Abstract
Prenatal nicotine exposure (PNE) is correlated with breathing abnormalities in humans and other animals. Despite evidence that this relationship results from alterations in nicotinic acetylcholine receptors (nAChRs), the mechanisms are poorly understood. Here, we hypothesize that PNE blunts nAChR-mediated respiratory-related motor output. We also hypothesize that the PNE-induced changes in nAChRs leads to secondary alterations in glutamatergic neurotransmission. To test these hypotheses, we used an in vitro brainstem-spinal cord preparation and recorded C4 ventral root (C4 VR) nerve bursts from 0 to 4-day-old rats that were exposed to either nicotine (6 mg kg−1 day−1) or saline (control) in utero. Nicotine bitartrate, nAChR antagonists, NMDA and AMPA were applied to the brainstem compartment of a “split-bath” configuration, which physically separated the medulla from the spinal cord. Nicotine (0.2 or 0.5 μM) increased peak C4 VR burst frequency by over 230% in control pups, but only 140% in PNE animals. The application of nAChR antagonists showed that these effects were mediated by the α4β2 nAChR subtype with no effect on α7 nAChRs in either group. We also show that AMPA-mediated excitatory neurotransmission is enhanced by PNE, but NMDA-mediated neurotransmission is unaltered. These data and the work of others suggest that the PNE may functionally desensitize α4β2 nAChRs located on the presynaptic terminals of glutamatergic neurons leading to less neurotransmitter release, which in turn up-regulates AMPA receptors on rhythm generating neurons.
Keywords: Nicotinic receptor, Control of breathing, Nicotine, Prenatal nicotine exposure, Respiratory rhythm generation
1. Introduction
In human and non-human animals the in utero exposure to exogenous nicotine has been linked to a variety of in vivo and in vitro ventilatory control abnormalities (for a thorough review of this literature see Hafstrom et al., 2005). This correlation is due to nicotine’s action as a potent neuroteratogen, which binds and modifies neuronal nAChRs throughout the brain (Pauly et al., 1991; Nordberg et al., 1992; Slotkin, 2004; Levin et al., 2006), including brainstem cardiorespiratory control regions (Huang et al., 2004a; Huang et al., 2004b; Kamendi et al., 2006). Neuronal nAChRs are often located presynaptically and are thought to modulate synaptic transmission by promoting the release of several neurotransmitters, including noradrenaline, GABA, glutamate, glycine and acetylcholine (Wonnacott, 1997; Vizi and Lendvai, 1999). When chronically exposed to nicotine, however, nAChRs may desensitize or become “functionally down-regulated” in a subtype specific manner (Gentry and Lukas, 2002; Giniatullin et al., 2005) leading to a persistent reduction of neurotransmitter release which can subsequently trigger compensatory changes in the expression of postsynaptic receptors (Mansvelder and McGehee, 2000; Mansvelder et al., 2002).
Consistent with this scenario, we have recently shown that prenatal nicotine exposure (PNE) leads to exaggerated decrements in the frequency of rhythmic respiratory-related motor output when agonists of GABAA and glycine receptors are applied to brainstem-spinal cord (BSSC) preparations from neonatal rats (Luo et al., 2004, 2007). These data show that PNE up-regulates inhibitory neurotransmission in medullary respiratory neurons, especially in the preBötzinger complex (preBötC) (Luo et al., 2007). Yet, how nAChRs may be involved is still poorly understood.
The mechanism by which PNE alters central ventilatory control presumably begins with its effects on nAChRs. Surprisingly, only a small number of studies have addressed how PNE evokes changes in the central nicotinic control of breathing, and no study has examined PNE-evoked alterations in fictive breathing frequency in response to exogenous nicotine. This is an important exclusion because nAChRs play a critical role in respiratory-related frequency modulation within brainstem rhythm generating networks (Shao and Feldman, 2001, 2002; Shao et al., 2008) and may also respond to state dependent acetylcholine release from cholinergic neurons in the intact animal (Bellingham and Ireland, 2002). Consequently, our goal was to examine the effects of PNE on central nAChR function in the BSSC preparation pioneered by Suzue (1984). We hypothesize that PNE functionally desensitizes neuronal nAChRs and depresses fictive respiratory-related motor output in response to exogenous nicotine applied to the medulla. We also tested whether PNE elicits these changes by altering the functional expression of specific nAChR subtypes (i.e., α4β2 and α7), which are known to be important for the modulation of respiratory frequency in the preBötC (Shao and Feldman, 2001, 2002, 2005; Shao et al., 2008).
Finally, nicotine is believed to increase respiratory frequency by stimulating nAChRs on the presynaptic terminals of glutamatergic neurons (Shao and Feldman, 2001, 2005). If chronic PNE desensitizes nAChRs on glutamatergic neurons that are presynaptic to respiratory-related neurons, we would expect a reduction in glutamate release and a concomitant increase in the expression of postsynaptic glutamate receptors (Fregosi and Pilarski, 2008). Thus, we also test the hypothesis that PNE is associated with a functional up-regulation of glutamatergic control of respiratory rhythm. Some of this work has been previously published in abstract form (Pilarski and Fregosi, 2008a, b) and as preliminary data in a recent invited review article in Respiration Physiology & Neurobiology (Fregosi and Pilarski, 2008).
2. Methods
2.1. Animals
We studied 94 Sprague–Dawley rats of either sex from birth through the first 5 days of life, i.e., postnatal day zero (P0) through postnatal day four (P4). This neonatal period corresponds to a gestational age of 29–34 weeks in humans (Ballanyi et al., 1999). The nicotine-exposed neonates were taken from 11 separate litters; the saline exposed neonates from nine different litters; and the unexposed from 12 litters. From each litter, 2–8 neonates were used. All neonatal rats were born via spontaneous vaginal delivery from pregnant adult female rats purchased from Simonsen Laboratories. Neonatal rat pups were housed together with their mothers and siblings until they were studied. Dams had access to food and water ad libitum. Animals were kept in a quiet room at 22 °C, 20–30% relative humidity, and a 12/12-h light/dark cycle. All procedures were approved in accordance with guidelines provided by the Institutional Animal Care and Use Committee (IACUC) at The University of Arizona.
2.2. Prenatal nicotine exposure
PNE was achieved by subcutaneous implantation of Alzet 1007D mini-osmotic pumps (Alzet Corp., CA., USA) into pregnant dams. Implantations were always performed on gestational day five under aseptic conditions, as described previously (Fregosi et al., 2004; Huang et al., 2004a; Luo et al., 2007). The osmotic pumps infused fluid into the subcutaneous space at a rate of 2.5 μl h−1 for 28 days. The pumps were filled with either physiological saline (control animals) or nicotine bitartrate. Based on this infusion rate, mean nicotine bitartrate delivery was 6 mg kg−1 day−1 throughout gestation; this dosing regimen produces plasma levels of free base nicotine of approximately 24 ng ml−1 (150 nM) in the neonates and 18 ng ml−1 (111 nM) in the dams (Chen et al., 2005). These nicotine concentrations are within the range (15–45 ng ml−1) found in the blood plasma of pregnant human mothers described as moderate smokers (Benowitz and Jacob, 1984) and in the amniotic fluid of prenatally smoke exposed human fetuses (Luck and Nau, 1985).
