Abstract
Aim:
To examine the effects of anisomycin on glioma cells and the related mechanisms in vitro.
Methods:
The U251 and U87 human glioblastoma cell lines were tested. The growth of the cells was analyzed using a CCK-8 cell viability assay. Apoptosis was detected using a flow cytometry assay. The expression of proteins and phosphorylated kinases was detected using Western blotting.
Results:
Treatment of U251 and U87 cells with anisomycin (0.01–8 μmol/L) inhibited the cell growth in time- and concentration-dependent manners (the IC50 values at 48 h were 0.233±0.021 and 0.192±0.018 μmol/L, respectively). Anisomycin (4 μmol/L) caused 21.5%±2.2% and 25.3%±3.1% of apoptosis proportion, respectively, in U251 and U87 cells. In the two cell lines, anisomycin (4 μmol/L) activated p38 MAPK and JNK, and inactivated ERK1/2. However, neither the p38 MAPK inhibitor SB203580 (10 μmol/L) nor the JNK inhibitor SP600125 (10 μmol/L) prevented anisomycin-induced cell death. On the other hand, anisomycin (4 μmol/L) reduced the level of PP2A/C subunit (catalytic subunit) in a time-dependent manner in the two cell lines. Treatment of the two cell lines with the PP2A inhibitor okadaic acid (100 nmol/L) caused marked cell death.
Conclusion:
Anisomycin induces glioma cell death via down-regulation of PP2A catalytic subunit. The regulation of PP2A/C exression by anisomycin provides a clue to further study on its role in glioma therapy.
Keywords: anisomycin, glioma, apoptosis, p38 MAPK, JNK, protein phosphatase 2A
Introduction
Anisomycin is an antibiotic that blocks protein synthesis by inhibiting peptidyl transferase activity in ribosomes1. It was reported to induce apoptosis in a variety of cell types, including colon adenocarcinoma cells, leukemia cells, Jurkat cells and ventricular myocytes2,3,4,5. Anisomycin was widely used as an agonist for p38 mitogen-activated protein kinase (p38 MAPK) and Jun-NH2 terminal kinase (JNK) in studies investigating the p38 and JNK signaling pathways. Anisomycin typically induces apoptosis through activation of the p38 MAPK and/or the JNK pathway. A recent novel finding demonstrated that, independent of its ability to activate p38 MAPK and JNK, anisomycin can also decrease FLIP (c-Fas — associated death domain-like interleukin-1 (IL-1) — converting enzyme-like inhibitory protein) protein synthesis in prostate cancer cells. This decrease in FLIP expression sensitizes cells to anoikis, which is the initiation of apoptosis that is triggered by a loss of contact with the extracellular matrix6.
In this study, we found that anisomycin induces U251 and U87 human glioblastoma cell death independent of its ability to activate p38 MAPK and JNK and their downstream kinases and transcription factors. Our data demonstrate that anisomycin suppressed U251 and U87 cell growth in a concentration dependent manner, and at 8 μmol/L, the cell viability was reduced to 18.4%±2.1% for U251 and 14.6%±1.3% for U87. However, neither p38 MAPK nor JNK inhibitors prevented anisomycin-induced U251 cell death. We also found that anisomycin induced U251 cell death independent of its role as an anoikis sensitizer. Interestingly, our study revealed that anisomycin down-regulates PP2A C subunit protein expression and almost totally inhibits PP2A/C expression after 48 h treatment. C subunit is the catalytic site of PP2A, and its downregulation can greatly decrease PP2A activity. Our results showed treatment of PP2A inhibitor okadaic acid (OA) causes significant cell death in U251 and U87 cells, indicating that anisomycin-induced glioma cell death may caused by downregulation of PP2A catalytic subunit. These findings may open new avenues for studying the mechanisms underlying p38 MAPK and JNK pathway-independent anisomycin-induced cell death and further research into the use of anisomycin as a glioma therapeutic agent.
Materials and methods
Agents and antibodies
Anisomycin, SB203580 and SP600125 were purchased from Sigma-Aldrich (St Louis, MO, USA). PP2A inhibitor OA was purchased from Beyotime Institute of Biotechnology (Shanghai, China). For cell treatment, agents were dissolved in dimethyl sulfoxide (DMSO) and then diluted in serum-supplemented media immediately before use. The final concentrations of SB203580 and SP600125 were 10 μmol/L, OA was 100 nmol/L. Anisomycin was used at the following concentrations: 0.01, 0.04, 0.1, 0.4, 1, 2, 4, or 8 μmol/L.
