin-attenuated P. chabaudi prbcs), and spleen cells from these and a naive mouse were collected 8 weeks after the last immunization and cultured with indicated antigens; uptake of 3H thymidine was determined after 72 hours as described in Methods. (C) C57BL/6 mice (5 per group) were vaccinated with 106P. chabaudi prbcs or equivalent nrbcs attenuated with centanamycin and sacrificed on day 5. Spleen cells were stimulated in vitro for 4 hours with PMA and ionomycin in the presence of BFA and stained for IFN-γ. Percentage that was IFN-γ positive was determined by subtracting isotype control and values from mice injected with saline for CD4 and CD8 populations. (D) Three A/J mice were immunized with 3 doses of vaccine (each, 106 centanamycin-attenuated P. chabaudi prbcs), and spleen cells from these mice were collected 50 days after the last immunization, cultured with indicated antigens, and after 72 hours culture, supernatants collected and use for cytokine bead analysis as described in Methods. (E) C57BL/6 mice (CD45.2, 5 per group) were adoptively transferred with OT-II T cells (CD45.1), and 1 group received ovalbumin peptide 323–339 mixed with LPS i.p. 24 hours later, mice were immunized i.v. with 106P. chabaudi prbcs or equivalent nrbcs attenuated with centanamycin. Mice were bled on day 6 after immunization and the phenotype of donor and recipient cells determined by flow cytometry. Percentages were calculated by subtracting values from mice receiving OT-II cells alone.
To determine whether the T cells were functional, splenic CD4+ and CD8+ T cells were examined for intracellular IFN-γ 5 days after vaccination with centanamycin-attenuated parasites, centanamycin-treated nrbcs, or saline. Cells were activated in vitro for 4 hours with PMA/ionomycin prior to staining (as described in ref. 23). There were significantly more cytokine-positive cells in the spleens of vaccinated mice compared with the other groups of mice (Figure 3C). Furthermore, spleen cells from vaccinated mice produced larger amounts of IFN-γ and MCP-1 ex vivo in response to prbcs compared with nrbcs or medium alone (Figure 3D).
To confirm that vaccination induced expansion and activation of antigen-specific CD4+ T cells, rather than bystander activation, we transferred congenic (CD45.1) OVA-TCR transgenic OTII cells into CD45.2 recipients, which we vaccinated with attenuated parasites or OVA peptide/LPS, and observed activation of the OTII cells following OVA peptide administration, but not following vaccination with attenuated parasites. Conversely, we observed activation of CD45.2 CD4+ T cells following vaccination with centanamycin-treated prbcs, but not following administration of centanamycin-treated nrbcs or OVA. These data demonstrate that the vaccine induced expansion and activation of antigen-specific CD4+ T cells, without activation of bystander T cells (Figure 3E).
To determine whether the vaccine-induced, antigen-specific CD4+ T cells were protective, immunized mice were given antibodies to clear more than 98% of their CD4+ T cells or more than 89% of CD8+ T cells (Supplemental Figure 7) and then challenged. Control mice that received normal rat immunoglobulin showed complete protection, but CD4+ T cell–depleted mice all succumbed, albeit more slowly than naive mice (Supplemental Figure 8). When CD8+ T cells were depleted from immune mice, we observed no change in their level of immunity. Thus, CD8+ T cells alone are not able to provide complete protection, but they or other cell types may contribute a minor role.
Although CD4+ T cells were critical for protection, we were not able to transfer protection with purified CD4+ T cells. Thus, 107 CD45.2 splenic CD4+ T cells from C57BL/6 mice immunized 3 times with centanamycin-attenuated P. chabaudi prbcs or equivalent numbers of naive CD45.2 T cells were transferred into CD45.1 congenic naive mice that had been pretreated 3 days earlier with 1 dose of anti-CD4 mAb to deplete most endogenous T cells. CD4+ T cell engraftment was successful, as shown by flow cytometry with between 5% and 12% of peripheral blood T cells being of donor origin. Mice were challenged 11 days after transfer, but no protection was observed, despite control animals vaccinated with centanamycin-treated prbcs being protected.
We next asked whether mice immunized with either 104 or 106 chemically attenuated parasites developed parasite-specific antibodies. Using an ELISA to whole parasite antigens, we observed that while sera taken from mice that experienced multiple infections each followed by drug cure contained antibodies, sera from mice immunized with attenuated parasites did not (Supplemental Figure 9A). In a repeat experiment to confirm protection in the absence of antibodies, we observed that not only were antibodies not detectable after vaccination, but that following challenge (during which parasites were not detectable by microscopy in any of the vaccinated recipients), antibodies were also not detected (Supplemental Figure 9B). In a further experiment to ascertain any role for antibodies, sera from 10 vaccinated A/J mice and from 10 control mice were transferred into naive recipients (0.5 ml on each of days –1, 0, and 1) that were challenged on day 0. Mice that received serum from immunized mice as well as mice that received serum from naive controls all succumbed without any evidence of protection, but the serum donor mice were protected (Supplemental Figure 9C).