2.3. Brainstem-spinal cord preparation
All experiments were done in the en bloc BSSC preparation originally described by Suzue (1984), and used by us in recent studies (Fregosi et al., 2004; Luo et al., 2004, 2007). The preparation was placed in a 10 ml Plexiglas recording chamber, with the neuraxis pinned ventral side up via dissection pins placed in the lumbar enlargement and the rostral medulla near the midline. The chamber was continuously superfused at a rate of 2.0–4.0 ml min−1 with modified Krebs solution [(in mM): 124 NaCl, 5 KCl, 2.4 CaCl2, 1.3 MgSO4, 26 NaHCO3, 1.2 KH2PO4 and 30 d-glucose] equilibrated with 95% O2/5% CO2 at a pH of 7.4. The temperature of the superfusate was maintained between 20 °C ± 5. In all experiments, a “split-bath” preparation was used to separate the brainstem compartment from the spinal cord compartment, as described in detail previously (Fregosi et al., 2004). This was done to study the effects of exogenous nicotine on brainstem neurons without concomitant activation of nAChR expressed on phrenic motor neurons (Dehkordi et al., 2004).
2.4. Electrophysiological recordings
The proximal ends of the severed fourth cervical ventral nerve roots (C4 VR) were used to record spontaneous extracellular respiratory-related inspiratory activity of phrenic motoneurons. The C4 VR electroneurograms were recorded with glass suction electrodes (outer diameter of the tip ~50–150 μm) filled with the modified Krebs superfusate and connected to a HI-Z differential pre-amplifier (Grass Instruments, Quincy, MA) via a Ag-AgCl wire. The Hi-Z probe output was amplified (gain = 1000–10000) and band pass filtered (0.3–3 kHz, Grass Instruments P 511, Quincy, MA). Analog signals were digitized at 5 kHz using Spike II software (Cambridge Electronic Design, Cambridge, UK) and stored for subsequent offline data analysis.
2.5. Experimental protocols
2.5.1. Protocol 1
In the first set of experiments (N = 59), we measured the effects of exogenous nicotine on C4 VR burst frequency and amplitude in unexposed, saline exposed, and PNE animals at two physiologically relevant concentrations. The protocol was as follows for all treatment groups: after 30 min of baseline recording, exogenous nicotine bitartrate (either 0.2 or 0.5 μM, in separate experiments) was delivered to the brainstem compartment of the split-bath for 30 min, and this was followed by a 30 min washout period using normal Kreb’s solution.
2.5.2. Protocol 2
In the second set of experiments (N = 35), we measured the effects of blocking different nAChR subunits on the nicotine-mediated increase in C4 VR burst frequency and amplitude. We used dihydro-β-erythriodine (DH-β-E) to block nAChRs containing the α4β2 nAChR subunit and alpha-bungarotoxin (α-Bgtx) to block receptors with the α7 subunit (Alkondon and Albuquerque, 1993; Shao and Feldman, 2002). The protocol was as follows in all treatment groups: following 30 min of baseline recording, 0.5 μM exogenous nicotine was superfused into the brainstem compartment for 30 min to increase C4 VR output. Then, the compartment was superfused with a mixture of 0.5 μM nicotine combined with one of the antagonists, either DH-β-E (0.2 μM) or α-Bgtx (0.2 μM). All drugs were acquired from Sigma–Aldrich (St. Louis, MO).
2.5.3. Protocol 3
Lastly, we measured the influence of PNE on glutamatergic control of C4 VR frequency and amplitude (N = 21) with bath application of either N-methyl-d-aspartate (NMDA, n = 11) or α-amino-3-hydroxy-5 methyl-4-isoxazolepropionic acid (AMPA, n = 10) to the medullary compartment in PNE and control animals. The protocol was as follows for NMDA application: following 15 min of baseline recording, 10 μM NMDA was superfused into the brainstem compartment for 15 min. The duration of NMDA superfusion was reduced (compared to above) in an attempt to minimize receptor desensitization. Finally, the compartment was washed for another 15 min. For AMPA application, control recordings lasted 15 min, followed by 30 min of AMPA superfusion at 2.5 μM, and 15 min of drug washout. All drugs were acquired from Sigma–Aldrich (St. Louis, MO).
2.6. Data analysis
For both protocols, C4 VR nerve burst activity was quantified offline by comparing the average frequency and amplitude in a stable, 2 min period within each 5 min epoch of the control, drug application and washout periods (e.g., Fig. 1C and D). To evaluate differences in respiratory frequency and amplitude, group means for all control and nicotine-exposed preparations at each of these epochs were compared using a repeated measures two-way ANOVA (main effects: time during each experiment, and treatment group). When the ANOVA revealed significant differences, post hoc analyses on all pair wise contrasts were performed with Tukey’s HSD procedure. We also quantified a single grand mean for each perturbation (e.g., baseline, nicotine application, and nicotine combined with one of the other drugs) and compared the results using repeated measures two-way ANOVA (main effects: time and treatment group). Tukey’s HSD procedure was again used where appropriate. A probability value of less than or equal to 0.05 was considered significant in all of our analyses. Reported values are means ± standard error of the mean (SEM). All statistical analyses were performed with JMP-IN software (SAS, Cary, SC).
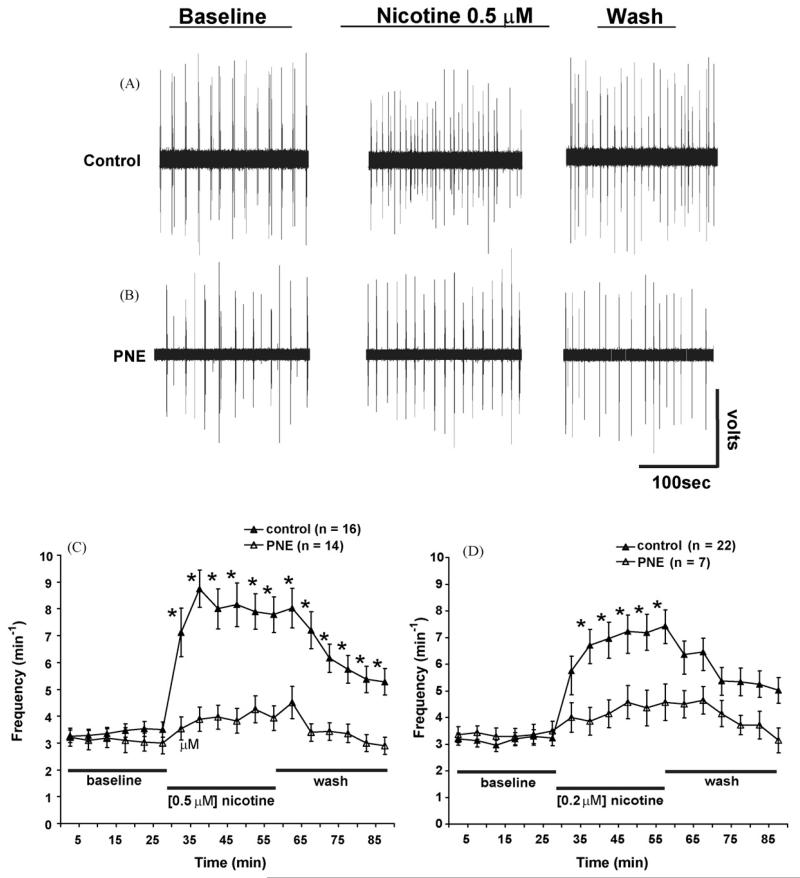
Fig. 1.