Antibodies against GAPDH, p38, phospho-p38 (Thr180/Tyr182), JNK, phospho-JNK (Thr183/Tyr185), ERK1/2, phospho-ERK1/2 (Thr202/Tyr204), phospho-ATF-2 (Thr71), phospho-Hsp27 (Ser15), phospho-c-JUN (Ser63 and Ser73), PP2A C subunit, caspase-8 and FLIP were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell culture
The U251 and U87 human glioblastoma cell lines were gifts from Professor Kun YAO (Department of Microbiology, Nanjing Medical University, Nanjing, China). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, glutamine, nonessential amino acids, and 1% penicillin/streptomycin (complete medium). The cells were grown at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Cell viability assay
A cell viability assay was performed using the cell counting kit-8 (CCK-8) according to the manufacturer's instructions (Dojindo Laboratories, Kumamoto, Japan). The cells were plated in 96-well plates in 200 μL of culture media with various concentrations of anisomycin. The cells were then cultured at 37 °C in a humidified incubator containing 95% air and 5% CO2. After 48 h, the CCK-8 solution was added to each well and incubated for 1 h in the incubator. The absorbance measurement was performed at 450 nm using an enzyme-linked immunosorbent assay plate reader (Bio-Rad Laboratories, Inc., Berkeley, CA, USA).
Apoptosis assay by flow cytometry
Cells were plated in 10-cm culture dishes, allowed to adhere for 8 h and then exposed to anisomycin for 48 h at 37 °C. After 48 h, the cells were collected by trypsinization, centrifuged (3500 r/min for 5 min), and washed twice with PBS. The cells were fixed in 1 mL of 70% ethanol, pelleted by centrifugation (3500 r/min for 5 min), rinsed twice with PBS, Then, cells were incubated for 15 min at room temperature with annexin V-FITC and propidium iodide before analysis with a FACSAria III flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blotting
The cells were lysed in an appropriate volume of lysis buffer (Cell Signaling Technology, Beverly, MA, USA). Cell lysates were heat denatured for 8 min and separated by SDS-PAGE. After gel electrophoresis, the proteins were transferred to an Immobilon-P Transfer membrane (Millipore Corporation, Bedford, MA, USA). Membranes were blocked for 1.5 h at room temperature in TBST (0.1% Tween-20+TBS) containing 5% (w/v) non-fat dry milk. After blocking, the membranes were probed overnight at 4 °C with the primary antibodies, followed by a 1-h incubation with the secondary antibodies at room temperature. Antibody detection was performed using the Super Signal West Pico chemiluminescent substrate (Pierce, Chicago, IL, USA) and the ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All data were analyzed by the Student-Newman-Keuls test and expressed as the mean±standard deviations (SD). The results at P<0.05 were considered to be statistical significant.
