Abstract
Biologics, including monoclonal antibodies (mAbs) and other therapeutic proteins such as cytokines and growth hormones, have unique characteristics compared to small molecules. This paper starts from an overview of the pharmacokinetics (PK) of biologics from a mechanistic perspective, the determination of a starting dose for first-in-human (FIH) studies, and dosing regimen optimisation for phase II/III clinical trials. Subsequently, typical clinical pharmacology issues along the corresponding pathways for biologics development are summarised, including drug-drug interactions, QTc prolongation, immunogenicity, and studies in specific populations. The relationships between the molecular structure of biologics, their pharmacokinetic and pharmacodynamic characteristics, and the corresponding clinical pharmacology strategies are summarised and depicted in a schematic diagram.
Keywords: biologics, pharmacokinetics, pharmacodynamics, clinical pharmacology, antibody
Introduction
Biologics development represents a major modern advancement in medicine because of their promise and huge success. The importance of biologics development is reflected by the numerous mergers and acquisitions for large pharmaceutical companies, including AstraZeneca, Merck, Pfizer, GSK, and Sanofi-Aventis, to acquire their biologics development platforms. Although still young, the biopharma industry in developing countries is exemplified by Chinese pharmas. China has been establishing industrial infrastructures at a fast pace with fiscal support from the government. Chinese biopharmas, such as Beijing Huasu Pharma, 3S Bio, Qilu Pharma, Baitai, Beijing SL Pharma, Quangang Pharma, and Anke Biotech, are mostly manufacturing-oriented, and their range of products includes interleukins, interferons, growth hormones, insulin, growth factor receptors, and monoclonal antibodies. However, few of these companies can produce monoclonal antibodies on a large scale. Nimotuzumab, manufactured by Biotech Pharma, is one of a few monoclonal antibody drugs that have been developed in Cuba but approved and produced in China. Other approved antibody drugs include a mouse anti-human CD3 product by the Wuhan Institute of Biological Products against organ transplant rejection, an anti-human interleukin-8 product by Dongguan Winnerway YES Biotech & Pharmaceutical Co against psoriasis, a recombinant human tumour necrosis factor receptor II fusion protein product by Shanghai CP Guojian Pharmaceutical Co against rheumatoid arthritis, and an iodine-conjugated tumour necrosis therapy product by Shanghai Meien Biotechnology Co against lung cancer. Research & Development activities (R&D) in China are normally characterised by close collaborations with top tier Chinese research institutions, small scale capital investments (5%–8% of revenue), and fast advancements in vaccine development (for example, China was the first country to manufacture an H1N1 vaccine).
Biologics are defined as a virus, therapeutic serum, toxin, antitoxin, vaccine, blood product, blood component or derivative, allergenic product (or any other analogous product), or an arsphenamine or derivative of arsphenamine (or any other trivalent organic arsenic compound) applicable to the prevention, treatment or cure of a disease/condition of human beings [United States Public Health Services Act 42 USC §262(i)]. Most biologics are large complex molecules/mixtures that are not easily identified or characterised. They are often generated from bacteria, yeast, insects, plants, or mammalian cells engineered with the gene of interest, but they can also be purified from natural sources. Philosophically speaking, the mechanism of action of most biologics falls into the Chinese Yin-yang theory: that is, using therapeutic proteins, such as monoclonal antibodies (Yang), to neutralise elevated antigen levels/over-expressed disease targets (Yin) to keep Yin and Yang in balance. In this paper, we will focus on the clinical pharmacology aspects of biologics, including pharmacokinetics (PK)/pharmacodynamics (PD), dose selection, modelling and simulation approaches, and general clinical pharmacology considerations in biologics drug development. The biologics discussed in this paper include monoclonal antibodies (mAbs), proteins and peptides. Vaccines and stem cell therapies are beyond the scope of this paper.
Mechanistic understanding of the pharmacokinetics of biologics
Compared to small molecule drugs, biologics have unique characteristics in absorption, distribution, metabolism, and elimination (ADME), which lead to significant differences in their development.
The primary distinctions between biologics and small molecule drugs are their sizes, structural complexity and how they are produced. The molecular weight of a small molecule drug is typically less than 1 kDa (20–100 atoms), whereas the molecular weights of biologics range from a few kDa to 1000 kDa (ie, IgM mAbs). The efficacy and safety of therapeutic proteins are also affected by their secondary, tertiary, and quaternary structures. Factors that need to be taken into account when considering the functionality of biologics include but not limited to protein folding, denaturation, amino acid substitution, deamidation, N- and C-terminal modifications, protein aggregation, oxidation, O/N-linked glycosylation, truncation, phosphorylation, sulphation, PEGylation, carbamylation/carboxylation/acetylation, multimer dissociation, mismatched S-S bonds, truncation, fatty acylation, gamma-carboxyglutamylation, formylation, and methylation.
Biologics are mainly delivered by parenteral administration, such as intravenous (IV), subcutaneous (SC) and intramuscular (IM) injections. Because of their large molecular size, biologics are absorbed slowly, with a longer time to peak concentration (Tmax) following SC and IM administration than small molecules. The longer Tmax is associated with slow lymphatic uptake, the major route of absorption for biologics, at the injection site1. The slow lymphatic absorption is due to the limited flow rate (1–2 mL·kg-1·h-1 in the thoracic duct)2. Based on the data published by the US Food and Drug Administration (FDA), the Tmax (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/) of 12 approved mAbs or fusion proteins administered by the SC route ranged from 2 to 14 d, and their bioavailability ranged from 50% to 80%. The oral bioavailability of therapeutic proteins is negligible because of their size, polarity, and enzymatic degradation in the gastrointestinal tract. Thus, oral dosing is not an appropriate route of administration for biologics. In comparison, oral dosing is the most common dosing route for small molecules with a Tmax in the order of hours.