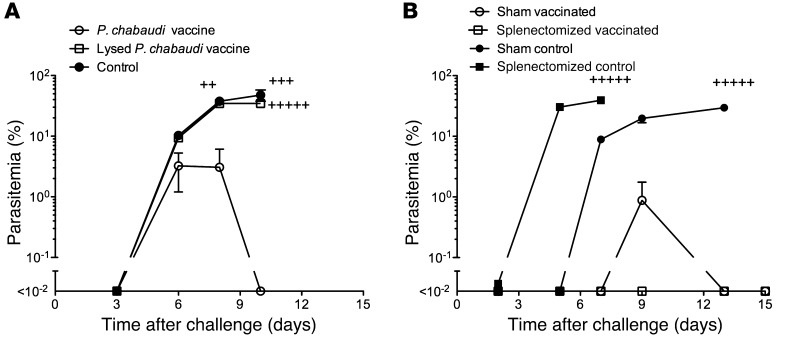
The above data suggest that antigen-presenting cells initiate an antiparasite CD4+ T cell response following uptake of rbcs containing attenuated parasites. To further understand the interactions between attenuated parasites and the cells of the immune system, we asked whether a preparation of lysed attenuated prbcs would also induce immunity. We immunized mice with intact or lysed attenuated prbcs and observed that when the rbc membranes were disrupted, immunity was not induced (Figure 4A). Therefore, the interaction between the attenuated parasites and the cells of the immune system requires the rbc membranes to remain intact. As the spleen is assumed to be a site where antiparasite immunity is induced (ref. 24 and Figure 2), we examined the importance of the spleen in the induction of protective immunity. We observed that, while eusplenic control mice could control parasite burden more effectively than splenectomized control mice, both groups of vaccinated mice could control parasite burden very effectively and to a similar level at 5 weeks after vaccination (Figure 4B). This demonstrated that short-term immunity could be induced and mediated in tissues other than the spleen, with one likely site being the liver (25, 26). However, in an experiment in which mice were challenged 12 weeks after vaccination, we observed that splenectomized mice were not protected, whereas sham-splenectomized mice were still protected. This may be due to the presence of spleen-resident memory cells mediating protection at later time points with effector cells, which may have a different tissue localization, mediating protection at earlier time points.
Figure 4. Vaccine protection requires an intact rbc membrane.
The role of the spleen early (5 weeks) after vaccination. (A) To determine whether the rbc membrane must remain intact for vaccine efficacy, cohorts of 5 A/J mice were vaccinated with a single dose of 106P. chabaudi prbcs attenuated with centanamycin or a vaccine lysed with distilled water, then returned to isotonicity with 2× PBS. Naive mice served as a control. Five weeks later, mice were challenged with 105P. chabaudi prbcs i.v. and parasitemia monitored. Plus signs indicate that mice succumbed to the infection. (B) To determine the importance of the spleen to immunity early after vaccination, A/J mice were splenectomized and others had a sham splenectomy. Four weeks later, half were immunized i.v. with 3 doses of 106P. chabaudi prbcs attenuated by treatment with centanamycin, each 2 weeks apart. The other half received no treatment. Five weeks after the final dose of vaccine, all mice were challenged with 105 prbcs i.v. and parasitemia monitored. Plus signs indicate that mice succumbed to the infection.
We next determined whether centanamycin treatment would result in attenuation of the major human parasite P. falciparum. It was previously observed that centanamycin could kill P. falciparum in vitro (27). To analyze this further, we measured RNA transcription and DNA replication after centanamycin treatment of a synchronized P. falciparum culture containing initially only rings (Supplemental Figure 10). We showed that, over a 64-hour time period, very few centanamycin-treated cells progressed through the cell cycle and that there was near complete arrest of transcription. This level of attenuation is consistent with our observations in vivo (above).
Discussion
We show that vaccination with chemically attenuated rodent parasites induces long-lived strain- and species-transcendent immunity via a mechanism that is CD4+ T cell dependent. At early times after vaccination (5 weeks), immunity can occur independently of the spleen, but the spleen appears to play a critical role at later time points (12 weeks) during the memory phase of the response. Vaccine efficacy requires the presence of intact rbc membranes.
Although T cells are critical for protection, we observed that splenic CD4+ T cells did not transfer protection. There are 2 likely explanations. First, the protective T cells of the donor mice were in the tissues and not resident in the spleen when the spleen cell transfer experiment was performed. As such, protective T cells may not be transferred in a spleen cell transfer experiment. The fact that the mature forms of this parasite (schizonts) reside in the tissues and are not found in peripheral blood lends support to the fact that the protective T cells may also reside in the deep tissues. While this is a possibility, we believe that this may be a less likely explanation since we have shown that T cells from the spleen of vaccinated mice can respond by proliferation and cytokine production to prbcs. Second, while T cells are critical for protection (Supplemental Figure 8), other cell types and/or other changes in the tissues may also be critical. For example, we know that immunized mice depleted of CD4+ T cells offer more resistance to infection than naive mice, suggesting that immune cells other than CD4+ T cells might also contribute to protection. Changes in splenic architecture have also been reported in mice following P. yoelii and P. chabaudi infection (28, 29), and we cannot rule out the possibility that vaccine-induced immunity occurs with structural changes in the spleen or other tissues. Furthermore, it is known that the liver can play an important role in protection (25, 26). Thus, vaccination with attenuated parasites might also alter tissue architecture in various tissues to promote antiparasitic immunity that cannot be replicated in a simple cell-transfer experiment in which normal unexposed mice are the recipients of immune cells. Further studies are underway to attempt to resolve this issue.