Results
Anisomycin inhibits U251 and U87 cell growth and induces apoptosis
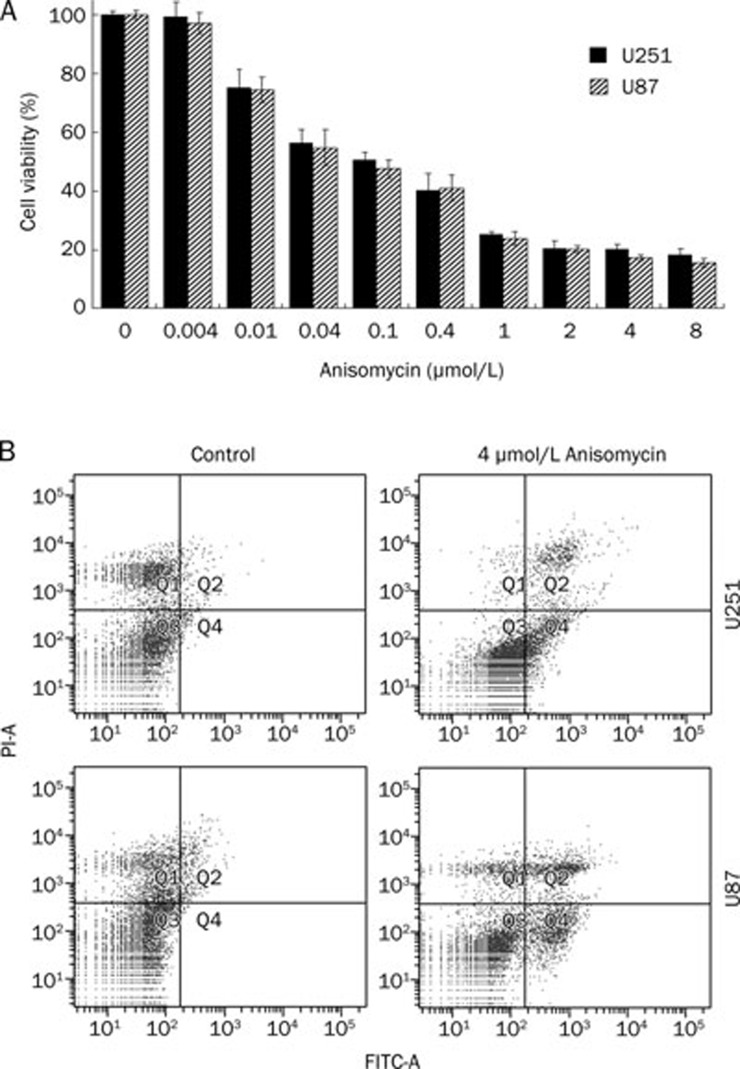
To study the effect of anisomycin on U251 and U87 cell growth, cells were treated with anisomycin at the following concentrations: 0.004, 0.01, 0.04, 0.1, 0.4, 1, 2, 4, or 8 μmol/L for 48 h. Cell viability was analyzed using a CCK-8 kit. The data demonstrated that U251 cell growth was suppressed by anisomycin at as low a concentration as 0.01 μmol/L (with cell viability at 75.3%±6.1% for U251 and 74.4%±4.3% for U87). At the high concentration of anisomycin (8 μmol/L), cell viability was reduced to 18.4%±2.1% for U251 and 15.6%±1.3% for U87 (Figure 1A). The IC50 at 48 h was measured as 0.233±0.021 μmol/L for U251 and 0.192±0.018 μmol/L for U87.
Figure 1.
Anisomycin inhibits U251 and U87 cell growth and induces cell apoptosis. (A) U251 and U87 cells were separately treated with anisomycin at concentrations of 0, 0.004, 0.01, 0.04, 0.1, 0.4, 1, 2, 4, or 8 μmol/L for 48 h. Cell viability was analyzed using CCK-8 kit. Anisomycin started to suppress U251 and U87 cell growth at concentration of 0.01 μmol/L (cell viability is 75.3%±6.1% for U251 and 74.4%±4.3% for U87). At 8 μmol/L, the cell viability is only 18.4%±2.1% for U251 and 15.6%±1.3% for U87. Anisomycin inhibits U251 and U87 cell growth in a concentration-dependent manner. (B) U251 or U87 cells were treated with 0 or 4 μmol/L anisomycin for 72 h, and double stained with annexin V-FITC conjugates and propidium iodide followed by analysing in a flow cytometer. The Q2 plus Q4 proportions indicated apoptosis cells, 4 μmol/L anisomycin caused 21.5%±2.2% of apoptosis proportion in U251 cells and 25.3%±3.1% in U87 cells.
We also detected the presence of apoptosis by flow cytometry. The cells were treated with 0 or 4 μmol/L anisomycin for 72 h, and double stained with annexin V-FITC conjugates and propidium iodide, which was followed by analysis with a flow cytometer. In this assay, the sub-G1 fraction represents apoptotic cells (indicated by black arrows in Figure 1B). We found that the Q2 plus Q4 proportions indicated apoptosis cells, 4 μmol/L anisomycin caused 21.5%±2.2% of apoptosis proportion in U251 cells and 25.3%±3.1% in U87 cells.