The mAbs, which could be considered the most important subset of biologics, bind to their targets by specific or nonspecific binding. The nonspecific binding function, including FcRn and Fcγ receptor binding, resides in the constant region (Fc) of the mAb. Specific binding mainly refers to the binding between the antigen binding site of the variable region and the targets (eg, soluble antigen/ligand or receptor). As a result, both the distribution and the clearance of mAb drugs exhibit very different patterns from those of small molecules.
In comparison to small molecules, mAbs have a relatively limited distribution, owing to their size, charge, and tight target binding. For example, the typical central volume of distribution for a mAb, normally derived from a two-compartment model, is approximately 2–4 L, which is similar to the total blood volume. In contrast, the distribution of small molecules is usually not confined to the central blood compartment and normally exhibits a much higher apparent volume of distribution. Prominent examples include theophylline and ethanol, which have distribution volumes that represent the distribution of ∼30 L total body water.
Even though the volume of distribution of a mAb is relatively small, mAb drugs are distributed to peripheral tissues by paracellular and/or transcellular movement following IV dosing or by absorption after parenteral injections3. Unlike small molecules, the paracellular movement of biologics is mainly via convective transport instead of passive diffusion4. In addition, a unique way of movement of mAbs is via transcellular trafficking. The initiation of transcellular movement of mAbs consists of three types of processes: 1) fluid-phase pinocytosis (ie, cells take up proteins from the surrounding fluid space), 2) receptor-mediated endocytosis (mainly via Fcγ receptor binding or through binding to cell surface antigens), and 3) phagocytosis. Fluid-phase pinocytosis is the main pathway by which mAbs enter endothelial cells. After cell entry, the FcRn recycling pathway can participate in the transcytosis step, in which mAbs can be bi-directionally transported to either the interstitial spaces or the vascular space5,6,7. The tissue distribution of mAbs may not be homogeneous because the tight binding between the mAb and its target can prohibit deeper penetration8,9,10,11. In contrast, this pattern is less prominent for small molecules, which can passively diffuse through a tissue.
Small molecule drugs are primarily cleared either through hepatic metabolism or through renal/biliary excretion. In contrast, mAbs are eliminated mainly via intracellular lysosomal proteolytic degradation which occurs throughout the entire body. One exception goes to IgA based antibodies, which are mainly eliminated by biliary secretion12. More than half of the mAbs on the market exhibit nonlinear pharmacokinetics. The linear portion of an antibody drug PK profile is mainly attributable to Fc-receptor mediated clearance and the nonlinear portion is attributable to the target-mediated drug disposition. Fc-mediated elimination is a nonspecific elimination pathway for both endogenous IgGs and exogenous therapeutic IgG mAbs involving either FcRn or Fcγ receptors.
The biologics with a molecular weight <69 kDa are mainly cleared by renal excretion. Therefore, the clearance of these biologics can be compromised in patients with renal impairment. Prominent cases include kineret, a non-glycosylated form of the human interleukin-1 receptor antagonist with a molecular weight of 17 kDa, and pegintron, PEGylated interferon alfa-2b with a molecular weight of 31 kDa. For kineret13, a renal impairment study revealed that its plasma clearance was incrementally reduced in patients with mild, moderate, or severe renal impairment, respectively. For pegintron, a renal impairment study indicated that its clearance decreased by 17% and 44% in patients with moderate or severe renal impairment, respectively. Thus, dose reductions were recommended for renal impaired patients for both kineret and pegintron. Similar results were observed with higher molecular weight such as basiliximab as reflected in its label. However, renal impairment doesn't not always affect the clearance of small biologics. For example, ranibizumab, a recombinant humanised IgG1 kappa isotype mAb fragment with a molecular weight of 48 kDa, showed no notable change in PK in renal impaired patients.
In absence of target-mediated drug clearance, most IgG-based mAbs (ie, IgG1, IgG2, and IgG4) exhibit long half-lives (∼3–4 weeks). This is primarily due to FcRn-mediated antibody recycling. Under acidic conditions, IgGs entering the endosome via fluid phase pinocytosis bind to the FcRn receptor and will not be transferred for lysosomal degradation, as opposed to unbound antibodies14,15. Fcγ receptors can be responsible for clearing soluble mAb-antigen immune complexes or cells opsonised by the mAb16. However, the exact mechanism of action of Fcγ receptors in antibody clearance is not fully understood.
Another distinction between small molecules and biologics is that biologics can be immunogenic. The formation of neutralising anti-drug antibodies (ADA) against the corresponding biologic or immune complexes that trigger proteolytic elimination in the reticuloendothelial system (RES) will cause increased clearance of a biologic. Infliximab, a chimeric IgG1κ monoclonal antibody (composed of human constant and murine variable regions) specific for human tumour necrosis factor-alpha (TNFα), was cleared more rapidly in patients who developed human anti-chimeric antibodies (HACAs)17. A decreased trough concentration with increasing immunogenicity was also reported for golimumab, a human IgG1κ monoclonal antibody specific for human tumour necrosis factor alpha (TNFα). The immunogenicity is often managed by co-medication of methotrexate (MTX) with the biologics that present significant immunogenicity issues18. In contrast to the notion that immunogenicity always increases drug clearance, changes in the elimination rate due to immunogenicity may be bi-directional. For example, an immune complex that does not trigger an RES response may slow down drug elimination by serving as a depot for the therapeutic proteins, which has been observed quite often for cytokines and hormones.
The degree of humanisation, route of administration, duration of therapy, and dose level can also impact immunogenicity. The incidence of immunogenicity is negatively correlated with the degree of humanisation of mAbs19. In the case of tositumomab, a murine antibody, the incidence of developing human anti-mouse antibody (HAMA) seropositivity was 70% in patients with low-grade non-Hodgkin's lymphoma20. In comparison, the reported incidence of immunogenicity for fully human, humanised and chimeric mAbs ranged from <1% to ∼10%4. The incidence of immunogenicity is positively correlated with the duration of therapy and the re-introduction of drugs20,21. It has been reported that immunogenicity is negatively related to the dose4. However, a firm conclusion regarding the relationship of immunogenicity to dose could not be reached. The potential to develop ADA could be higher following SC or IM administration than IV administration because phagocytes and NK cells, which are responsible for the initial, innate, immune response, are found under the skin and in the mucosal epithelia. However, the above hypothesis has not yet been confirmed with human data, and statistical comparison between the different routes of administration is not available.