The seco-cyclopropyl pyrrolo indole compounds are known to bind within the minor groove of parasite DNA in AT rich sequences (19, 27), which are highly abundant in Plasmodium. While we cannot exclude that these chemicals may have other effects on the parasite, the data suggest that the primary role of the seco-cyclopropyl pyrrolo indoles is to alkylate the parasite’s AT-rich DNA, severely compromising its ability to replicate. It is possible that some parasites may escape complete attenuation and continue a limited replicative cycle in vivo; the data showing persisting but fluctuating levels of parasite DNA in vivo support the hypothesis that active parasite replication may be occurring at a very low level. However, any such replication would appear to be limited, as transfer of blood from vaccinated mice to naive recipients (xenodiagnosis) failed to establish a patent infection. There are potential concerns regarding a replicating parasite vaccine, including whether a more virulent phenotype would emerge and whether or not transmission might occur to another individual. While our data suggest that these will not be of concern, this nevertheless is something that will need to be carefully studied in clinical trials.
The results presented here strongly suggest that antigens expressed during the ring stage are responsible for the induction of protective immunity. We cannot exclude the possibility that a vaccine based on other stages (e.g., trophozoites) would also be effective. We chose ring-stage parasites for these studies because they are readily harvested at more than 98% purity from the blood of infected mice, whereas the mature forms are sequestered in deep tissues. Whether the immune response induced by the ring-stage vaccine responds to all stages or simply the ring stages is not known. However, it is known that many antigens are expressed throughout the various rbc stages ( www.PlasmoDB.org and ref. 30). It seems likely that vaccine-induced CD4+ T cells are reactivated by the different blood stages of Plasmodium at the start of an infection and that they then promote a cell-mediated immune response that prevents further parasite growth. Evidence from other studies (e.g., refs. 1, 2, 31, 32) collectively suggests that these CD4+ T cell–dependent, cell-mediated mechanisms include macrophage activation, phagocytosis, and the release of small inflammatory molecules such as nitric oxide.
A critical question for vaccine development is whether the data observed in a rodent model using a P. chabaudi vaccine will translate to an efficacious and safe multispecies vaccine for humans. While this question will only be satisfactorily answered following human clinical trials, the data presented here, and elsewhere (11, 12), suggest that this approach will be successful. We show that the vaccine is effective in different strains of mice, that immunity is cross-species protective, and that attenuated parasites prepared using a different rodent malaria species, P. yoelii, are also highly effective. This is the first time, to our knowledge, that any vaccine preparation has shown protection against the highly virulent P. vinckei (24). We previously demonstrated that deliberate infection of human volunteers with a subpatent P. falciparum infection (in which the volunteers are drug treated before the microscopic appearance of parasites and clinical symptoms) induced profound T cell immunity to the parasite (11). Here, we extend this observation markedly by attenuating the parasites prior to administration and thus converting the original observation into a vaccine strategy. Collectively, these data are consistent with the hypothesis that the target antigens for T cells in Plasmodium are highly conserved across species (14, 16), and because of this, a human parasite vaccine is likely to be effective.
Although a whole parasite vaccine without any human rbc material may be preferable, we show that the intact rbc membrane is essential for efficacy. Our data suggest that the role of the rbc membrane is to target the parasite to the antigen-presenting cells, as has been shown for P. falciparum (33), thereby initiating the antiparasite immune response. The issue of safety in the preparation of a human vaccine using human rbcs will be addressed in part by preparing a P. falciparum vaccine in “universal donor” (transfusion-grade blood group O negative) rbcs. This topic has been discussed (34–37) looking at the recent inoculations of healthy volunteers with infected rbcs to study immunity and the inoculations of syphilis patients who received malaria therapy in the early 20th century and of volunteers in the latter half of the century who received deliberate infections to test schizonticides. No adverse side effects were reported in these studies. Today, stringent quality assurance makes blood transfusions extremely safe.
Another question relevant to vaccine development is whether sufficient vaccine can be prepared at scale in vitro. While the dose of attenuated parasites required to induce immunity in humans is not known, we are encouraged by the ability of extremely low parasite densities to activate human T cells (11), which suggests that very low doses of attenuated parasites may suffice. In those studies, volunteers received approximately 30 infected rbcs and the infection was curtailed after one week.