Anisomycin activates p38 MAPK and JNK but inactivates ERK1/2 in U251 and U87 cells
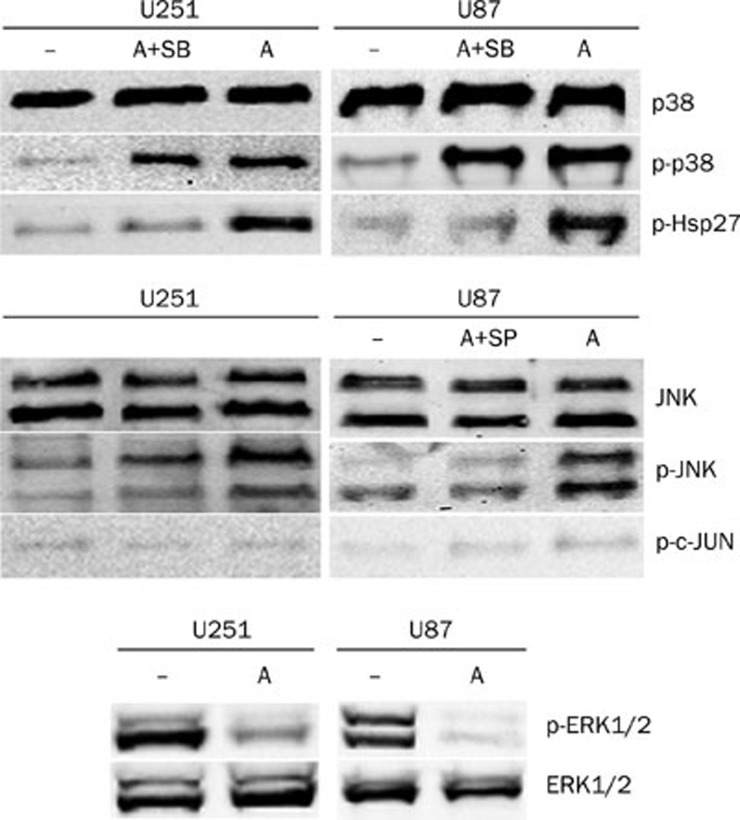
The phosphorylation of p38 MAPK, JNK, and ERK1/2 was detected in U251 and U87 cells either untreated or treated with 4 μmol/L anisomycin for 30 min. As shown in Figure 2, anisomycin-induced phosphorylation of p38 MAPK at Thr180/Tyr182 and JNK at Thr183/Tyr185. To investigate the effect of anisomycin on the downstream substrates of p38 and JNK, we detected the phosphorylation of Hsp27, c-JUN, and ATF-2 by Western blotting. We found that anisomycin activated both Hsp27 (Ser15), which is phosphorylated by MAPKAP kinase 2 as a result of the activation of the p38 MAPK pathway, and ATF-2 (Thr71), which can be phosphorylated by both JNK and p38 MAPK. However, c-JUN (Ser63 or Ser73), the substrate of JNK, was not activated in anisomycin treated U251 or U87 cells, compared with control treated cells.
Figure 2.
Anisomycin activates p38MAPK and JNK but inactivates ERK1/2 in U251 and U87 cells. Cells were non-treated (–) or treated with 4 μmo/L anisomycin for 30 min, or pre-treated with SB203580 or SP600125 for 1 h and then treated with 4 μmol/L anisomycin for 30 min. The phosphorylated and unphosphorylated p38MAPK, JNK, and ERK1/2 were detected by Western blotting using corresponding antibodies. The activations (phosphorylations) of the downstream substrates of p38 and JNK, Hsp27, and c-JUN were also detected. The unphosphorylated MAPKs served as loading control. A: Anisomycin; SB: SB203580; SP: SP600125.
ERK1/2 is another well-studied member of the MAPKs, but its function is usually distinct from the other two members, p38, and JNK. Our results demonstrated that activated ERK1/2 expression was high in untreated U251 or U87 cells but very low in anisomycin treated cells (Figure 2).
Neither p38 MAPK nor JNK inhibitors prevented anisomycin-induced U251 cell death
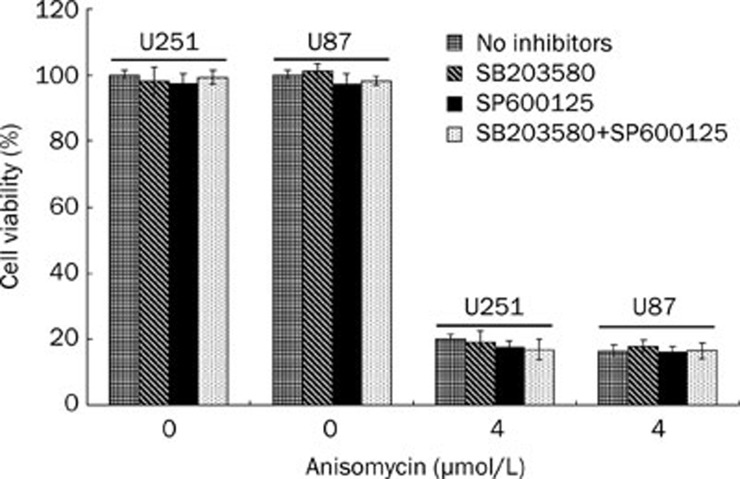
To investigate whether the p38 MAPK or the JNK pathway was mediating anisomycin-induced U251 cell death, the p38 MAPK inhibitor SB203580 and the JNK inhibitor SP600125 were used to pre-treat U251 or U87 cells. First of all, the inhibitory effects of SB203580 and SP600125 on anisomycin treated cells were analyzed (Figure 2). Our results showed SB203580 significantly inhibits activation of Hsp27 which is the downstream substrate of p38MAPK but does not inhibit the phosphorylation of p38MAPK, indicating that SB203580 inhibit p38MAPK activity but not its activation. For SP600125, we demonstrated it significantly inhibits the phosphorylation of JNK (Figure 2). Cells were divided into four groups and pre-treated with either DMSO vehicle control, 10 μmol/L SB203580, 10 μmol/L SP600125 or the combination of 10 μmol/L SB203580 and 10 μmol/L SP600125. After 1 h, the pre-treated cells were treated with with 0 or 4 μmol/L anisomycin for 48 h. A CCK-8 kit was used to detect the quantity of viable cells and determine cell viability. As the results show in Figure 3, neither SB203580 nor SP600125 prevented anisomycin-induced U251 or U87 cell death (P>0.05).
