Abstract
The vocal folds, which are located in the larynx, are the main organ of voice production for human communication. The vocal folds are under continuous biomechanical stress similar to other mechanically active organs, such as the heart, lungs, tendons and muscles. During speech and singing, the vocal folds oscillate at frequencies ranging from 20 Hz to 3 kHz with amplitudes of a few millimeters. The biomechanical stress associated with accumulated phonation is believed to alter vocal fold cell activity and tissue structure in many ways. Excessive phonatory stress can damage tissue structure and induce a cell-mediated inflammatory response, resulting in a pathological vocal fold lesion. On the other hand, phonatory stress is one major factor in the maturation of the vocal folds into a specialized tri-layer structure. One specific form of vocal fold oscillation, which involves low impact and large amplitude excursion, is prescribed therapeutically for patients with mild vocal fold injuries.
Although biomechanical forces affect vocal fold physiology and pathology, there is little understanding of how mechanical forces regulate these processes at the cellular and molecular level. Research into vocal fold mechanobiology has burgeoned over the past several years. Vocal fold bioreactors are being developed in several laboratories to provide a biomimic environment that allows the systematic manipulation of physical and biological factors on the cells of interest in vitro. Computer models have been used to simulate the integrated response of cells and proteins as a function of phonation stress. The purpose of this paper is to review current research on the mechanobiology of the vocal folds as it relates to growth, pathogenesis and treatment as well as to propose specific research directions that will advance our understanding of this subject.
Keywords: Vocal folds, Mechanobiology, Systems biology
Introduction
The vocal folds, which are located within the larynx, are the main organ of voice production for human communication. During normal phonation, the vocal folds undergo oscillations at frequencies ranging from 20 Hz to 3kHz with amplitudes of a few millimeters [1]. During phonation, various mechanical stresses including tensile (~1.0 MPa), contractile (~100 kPa), aerodynamic (~1–10 kPa), inertia (~1–2 kPa), impact (~0.5–5.0 kPa) and shear stresses (~0.8 kPa) act on the mucosa or muscles of human vocal folds [2–5]. To withstand large repetitive mechanical stresses, the vocal folds have a distinctive geometry, histology and viscoelasticity that result in efficient oscillations during phonation [6–12]. The human vocal folds possess three anatomically distinctive layers, namely, the epithelium (0.05 – 0.1 mm thick), the lamina propria (1.5 – 2.5 mm thick) and the vocalis muscle (7–8 mm thick). The epithelium and lamina propria are connected to each other by a very thin layer of the basement membrane [8,13] and are the major vibratory tissue during phonation. The lamina propria is a hypocellular composite of extracellular matrix (ECM) molecules, including proteoglycans, collagen, elastin and hyaluronic acid. Cells including myofibroblasts, macrophages and fibroblasts are distributed sparsely across the lamina propria [14].
The lamina propria has three layers, each one characterized by a particular ECM composition [10–12,15,16]. The superficial lamina propria contains sparse elastin and collagen fibers that make this layer pliable for mucosal oscillation. The intermediate lamina propria contains more elastin and collagen fibers. The deep lamina propria contains less elastin but more collagen fibers in relation to the superficial and intermediate layers. The intermediate and deep lamina propria constitute the vocal ligament, providing elasticity and stiffness for the vocal folds. With this specialized structure, the vocal fold lamina propria is pliable enough for oscillation yet strong enough to withstand the phonatory stress [6,7,9,12,17–19].
Mechanical forces are known to alter cell identity and activity [20]. Depending on their type and magnitude, such forces can cause cell damage or stimulate proliferation or repair [21]. The best examples of these interactions are from stem cell studies. Stems cells will likely differentiate into bone cells when subjected to compressive forces because bones nearly constantly experience compressive forces. When stem cells are exposed to stretching forces, they tend to differentiate into more muscle-like cells. In other words, the specific magnitude, distribution, and orientation of the mechanical forces applied to the cells could play a key role in integrating stem cells into the body in a useful and functional way [22–24]. Vocal fold fibroblasts, the most abundant cells in the vocal fold lamina propria, exhibit similar characteristics and functions as mesenchymal stem cells [25].