Influence of prenatal nicotine exposure on nicotine-induced excitation of rhythmic respiratory-related motor output. (A and B) Upper and middle panels show 200 ms representative extracellular recordings from the fourth cervical ventral root (C4 VR) during similar times within each experimental period. (A) Following baseline recording in a control animal, bath applied nicotine potentiated C4 VR burst frequency, which was then reduced during the wash out period. (B) In this PNE animal, the typical nicotine-induced potentiation of rhythmic respiratory-related motor output was markedly reduced (see Section 3 for more details). (C and D) Time course of C4 VR burst frequency recorded in the presence of bath applied 0.5 μM (C) and 0.2 μM (D) exogenous nicotine in control (▲) and PNE animals (△). Two-way ANOVA showed that mean C4 VR burst frequency significantly increased in control but not PNE animals at both nicotine concentrations. All drugs were applied to the medullary superfusion chamber only (see Section 2 for details). PNE = prenatal nicotine exposure. *Indicates significance (P < 0.05) between PNE and control animals. Values are means ± S.E.M. Redrawn from Fregosi and Pilarski (2008).
3. Results
3.1. Effects of exogenous nicotine on rhythmic respiratory-related motor output in PNE and control animals
We studied separate groups of unexposed (i.e., pups born to mothers that had no implant surgery) and saline exposed neonatal rats (i.e., sham surgery, see Section 2). Since we found no significant C4 VR burst activity differences between these two control groups under any of the conditions, these data were pooled into one control group for comparison with PNE animals. Throughout the remainder of the manuscript “control” indicates grouped saline exposed sham animals and unexposed control animals.
Fig. 1A and B show representative extracellular C4 VR recordings under baseline conditions, in response to exogenous nicotine, and during the washout period. In the control animal (Fig. 1A), 0.5 μM nicotine increased C4 VR frequency from 3.6 to 9.0 bursts min−1; frequency returned to 7.2 bursts min−1 after 30 min of washout with the drug-free Kreb’s superfusate. In contrast, the nicotine-induced frequency changes in the PNE animals (Fig. 1B) were much less. For example, C4 VR burst frequency increased from 3.9 bursts min−1 at baseline to 5.4 bursts min−1 with nicotine and returned to 4.8 bursts min−1 during the drug-free washout period. Average data for these initial experiments are shown in Fig. 1C and 1D. PNE had no effect on baseline C4 VR burst frequency (Fig. 1C and D), and the response to either 0.2 (Fig. 1C) or 0.5 μM nicotine (Fig. 1D) was markedly attenuated in PNE animals. The nicotine concentrations we used are near the EC50 range for the α7 and α4β2 nAChR subtypes (Gentry and Lukas, 2002); these doses are also in the concentration range found in the arterial blood of smokers following a single cigarette (Henningfield et al., 1993).
3.2. Effects of nAChR antagonism on the nicotine-induced potentiation of respiratory-related rhythmic motor output in PNE and control animals
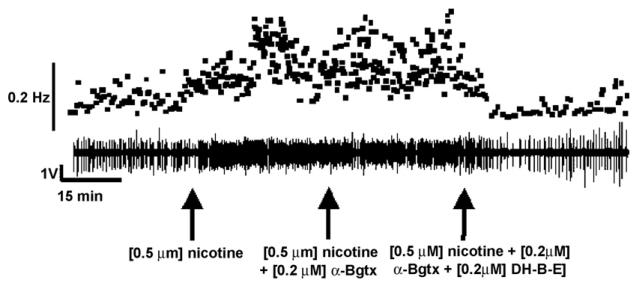
Fig. 2 shows a >90 min C4 VR recording from one control animal during an experiment in which nAChR antagonists specific for the α7 and α4β2 subtypes were administered to the medullary superfusate in succession. First, following baseline recordings, 0.5 μM nicotine was bath applied at t = 30 min (left-hand arrow in Fig. 2) and respiratory frequency increased. Then, a mixture of 0.2 μM α-Bgtx and 0.5 μM nicotine was given at t = 60 min (middle arrow of Fig. 2) to block α7 nAChRs, but there was no discernible effect on frequency. We then introduced a mixture of 0.5 μM nicotine, 0.2 μM α-Bgtx and 0.2 μM DH-β-E to the bath at t = 90 min, which produced a clear and substantial decrease in C4 VR burst frequency. Fig. 3 shows the average C4 VR burst frequency for PNE and control animals as a function of time in response to nicotine and its antagonists. These data are consistent with the recording shown in Fig. 2 in that DH-β-E (Fig. 3A), but not α-Bgtx (Fig. 3B), significantly blocked the nicotine-induced increase in C4 VR burst frequency in control animals (P < 0.05). These observations suggest that the α4β2 nAChR subtype underlies the nicotine-induced increase in C4 VR frequency in this preparation.
Fig. 2.
Representative experiment showing the influence of α7 and α4β2 nAChR subtype antagonists on nicotine-induced excitation of rhythmic respiratory-related motor output. Following baseline C4 VR recording, 0.5 μM nicotine (left arrow) was bath applied to the medulla at t = 30 min, potentiating C4 VR burst frequency. At t = 60 min, a cocktail containing 0.2 μM of the α7 nAChR subtype antagonist α-bungarotoxin (α-Bgtx), plus 0.5 μM nicotine was added to the bath (middle arrow), and had little effect on C4 VR burst frequency. At t = 90 min (right arrow), a mixture of 0.2 μM of the α4β2 nAChR subtype antagonist dihydro-β-erythriodine (DH-β-E) and 0.5 μM nicotine was added to the bath, resulting in the complete reversal of the nicotine-induced excitation of C4 VR burst frequency. The upper panel shows instantaneous C4 VR burst frequency corresponding to the extracellular C4 VR recording in the lower panel.
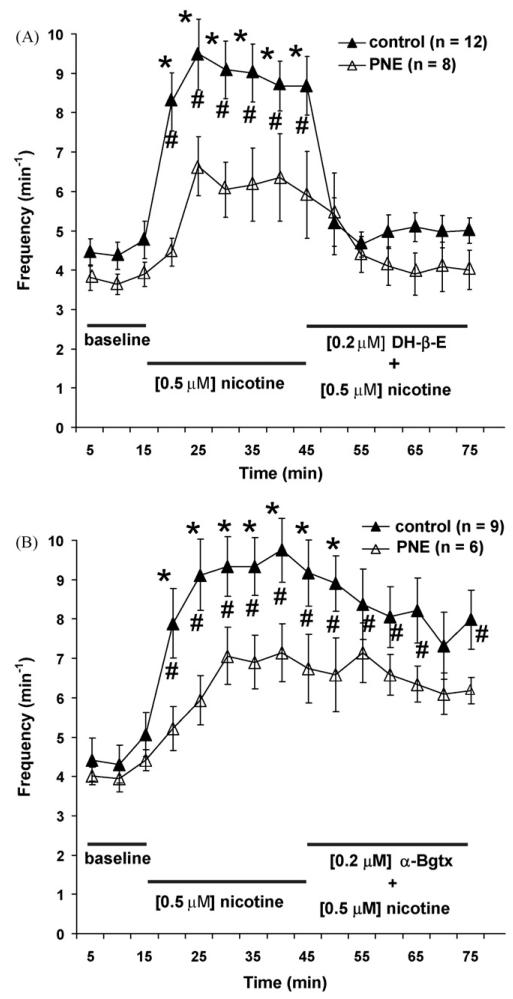
Fig. 3.