Figure 3.
Neither SB203580 nor SP600125 prevents anisomycin-induced U251 or U87 cell death. U251 or U87 cells were divided into four groups and separately pre-treated with DMSO vehicle, 10 μmol/L SB203580, 10 μmol/L SP600125, or 10 μmol/L SB203580 plus 10 μmol/L SP600125. After 1 h, the pre-treated cells were treated with 0 or 4 μmol/L anisomycin for 48 h. Then, cell viability was detected using CCK-8 kit. In groups that pre-treated with 10 μmol/L SB203580, 10 μmol/L SP600125, or 10 μmol/L SB203580 plus 10 μmol/L SP600125, anisomycin-induced cell death was not prevented (P>0.05).
Anisomycin induces U251 and U87 cell death independent of its ability to sensitize cells to anoikis
Caspase-8 and the caspase-8 inhibitor FLIP have been reported to participate in anisomycin-induced apoptosis6. Anisomycin can induce apoptosis by decreasing levels of FLIP, resulting in a sensitization of cells to anoikis. We detected the levels of full-length caspase-8, cleaved caspase-8 and FLIP by Western blotting lysates from U251 and U87 cells that were either untreated or treated with anisomycin. We found that full-length caspase-8 and FLIP were both undetectable in untreated U251 and U87 cells. In the cells treated with anisomycin, no activated cleaved caspase-8 was detected (data not shown). Our results suggest that anisomycin-induced U251 and U87 cell death is independent of its role as an anoikis sensitizer.
Anisomycin down-regulates PP2A C subunit expression in U251 and U87 cells
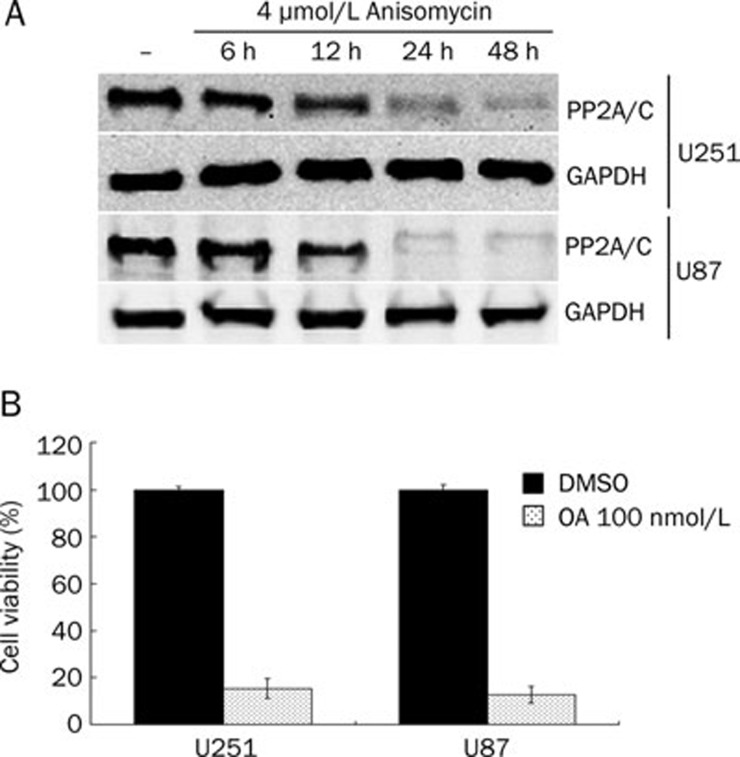
Depletion of the PP2A C subunit has been shown to be lethal in cells. We detected the effect of anisomycin on the expression of the PP2A C subunit in U251 and U87 cells by Western blotting. We found that anisomycin (4 μmol/L) down-regulates PP2A C subunit protein expression in a time-dependent manner and almost completely inhibits its expression after 48 h of treatment (Figure 4). C subunit is the catalytic part of PP2A, and its downregulation can greatly decrease PP2A activity. To investigate whether inhibition of PP2A activity could cause U251 or U87 cell death, we used PP2A inhibitor OA 100 nmol/L to treat U251 and U87 cells for 48 h and detected the cell viability by CCK-8. In OA treated cells, the cell viability was 15.3%±4.2% for U251 and 12.8%±3.5% for U87. We demonstrated that PP2A inhibition in glioblastoma cells causes significant cell death (Figure 4B). These results indicate that anisomycin may induce U251 and U87 cell death by downregulation of PP2A catalytic subunit.