First-in-human (FIH) starting dose determination for biologics
Based on both the FDA and the European Medicines Agency (EMA) guidance, the starting dose for a FIH trial is normally a dose that results in no pharmacological or toxicological effects. As a result, the commonly adopted method to determine the FIH starting dose for new molecular entities is the no observed adverse effect level (NOAEL) approach. The no observed effect level (NOEL) approach has also been practised to a lesser extent. The NOAEL approach normally includes three steps, and the approach is the same for both small molecules and biologics. The first step is the determination of a NOAEL dose in the most sensitive or most clinically relevant nonclinical species. The second step is to convert the animal NOAEL dose to a human equivalent dose (HED) by applying a Body Surface Area Conversion Factor (BSA-CF). The third step is to propose the maximum recommended starting dose (MRSD) after adjusting the HED with a safety factor (MRSD=HED* Safety Factor). However, the NOAEL-to-HED conversion step is not needed to calculate MRSD for biologics as compared to small molecules. To calculate MRSD for biologics, the safety factor can be directly applied to the body weight normalized NOAEL dose.
Additional considerations for proposing an MRSD are sometimes taken into account under special circumstances. For example, when cross-reactivity between human and animal models cannot be established for the biologics in development, especially for mAbs, a surrogate antibody may need to be developed for the animal study if a toxicology study in chimpanzees is prohibited. Furthermore, an MRSD derived from an animal NOAEL approach may not be reliable because of potential target/antigen binding, distribution and capacity differences between humans and animals. Therefore, all potential factors leading to changes in PK exposure, efficacy, and safety responses should be taken into account when extrapolating a dose from animals to humans. The relevant extrapolation techniques include allometric scaling, target-mediated drug disposition modelling, and physiologically based PK (PBPK) modelling.
As triggered by the TGN1412 tragedy, for high risk biologics, the EMA has proposed the minimum anticipated biological effect level (MABEL) approach for FIH dose selection. The MABEL approach is considered for biologic agonists in particular, such as biologics with cellular targets that may activate downstream intracellular pathways and trigger cytokine release. The determination of a human MABEL dose depends on PK-PD relationships. Such relationships can be based on in vitro and/or in vivo studies involving human cells or animal species. Animal-human differences in PK exposure, target binding affinity, and PD potency should all be taken into consideration22. Recent examples illustrating the MABEL dose determination include an antibody compound that targets a blood cell surface receptor23. In general, the MABEL dose is usually a more conservative approach than the starting dose derived by the NOAEL approach, as it is normally one order of magnitude lower. Sometimes, MRSDs determined from the NOEL, the pharmacologically active dose (PAD), and/or the MABEL approaches are evaluated against the MRSD dose determined from the NOAEL to make the final FIH recommendation.
Regulatory agencies in other countries have taken similar approaches to those adopted by the FDA and EMA. For example, the State Food and Drug Administration (SFDA), the Chinese regulatory agency, uses similar language in its guidance regarding MRSD to that adopted by the FDA and EMA but with fewer details.
Model-based drug development for biologics
Fixed dosing vs body size-adjusted dosing
Fixed dosing is the most common dosing approach for small molecule drugs in adult patients. However, biologic products are often dosed based on body size. Whether a drug should be administered based on a patient's body size, such as body weight (BW) and body surface area (BSA), mainly depends on the effect of the body size on the pharmacokinetics (PK) and pharmacodynamics (PD) of the drug, as well as its therapeutic window (Table 1). A good dosing strategy should provide reduced inter-patient variability in PK and/or PD and ultimately optimise therapeutic outcomes.
Table 1. Selected therapeutic peptides and proteins and their dosing approaches for adult patients. Reprinted from25.
| Generic name | Brand name | Approval date | MW (Da) | Type | Target | Dosing approach |
|---|---|---|---|---|---|---|
| Abatacept | Orencia | 2005 | 92 300 | Fusion protein | CD80/CD86 | mg/kg |
| Daptomycin | Cubicin | 2004 | 1 620 | Peptide | LTA synthesis | mg/kg |
| Darbepoetin alfa | Aranesp | 2001 | 37 100 | Protein | EpoR | μg/kg |
| Degarelix | Firmagon | 2008 | 1 632 | Peptide | GnRHR | mg |
| Emfilermin | Discontinued | 22, 007 | Protein | LIFR | μg/kg | |
| Enfuvirtide | Fuzeon | 2003 | 4 492 | Peptide | gp41 | mg |
| Erythropoietin alpha | EPOGEN | 1989 | 30, 400 | Protein | EpoR | Units/kg |
| Erythropoietin beta | NeoRecormon | 1993 | 30 000 | Protein | EpoR | μg/kg |
| Etanercept | Enbrel | 1999 | 150 000 | Fusion protein | TNF | mg |
| Hematide | In development | NR1 | Pegylated peptide | EpoR | mg/kg | |
| Lanreotide autogel | Somatuline | 2007 | 1 096 | Peptide | IGF-1 | mg |
| Octreotide acetate | Sandostatin | 1988 | 1 019 | Peptide | SSTR2/5 | μg |
| Onercept | Discontinued | 18 000 | Fusion protein | TNFR | mg/kg | |
| PEGinterferon alpha-2b | PEG-Intron A | 2001 | 19 271 | Protein | IFNAR1/2 | μg/kg |
| Plitidepsin | Aplidin | 2004 | 1 110 | Peptide | EGFR | mg/m2 |
| Recombinant Factor VIIa | NovoSeven | 1999 | 50 000 | Protein | TF | μg/kg |
| rhGH1 | Norditropin | 1987 | 22 000 | Protein | GH receptor | mg/kg |
| u-hFSH1 | Metrodin HP | Discontinued | 30 000 | Protein | FSH receptor | IU |
1Abbreviation: NR, not reported; rhGH, recombinant human growth hormone; u-hFSH, Urinary human follicle stimulating hormone.