Time course showing the influence of α7 and α4β2 nAChR subtype antagonists on nicotine-induced excitation of rhythmic respiratory-related motor output. (A and B) Two-way ANOVA showed that mean C4 VR burst frequency as a function of time was significantly increased in control (▲; P < 0.05) but not PNE (△) animals when 0.5 μM exogenous nicotine was added to the superfusate at t = 15 min. (A) In control animals, addition of a cocktail containing the α4β2 nAChR subtype antagonist dihydro-β-erythriodine (DH-β-E) and 0.5 μM nicotine eliminated the nicotine-induced C4 VR burst frequency. (B) Bath application of a mixture of the α7 nAChR subtype antagonist α-bungarotoxin (α-Bgtx) and 0.5 μM nicotine at t = 15 min had no effect on mean C4 VR burst frequency in control animals. #indicates statistical significance (P < 0.05) within each treatment group and *indicates significance (P < 0.05) between PNE and control animals. Values are means ± S.E.M.
Similar to data shown in Fig. 1, the increase in C4 VR burst frequency with nicotine in the PNE animals was small and did not reach statistical significance (Fig. 3A and B). As a result, we were not able to conduct statistical tests on the effects of blocking α7 and α4β2 nAChRs on C4 VR frequency in the PNE animals. However, Fig. 3A shows that in control animals bath applied nicotine significantly increased C4 VR burst frequency and this effect was abolished by the addition of 0.2 μM DH-β-E. In contrast, Fig. 3B shows that 0.2 μM α-Bgtx was an ineffective blocker of the nicotine-mediated increase in C4 VR burst frequency. We also computed a single mean value for each of the three experimental conditions [i.e., baseline, nicotine and (nicotine + antagonist)] to quantify the effects of blocking α7 or α4β2 nAChRs on C4 VR frequency in control and PNE treatment groups. Differences in these average data between control and PNE pups were then compared statistically, with two-way ANOVA (see Section 2). Using this approach, we again found that PNE had no effect on C4 VR burst frequency under baseline conditions (Fig. 4A and B, left-most bars). Although exogenous nicotine significantly increased C4 VR burst frequency in both groups (Fig. 4A and B, middle bars; (P < 0.05)), the increase was much less in PNE animals than controls. Fig. 4A (right-most bars) also shows that the addition of 0.2 μM DH-β-E reduced the nicotine-mediated increase in C4 VR burst frequency by 94% in control animals (P < 0.05) and 78% in PNE animals (P < 0.05). Fig. 4B (right-most bars) confirms that 0.2 μM α-Bgtx was ineffective as a blocker of the nicotine-mediated increase in C4 VR burst frequency in both PNE and control animals.
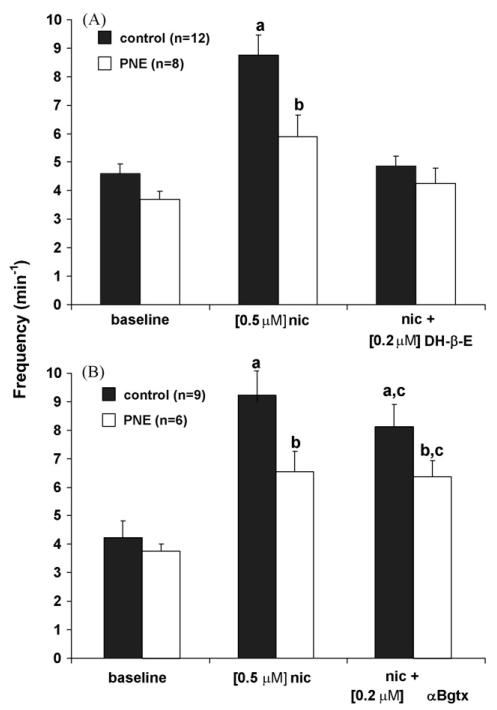
Fig. 4.
Summary data for each treatment showing the influence of α7 and α4β2 nAChR subtype antagonists on nicotine-induced excitation of rhythmic respiratory-related motor output. (A) 0.5 μM exogenous nicotine (nic) significantly potentiated C4 VR burst frequency compared to baseline values and when PNE and control groups are compared. Bath application of a mixture containing the α4β2 nAChR subtype antagonist dihydro-β-erythriodine (DH-β-E) and 0.5 μM nicotine eliminated the nicotine-induced increase in C4 VR burst frequency in both PNE and control animals. (B) As in (A) 0.5 μM exogenous nicotine (nic) significantly increased C4 VR burst frequency in control and PNE animals. However, a mixture containing the α7 nAChR subtype antagonist α-bungarotoxin (α-Bgtx) and 0.5 μM nicotine had no effect on nicotine-induced C4 VR burst frequency in either PNE or control animals, except that the difference between PNE and control animals disappeared. a,b,cIndicates where statistical significance was found (P < 0.05). Values are means ± S.E.M.
3.3. Effects of PNE on glutamatergic neurotransmission and the control of breathing
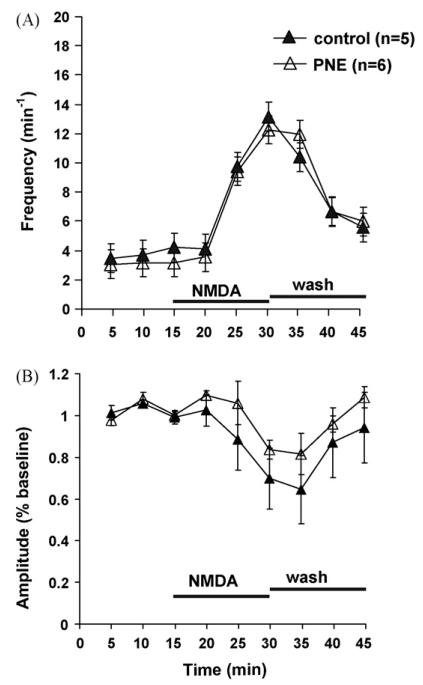
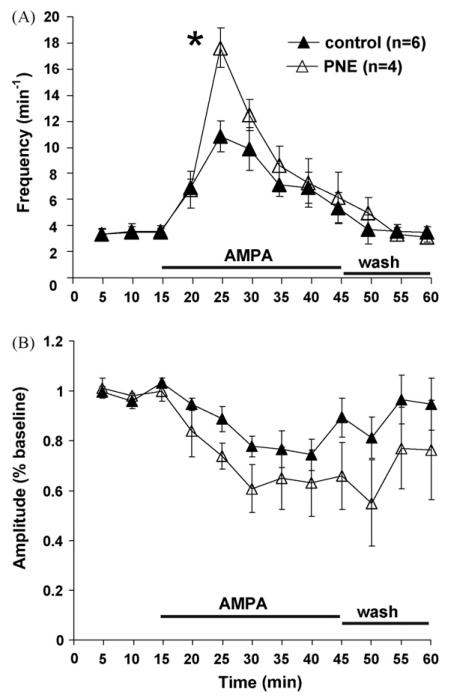
Fig. 5A shows that bath applied NMDA increased C4 VR burst frequency in both PNE and control animals, but there was no treatment effect. There was also no difference in C4 VR burst amplitude between PNE and control animals, although there was a non-significant trend toward a decrease in burst amplitude at the highest burst frequencies within each treatment group (Fig. 5B). In contrast, AMPA caused a greater increase in C4 VR burst frequency in PNE compared to control animals (Fig. 6A; P < 0.05). Similar to NMDA administration, AMPA evoked a non-significant trend toward decreased C4 VR burst amplitude within each treatment group, but there was no treatment effect when PNE and control animals were compared (Fig. 6B).