Figure 4.
Anisomycin down-regulates PP2A C subunit level in U251 and U87 cells. (A) U251 and U87 cells were non-treated (–) or treated with 4 μmol/L anisomycin for 6, 12, 24, or 48 h. PP2A C subunit level was detected by Western blotting. GAPDH served as loading control. (B) U251 and U87 cells were treated with 0 or 100 nmol/L okadaic acid (OA) for 48 h, then the cell viability were detected by using CCK-8 kit. In OA treated cells, the cell viability was 15.3%±4.2% for U251 and 12.8%±3.5% for U87.
Discussion
Anisomycin is an agonist of p38 and JNK and is widely used as a research tool to study these two pathways in various cellular processes. Anisomycin is also well known as a protein synthesis inhibitor that is involved in apoptosis7,8,9. In this study, we investigated the effects of anisomycin on U251 and U87 human glioblastoma cells. We first detected the effects of anisomycin on U251 and U87 cell growth and found that anisomycin efficiently induced cell death (at 8 μmol/L, cell viability was reduced to 18.4%±2.1%). Previous studies suggest that MAPK plays a major role in anisomycin-induced apoptosis10,11,12. Stress stimuli, including UV irradiation, anoxia and translation inhibitors like anisomycin, can activate p38 and JNK pathways and induce apoptosis13. ERK1/2 is typically activated by growth factors and phorbol esters and contributes to cell proliferation, differentiation, and survival. These three MAPK pathways interact with each other by cross-talking to influence the cellular outcome14,15. Thus, we investigated the effects of anisomycin on the phosphorylation of the three major MAPKs: p38, JNK, and ERK1/2. Our results demonstrated that p38 and JNK, as well as their downstream effectors Hsp27 and ATF-2, were activated by anisomycin, whereas ERK1/2 was dephosphorylated after anisomycin treatment. To confirm whether the p38 and JNK pathways played a role in anisomycin-induced cell death, we introduced their inhibitors, SB203580 and SP600125, into this study. We found that neither of the two inhibitors could prevent anisomycin-induced cell death, even when used in combination. These data indicate that anisomycin induces U251 and U87 cell death independent of its ability to activate p38 MAPK and JNK. These data led to the following important question: through which pathway does anisomycin induce U251 cell death?
A recent study addressed a similar question6. Using a chemical screen, the authors identified a novel mechanism by which anisomycin induces apoptosis. They demonstrated that, independent of the ability to activate p38 MAPK and JNK, anisomycin can also induce apoptosis by decreasing expression of the caspase-8 inhibitor FLIP, resulting in cells being sensitized to anoikis. The term “anoikis” refers to a self-initiated process, during which nonmalignant cells undergo apoptosis upon detachment from the extracellular matrix (ECM)16,17. More recently, it was found that not only endothelial cells and nonmalignant epithelial cells but also malignant epithelial cells could undergo anoikis through self-initiated activation of the death receptor signaling pathway17,18,19,20. The results from these studies suggested that anisomycin initiates anoikis in a caspase-8-dependent manner, due to its inhibition of FLIP protein synthesis, and independent of its ability to activate JNK and p386. To investigate whether anisomycin initiates anoikis in U251 and U87 cells, we detected the expression of full-length caspase-8, cleaved caspase-8 and FLIP protein by Western blotting. The results showed that full-length caspase-8 and FLIP protein were both undetectable in untreated U251 and U87 cells, and in anisomycin treated cells, active cleaved caspase-8 was also negative. Ashley et al21 reported similar results in 2005. They demonstrated that caspase-8 is absent or low in many gliomas, and almost no gliomas express significant amounts of cellular FLIP. Taken together, these data suggest that initiation of anoikis cannot be the mechanism by which anisomycin induces U251 or U87 cell death.