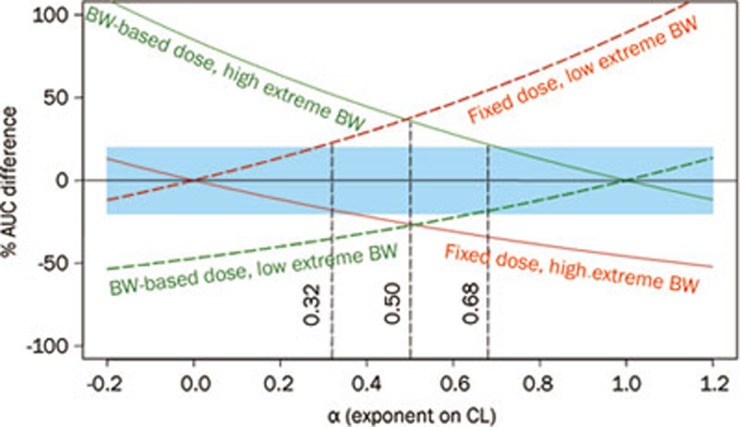
Two retrospective studies evaluated the potential benefits of fixed dosing and body size-based dosing by comparing the ability of each of the two approaches to reduce pharmacokinetic (PK) and/or pharmacodynamic (PD) variability in adults for 30 biologics with published population PK and/or PD models24,25. Of these 30 biologics, 1 2 were mAbs24, and 18 were not mAbs (these included therapeutic proteins and peptides)25. At the population level, the inter-subject variability in exposure (AUC and Cmax) was examined for 1000 subjects, for both dosing approaches. At the individual level, the difference between the exposure of patients with extreme body sizes and the typical exposure following both approaches was compared. The results, as illustrated by a representative plot (Figure 1), show that the two dosing approaches perform similarly across the biologics investigated, with fixed dosing being better for some biologics and body size-based dosing being better for the others. Based on these findings, fixed dosing is recommended for FIH adult studies because it offers advantages, including the ease of preparation, reduced costs and a reduced chance of dosing errors. When sufficient data become available, a full assessment of the body size effect on PK/PD should be conducted to determine the optimal dosing approach for phase 3 trials.
Figure 1.
The % difference of AUC for patients with extremely low body weight (BW) (40 kg, colored broken lines) and extremely high BW (140 kg, colored solid lines) from those for patients with a median BW of 75 kg as a function of the α values following a fixed (red) and a BW-based (green) dose, assuming 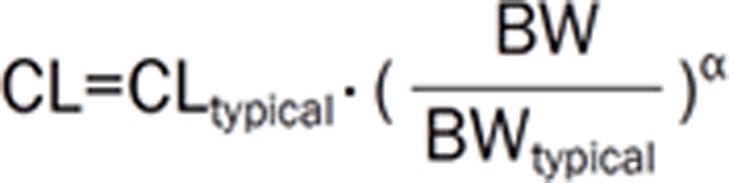 . The shaded area represents AUC values within 100%±20% of typical AUC. Reprinted from25.
. The shaded area represents AUC values within 100%±20% of typical AUC. Reprinted from25.
Dosing regimen optimisation
PK-PD-clinical response models play a central role in dosing regimen determination. There are different types of models, such as mechanism-based models, physiologically based models, empirical models, semi-empirical models, and meta-analysis for biologics of similar molecular structure and the same target. All of these models can be used to analyse different types of relationships, such as exposure/biomarker, exposure/response, and biomarker/response relationships. Empirical exposure-response models have gained popularity mainly because of their practicality and convenience, as many of the downstream actions after a drug binds to its target remain unknown. In this regard, empirical exposure-response models used for small molecules can be directly adapted to characterise the drug effects of biologics. It should be noted that predictive empirical models, as well as all other types of models, rely on sufficient high-quality data being available, which calls for well designed studies with prospectively defined PK, PD and clinical response endpoints. Another modelling tool that has been increasingly used is meta-analysis. Meta-analysis synthesises reported clinical data from drugs in the same class to enrich the information for the dose/exposure response of the drug candidate in development26,27,28,29 and also provides a benchmark for comparison purposes. Simultaneous modelling of exposure-PD response, PD response-clinical response, and exposure/PD response-clinical response has been considered to be an ideal approach for dosing regimen justification. However, the data requirements, the difficulty in identifying measurable biomarkers, and the lack of a relationship between PD response and clinical response have limited the application of PK-PD-clinical response models in certain therapeutic areas.
Mechanism- and physiologically-based models for mAbs
Mechanism-based models provide a unique advantage for understanding drug efficacy and safety, through mathematically describing the underlying biological and pharmacological processes as realistically as possible. Antibody drug development starts from the selection of a target that is responsible for disease pathophysiology. Therefore, the common feature of the frequently used mechanism-based models is to characterise the relationship between drug exposure and target suppression.
Because of the large size of mAbs, it is of particular importance to understand their distribution at organs/tissues of interest in order to better establish the exposure/response relationship, as the exposure in the blood stream may not reflect the exposure in the targeted tissues. This may be achieved by PBPK models. The concept of physiologically based PK/PD modelling is to use a system of differential equations to describe drug absorption, distribution, metabolism, and elimination (ADME) dynamics with human physiological parameters. A typical whole-body PBPK model involves modelling the whole human body as a closed circulatory system with compartmentalised and interconnecting organs or specific tissues. The drug mass transfer for each compartment is described by a turnover model using organ- and tissue-specific blood flows as the corresponding input to and output from the compartment. In a sense, a PBPK model is a fluid flow anatomy of the human body. In this regard, the arterial and venous blood connects most organs, whereas the flow from the gastrointestinal tract, spleen and pancreas goes to the liver via the portal vein before it reaches the venous side. All of the blood that flows from various organs will converge at the lungs and then return to the blood compartment to complete a cycle. In a PBPK model, a flow balance is kept by ensuring that the sum of input flows (ie, blood+lymphatic flow) is equal to the sum of output flows for each compartment. A schematic chart of a PBPK model can be found in many publications12,30,31.