Fig. 5.
Time course showing the influence of NMDA on rhythmic respiratory-related motor output in PNE and control animals. (A) Mean C4 VR burst frequency as a function of time was significantly increased in control (▲) and PNE (△) animals when 10 μM N-methyl-d-aspartate (NMDA) was added to the superfusate for 15 min beginning at t = 15, but no PNE effect was observed. (B) Mean C4 VR burst amplitude as a function of time was unaltered following NMDA administration despite a trend toward lower values corresponding to potentiated C4 VR burst frequencies shown in (A). Statistical analysis was performed on raw data. Values are means ± S.E.M.
Fig. 6.
Time course showing the influence of AMPA on rhythmic respiratory-related motor output in PNE and control animals. (A) Mean C4 VR burst frequency as a function of time was significantly increased in control (▲) and PNE (△) animals when 2.5 μM α-amino-3-hydroxy-5 methyl-4-isoxazolepropionic acid (AMPA) was added to the superfusate for 30 min beginning at t = 15. Peak C4 VR was also increased in PNE animals compared to control at t = 25 min. (B) Mean C4 VR burst amplitude as a function of time was unaltered following AMPA administration despite a trend toward lower values corresponding to potentiated C4 VR burst frequency shown in (A). Statistical analysis was performed on raw data. Values are means ± S.E.M. *Indicates significance (P < 0.05) between PNE and control animals. Values are means ± S.E.M.
4. Discussion
When we applied exogenous nicotine to the isolated neonatal medulla, respiratory frequency increased by more than 200% in control animals without effect on nerve burst amplitude, consistent with other in vitro studies (Shao and Feldman, 2001, 2005; Shao et al., 2008). Following PNE, however, we found a significant attenuation (140%) of the nicotine-evoked increase in respiratory frequency, supporting our hypothesis that PNE produces a loss of nAChR function that normally contributes to frequency modulation in respiratory rhythm generating neurons. We also confirmed that the central nicotine-induced frequency modulation acts through the α4β2 nAChR subtypes, because the increase in frequency was abolished by its antagonist, DH-β-E. In contrast, the α7 nAChR antagonist α-bungarotoxin was without significant affect. These data are consistent with most current pharmacological and physiological data on the respiratory-related functions of α4β2 and α7 nAChR subunits in respiratory neurons, especially the respiratory rhythm generating preBötzinger region (Bradley and Lucy, 1983; Bohmer et al., 1987; Wada et al., 1989; Dominguez del Toro et al., 1994; Shao and Feldman, 2002, 2005; Hatori et al., 2006; Shao et al., 2008). As in control animals, α4β2 nAChRs appeared exclusively responsible for the nicotine-evoked increase in respiratory frequency in PNE animals. In other words, we did not detect a PNE-mediated shift in the contribution of α4β2 and α7 nAChR subunits to the nicotine-evoked increase in respiratory frequency following PNE, despite different propensities of these receptor subtypes to desensitize and proliferate (Gentry and Lukas, 2002; McKay et al., 2007). Lastly, we showed that PNE induced an enhancement of peak AMPAergic, but not NMDA-mediated C4 VR burst frequency. The latter data suggest that AMPA receptors, which are known to be necessary for the neurogenesis and maintenance of respiratory rhythm in preBötC inspiratory neurons (Ge and Feldman, 1998; Shao et al., 2003; Pace and Del Negro, 2008), may be over-expressed in response to the PNE (see Fig. 7 and below for more details).
Fig. 7.
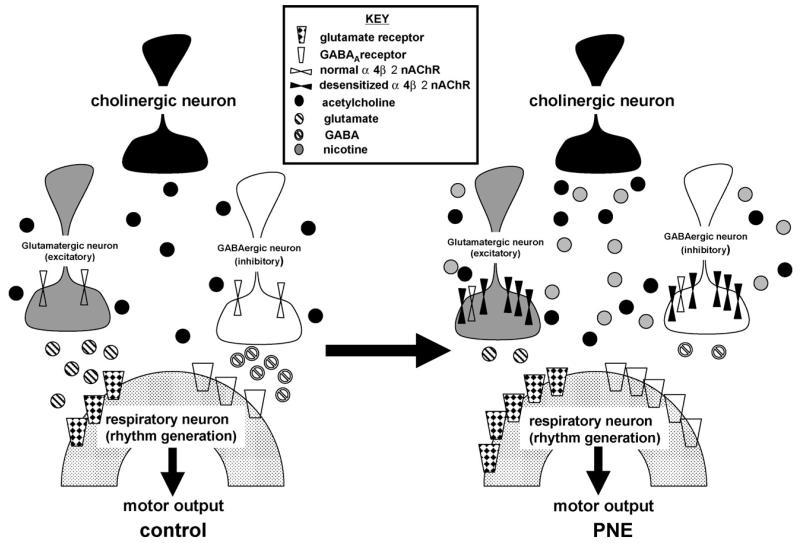
Conceptual model summarizing physiological and anatomical changes that might occur in respiratory rhythm generating circuits following prenatal nicotine exposure (PNE). The PNE-induced persistent excitation of nicotinic acetylcholine receptors (nAChRs) initially results in an up-regulation of these receptors, located presynaptically to rhythm generating neurons, on both GABAergic and glutamatergic neurons. Prolonged excitation of presynaptic nAChRs subsequently leads to desensitization and a diminution of GABA and glutamate release onto rhythm generating neurons in the ventrolateral medulla. The reduction in GABAergic and glutamatergic neurotransmission is followed by an up-regulation of GABAA and glutamate receptors on postsynaptic neurons, and, as a consequence, endogenous stressors associated with an increase in GABA or glutamate release (e.g., hypoxia) would generate an exaggerated postsynaptic response. A similar version of this working model was recently published (Fregosi and Pilarski, 2008), and the present data documents the premise that PNE functionally desensitizes nAChRs and potentiated AMPA-mediated neurotransmission, extending and strengthening the model. Redrawn from Fregosi and Pilarski (2008).