While whole parasite vaccines were not considered a viable and effective option for malaria until 10 years ago (38), there are now concerted efforts to develop a whole parasite sporozoite vaccine using either irradiated parasites (39), genetically attenuated organisms (40), or via sporozoite exposure and drug treatment (41), with all these approaches entering clinical trials. This has been driven in large part by the limited efficacy of the leading subunit sporozoite vaccine, RTS,S (42). Despite a multitude of blood-stage subunit vaccine candidates, none have progressed to late-stage trials, underscoring the importance of considering whole parasite vaccines as alternate strategies for the blood stages as well. Thus, we present data that we believe for the first time show that a chemically attenuated blood-stage malaria vaccine is highly efficacious and exerts its effect via a different mechanism from that of subunit vaccine candidates. This makes it worthy of serious consideration as a new approach to developing a malaria vaccine. Furthermore, the strategy of chemical attenuation and vaccine development could prove very useful for other organisms, many of which are known to be susceptible to attenuation by seco-cyclopropyl pyrrolo indoles (19).
Methods
Mice.
Four- to six-week-old female A/J mice (H-2a), BALB/c congenic SCID (H-2d) and C57BL/6 (H-2b), and B6.SJL-PtprcaPep3b/BoyJArc (Ptprca), were purchased from the Animal Resource Centre (Willeton, Western Australia). Animals were maintained in a pathogen-free environment at the Griffith University Animal Facility.
Parasites.
Cloned lines of P. chabaudi isolates AS and AJ, P. yoelii, and P. vinckei were obtained from The Queensland Institute of Medical Research from stock originally provided by Richard Carter (University of Edinburgh, United Kingdom). Stabilates were maintained by i.p. passage of 105 prbcs into naive recipients. PCR amplification of the MSP1 genes was used to confirm parasite identity. Challenge infections were performed by i.v. injection of 105 prbcs. P. falciparum NF54 strain was obtained from Sanaria Inc. Parasitemia was monitored from thin blood smears stained with Giemsa or Diff-Quick (Bacto).
Complete medium for lymphocyte culture.
RPMI 1640 (Gibco; Invitrogen) was supplemented with 10% heat inactivated FBS, penicillin (2 mM), streptomycin (1,000 U/ml), and 2-mercaptoethanol (0.05 mM) (Gibco; Invitrogen) to make complete medium.
PCR.
For sample preparation and DNA extraction, 10, 20, or 50 μl of infected blood was collected at different time points in 200 μl of 0.9% saline. Blood samples were centrifuged at 11,000 g for 5 minutes to pellet the erythrocytes, and the supernatant was removed. Samples were frozen at –80°C prior to DNA extraction. Genomic DNA (gDNA) was extracted using a High Pure PCR Template Preparation Kit (Roche) following the manufacturer’s instructions. gDNA was eluted in 30 μl elution buffer.
DNA standards were prepared from a whole blood sample from an outbred Quackenbush mouse infected with P. chabaudi AS. Infected blood was collected through cardiac puncture. 98% of prbcs were at the ring stage. The parasite density was measured by microscopy, diluted to a concentration of 9.5 × 108 prbcs/ml, aliquoted, and stored at –80°C. gDNA was extracted fresh for each PCR run from 10 μl of whole blood and serially diluted to 9.5 prbcs/ml. The data from all curves were analyzed using Bio-Rad CFX manager 2.1 software. The log10 of each known concentration in the dilution series was plotted against the quantification cycle (Cq values) in a linear regression model.
qPCR was performed using a Bio-Rad CFX96 Real-Time PCR System. A 68-bp region of the 18S ribosomal RNA (18S rRNA) gene of P. chabaudi AS (GenBank DQ241815.1) that does not contain any putative centanamycin-binding sites (AAA[A/T]) was selected as the amplification target. PCR primers and a TaqMan MGB probe were designed as follows: 18SNCB-forward: 5′-ACTTCCATTAATCAAGAACGAAAGTT-3′, 18SNCB-reverse: 5′-TGGTTAAGATTACGATCGGTATCTGA-3′ and 18SNCB-probe: FAM 5′-AAGGGAGTGAAGACGA-3′ MGBNFQ (Applied Biosystems). The PCR mix consisted of 10 μl SsoFast Probes Supermix (Bio-Rad), 0.2 μM of each primer, and TaqMan MGB probe and 5 μl of template DNA in a 20 μl final reaction mix under the following conditions: 2 minutes incubation at 95°C, followed by 45 cycles of 95°C for 15 seconds and 60°C for 45 seconds. The standards and samples were run in triplicate.
rbc lysis.
For analysis of the need for rbc membranes to remain intact for immunogenicity, the packed rbc volume was measured and 4 times the volume of sterile distilled water was added to the packed cells. The cells were resuspended for 2 minutes; then double-strength PBS was added to restore tonicity. Sample was reconstituted to 5 × 106 equivalent prbcs/ml.
Attenuation and immunization.