Information about the relationships between cells, proteins and biomechanical simulation is sparse in the vocal fold literature. Progress over the past ten years includes in vivo evidence of how phonatory forces affect vocal fold physiological and pathophysiological processes. Cell culture devices and bioreactors are used to determine the relationship between phonation-relevant mechanical stimulation and cell response. Ongoing and future research on systems biology may lead to a better understanding of vocal fold mechanobiology.
In vivo Evidence of the Role of Phonatory Forces in Vocal Fold Physioslogy and Pathophysiology
Effecs of phonatory forces on vocal fold development
Newborn vocal folds do not have a layered structure typical of the lamina propria [14,26–28]. The superficial lamina propria was observed to appear between 7 and 12 years of age [14,27]. A clear demarcation between the intermediate and the deep lamina propria in terms of cell density and population was assured at the age of 7. A differentiated layer organization of collagen and elastic fibers was observed in the lamina propria around the age of 13 [29]. These observations suggest that the vocal folds do not possess an intrinsic layered structure. A maturation process is involved in the development of vocal fold layers. These findings naturally lead to questions about whether phonatory forces are involved in vocal fold maturation and whether other factors, such as hormones, could also contribute to the process.
Indirect evidence of the role of phonation in vocal fold maturation can be gleaned from investigations in adult patients with cerebral palsy who were unphonated since their birth [30–32]. Histological results uniformly showed that the vocal folds from these unphonated patients looked hypoplastic and homogeneous without differentiable vocal fold ligament and Reinke’s Space. The cells also exhibited signs of degeneration with few vesicles in the cytoplasm. Cells did not produce vocal fold ECM proteins and expressed minimal hyaluronan receptors. Results suggested that phonation after birth might be necessary to signal or activate vocal fold fibroblasts to synthesize and organize ECM proteins for normal vocal fold growth and maturation.
Further evidence showed that biomechanical stimulation is necessary for both the maturation and the maintenance of the layered ECM organization in the vocal folds. A 62-year-old male was unphonated for 11 years and 2 months after a cerebral hemorrhage [33]. His vocal fold mucosa showed signs of atrophy with a uniform structure and undifferentiated layers in the lamina propria. These changes from the normal structure suggest that vocal fold fibroblasts may need constant biomechanical stimulation for ECM synthesis to maintain vocal fold tissue homeostasis. Furthermore, the gradient and heterogeneous structure of the vocal fold lamina propria might be the result of variations in the magnitude of forces across the thickness of the lamina propria.
Relationship between phonatory forces and vocal fold injury
Phonatory forces can alter the vocal fold tissue’s physical structure by disrupting the intracellular adhesion and cellular structure as well as by inducing a cell-mediated response to tissue damage. Animal studies using rabbits showed that either transient (30 minutes) or prolonged (3 hours) experimentally induced, raised-intensity phonation could significantly increase the mRNA expression of interlukin (IL)-1β, an inflammatory cytokine compared to controls [34,35]. Other cytokines related to ECM remodeling, such as matrix metalloproteinase (MMP)-1 and transforming growth factor beta (TGF-β)-1, were also found in greater concentration following raised phonation [33,34]. A human subject study reported that one hour of continuous loud phonation induced a marked increase in secretion protein concentrations of the pro-inflammatory cytokines IL-1β, tumor necrosis factor (TNF)-alpha and MMP-8 from baseline to the 10-minute post-phonation time point. Subtle vocal fold edema was also noticed concomitantly under the laryngoscope [36]. Acute edema may be the outcome of submucosal capillary rupture, vasodilation, leakage of blood plasma into the extravascular compartment, and inflammatory cytokine release [37]. Clinically, vocal fold hemorrhage or acute laryngitis is often manifested after an acute episode of loud phonation.