Overall, model-based drug development takes advantage of a series of quantitative approaches, such as mechanism-based PK, PBPK, PK-PD, exposure-response, and PK-PD-clinical response models. These models offer a unique edge for developing biologic drugs. With the advancement of new quantitative tools32,33, drug developers and researchers can gain powerful insights into designing the most effective therapeutic regimens.
Common clinical pharmacology issues in biologics development
From a global perspective, the US FDA and EMA are the two leading agencies in the development of guidelines to regulate biologics development. The relevant clinical pharmacology guidance documents published by the US FDA are deposited at http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064982.htm; the clinical pharmacology guidelines published by the EMA are deposited at http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000370.jsp&mid=WC0b01ac0580032ec5. However, many development issues encountered in practice are not covered or elaborated on clearly in these publications. The following sections are a collection of both development norms and regulatory guidance for common clinical pharmacology pathways in biologics development. Caveat should be given that the current development strategies are subject to change with advancements in both technology and regulatory sciences. For example, although the characterisation of metabolism was not required for the approval of the early pioneering protein-based drugs, regulatory expectations and guidance have recently evolved. Future pharmaceutical sponsors may be expected to characterise the metabolism of newer biologics34.
Considerations for drug-drug interactions (DDI) potential for biologics
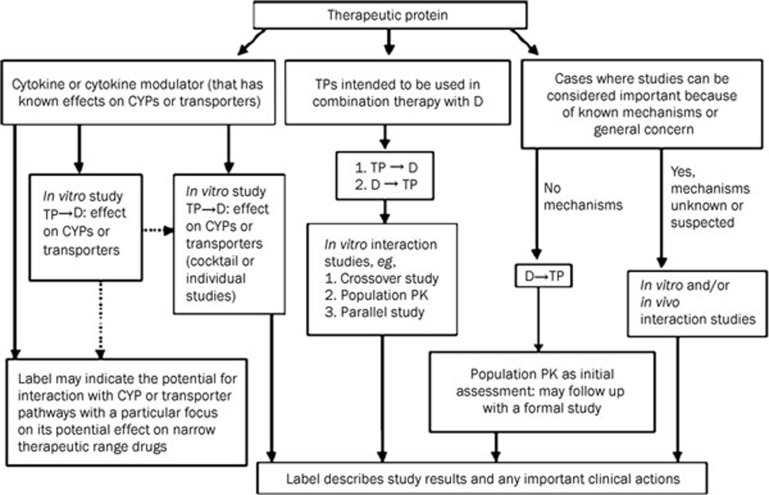
Although DDIs between biologics and small molecules have been reported, they are mostly mild and are less common than those between small molecules, owing to the difference in the clearance mechanisms. Cytokine-mediated changes in drug-metabolising enzymes are the most well documented therapeutic DDI mechanisms for biologics. Because of the lack of predictive in vitro and preclinical animal models for addressing DDIs, clinical study is the routine approach for biologic DDI assessment. Clinical investigations on biologics as a victim of DDI include the impact of altered target protein levels by the concomitant medication on the clearance of therapeutic proteins, the displacement of therapeutic proteins from binding proteins, and the modulation of Fcγ receptor expression. When designing a DDI study for biologics, factors such as patient population, disease status, medications that are likely to be coadministered in that population, clearance mechanisms of a therapeutic protein and concomitant drugs, and effect of biologics on P450 activities, among other factors, should be taken into account to determine the potential for DDIs. While the crossover study design is the most often used approach for small molecule DDI assessment, it is not a feasible approach for most biologics because of their long half-lives. Even for evaluating the effect of biologics on small molecules, a sequential study design (small molecule drug administered in period 1 or lead-in phase of a Phase II or III study, small molecule+biologics administered in period 2 or Day 1 of a Phase II or III study) is often used to avoid long washout period for biologics. In addition, DDI assessment for biologics is often conducted in patients instead of healthy subjects. This is mainly due to 1) potential difference in PK and PD between patients and healthy subjects; 2) toxicities of the biologics and small molecules especially for oncology compounds that prohibit evaluation of DDI in healthy subjects; 3) immunogenicity issues. All the factors discussed above pose difficulties to conduct dedicated DDI assessment for biologics, as an alternative, population PK method can be used for confirmatory DDI assessment. Population PK approach allows less intensive sampling in patients, incorporating DDI assessment in larger Phase II/III trials, and integrating data generated across multiple studies during different development phases. DDI findings identified by population PK approach have already been exemplified in current labels (eg pregabalin, pramipexole, Tocilizumab, sildenafil, cilostazol, and etc). It should be noted that biologics and small molecules share many of the same principles in terms of the DDI data analysis method and labelling output regarding dosing and DDI potential35. Figure 2 shows the decision tree and steps involved in DDI evaluations of biologics.
Figure 2.
Decision tree for DDI study for biologics proposed by Huang et al. TP, therapeutic protein; D, small-molecule drug; TP→D, an evaluation of the effect of TP on D; D→TP, an evaluation of the effect of D on TP; Broken lines, the limited use of in vitro studies for informing in vivo study design or labeling; CYP, cytochrome P450. Reprinted from35.
QTc prolongation by biologics
The QT interval measures the time from the start of the Q wave to the end of the T wave in a heart's electrical cycle. QTc represents the heart rate-corrected QT interval because QT is heart rate dependent. In general, a thorough QTc study is not required for mAb drugs. However, it is recommended that extensive ECG monitoring be undertaken in the early phase of clinical development to monitor cardiac safety and to confirm that a thorough QT (TQT) study is not necessary. Particular attention should be paid to small biotherapeutics (<5 kDa), biologics with heart and/or vasculature targets or targets with the same nature, and compounds with positive preclinical cardiovascular safety signals. A TQT study may be needed on a case by case basis, depending on the information gained from the on-going clinical development.