4.1. A working model of PNE-induced neuroplasticity in brainstem respiratory neurons
Our data are consistent with a large body of literature showing that the persistent activation of nAChRs desensitizes or functionally down-regulates α4β2 nAChRs (Gentry and Lukas, 2002), leaving the network incapable of adequately responding to exogenous nicotine and, most likely, to endogenous acetylcholine as well. Although other nAChR subtypes likely play a role (Clarke et al., 1984), we could abolish the excitatory effects of bath applied nicotine with DH-β-E, strongly suggesting that if other subtypes play a role, it is a minor one. In an attempt to explain how PNE may alter the control of central respiratory rhythm through inhibitory and excitatory neurotransmitter systems, we recently published a working model that is based on identified and proposed PNE-mediated alterations of brainstem nAChRs (Fregosi and Pilarski, 2008). The present study tested two aspects of this working model: (1) Does PNE reduces the respiratory motor response to exogenous nicotine? And (2) Does PNE evoke changes in glutamatergic modulation of the respiratory motor response? The model is based on the premise that PNE desensitizes or functionally down-regulates nAChRs that are located on presynaptic terminals of GABAergic (Yang et al., 1996), serotonergic (McGehee and Role, 1995) and glutamatergic (McGehee et al., 1995) neurons. In respiratory rhythm generating neurons, this presynaptic location can be inferred from data showing that preBötC inspiratory neurons do not respond to exogenous nicotine when the neurons are synaptically isolated with tetrodotoxin (Shao and Feldman, 2001). Presynaptic nAChR desensitization, in turn, reduces the release of neurotransmitter onto the postsynaptic neuron, and, in response, the postsynaptic cell increases receptor density in order to maintain function [see Fig. 7 and (Fregosi and Pilarski, 2008) for more detail]. In another recent test of this model, Luo et al. (2004, 2007) showed that bath application or microinjection of GABAergic or glycinergic agonists into the preBötzinger complex results in more profound slowing of C4 VR frequency in brainstem-spinal cord preparations from PNE compared to control preparations, suggesting that inhibitory neurotransmission is enhanced.
Our model also predicts that PNE evokes an up-regulation of glutamatergic (i.e., AMPA or NMDA) excitation of respiratory rhythm by the same mechanisms outlined above; therefore, we tested this hypothesis by applying the excitatory glutamatergic receptor agonists NMDA and AMPA to the brainstem compartment. PNE animals showed a significantly increased peak C4 VR burst frequency in the presence of exogenous AMPA (Fig. 6A), but not NMDA (Fig. 5A). These data suggest that PNE induced an up-regulation of AMPA receptors within rhythm generating networks (presumably on preBötzinger inspiratory neurons) secondary to nAChR desensitization, as predicted by our working model (Fig. 7). These data are also consistent with previous reports showing that AMPA receptor function, but not NMDA receptor function, is critical for respiratory rhythm generation in the preBötzinger coplex, at least in the presence of voltage-dependent Mg2+ block (Morgado-Valle and Feldman, 2007). Although the working model in Fig. 7 implies similar PNE-mediated changes in excitatory and inhibitory neurotransmission, data suggest that PNE alters inhibitory neurotransmission more than excitatory neurotransmission (this study; Luo et al., 2004, 2007). In future investigations, it will be important to confirm the specific medullary locations of neurons affected by PNE. Despite our speculation that these effects are due to PNE-induced alterations in the preBötzinger complex, at present we cannot rule out other medullary respiratory-related regions, such as the paraFacial respiratory group (pFRG), which may also play a role in the cholinergic/nicotinic control of respiratory frequency in reduced brainstem preparations (Hatori et al., 2006).
4.2. PNE and the central control of respiratory motor pattern
Differences in the amplitude of C4 VR nerve bursts were not observed as a function of condition (i.e., control, nicotine application or nAChR antagonism) or treatment (i.e., PNE vs. control animals). In reduced preparations C4 VR nerve burst amplitude serves as a surrogate for changes in phrenic motoneuron recruitment, firing rate, and, by extension, tidal volume. The absence of a nicotine-induced effect on peak C4 VR burst amplitude in our split-bath BSSC preparation suggests that medullary nAChRs regulate the frequency of rhythmic motor output, but have no role in modulation of the recruitment or firing rate of phrenic motoneurons. Similarly, in more reduced medullary slice preparations, nicotine injected into the preBötzinger complex increases the frequency of rhythmic motor (hypoglossal) output, but does not change burst amplitude (Shao and Feldman, 2001). In contrast, nicotine injection into the hypoglossal motor nucleus increases XII nerve burst amplitude, but not nerve burst frequency (Shao and Feldman, 2001; Robinson et al., 2002), suggesting local nicotinic modulation of motoneuron recruitment. Based on these observations, we applied nicotine to the spinal cord chamber in three preparations to determine if to see if nicotine could increase local recruitment of phrenic motoneurons, as reflected by changes in C4 VR burst amplitude. C4 VR burst amplitude (and also frequency) failed to increase when nicotine was applied to the spinal cord compartment, although tonic activity doubled (data not shown). These data suggest that central nicotinic receptors do not play a role in the recruitment of phrenic motoneurons, especially during phasic respiratory-related bulbospinal drive, despite their expression in the phrenic motor nucleus (Dehkordi et al., 2004). In the phrenic motor nucleus, nAChRs presumably reflect nicotine-mediated Renshaw cell inhibitory neurotransmission (Curtis and Ryall, 1966).
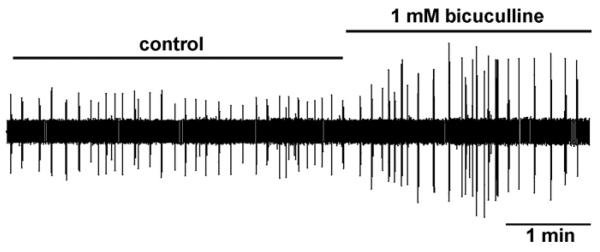
We also considered the possibility that in this preparation the phrenic motoneuron pool is already maximally activated under baseline conditions (St John, 1996, 1999), precluding any further increase in bulbospinal excitatory drive. To address this issue, we tested whether C4 VR burst amplitude could be increased by blocking GABAergic inhibition with the GABAA receptor antagonist bicuculline delivered to the spinal cord. Fig. 8 shows that bicuculline applied to the spinal cord increased peak phasic C4 VR burst amplitude by over 100% in our split-bath preparation. This is consistent with the increase in phrenic motoneuron discharge observed when bicuculline is microinjected into the phrenic motor nucleus (Parkis et al., 1999). These data support our findings that nAChR-mediated excitation of medullary bulbospinal pathways has little influence on phrenic motoneuron recruitment or discharge rate modulation in the BSSC preparation because the phrenic motor pool can still respond to an increase in overall excitation. Interestingly, Kopczynska and Szereda-Przestaszewska (1999) showed that intravenous nicotine infusion increased both frequency and tidal volume when the carotid bodies were intact, but following carotid body denervation only frequency increased, providing further evidence that the central respiratory response to nicotine is dominated by frequency control.
Fig. 8.
Effects of bicuculline administered to the spinal cord chamber in the split-bath brainstem-spinal cord preparation. Following baseline C4 VR recording, bicuculline was bath applied to the spinal cord compartment, increasing peak C4 VR inspiratory burst amplitude by over 200% of control.