Role of phonatory forces in vocal fold rehabilitation
A specific form of vocal fold oscillation called “resonant voice” is prescribed clinically for patients with mild vocal fold injury. The assumption is that the biomechanical stresses associated with resonant voice are beneficial to vocal fold repair. Resonant voice involves large-amplitude but low-impact vocal fold vibrations [38–40], relative to normal speech. Research has shown that mechanical force amplitude regulates vocal fold cytokine response [41,42].
The first study used an in vitro model to evaluate the effects of cyclic equibiaxial tensile strain (CTS) on rabbit vocal fold fibroblast cultures [41]. In this in vitro study, CTS was applied for varying excitation time periods (4–36 continuous hours), force magnitudes (0 – 18%) and frequencies (static – 0.5 Hz) in fibroblast cultures in the presence or absence of IL-1β. A key pro-inflammatory cytokine, IL-1β, induces numerous pro-inflammatory mediators, such as inducible-nitric oxide synthase (iNOS), nitric oxide (NO), cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2) and MMPs. The excessive synthesis of pro-inflammatory markers generally leads to unfavorable healing outcomes [43–45]. Results showed that a low magnitude of CTS significantly blocked COX-2, MMP-1 and PGE2 synthesis up to 24 hours and NO up to 36 hours in the IL-1β-induced inflamed cultures. Although the 0.5 Hz frequency is not within human phonation range, this study provided the very first data to show a threshold cell responses to vibration magnitude.
Another study investigated the biological effects of voice rest versus two different forms of tissue mobilization (i.e., resonant voice exercises and spontaneous speech) for experimentally-induced acute vocal fold inflammation in human subjects [42]. Voice rest, resonant voice exercises and spontaneous speech can be considered on a continuum of tissue mobilization and especially vocal fold impact stress magnitude: (1) none for voice rest, (2) normal- to large-amplitude vocal fold oscillations and low-impact stress for resonant voice exercises and (3) normal-to large-amplitude oscillations with potentially larger impact stress for spontaneous speech. Nine vocally healthy human participants were subjected to a vocal loading task involving 45 minutes of loud voice phonation (75–90 dB SPL at 15 cm microphone-to-mouth distance) during a one-hour period. Participants randomly assigned to one of three treatment groups-voice rest, resonant voice exercises or spontaneous speech-were monitored for four hours in the clinic. After a four-hour treatment period, participants were discharged with instructions to continue to follow their corresponding treatment condition. Laryngeal secretions were sampled from the vocal fold surfaces at the following time points: pre-loading (baseline), immediately post-loading, 4-hour post-treatment onset, and 24-hour post baseline. Enzyme-Linked Immuno Sorbent Assays (ELISA) were then used to measure the concentrations of cytokines in the secretions.
Differentiated cytokine profiles were noted across treatment groups. Protein concentrations of pro-inflammatory mediators (IL-1β, IL-6 and MMP-8) were lowest following resonant voice exercises and highest following the spontaneous speech condition at the 24-hour post baseline time point. The concentration of the anti-inflammatory cytokine IL-10 showed an opposite trend at the 24-hour time point, i.e., concentrations for this marker were highest following resonant voice exercises and lowest following voice rest. These preliminary findings suggest that large-amplitude, low-impact vocal fold tissue mobilization, as reported for resonant voice exercises, may optimize the quality of the healing response for acute and mild vocal fold injury by attenuating pro-inflammatory and stimulating anti-inflammatory responses.
In vitro studies of vocal fold bioreactor
In vitro bioreactors have been proposed to create a dynamic and biomimetic vocal fold vibratory microenvironment that allows the systematic investigation of the relationship between vocal fold cells and phonation-relevant mechanical stimulation (Table 1 for summary). Most existing bioreactors are mechanically driven [46–50], i.e. they use electromagnetic voice coil actuators to apply vibratory excitations onto the cells via moving bars [47,48,50] or sample holders [49]. An alternative bioreactor design using aerodynamic forces to generate vibrations was recently proposed [51]. Porous substrates (such as Tecoflex) or hydrogel were used for cell seeding in most studies. Strain and frequency of vibration are the primary variables, which were controlled by the computer software (such as Labview), during the mechanical testing using these bioreactors. The degree of substrate displacement was measured as a function frequency of applied voltage using digital image correlation technique [49] or laser dopper vibrometer coupled with high-speed digital imaging [51]. Local mechanical forces exerted on the cells were not reported. Finite element models will be required to quantify all components of mechanical stresses and strains with known material properties of the substrate (see Future Prospects for Vocal Fold Mechanobiology for further discussion).