Immunogenicity
Owing to the size of biologics and human body's mechanisms for protecting against foreign invasion, use of biologics has the concern of immunogenicity. The possible consequences of immunogenicity include the loss of therapeutic efficacy and severe life threatening adverse events. The safety events can originate from an intensified general immune response or cross-reactivity of the ADA with endogenous substances that are critical for maintaining physiological function.
In the study protocol for biologics, a risk mitigation strategy for potential immunogenicity-mediated adverse events should be developed and implemented starting in the early phases. The risk mitigation strategy could also evolve at different stages of drug development based on the developing understanding of the risk factors for immunogenicity and the degree of its impact on drug safety and efficacy. A typical mitigation plan is formulated by considering the protein structure (eg, its similarity to endogenous substances), the manufacturing process, and the target population. It normally includes a bioanalytical assay strategy for both preclinical and clinical samples, a tittered testing approach to identify the types of ADA responses, a plan for medical treatment in anticipation of potential immunogenicity mediated safety events, and an appropriate study stopping criteria.
Specific populations
Paediatrics
The clinical programme in the paediatric population is the same for both biologics and small molecules. The principle that underlies current regulatory guidance for paediatric trials is to minimise the trial burden in the paediatric population for paediatric indications36. For example, it is recommended that the relative bioavailability of paediatric oral formulations compared to adult oral formulations is evaluated in the adult population. To conduct a paediatric PK study, the use of population pharmacokinetics and sparse sampling based on the optimal sampling theory is recommended.
However, definitive pharmacokinetic studies for dose selection across the age ranges of paediatric patients in whom the medicinal product is likely to be used should be conducted in the paediatric population. For biologics that exhibit nonlinear pharmacokinetics in adults, probably as a result of TMDD, a steady state study in the paediatric population is normally needed.
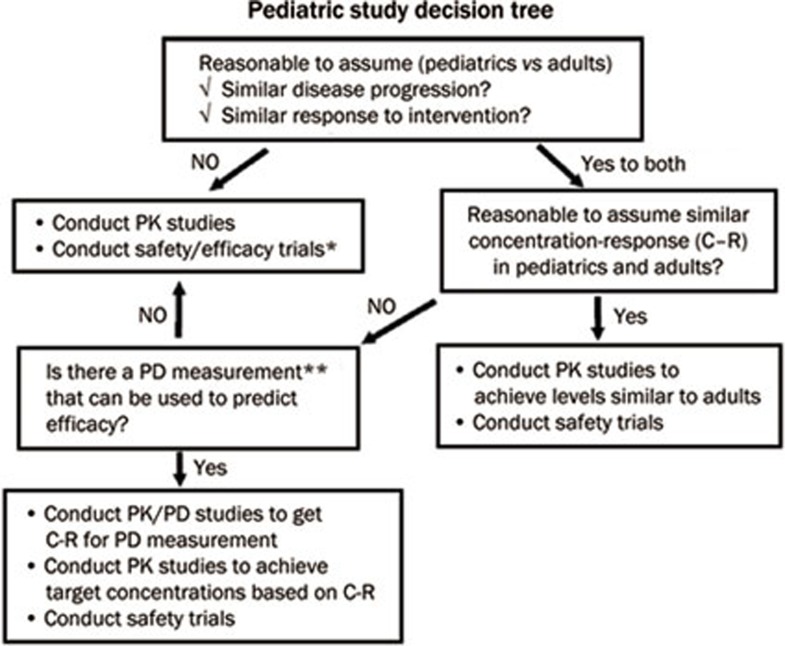
Based on similarities in disease progression and the exposure-response between children and adults, a different clinical development plan is needed. In the event of a different disease progression pattern, it is expected that both safety and efficacy trials will be carried out in the paediatric population (Figure 3). The volume of blood withdrawn should be minimised in paediatric studies37. If a paediatric study is needed, current recommendations include the use of the patient population instead of healthy volunteers, body size-based dosing (ie, body weight- or body surface area-based dosing even if fixed dosing is used for adults), conservative dosing in anticipation of potential differences in pharmacokinetic parameters, and the use of a formulation and vial strength suitable for the paediatric population38.
Figure 3.
Pediatric study decision tree. Reprinted from36.
Renal Impairment: Based on the molecular size constraint for glomerular filtration, for biologics with molecular weights >69 kDa, such as mAbs, current guidelines do not require an evaluation of the effect of renal impairment for their licensing applications13.
The effect of renal impairment on the exposure of therapeutic proteins with molecular weight <69 kDa is not consistent across different proteins. Renal impairment has been shown to potentially increase exposure for cytokines or cytokine modulators with a molecular weight of <69 kDa. The examples include anakinra, peginterferon α-2A/B, and oprelvekin. A dose reduction is required for these therapeutic proteins in renal impairment patients. However, there is “no clinical consequence” of reduced renal function for digibind and ranibiumab, both are antibody fragments, as indicated in their product labelling.
Specific studies to investigate the impact of renal impairment on pharmacokinetics have generally been recommended for small proteins with presumed or known renal clearance as the dominant pathway. Similar to the approach undertaken for small molecules, the initial study should be a single dose in a renal impaired patient population, followed by additional studies (eg, multiple doses in patient population with moderate/severe impairment) if warranted.
Hepatic Impairment: Hepatic impairment is not likely to affect the exposure to biologics. It is unlikely that >20% of a dose of biologics can be catabolised in the liver and there have been very few reports of specific studies investigating the impact of hepatic impairment on the pharmacokinetics of biologics. One exception is Mylotarg, a cytotoxic antitumour antibiotic linked to an antibody for targeted delivery. The metabolism of Mylotarg has been investigated in human liver microsomes, human liver cytosol and human leukaemia cells. After incubation with Mylotarg, a total of 11 metabolites were found. As a result, the Mylotarg label notes that “extra caution should be taken when administering Mylotarg in patients with hepatic impairment”. A specific study to evaluate hepatic clearance in patients with impaired hepatic function is normally not required for biologics.