In our study, we present a novel finding that may provide a new perspective for investigating anisomycin-induced apoptosis. We detected the protein expression of the PP2A C subunit in anisomycin treated U251 and U87 cells. The results showed that anisomycin down-regulates PP2A/C expression in a time-dependent manner. After 48 h of treatment with anisomycin, almost no PP2A/C protein could be detected. PP2A/C is the catalytic subunit, and its downregulation can greatly decrease PP2A activity. We hypothesize that in anisomycin-induced U251 and U87 cell death, PP2A/C may be a target that initiates apoptosis. To investigate whether inhibition of PP2A activity could cause U251 or U87 cell death, we introduced a PP2A inhibitor Okadaic acid (OA). U251 and U87 cells were treated with 100 nmol/L OA and then the cell viability were evaluated by using CCK-8. Our results demonstrated that PP2A inhibition in U251 and U87 cells causes significant cell death. This indicated that anisomycin-induced U251 and U87 cell death may directly caused by downregulation of PP2A catalytic subunit. Lots of previous studies have proven the direct effects of PP2A/C on apoptosis, although none of these studies investigated anisomycin-induced apoptosis. Deletion of the PP2A/Cα subunit in homozygous null mutant mice is embryonically lethal22. Silencing of the PP2A/C gene was found to greatly decrease PP2A activity and lead to localized death in plant stems and cells23. Knockdown of the PP2A/C led to concurrent loss of nontargeted PP2A subunits, and caused cell death with the morphological and biochemical changes characteristic of apoptosis in cultured S2 cells24. Wong et al found that the introduction of siPP2A/Cα to cells caused a noticeably accelerated cell death in both the BT474 and the SKBR3 cell lines25. In future studies, PP2A/C would be the focus of our research, not only because of its potential role in mediating anisomycin-induced U251 cell death but also because of its potential role as a target for glioma therapy.
In conclusion, our findings demonstrate that anisomycin can induce U251 cell death independent of its ability to activate p38 MAPK and JNK or its ability to sensitize cells to anoikis. The novel finding that anisomycin sharply down-regulates PP2A/C protein expression may provide new perspectives for anisomycin-induced apoptosis and further studies with anisomycin in glioma therapy.
Author contribution
Jun-yang LI and Wei-xing HU designed the research; Jun-yang LI, Jia-yuan HUANG, Meng LI, Han ZHANG, Biao XING, Gong CHEN, Dong WEI, and Pei-yuan GU performed the research; Jun-yang LI, Jia-yuan HUANG, Meng LI, and Wei-xing HU analyzed the data; Jun-yang LI wrote the paper; and Wei-xing HU revised the paper.
Acknowledgments
We thank Professor Kun YAO for providing the U251 and U87 human glioblastoma cell line. This work was supported by grants from the Jiangsu Province Natural Science Foundation (No BK2007257).
References
- Gold PE. Protein synthesis inhibition and memory: formation vs amnesia. Neurobiol Learn Mem. 2008;89:201–11. doi: 10.1016/j.nlm.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Choi HJ, Park SH, Kim JS, Moon Y. Macrophage inhibitory cytokine-1 (MIC-1) and subsequent urokinase-type plasminogen activator mediate cell death responses by ribotoxic anisomycin in HCT-116 colon cancer cells. Biochem Pharmacol. 2009;78:1205–13. doi: 10.1016/j.bcp.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Lunghi P, Tabilio A, Pinelli S, Valmadre G, Ridolo E, Albertini R, et al. Expression and activation of SHC/MAP kinase pathway in primary acute myeloid leukemia blasts. Hematol J. 2001;2:70–80. doi: 10.1038/sj/thj/6200095. [DOI] [PubMed] [Google Scholar]
- Caricchio R, D'Adamio L, Cohen PL. Fas, ceramide and serum withdrawal induce apoptosis via a common pathway in a type II Jurkat cell line. Cell Death Differ. 2002;9:574–80. doi: 10.1038/sj.cdd.4400996. [DOI] [PubMed] [Google Scholar]