Table 1.
Summary of the Vocal Fold Mechanobiological in vitro Studies (in chronological orders).
| Study | Mechanobiological Device | Cell Type | Stimulation Condition |
Major Gene/ Protein Measures | Results |
|---|---|---|---|---|---|
| Titze et al. (2004) | Mechanically driven bioreactor. Cells were seeded on Tecoflex porous substrates. | Human VFF | 20% static or cyclic axial strain (at 100 Hz) for 6 hours | mRNA expressions of elastin, procollagen I, fibronectin, fibromodulin, decorin, hyaluronic acid synthase 2 (HAS2), receptor for hyaluronan-mediated motility (RHAMM), CD44, matrix metalloprotease-1 (MMP-1), and hyaluronidase. | Compared to no-tension controls, elastin, procollagen I and fibronectin levels were significantly increased under the static axial strain condition. Cyclic axial strains further increased fibronectin and MMP-1 levels but significantly decreased procollagen I level. No significant changes were found in proteoglycan and HA-associated gene levels under the static axial strain condition. Cyclic axial strain produced significant increases in HAS2, CD 44, fibromodulin, and decorin levels. |
| Webb et al. (2006) | Mechanically driven bioreactor. Cells were seeded on Tecoflex porous substrates. | Human tracheal fibroblasts | 10% static or cyclic axial strain (at 0.25 Hz) for up to 8 hrs per day over 7 days | DNA content; mRNA expressions of procollagen I, fibronectin, MMP-1, transforming growth factor-beta 1 (TGF-β1) and connective tissue growth factor (CTGF) | Compared to static strain controls, cyclic strain significantly increased fibroblast DNA content after 7 days as well as levels of procollagen I, TGF-β1, and CTGF after 6 hrs of stimulation. |
| Branski et al. (2007) | Collagen type- I coated Bioflex II plates | Rabbit VFF with or without IL-1β treatment | 3–18% static or cyclic equibiaxial tensile strain (at 0.005–0.5 Hz) for up to 36 hrs | mRNA expressions of interleukin- 1beta (IL-1β), Inducible nitric oxide synthase (iNOS), cyclo-oxygenase-2 (COX-2), prostaglandin E (PGE-2), MMP-1 procollagen I | Compared to the CTS (6% and 0.5 Hz) alone condition, COX-2, MMP-1 and PGE2 expressions decreased up to 24 hrs and NO up to 36 hrs in the IL-1β-treated and CTS- stimulated cultures. Compared to IL-1β plus static conditions, procollagen I expression was significantly increased in the CTS only and CTS plus IL-1β conditions at 24 hrs and 48 hrs. |
| Wolchok et al. (2009) | Mechanically driven bioreactor. Cells were seeded on Tecoflex porous substrates. | Human laryngeal fibroblasts | 100 Hz vibration with 15 min over a 6-hr period followed by 18- hr rest for up 21 days. | DNA microarray and protein expression of TGF-β1 and monocyte chemotactic protein-1 (MCP-1). | After 3 days of vibration, the gene expression ratios (vibrated: static control) ranged from 1.5 to 4.2 in collagen I and IX, syndecan, laminin, tissue inhibitor of metalloproteinase (TIMP) 1 and 3, CTGF and platelet-derived growth factor (PDGF). Compared to static controls, TGF-β1 and MCP-1 protein levels were significantly increased and decreased, respectively, after 24 hrs of vibration. |
| Kutty et al. (2010) | Mechanically driven bioreactor. Cells were encapsulated in hyaluronic acid hydrogels crosslinked with Tecoflex films. | Human dermal fibroblasts | 100 Hz in a 2s on- 2s off regimen for 4 hrs per day for up to 10 days | mRNA expressions of collagen I, elastin, HAS2, decorin, fibromodulin and MMP-1. Protein expression of sulphated glycosaminoglycan (GAG) and hydroxyproline. | Compared to static controls, gene expressions of HAS2, decorin and MMP-1 were significantly increased whereas collagen and elastin were significantly decreased in the vibration group at Day 5. Compared to static controls, protein expressions of GAG and hydroxyproline were significantly increased and decreased respectively at both Day 5 and Day 10 in the vibration group. |