Elderly
Reports on clinical pharmacology studies of biologics in elderly subjects have been scarce. However, there are examples where age has an effect on PK parameters of certain biologics. For instance, canakinumab, an IgG1-based antibody drug, has been found to have a slightly reduced absorption rate, but not a reduced overall drug exposure, in the elderly39. Levemir, a recombinant long-acting basal insulin with a molecular weight of ∼6 kDa, shows a higher exposure in the elderly than younger subjects but no difference in overall safety or effectiveness. In product labeling, PK parameters can be summarised by different age groups if clinical significant age effect is identified. However, sufficient number of patient in each age group is needed to warrant a labelling claim. Under most circumstances, age effect is evaluated as a covariate (eg, for panitumumab) in their corresponding population PK models.
Race/gender
The effect of race or gender on the exposure of biologics is often insignificant after the difference in body weight has been taken into account. Race and gender effects have often been investigated as a covariate on PK parameters using population PK modelling. For examples, age was found not to be a significant covariate on PK parameters for panitumumab and natalizumab. Definitive statements regarding the effect of race or gender on drug exposure are rarely found on biologics labels. However, pharmaceutical sponsors may wish to facilitate quick entry to other ethnic regions and cross the regulatory barriers early, by conducting bridging studies to investigate potential differences in drug exposure in different races.
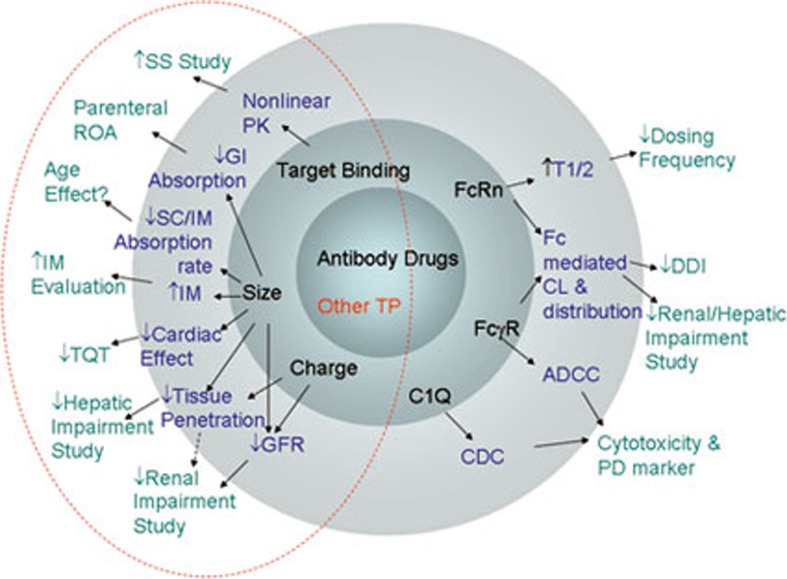
In summary, general clinical pharmacology considerations for biologics are depicted in Figure 4. Ultimately, it is the size of the biologics that distinguishes the clinical development programme of biologics from that of small molecules. The inherent characteristics of mAbs, such as specific functions associated with their FcRn, FcγR, and C1Q domains, differentiate them from other therapeutic proteins in many respects, such as PK, PD, and pharmacological functions. Consequently, in addition to the general considerations for biologics, special considerations should be given to each individual biological product based on its own characteristics.
Figure 4.
Grand view of clinical pharmacology aspects for biologics development and their origins. Common clinical pharmacology considerations are shown in the un-shaded area. Typical PK, PD, physiological, and pharmacological properties of biologics are shown in the third layer of the shaded area. Biochemical structural properties are shown in the second shaded layer. The two common classes of biologics, antibody drugs and other therapeutic proteins (TP), are shown in the inner layer. The broken circle highlights the features common to all TPs. TP, therapeutic protein; DDI, drug-drug interaction; CDC, Complement dependent cytotoxicity; ADCC: Antibody dependent cellular cytotoxicity; SS, Steady state; TQT, Thorough QT prolongation; GFR, Glomerular; GI, Gastrointestinal; ROA, Route of administration; IM, Intramuscular; SC, Subcutaneous.
Disclaimer
The views expressed in this chapter are those of the author and do not reflect the official policy of the FDA. No official support or endorsement by the FDA is intended or should be inferred.
References
- Roskos L, Ren S, Robbie G.Application of modeling and simulation in the development of protein drugsIn Kimko H and Peck C, editors. Clinical trial simulations AAPS advances in pharmaceutical sciences SeriesNew York: Springer Science+Business Media, LLC; 2011. p361–96.
- O'Driscoll C.Anatomy and physiology of the lymphaticsIn Charman W, Stella V, editors. Lymphatic transport of drugsBoca Raton (FL): CRC Press; 1992. p1–36.
- Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–68. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–58. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- Antohe F, Radulescu L, Gafencu A, Ghetie V, M. Simionescu M. Expression of functionally active FcRn and the differentiated bidirectional transport of IgG in human placental endothelial cells. Hum Immunol. 2001;62:93–105. doi: 10.1016/s0198-8859(00)00244-5. [DOI] [PubMed] [Google Scholar]
- Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–11. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Yoong Y, Simister NE. Bidirectional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: a system to study protein transport across epithelia. J Cell Sci. 2000;113:1277–85. doi: 10.1242/jcs.113.7.1277. [DOI] [PubMed] [Google Scholar]
- Bill A. Plasma protein dynamics: albumin and IgG capillary permeability, extravascular movement and regional blood flow in unanesthetized rabbits. Acta Physiol Scand. 1977;101:28–42. doi: 10.1111/j.1748-1716.1977.tb05980.x. [DOI] [PubMed] [Google Scholar]
- Hedger MP, Hettiarachchi S. Measurement of immunoglobulin G levels in adult rat testicular interstitial fluid and serum. J Androl. 1994;15:583–90. [PubMed] [Google Scholar]
- Juweid M, Strauss HW, Yaoita H, Rubin RH, Fischman AJ. Accumulation of immunoglobulin G at focal sites of inflammation. Eur J Nucl Med. 1992;19:159–65. doi: 10.1007/BF00173275. [DOI] [PubMed] [Google Scholar]
- Weinstein JN, Eger RR, Covell DG, Black CD, Mulshine J, Carrasquillo JA, et al. The pharmacology of monoclonal antibodies. Ann N Y Acad Sci. 1987;507:199–210. doi: 10.1111/j.1749-6632.1987.tb45802.x. [DOI] [PubMed] [Google Scholar]
- Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn. 2007;34:687–709. doi: 10.1007/s10928-007-9065-1. [DOI] [PubMed] [Google Scholar]
- US Food and drug administration. Guidance for industry: Pharmacokinetics in patients with impaired renal function — Study design, data analysis, and impact on dosing and labeling (Draft); 2010
- Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, et al. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol. 2002;169:5171–80. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]
- Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, et al. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol. 1997;15:637–40. doi: 10.1038/nbt0797-637. [DOI] [PubMed] [Google Scholar]
- Murphy K, Travers P, Walport M.Janeway's ImmunobiologyGarland Science; 2007
- De Vries MK, Brouwer E, van der Horst-Bruinsma IE, Spoorenberg A, van Denderen JC, Jamnitski A, et al. Decreased clinical response to adalimumab in ankylosing spondylitis is associated with antibody formation. Ann Rheum Dis. 2009;68:1787–8. doi: 10.1136/ard.2009.109702. [DOI] [PubMed] [Google Scholar]
- Simponi (golimumab) injection, solution: US prescribing information. Horsham (PA): Janssen Biotech, Inc; 2011
- Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24:1720–40. doi: 10.1016/s0149-2918(02)80075-3. [DOI] [PubMed] [Google Scholar]
- BEXXAR (tositumomab) kit: US prescribing information. Research Triangle Park (NC): GlaxoSmithKline LLC; 2011
- Remicade (infliximab) injection: US prescribing information. Horsham (PA): Janssen Biotech, Inc; 2011
- Muller PY, Milton M, Lloyd P, Sims J, Brennan FR. The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies. Curr Opin Biotechnol. 2009;20:722–9. doi: 10.1016/j.copbio.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Yu J, Karcher H, Feire AL, Lowe PJ. From target selection to the minimum acceptable biological effect level for human study: use of mechanism-based PK/PD modeling to design safe and efficacious biologics. AAPS J. 2011;13:169–78. doi: 10.1208/s12248-011-9256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49:1012–24. doi: 10.1177/0091270009337512. [DOI] [PubMed] [Google Scholar]
- Zhang S, Shi R, Li C, Parivar K, Wang DD. Fixed dosing versus body size-based dosing of therapeutic peptides and proteins in adults. J Clin Pharmacol. 2012;52:18–28. doi: 10.1177/0091270010388648. [DOI] [PubMed] [Google Scholar]
- Mandema JW, Gibbs M, Boyd RA, Wada DR, Pfister M. Model-based meta-analysis for comparative efficacy and safety: application in drug development and beyond. Clin Pharmacol Ther. 2011;90:766–9. doi: 10.1038/clpt.2011.242. [DOI] [PubMed] [Google Scholar]
- Mandema JW, Boyd RA, DiCarlo LA. Therapeutic index of anticoagulants for prevention of venous thromboembolism following orthopedic surgery: a dose-response meta-analysis. Clin Pharmacol Ther. 2011;90:820–7. doi: 10.1038/clpt.2011.232. [DOI] [PubMed] [Google Scholar]
- Mandema JW, Salinger DH, Baumgartner SW, Gibbs MA. A dose-response meta-analysis for quantifying relative efficacy of biologics in rheumatoid arthritis. Clin Pharmacol Ther. 2011;90:828–35. doi: 10.1038/clpt.2011.256. [DOI] [PubMed] [Google Scholar]
- Mandema JW, Cox E, Alderman J. Therapeutic benefit of eletriptan compared to sumatriptan for the acute relief of migraine pain — results of a model-based meta-analysis that accounts for encapsulation. Cephalalgia. 2005;25:715–25. doi: 10.1111/j.1468-2982.2004.00939.x. [DOI] [PubMed] [Google Scholar]
- Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55:4611–22. [PubMed] [Google Scholar]
- Thygesen P, Macheras P, Van PA. Physiologically-based PK/PD modelling of therapeutic macromolecules. Pharm Res. 2009;26:2543–50. doi: 10.1007/s11095-009-9990-3. [DOI] [PubMed] [Google Scholar]
- Zhao L. New developments in using stochastic recipe for multi-compartment model: inter-compartment traveling route, residence time, and exponential convolution expansion. Math Biosci Eng. 2009;6:663–82. doi: 10.3934/mbe.2009.6.663. [DOI] [PubMed] [Google Scholar]
- Zhao L, Li N, Yang H. A new stochastic approach to multi-compartment pharmacokinetic models: probability of traveling route and distribution of residence time in linear and nonlinear systems. J Pharmacokinet Pharmacodyn. 2011;38:83–104. doi: 10.1007/s10928-010-9179-8. [DOI] [PubMed] [Google Scholar]
- Hamuro LL, Kishnani NS. Metabolism of biologics: biotherapeutic proteins. Bioanalysis. 2012;4:189–95. doi: 10.4155/bio.11.304. [DOI] [PubMed] [Google Scholar]
- Huang SM, Zhao H, Lee JI, Reynolds K, Zhang L, Temple R, et al. Therapeutic protein-drug interactions and implications for drug development. Clin Pharmacol Ther. 2010;87:497–503. doi: 10.1038/clpt.2009.308. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. Guidance for industry: General considerations for pediatric pharmacokinetic studies for drugs and biological products (Draft); 1998
- US Food and Drug Administration. Guidance for industry: E11 clinical investigation of medicinal products in the pediatric population; 2000 [PubMed]
- US Food and Drug Administration. Guidance for Industry. Exposure-response relationships — study design, data analysis, and regulatory applications; 2003
- European Medicines Agency. CHMP Assessment Report for Ilaris; 2009