Infected blood was collected by cardiac puncture when parasitemia was between 30%–40% and 100 μl diluted to 10 ml in warm serum-free RPMI. AS-I-145 (Centanamycin) (43), TH-III-149 (tafuramycin A) (44), and ASVIII-104 (45) have been previously described. These were dissolved in DMSO at 10,000× the desired attenuating concentration. Stock solutions of PET (polyethylene glycol 400, absolute ethanol, and Tween 80 in 6:3:1 portions) and 5% glucose were made and mixed 1:2. The DMSO solution was then added at 10% volume to the PET/glucose mix. Attenuating agents were diluted to a 20-μM final concentration in RPMI and then infected blood added in RPMI to give a final concentration of 2 μM. The mixture was then incubated at 37°C in a 5% CO2 incubator for 40 minutes. The cells were washed 3 times and counted, and the immunizing dose was calculated. Mice were immunized i.v. Control mice received treated nrbcs, saline, or no treatment, as described.
Analysis of P. falciparum attenuation.
P. falciparum NF54 parasites were cultivated at 37°C with 5% carbon dioxide/5% oxygen in air using O+ human erythrocytes (Australian Red Cross Blood Service) in RPMI 1640 containing 25 mM HEPES, 10% O+ human serum (Australian Red Cross Blood Service), l-glutamine, and 25 mg/ml gentamycin (complete culture medium). Parasites were synchronized with sorbitol treatment and ring-stage parasites prepared (1.8 to 2.0% parasitemia). Parasites were treated with 2 μM centanamycin at 37°C for 40 minutes and then washed with serum free RPMI 1640 medium. WT or centanamycin-treated P. falciparum parasites were grown in complete medium for 64 hours and parasites collected every 16 hours. The culture medium was replaced every 24 hours.
For RNA and DNA staining (46), rbcs were pelleted and resuspended in 400 μl of Retic-COUNT Reagent (thiazole orange [TO]; BD), and then HO 34580 added at a 4-mM final concentration. A total of 100,000 cells were collected by the LSRFortessa flow cytometer, and data were analyzed by FACSDiva software. HO and TO signals were detected by 450/50 (excited by 405 nm) and 530/30 (excited by 488 nm) filters, respectively.
Immunodepletion.
Anti-CD4 antibodies were generated from the culture supernatant of rat anti-mouse CD4, clone GK1.5 (ATTC). Rat anti-mouse CD8β was generated from clone 53.5.8 (ATCC). Both were purified over a protein G–sepharose column (Thermo Scientific). 500 μg of anti-CD4 was administered on days –4, –3, –2, –1, 0, and +1 relative to challenge day. 500 μg of rat anti-CD8β was administered i.p. on days –4, –3, –2, –1, 0, +1, +3, and +5 relative to the day of challenge. 500 μg of rat IgG (Sigma-Aldrich) was used as a control antibody. CD4 depletion was shown to be 99% effective, and CD8 depletion was shown to be 98% complete using flow cytometry.
Lymphocyte proliferation assays.
Mouse spleens were processed to obtain a single-cell suspension, diluted to 5 × 106 cells/ml in RPMI 1640 complete medium, and aliquoted into 96-well plates at 5 × 105 cells/well. Triplicate wells were cultured with fresh prbcs or nrbcs at 5 × 105/well. Cells were pulsed with 0.25 μCi of 3[H]-Thymidine (PerkinElmer)/well during the last 18 hours of a 72-hour culture and harvested onto fiberglass mats; 3H incorporation was measured on a PerkinElmer MicroBeta2 β counter.
Analysis of circulating T cells.
Approximately 50 μl of blood was obtained from the tail of each mouse. rbcs were lysed with ACK and Fc-receptors blocked with 100 μg/ml heat-inactivated rat IgG for 10 minutes on ice. Samples were stained for 20 minutes on ice with a biotinylated anti-CD49d, followed by washing and staining with anti-CD4 V500 (clone RM 4-5), anti-CD8 PerCP-Cy5.5 (clone 53-6.7), anti-CD11a FITC (clone 2D7), and streptavidin-APC (all from BD). Samples were fixed with 4% formaldehyde and run on a BD LSRFortessa. Data were analyzed with FlowJo; saline means were calculated and subtracted from samples using Excel and graphed using Prism 5 for Mac OS X. One-sample t tests (2 tailed) were performed with Graph Pad QuickCalcs software ( http://www.graphpad.com/quickcalcs/).
Adoptive transfer.
Spleens from 2 female B6.OT-II.Ptprca (CD45.1) mice were placed into a 100-mm dish containing medium and a 70-μm nylon cell strainer. The plastic end of a 5-ml syringe was used to gently mash the spleens, and the cell suspension was strained through the same 70-μm strainer. The petri dish was rinsed with additional medium. The cell suspension was centrifuged at 300 g for 5 minutes and supernatant aspirated. rbcs were lysed using 1× Vitalyse buffer for 5 minutes at room temperate and quenched with excess medium. Cells were centrifuged as above and isolated using the CD4+ T cell Isolation Kit II (Miltenyi Biotec) according to the manufacturer’s recommendations. Cells were resuspended in 0.9% saline and injected i.v. with either 500,000 or 100,000 cells (for I-Ab restricted chicken ovalbumin peptide 323–339, sequence ISQAVHAAHAEINEAGR, +LPS group) per mouse. The ovalbumin peptide group received 10 μg of LPS mixed with 30 μg of peptide administered i.p. After 24 hours, mice received either nothing, 1 × 106P. chabaudi AS centanamycin attenuated prbcs, or equivalent numbers of centanamycin-attenuated naive rbcs administered i.v. Mice were tail bled approximately 50 μl on day 6 after immunization (day 7 after adoptive transfer) and the phenotype of donor (OT-II, CD45.1+) and host (B6, CD45.2+) were assessed via flow cytometry. Cells were stained with antibodies against CD3, CD4, CD11a, CD49d, CD45.1, and CD45.2. The phenotype and purity of the adoptively transferred cells was also assessed and was found to be 80% CD3+CD4+.