| Farran et al. (2011) | Aerodynamically driven bioreactor. Cells were seeded on silicone membranes. | Human neonatal foreskin fibroblasts | 60, 110 or 300 Hz with varying vibration amplitude (1, 5, 10 and 30µm) for 1 hr followed by a 6-hr rest | mRNA expressions of collagen type I, fibronectin, MMP-1, TIMP-1, HAS3, hyaluronidase 1 (HYAL1) and CD44 | At 60 Hz, collagen I level was significantly higher at 1µm than 10µm. HYAL1 level was significantly lower at 1µm than 5µm. At 110 Hz/ 30µm, levels of collagen I, MMP-1 was lower than the static controls. CD44 level was significantly lower at 1µm than 30µm. |
| Gaston et al. (2012) | Mechanically driven bioreactor. Cells were seeded on Tecoflex porous substrates | Human VFF and BM-MSC | 200 Hz vibration at 20% strain for 8 hrs | mRNA expressions of collagen type I, fibronectin, TGF-β1 and α-smooth muscle actin (SMA) | Expressions of all genes were not significantly different between vibrated and non-vibrated controls in either cell types. |
VFF = vocal fold fibroblasts; BM-MSC = bone marrow mesenchymal stem cells.
Mechanically driven bioreactors
Titze et al. [46] first used a bioreactor that was able to generate vibratory regimes comparable to vocal fold oscillations with 0–1 mm amplitudes, 20–200 Hz frequencies and on-off stress cycles [46]. The bioreactor was controlled by two motor drivers: one for low frequency or static strains and one for high-frequency vibrational strains. In this study, human vocal fold fibroblasts were seeded in 3D porous polyurethane and subjected to 100 Hz vibration at 20% axial strain for 6 hours. Results indicated vibration increased mRNA expressions of ECM-related genes, including MMP-1, HA synthase 2, CD44, fibronectin, fibromodulin, and decorin, compared to the static controls without vibration. Further studies using a similar bioreactor design were reported [47,48]. Adult normal dermal fibroblasts were encapsulated in hydrogel samples that were crosslinked to Tecoflex films. Cells were subjected to 100 Hz vibrations with a uniaxial displacement of 1-mm amplitude for up to 10 days. Compared to static controls, real PCR data showed that vibrations increased mRNA expressions of HA synthase 2, decorin, fibromodulin, and MMP-1, while collagen and elastin expression was relatively unchanged. Gene expression levels were highest on Days 3 and 5 after vibratory stimulation and lowest on Day 10. Accumulated ECM protein levels, GAG and collagen were also measured in the hydrogel samples. Sulfated GAG increased and collagen decreased significantly compared to static controls after 5 and 10 days of vibratory stimulation, respectively.
Wolchok et al. [49] reported another mechanically driven bioreactor study that applied mechanical stimulation to human vocal fold fibroblasts at 100 Hz for 21 days. Instead of moving strips, cells were seeded on a porous polyurethane foam sheet housed in commercial multi-well culture plates. Cytokine proteins of TGF-β1 and monocyte chemoattractant protein (MCP)-1 were measured in the culture medium sample after 1 day of vibration. Compared to static controls, TGF-β1 and MCP-1 levels were significantly increased and decreased, respectively, in the culture medium. By the end of the experiment (i.e., Day 21), a significant accumulation of fibronectin and collagen type 1 proteins was found in the porous substrate. The resulted substrate was significantly stiffer than the static controls.