- Clerk A, Sugden PH. Cell stress-induced phosphorylation of ATF2 and c-Jun transcription factors in rat ventricular myocytes. Biochem J. 1997;325:801–10. doi: 10.1042/bj3250801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawji IA, Simpson CD, Gronda M, Williams MA, Hurren R, Henderson CJ, et al. A chemical screen identifies anisomycin as an anoikis sensitizer that functions by decreasing FLIP protein synthesis. Cancer Res. 2007;67:8307–15. doi: 10.1158/0008-5472.CAN-07-1687. [DOI] [PubMed] [Google Scholar]
- Piekarski DJ, Seto T, Zucker I. The protein synthesis inhibitor anisomycin reduces sex behavior during a critical period after testosterone treatment in male Syrian hamsters. Physiol Behav. 2011;105:215–9. doi: 10.1016/j.physbeh.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Sadowski RN, Canal CE, Gold PE. Lidocaine attenuates anisomycin-induced amnesia and release of norepinephrine in the amygdala. Neurobiol Learn Mem. 2011;96:136–42. doi: 10.1016/j.nlm.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysheva MV, Grigoryev AA, Bulycheva TI, Zatsepina OV. Enhanced sensitivity of nucleoli in human proliferating cells to inhibition of protein synthesis with anisomycin. Bull Exp Biol Med. 2010;150:258–62. doi: 10.1007/s10517-010-1118-6. [DOI] [PubMed] [Google Scholar]
- Croons V, Martinet W, Herman AG, Timmermans JP, De Meyer GR. The protein synthesis inhibitor anisomycin induces macrophage apoptosis in rabbit atherosclerotic plaques through p38 mitogen-activated protein kinase. J Pharmacol Exp Ther. 2009;329:856–64. doi: 10.1124/jpet.108.149948. [DOI] [PubMed] [Google Scholar]
- Desgranges B, Levy F, Ferreira G. Anisomycin infusion in amygdala impairs consolidation of odor aversion memory. Brain Res. 2008;1236:166–75. doi: 10.1016/j.brainres.2008.07.123. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang JY, Liu SJ, Li HL. Overactivated mitogen-activated protein kinase by anisomycin induces tau hyperphosphorylation. Sheng Li Xue Bao. 2008;60:485–91. [PubMed] [Google Scholar]
- Qiao S, Murakami K, Zhao Q, Wang B, Seo H, Yamashita H, et al. Mimosine-induced apoptosis in C6 glioma cells requires the release of mitochondria-derived reactive oxygen species and p38, JNK activation. Neurochem Res. 2012;37:417–27. doi: 10.1007/s11064-011-0628-6. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–31. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Mawji IA, Simpson CD, Hurren R, Gronda M, Williams MA, Filmus J, et al. Critical role for Fas-associated death domain-like interleukin-1-converting enzyme-like inhibitory protein in anoikis resistance and distant tumor formation. J Natl Cancer Inst. 2007;99:811–22. doi: 10.1093/jnci/djk182. [DOI] [PubMed] [Google Scholar]
- Frisch SM. Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr Biol. 1999;9:1047–9. doi: 10.1016/s0960-9822(99)80455-2. [DOI] [PubMed] [Google Scholar]
- Rosen K, Shi W, Calabretta B, Filmus J. Cell detachment triggers p38 mitogen-activated protein kinase-dependent overexpression of Fas ligand. A novel mechanism of Anoikis of intestinal epithelial cells. J Biol Chem. 2002;277:46123–30. doi: 10.1074/jbc.M207883200. [DOI] [PubMed] [Google Scholar]
- Ashley DM, Riffkin CD, Muscat AM, Knight MJ, Kaye AH, Novak U, et al. Caspase 8 is absent or low in many ex vivo gliomas. Cancer. 2005;104:1487–96. doi: 10.1002/cncr.21323. [DOI] [PubMed] [Google Scholar]
- Gotz J, Probst A, Ehler E, Hemmings B, Kues W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha. Proc Natl Acad Sci U S A. 1998;95:12370–5. doi: 10.1073/pnas.95.21.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Anderson JC, del Pozo O, Gu YQ, Tang X, Martin GB. Silencing of subfamily I of protein phosphatase 2A catalytic subunits results in activation of plant defense responses and localized cell death. Plant J. 2004;38:563–77. doi: 10.1111/j.1365-313X.2004.02073.x. [DOI] [PubMed] [Google Scholar]
- Li X, Scuderi A, Letsou A, Virshup DM. B56-associated protein phosphatase 2A is required for survival and protects from apoptosis in Drosophila melanogaster. Mol Cell Biol. 2002;22:3674–84. doi: 10.1128/MCB.22.11.3674-3684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LL, Zhang D, Chang CF, Koay ES. Silencing of the PP2A catalytic subunit causes HER-2/neu positive breast cancer cells to undergo apoptosis. Exp Cell Res. 2010;316:3387–96. doi: 10.1016/j.yexcr.2010.06.007. [DOI] [PubMed] [Google Scholar]