T cell transfer for protection.
Ten female Ptprca (CD45.1) mice received a single dose of 100 μg anti-CD4 monoclonal antibody (clone YTS191.1) i.v. on day 3 prior to adoptive transfer. Spleens from 5 female C57BL/6 mice 3 days after the third immunization with 106P. chabaudi AS centanamycin–attenuated prbcs or from naive C57BL/6 mice were processed (as described above for adoptive transfer) and CD4+ T cells isolated. 107 CD4+ T cells were injected i.v. per CD4-depleted Ptprca mouse. Mice were challenged with 105P. chabaudi AS prbcs i.v. on day 11 after transfer. Additionally, naive Ptprca mice and thrice immunized C57BL/6 that received either centamycin-attenuated nrbcs or P. chabaudi AS prbcs were all also challenged on the same day. Parasitemias and clinical scores were monitored. On day 7 after challenge, approximately 50 μl of blood was collected via tail from Ptprca mice that received no cells or CD4+ T cells from naive or immunized mice and evaluated for phenotype of host and recipient cells. Percent chimerism was calculated as the percentage of leukocytes that were CD3+CD4+CD45.2+CD45.1–.
Intracellular staining for IFN-γ.
Spleens were individually processed in a well of a 6-well plate containing 5 ml medium and a 70-μm nylon cell strainer. The rubber end of a 10-ml syringe was used to gently mash each spleen, and the cell suspensions were strained through their respective 70-μm strainers. Each well was rinsed with additional medium. The cell suspension was spun at 400 g for 5 minutes and supernatant aspirated. rbcs were lysed using 1× Vitalyse buffer for 5 minutes at room temperate and quenched with excess medium. Cells were spun as above and resuspended to 107 cells per ml and 100 μl (106) cells added per well into a round-bottomed 96-well plate. All wells received brefeldin A (BFA) at a final concentration of 10 μg/ml or with the addition of 5 × 106P. chabaudi AS prbcs or equivalent nrbcs or with final concentrations of 0.1 μg/ml PMA and 0.1 μM ionomcyin (all from Sigma-Aldrich). Cells were cultured for 4 hours at 37°C and centrifuged at 400 g for 5 minutes; supernatants were removed by flicking. Cells were washed with PBS and resuspended in PBS containing yellow live/dead fixable stain (Invitrogen) (1:100) and incubated for 30 minutes on ice protected from light. Cells were washed with PBS and Fc receptors blocked by incubating for 10 minutes on ice with 2.4 G2 antibody supernatant. Cells were centrifuged and resuspended in 50 μl of surface antibodies (CD4,CD8,CD11a,CD49d) in PBS and incubated on ice for 20 minutes. Cells were washed three times and resuspended in BD Fix/Perm buffer according to the manufacturer’s recommendations. Cells were subsequently stained with anti–IFN-γ or rat IgG1 isotype control antibodies in fix/perm wash buffer for 20 minutes on ice. After washing, cells were resuspended in 1% paraformaldehyde in PBS and kept at 4°C protected from light until run on a BD LSRFortessa cytometer.
Cytokine bead array.
Three A/J mice were immunized with 3 doses of vaccine (each consisting of 106 centanamycin-attenuated P. chabaudi prbcs), and spleen cells from these mice were collected 50 days after the last immunization, cultured with indicated antigens (as described above for proliferation assay); after 72 hours, culture supernatants were collected. Supernatants from duplicate wells were used for the Mouse Inflammation Kit Cytometric Bead Array (BD) according to the manufacturer’s recommendations, except that volumes of samples and standards were scaled down to 10 μl and 2 μl of each capture bead was used. Samples were run on a BD LSRFortessa cytometer and data analyzed with FCAP array (v1.01 for Windows) software (BD).
ELISA.
To prepare crude parasite antigen, blood from mice infected with P. chabaudiAS at peak parasitemia was collected, washed in PBS, and incubated with 0.01% (wt/vol) saponin (Sigma-Aldrich) at 37°C for 20 minutes. The pellet was washed in PBS, resuspended in PBS, and sonicated. 96-well MaxiSorp Nunc immunoplates (Nunc) were coated overnight at 4°C with 10 μg of parasite antigen/ml in bicarbonate coating buffer (pH 9.6). Wells were blocked for 2 hours at room temperature with PBS/5% BSA. 100 μl of sera diluted 1/20 were added in triplicate, incubated 1 hour at room temperature, washed 5 times with PBS Tween 20 before HRP-conjugated total Ig, IgG1, or IgG2a (BD) were added, and incubated for 1 hour at room temperature. TMB (OptEIA substrate reagent set BD) was added and incubated at room temperature for 30 minutes before adding 1N H2SO4 and reading optical density at 405 nm, using an ELISA reader.