Gaston et al. [50] advanced the bioreactor design by reproducing phonation-relevant vibrations and making the vibratory strips contact each other during operation. Physiologically, when the vocal folds oscillate, contact between certain areas of the two sides occurs depending on the frequency, amplitude and type of oscillation. The bioreactor had three computer-controlled motors that could generate three independent mechanical stimuli: a linear voice-coil actuator for vibration (0–2727 Hz), a linear stepper motor for stretch (0–100% of elongation), and a rotary stepper motor for angle change (0–39°). Modified T-cell culture flasks were fastened to the bioreactor base. Cell-seeded Tecoflex strips were held in the T-flasks during the experiment. In this study, the bioreactor was used to characterize the response of functional phenotypes of human vocal fold fibroblasts (hVFF) and bone marrow mesenchymal stem cells (BM-MSC) to mechanical vibrations. Cells were subjected to 200 Hz vibration and 20% strain for 8 hours. Both hVFF and BM-MSC were viable (96%) after being subjected to the prescribed mechanical vibrations. Interestingly, semi-quantitative RT-PCR results showed that both hVFF and BM-MSC vibration groups had comparable gene expression levels of TGF-β1, collagen I and fibronectin, compared to the static controls. Results showed that hVFF had similar mechanobiological responses to those of BM-MSC in terms of ECM-related gene expressions. This finding contradicts previous bioreactor studies that showed that fibroblasts were mechano-sensitive in their gene expression following a brief exposure (e.g., 60 minutes) of mechanical stimulation [46].
Aerodynamically driven bioreactors
Vocal fold oscillation are airflow-induced in reality. Conventional, mechanically driven bioreactors apply idealized loading that may or may not be representative of human phonation. One limitation of the mechanically-driven bioreactors is that the vibratory forces are transferred mechanically. Cells may be agitated by unwanted mechanical or fluid perturbations [51]. Farran et al. [51] developed a bioreactor composed of a power amplifier, an enclosed loudspeaker and a function generator. Cells were seeded on the silicone membranes and exposed to acoustic pressure fluctuations. The bioreactor was able to generate vibrations within a frequency range of 60–300 Hz and an amplitude range of 1–30 mm, which is within the physiological range of human vocal fold oscillations. Human neonatal foreskin fibroblasts (NFFs) were subjected to an hour vibration at 60, 110 and 300 Hz followed by a 6-hour rest. Vibrations at 110 Hz increased cell proliferation compared to other mechanical testing regimes. Quantitative polymerase chain reaction (qPCR) data showed that ECM-related gene expressions of collagen type I, CD44, MMP-1 and tissue inhibitor of metalloproteinase 1 (TIMP1) were dependent on the vibratory frequency and amplitude. These results again confirmed that mechanical forces involved in human phonation are a critical epigenetic factor modulating fibroblast functions in ECM production and degradation. A uniform pressure also seems to induce a more realistic cell response than more complex shear and normal stress loading associated with axial testing. The acoustic excitation in this bioreactor design fails to mimic the collision between the vocal folds.
In general, direct comparison of gene expression data among these bioreactor studies was not practical due to the differences in the vibration regimens, cell types and the substrates that cells were seeded on. Overall, collagen type I seemed to be the most sensitive gene to mechanical stimulation in fibroblast cultures although the response varied with the mechanical regimens applied.
Future Prospects for Vocal Fold Mechanobiology
Mechanobiology is a multi-scale biological problem. A systems-biology based analysis is required to bridge the mechanical and chemical signals at molecular, cellular, tissue and organ levels to understand this complex problem in a tractable and effective manner. Agent-based computational models (ABMs) have been used to integrate biological data and predict concentrations of vocal fold cells and ECM as a function of phonatory stress [52,53]. The ABMs simulate the interactions among (1) platelets, (2) cells, specifically neutrophils, macrophages and fibroblasts, (3) growth factors, specifically transforming growth factor [TGF]-β1, basic fibroblast growth factor [bFGF]), (4) cytokines, specifically interleukin [IL]-1β, IL-6, IL-8, IL-10, and TNF-α), (5) collagenase (MMP-8), (6) ECM substances, specifically collagen type I, elastin and HA) and (7) a damage associated molecular pattern (DAMP) variable [52,54–56]. The models have an interface to input a person’s biomarker profile consisting of inflammatory mediators (IL-1β, IL-6, IL-8, IL-10, TNF-α and MMP-8) [52,54–56], representing vocal fold health. Based on initial inputs, the models predict the long-term wound healing response (future biomarker profile) for the subject of the investigation.