4.3. Comparison with other studies
Previous studies have shown that chronic nicotine exposure influences the density and/or function of neuronal nAChRs in both neonates and adult animals (Yates et al., 1995; Wonnacott, 1997; Slotkin et al., 2002; Slikker et al., 2005; Ochoa and Lasalde-Dominicci, 2007; Slotkin et al., 2008), including the ventrolateral medulla (Nachmanoff et al., 1998; Duncan et al., 2008), but only a few studies have addressed the biological ramifications of chronic nAChR excitation on central respiratory-related neurons (Robinson et al., 2002; Huang et al., 2004b, 2005; Kamendi et al., 2006; Eugenin et al., 2008) and only two besides the present study have examined the nicotinic control of breathing pattern following PNE (Robinson et al., 2002; Eugenin et al., 2008). Using a brainstem slice and BSSC preparation Robinson et al. (2002) and Eugenin et al. (2008), respectively, both demonstrated PNE-induced alterations in the function of central respiratory neurons, but neither applied exogenous nicotine (or other nicotinic receptor agonists) to brainstem regions that control breathing frequency. Exogenous nicotine mimics endogenous acetylcholine release in vivo and it is noteworthy that endogenous release of acetylcholine may be lacking in reduced preparations that are devoid of the pons and therefore the major sources of acetylcholine release (Woolf, 1991; Wonnacott, 1997). Without exciting nAChRs experimentally, decrements in nAChR frequency modulation would likely be absent. Here we addressed this important omission by systematically applying exogenous nicotine to excite nAChRs in the isolated medulla of control and PNE animals and show that PNE alters the central control of breathing in complex ways. An interesting difference between the present study and Eugenin et al. (2008) is the lower baseline frequency they report in PNE animals. This inconsistency might reflect differences in species (mouse vs. rat) and/or differences in the preparation that could influence the release of endogenous neuromodulators. More data are needed to determine if and by what mechanism PNE alters the eupneic breathing pattern in neonates.
4.4. Conclusions
Consistent with our working model (Fig. 7), this and previous studies show that PNE leads to alterations in cholinergic, GABAergic, glycinergic, and AMPAergic neurotransmission in brainstem respiratory neurons (this study; Luo et al., 2004, 2007). Data also suggest that the respiratory-related PNE-induced neuroplasticity begins with the functional alteration of nAChRs, specifically the α4β2 subtype. Nevertheless, we do not yet know where these receptors are located (i.e., presynaptic, postsynaptic, or both) and how receptor expression is altered by chronic PNE. Data from other brain regions indicate that the mechanisms may be diverse, as each type of nAChR has unique properties, such as variable Ca2+ permeability and the propensity to desensitize (Wonnacott, 1997).
Although the concomitant increase in inhibitory and excitatory neurotransmission that we see in PNE animals seems paradoxical, this situation may result from competing mechanisms that function to balance excitatory and inhibitory synaptic activity within rhythm generating neural circuits, especially when they are perturbed (Turrigiano, 1999, 2008; Kline et al., 2007). For example, it is possible that the PNE-induced increase in excitatory neurotransmission results in a secondary increase in inhibitory neurotransmission, or vice versa. This makes intuitive sense because rhythm generating networks must be active and stable at parturition. While this might aid in the prevention of some respiratory pathologies, such scaling of synaptic inputs may lead to respiratory instabilities when one of the pathways is preferentially recruited, such as during hypoxia, wherein GABA release is greatly increased (Melton et al., 1990). It is clear that future studies need to address the location and identity of neurotransmitter systems targeted by PNE, as well as how compensatory homeostatic adjustments are orchestrated.
Acknowledgements
The authors would like to thank Amber Rice, Seres Costy-Bennet, and Hilary Wakefield for expert technical support, and Katherine Promer for assistance with data analysis. These studies were generously funded by the Pacific Mountain Affiliate of the American Heart Association (Award 0555547Z).
References
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J. Pharmacol. Exp. Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog. Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir. Physiol. Neurobiol. 2002;131:135–144. doi: 10.1016/s1569-9048(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., III Daily intake of nicotine during cigarette smoking. Clin. Pharmacol. Ther. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Bohmer G, Schmid K, Schmidt P, Stehle J. Cholinergic effects on spike-density and burst-duration of medullary respiration-related neurones in the rabbit: an iontophoretic study. Neuropharmacology. 1987;26:1561–1572. doi: 10.1016/0028-3908(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Bradley PB, Lucy AP. Cholinoceptive properties of respiratory neurones in the rat medulla. Neuropharmacology. 1983;22:853–858. doi: 10.1016/0028-3908(83)90131-4. [DOI] [PubMed] [Google Scholar]
- Chen H, Parker SL, Matta SG, Sharp BM. Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. Eur. J. Neurosci. 2005;22:380–388. doi: 10.1111/j.1460-9568.2005.04229.x. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Pert CB, Pert A. Autoradiographic distribution of nicotine receptors in rat brain. Brain Res. 1984;323:390–395. doi: 10.1016/0006-8993(84)90320-2. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Ryall RW. The acetylcholine receptors of Renshaw cells. Exp. Brain Res. 1966;2:66–80. doi: 10.1007/BF00234361. [DOI] [PubMed] [Google Scholar]
- Dehkordi O, Haxhiu MA, Millis RM, Dennis GC, Kc P, Jafri A, Khajavi M, Trouth CO, Zaidi SI. Expression of alpha-7 nAChRs on spinal cord-brainstem neurons controlling inspiratory drive to the diaphragm. Respir. Physiol. Neurobiol. 2004;141:21–34. doi: 10.1016/j.resp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Dominguez del Toro E, Juiz JM, Peng X, Lindstrom J, Criado M. Immunocytochemical localization of the alpha 7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. J. Comp. Neurol. 1994;349:325–342. doi: 10.1002/cne.903490302. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Randall LL, Belliveau RA, Trachtenberg FL, Randall B, Habbe D, Mandell F, Welty TK, Iyasu S, Kinney HC. The effect of maternal smoking and drinking during pregnancy upon (3)H-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high risk population. Brain Pathol. 2008;18:21–31. doi: 10.1111/j.1750-3639.2007.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin J, Otarola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, von Bernhardi R. Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J. Neurosci. 2008;28:13907–13917. doi: 10.1523/JNEUROSCI.4441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir. Physiol. Neurobiol. 2008 doi: 10.1016/j.resp.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Luo Z, Iizuka M. GABAA receptors mediate postnatal depression of respiratory frequency by barbiturates. Respir. Physiol. Neurobiol. 2004;140:219–230. doi: 10.1016/j.resp.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ge Q, Feldman JL. AMPA receptor activation and phosphatase inhibition affect neonatal rat respiratory rhythm generation. J. Physiol. 1998;509(Pt 1):255–266. doi: 10.1111/j.1469-7793.1998.255bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr. Drug Targets CNS Neurol. Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signalling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Hafstrom O, Milerad J, Sandberg KL, Sundell HW. Cardiorespiratory effects of nicotine exposure during development. Respir. Physiol. Neurobiol. 