Splenectomy.
Mice were anesthetized with Buprenorphine analgesic (0.05 mg/kg) given subcutaneously and splenectomy performed by Hamish McMath (Animal Facilities Manager, Griffith University).
Statistics.
Unpaired t tests were performed (2 tailed), and values were considered significantly different at P < 0.05.
Study approval.
All experiments were performed with the consent of the Griffith University Animal Ethics Committee. The use of human blood products for culture of P. falciparum was approved by the Griffith University Human Research Ethics Committee (approval # BDD/03/10/HREC). As part of standard Australian Red Cross Blood Service practice, donors consent each time they donate blood or blood products to the use of their donation for research purposes.
Supplementary Material
Acknowledgments
We thank Hamish McMath for performing the splenectomy and sham splenectomy operations, Xue Liu for assistance in parasite culture, Noah Butler for advice on adoptive transfer experiments and flow cytometry studies, and Chris Davis for many useful discussions. M.F. Good acknowledges grant support from an NHMRC Australia Fellowship and Program Grant from Griffith University.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article:J Clin Invest. 2013;123(8):3353–3362. doi:10.1172/JCI66634.
References
- 1.Stevenson MM, Riley EM. Innate immunity to malaria. Nat Rev Immunol. 2004;4(3):169–180. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 2.Langhorne J, Ndungu F, Sponaas A, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9(7):725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 3.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120(12):4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genton B, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis. 2002;185(6):820–827. doi: 10.1086/339342. [DOI] [PubMed] [Google Scholar]
- 5.Sagara I, et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine. 2009;27(23):3090–3098. doi: 10.1016/j.vaccine.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogutu BR, et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. Plos One. 2009;4(3):e4708. doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry AE, Schultz L, Buckee CO, Reeder JC. Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS One. 2009;4(12):e8497. doi: 10.1371/journal.pone.0008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirunpetcharat C, et al. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J Immunol. 1997;159(7):3400–3411. [PubMed] [Google Scholar]
- 9.Singh S, et al. Immunity to recombinant plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect Immun. 2006;74(8):4573–4580. doi: 10.1128/IAI.01679-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77(6 suppl):79–87. [PMC free article] [PubMed] [Google Scholar]
- 11.Pombo DJ, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360(9333):610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 12.Elliott SR, Kuns RD, Good MF. Heterologous immunity in the absence of variant-specific antibodies after exposure to subpatent infection with blood-stage malaria. Infect Immun. 2005;73(4):2478–2485. doi: 10.1128/IAI.73.4.2478-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edstein MD, et al. Lengthy antimalarial activity of atovaquone in human plasma following atovaquone-proguanil administration. Antimicrob Agents Chemother. 2005;49(10):4421–4422. doi: 10.1128/AAC.49.10.4421-4422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makobongo MO, et al. The purine salvage enzyme hypoxanthine guanine xanthine phosphoribosyl transferase is a major target antigen for cell-mediated immunity to malaria. Proc Natl Acad Sci U S A. 2003;100(5):2628–2633. doi: 10.1073/pnas.0337629100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, et al. The mechanism and significance of deletion of parasite-specific CD4(+) T cells in malaria infection. J Exp Med. 2002;195(7):881–892. doi: 10.1084/jem.20011174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinzon-Charry A, et al. Low doses of killed parasite in CpG elicit vigorous CD4+ T cell responses against blood-stage malaria in mice. J Clin Invest. 2010;120(8):2967–2978. doi: 10.1172/JCI39222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aly AS, Downie MJ, Mamoun CB, Kappe SH. Subpatent infection with nucleoside transporter 1-deficient Plasmodium blood stage parasites confers sterile protection against lethal malaria in mice. Cell Microbiol. 2010;12(7):930–938. doi: 10.1111/j.1462-5822.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 18.Annoura T, et al. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine. 2012;30(16):2662–2670. doi: 10.1016/j.vaccine.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Yanow SK, Purcell LA, Lee M, Spithill TW. Genomics-based drug design targets the AT-rich malaria parasite: implications for antiparasite chemotherapy. Pharmacogenomics. 2007;8(9):1267–1272. doi: 10.2217/14622416.8.9.1267. [DOI] [PubMed] [Google Scholar]