The ABMs were calibrated and validated with empirical data covering a panel of cytokines obtained from laryngeal secretions in a human subject study [57]. The ABM [52,54,55] also allowed testing of the biological effects of three behavioral treatment options, that is voice rest, resonant voice exercise and spontaneous speech, for acute vocal fold injury in silico. The fundamental difference among the treatments is the putative magnitude of mechanical forces in the vocal fold tissue. Algorithms regarding biological effects of mechanical-based treatments were implemented based on the literature in exercise physiology [58–60], then the model was calibrated with individual cytokine levels in laryngeal secretions from the human study [57]. The model reproduced individual-specific mediator levels at 24 hours post-injury with 73% accuracy (22/30 cases in the 95% confidence interval). This work successfully incorporated the treatment effects of mechanical stresses into a biology-based model. This new research direction will ultimately accelerate the understanding of the mechanobiological pathways underlying phonation and tissue response in the vocal folds.
Systems-Based Research in Vocal Fold Mechanobiology
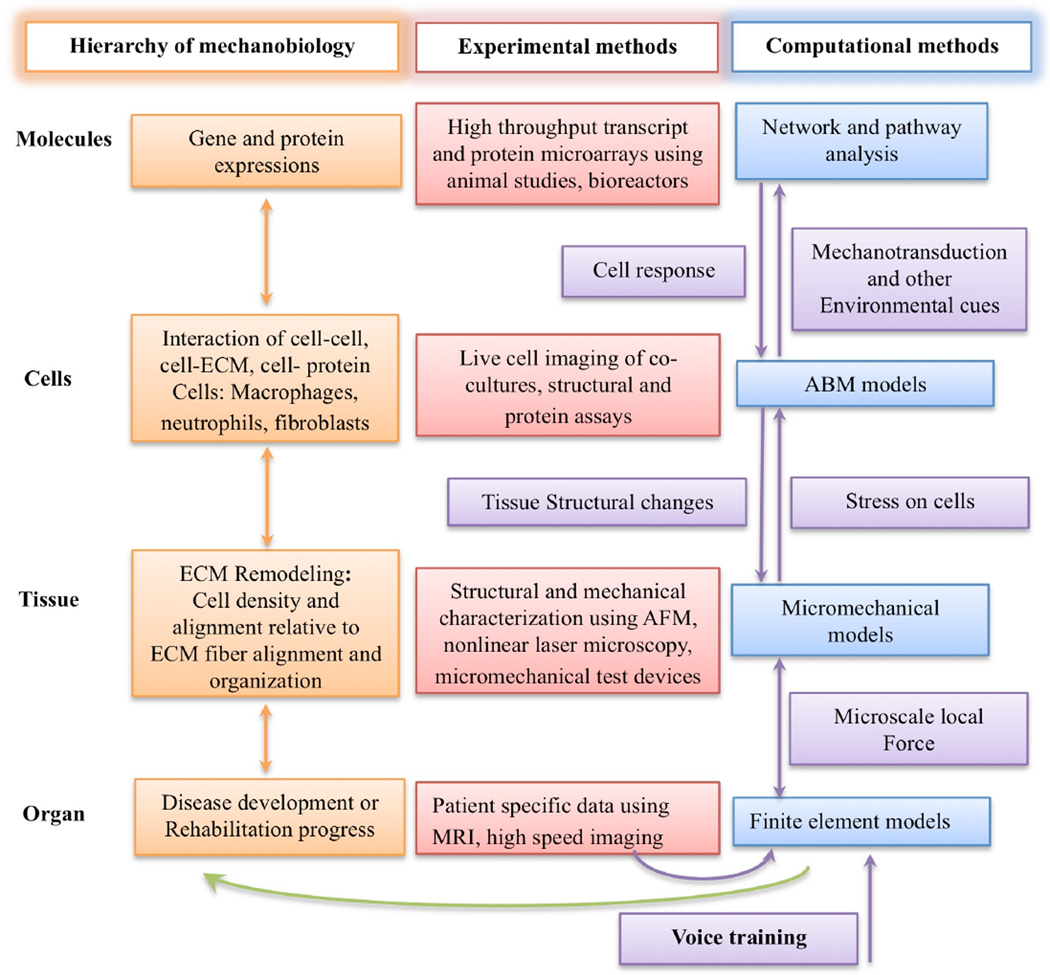
Comprehensive studies that integrate in vitro, in vivo and in silico approaches are needed to advance the systems-biology research in vocal fold mechanobiology (Figure 1). Cross-scale empirical data are required to develop integrative computational models.