2005;149:325–341. doi: 10.1016/j.resp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hatori E, Sakuraba S, Kashiwagi M, Kuribayashi J, Tsujita M, Hosokawa Y, Takeda J, Kuwana S. Association of nicotinic acetylcholine receptors with central respiratory control in isolated brainstem-spinal cord preparation of neonatal rats. Biol. Res. 2006;39:321–330. doi: 10.4067/s0716-97602006000200014. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug. Alcohol Depend. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Huang YH, Brown AR, Costy-Bennett S, Luo Z, Fregosi RF. Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir. Physiol. Neurobiol. 2004a;143:1–8. doi: 10.1016/j.resp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Wang X, Dergacheva O, Mendelowitz D. Prenatal nicotine exposure recruits an excitatory pathway to brainstem parasympathetic cardioinhibitory neurons during hypoxia/hypercapnia in the rat: implications for sudden infant death syndrome. Pediatr. Res. 2005;58:562–567. doi: 10.1203/01.PDR.0000179380.41355.FC. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Wang X, Evans C, Gold A, Bouairi E, Mendelowitz D. Prenatal nicotine exposure alters the types of nicotinic receptors that facilitate excitatory inputs to cardiac vagal neurons. J. Neurophysiol. 2004b;92:2548–2554. doi: 10.1152/jn.00500.2004. [DOI] [PubMed] [Google Scholar]
- Kamendi H, Stephens C, Dergacheva O, Wang X, Huang ZG, Bouairi E, Gorini C, McIntosh JM, Mendelowitz D. Prenatal nicotine exposure alters the nicotinic receptor subtypes that modulate excitation of parasympathetic cardiac neurons in the nucleus ambiguus from primarily alpha3beta2 and/or alpha6betaX to alpha3beta4. Neuropharmacology. 2006;51:60–66. doi: 10.1016/j.neuropharm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J. Neurosci. 2007;27:4663–4673. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopczynska B, Szereda-Przestaszewska M. Response of respiratory muscles to intravenous nicotine challenge in anaesthetized cats. Respir. Physiol. 1999;116:145–157. doi: 10.1016/s0034-5687(99)00049-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol. Biochem. Behav. 2006;85:669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck W, Nau H. Nicotine and cotinine concentrations in serum and urine of infants exposed via passive smoking or milk from smoking mothers. J. Pediatr. 1985;107:816–820. doi: 10.1016/s0022-3476(85)80427-3. [DOI] [PubMed] [Google Scholar]
- Luo Z, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure increases the strength of GABA(A) receptor-mediated inhibition of respiratory rhythm in neonatal rats. J. Physiol. 2004;561:387–393. doi: 10.1113/jphysiol.2004.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, McMullen NT, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure alters glycinergic and GABAergic control of respiratory frequency in the neonatal rat brainstem-spinal cord preparation. Respir. Physiol. Neurobiol. 2007;157:226–234. doi: 10.1016/j.resp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2007;74:1120–1133. doi: 10.1016/j.bcp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton JE, Neubauer JA, Edelman NH. GABA antagonism reverses hypoxic respiratory depression in the cat. J. Appl. Physiol. 1990;69:1296–1301. doi: 10.1152/jappl.1990.69.4.1296. [DOI] [PubMed] [Google Scholar]
- Morgado-Valle C, Feldman JL. NMDA receptors in preBotzinger complex neurons can drive respiratory rhythm independent of AMPA receptors. J. Physiol. 2007;582:359–368. doi: 10.1113/jphysiol.2007.130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmanoff DB, Panigrahy A, Filiano JJ, Mandell F, Sleeper LA, Valdes-Dapena M, Krous HF, White WF, Kinney HC. Brainstem 3H-nicotine receptor binding in the sudden infant death syndrome. J. Neuropathol. Exp. Neurol. 1998;57:1018–1025. doi: 10.1097/00005072-199811000-00004. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Alafuzoff I, Winblad B. Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. J. Neurosci. Res. 1992;31:103–111. doi: 10.1002/jnr.490310115. [DOI] [PubMed] [Google Scholar]
- Ochoa EL, Lasalde-Dominicci J. Cognitive deficits in schizophrenia: focus on neuronal nicotinic acetylcholine receptors and smoking. Cell Mol. Neurobiol. 2007;27:609–639. doi: 10.1007/s10571-007-9149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Del Negro CA. AMPA and metabotropic glutamate receptors cooperatively generate inspiratory-like depolarization in mouse respiratory neurons in vitro. Eur. J. Neurosci. 2008;28:2434–2442. doi: 10.1111/j.1460-9568.2008.06540.x. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Dong X, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. J. Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J. Pharmacol. Exp. Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Pilarski JQ, Fregosi RF. Influence of prenatal nicotine exposure on in vitro central respiratory-related cholinergic neurotransmission. FASEB J. 2008a;22(954):910. [Google Scholar]
- Pilarski JQ, Fregosi RF. Prenatal nicotine exposure increases the strength of glutamatergic receptor-mediated excitation of respiratory rhythm in neonatal rats. Soc. Neurosci. Abstr. 2008b;538:5. [Google Scholar]
- Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J. Physiol. 2002;538:957–973. doi: 10.1113/jphysiol.2001.012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Mechanisms underlying regulation of respiratory pattern by nicotine in preBotzinger complex. J. Neurophysiol. 2001;85:2461–2467. doi: 10.1152/jn.2001.85.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Pharmacology of nicotinic receptors in preBotzinger complex that mediate modulation of respiratory pattern. J. Neurophysiol. 2002;88:1851–1858. doi: 10.1152/jn.2002.88.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Cholinergic neurotransmission in the preBotzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience. 2005;130:1069–1081. doi: 10.1016/j.neuroscience.2004.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Ge Q, Feldman JL. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBotzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J. Physiol. 2003;547:543–553. doi: 10.1113/jphysiol.2002.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, Feldman JL. Alpha4* nicotinic receptors in preBotzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J. Neurosci. 2008;28:519–528. doi: 10.1523/JNEUROSCI.3666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Jr., Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction—developmental neurotoxicity of nicotine. Crit. Rev. Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ. Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Res. Bull. 2008;76:152–165. doi: 10.1016/j.brainresbull.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Auman JT, Qiao D, Seidler FJ. Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Brain Res. Dev. Brain Res. 2002;133:175–179. doi: 10.1016/s0165-3806(02)00281-x. [DOI] [PubMed] [Google Scholar]
- St John WM. Medullary regions for neurogenesis of gasping: noeud vital or noeuds vitals? J. Appl. Physiol. 1996;81:1865–1877. doi: 10.1152/jappl.1996.81.5.1865. [DOI] [PubMed] [Google Scholar]
- St John WM. Rostral medullary respiratory neuronal activities of decerebrate cats in eupnea, apneusis and gasping. Respir. Physiol. 1999;116:47–65. doi: 10.1016/s0034-5687(99)00030-4. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J. Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi ES, Lendvai B. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res. Brain Res. Rev. 1999;30:219–235. doi: 10.1016/s0165-0173(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J. Comp. Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog. Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Breese GR. Nicotine-induced inhibition in medial septum involves activation of presynaptic nicotinic cholinergic receptors on gamma-aminobutyric acid-containing neurons. J. Pharmacol. Exp. Ther. 1996;276:482–489. [PubMed] [Google Scholar]
- Yates SL, Bencherif M, Fluhler EN, Lippiello PM. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochem. Pharmacol. 1995;50:2001–2008. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]