- 20.Purcell LA, Yanow SK, Lee M, Spithill TW, Rodriguez A. Chemical attenuation of Plasmodium berghei sporozoites induces sterile immunity in mice. Infect Immun. 2008;76(3):1193–1199. doi: 10.1128/IAI.01399-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell LA, Wong KA, Yanow SK, Lee M, Spithill TW, Rodriguez A. Chemically attenuated Plasmodium sporozoites induce specific immune responses, sterile immunity and cross-protection against heterologous challenge. Vaccine. 2008;26(38):4880–4884. doi: 10.1016/j.vaccine.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu SC, et al. Red cell membrane remodeling in sickle cell anemia. Sequestration of membrane lipids and proteins in Heinz bodies. J Clin Invest. 1996;97(1):29–36. doi: 10.1172/JCI118402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler NS, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13(2):188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Good MF, Dontfraid F, Vinetz JM, Miller LH. Interdependence of CD4+ T cells and malarial spleen in immunity to Plasmodium vinckei vinckei. Relevance to vaccine development. J Immunol. 1989;143(6):2017–2023. [PubMed] [Google Scholar]
- 25.Playfair JH, De Souza JB, Dockrell HM, Agomo PU, Taverne J. Cell-mediated immunity in the liver of mice vaccinated against malaria. Nature. 1979;282(5740):731–734. doi: 10.1038/282731a0. [DOI] [PubMed] [Google Scholar]
- 26.Dockrell HM, de Souza JB, Playfair JH. The role of the liver in immunity to blood-stage murine malaria. Immunology. 1980;41(2):421–430. [PMC free article] [PubMed] [Google Scholar]
- 27.Yanow SK, et al. Potent antimalarial and transmission-blocking activities of centanamycin, a novel DNA-binding agent. J Infect Dis. 2008;197(4):527–534. doi: 10.1086/526788. [DOI] [PubMed] [Google Scholar]
- 28.Weiss L, Geduldig U, Weidanz W. Mechanisms of splenic control of murine malaria: reticular cell activation and the development of a blood-spleen barrier. Am J Anat. 1986;176(3):251–285. doi: 10.1002/aja.1001760303. [DOI] [PubMed] [Google Scholar]
- 29.Alves HJ, Weidanz W, Weiss L. The spleen in murine Plasmodium chabaudi adami malaria: stromal cells, T lymphocytes, and hematopoiesis. Am J Trop Med Hyg. 1996;55(4):370–378. doi: 10.4269/ajtmh.1996.55.370. [DOI] [PubMed] [Google Scholar]
- 30.Florens L, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419(6906):520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson M, Zavala F. Immunology of malaria infections. Parasite Immunology. 2006;28(1–2):1–4. doi: 10.1111/j.1365-3024.2006.00797.x. [DOI] [PubMed] [Google Scholar]
- 32.Good MF, Xu H, Wykes M, Engwerda CR. Development and regulation of cell-mediated immune responses to the blood stages of malaria: implications for vaccine research. Annu Rev Immunol. 2005;23:69–99. doi: 10.1146/annurev.immunol.23.021704.115638. [DOI] [PubMed] [Google Scholar]
- 33.Urban BC, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400(6739):73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 34.Sanderson F, Andrews L, Douglas AD, Hunt-Cooke A, Bejon P, Hill AV. Blood-stage challenge for malaria vaccine efficacy trials: a pilot study with discussion of safety and potential value. Am J Trop Med Hyg. 2008;78(6):878–883. [PubMed] [Google Scholar]
- 35.McCarthy JS, Good MF. Whole parasite blood stage malaria vaccines: a convergence of evidence. Hum Vaccin. 2010;6(1):114–123. doi: 10.4161/hv.6.1.10394. [DOI] [PubMed] [Google Scholar]
- 36.Cheng Q, et al. Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am J Trop Med Hyg. 1997;57(4):495–500. doi: 10.4269/ajtmh.1997.57.495. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence G, et al. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine. 2000;18(18):1925–1931. doi: 10.1016/S0264-410X(99)00444-2. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman SL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 39.Epstein JE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science. 2011;334(6055):475–480. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 40.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433(7022):164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 41.Roestenberg M, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361(5):468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 42.Agnandji ST, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365(20):1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 43.Sato A, et al. A novel class of in vivo active anticancer agents: achiral seco-amino- and seco-hydroxycyclopropylbenz[e]indolone (seco-CBI) analogues of the duocarmycins and CC-1065. J Med Chem. 2005;48(11):3903–3918. doi: 10.1021/jm050179u. [DOI] [PubMed] [Google Scholar]
- 44.Howard TT, et al. Novel furano analogues of duocarmycin C1 and C2: design, synthesis, and biological evaluation of seco-iso-cyclopropylfurano[2,3-e]indoline (seco-iso-CFI) and seco-cyclopropyltetrahydrofurano[2,3-f]quinoline (seco-CFQ) analogues. Bioorg Med Chem. 2002;10(9):2941–2952. doi: 10.1016/S0968-0896(02)00157-8. [DOI] [PubMed] [Google Scholar]
- 45.Chavda S, et al. A novel achiral seco-cyclopropylpyrido[e]indolone (CPyI) analog of CC-1065 and the duocarmycins: synthesis, DNA interactions, in vivo anticancer and anti-parasitic evaluation. Bioorg Med Chem. 2010;18(14):5016–5024. doi: 10.1016/j.bmc.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 46.Grimberg BT, Erickson JJ, Sramkoski RM, Jacobberger JW, Zimmerman PA. Monitoring Plasmodium falciparum growth and development by UV flow cytometry using an optimized Hoechst-thiazole orange staining strategy. Cytometry A. 2008;73(6):546–554. doi: 10.1002/cyto.a.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.