Figure 1.
Systems biology of the vocal fold mechanobiology. At the organ level, finite element modeling is used to predict the average mechanical stress and strain in vocal fold tissue. Subject specific information, such as vocal fold geometry, lamina propria structural organization and high-speed imaging can provide information for the modeling and validation of a finite element model capable of predicting average stress and strain in the tissue down to a level where continuum assumptions are no longer valid. At the tissue level, structural and mechanical characterization of tissue constituents provides inputs to micromechanical models to predict the amount of stress on a single cell. The mechanical stress on a single cell can induce an inflammatory response involving other cell types. Co-culture of cells inside ex vivo vocal fold tissue can elucidate the complex interaction between cells as well as their migration speed and dynamics, which are the essential data for the agent-based models (ABMs). In addition to the rules measureable at the cellular level using microscopic techniques, the cues that initiate the migration of these cells are the result of complex interactions inside the cells. Bioreactors can provide sufficient RNA and protein samples for genomic and proteomic analysis respectively for complex cell-cell or cell-protein interactions. The use of computer science-based pattern recognition techniques, such as pathway and network analysis, can identify and predict events at the cellular level. Integration of all the data at the molecule, cell, tissue and organ levels can constitute a multi-scale model of vocal fold mechanobiology. The clinical application of such a model is, for example, to predict the biological effect of phonation or specific voice training on vocal fold injury and healing.
Finite Element Model of Mechanical Stress and Strain Distribution
At the organ level, computational models such as finite element analysis can quantify mechanical stress and strain at any location within the lamina propria, assuming that the local mechanical properties of the vocal fold tissue is known through mechanical testing of the tissue. Finite element models treat the tissue as a continuum. They yield average mechanical stresses at the sub-millimeter scale. At the micrometer scale, the tissue can be considered a representative volume element (RVE), composed of fibrils, attached cells, and fibers, wherein the assumption of continuity is no longer valid. Given that we can predict the amount of stress and stain on these RVEs, micromechanical models can be developed to relate the stress and strain acting on the RVE to the amount that is exerted on each cell. To create such micromechanical models, the structural and mechanical properties of each fibril as well as those of cells need to be known. Structural data can be obtained using a variety of microscopy imaging techniques such as confocal scanning laser microscopy, nonlinear laser microscopy [61], and atomic force microscopy.
Atomic Force Microscopy of Cell and Fibril Elasticity
The elastic properties of fibers and cells can be measured using techniques such as atomic force microscopy (AFM), magnetic and optical tweezers, and particle tracking microrheology [62,63]. Atomic force microscope is the most commonly used tool to study the micro and nano mechanics of biological materials. Both nanotopographical and mechanical properties can be obtained, allowing correlation to be drawn from these properties. The AFM probe is composed of a tip attached to a cantilever that bends under force. The probe and the sample can be moved with respect to each other. The main advantage of AFM over other scanning techniques is that a sample can be imaged under physiological conditions in an aqueous environment without fixation or dehydration. The AFM microscopy allows force measurements at micrometer and nanometer scales. Force versus deflection is obtained through indentation and the elastic properties are calculated based on Hertzian theory.
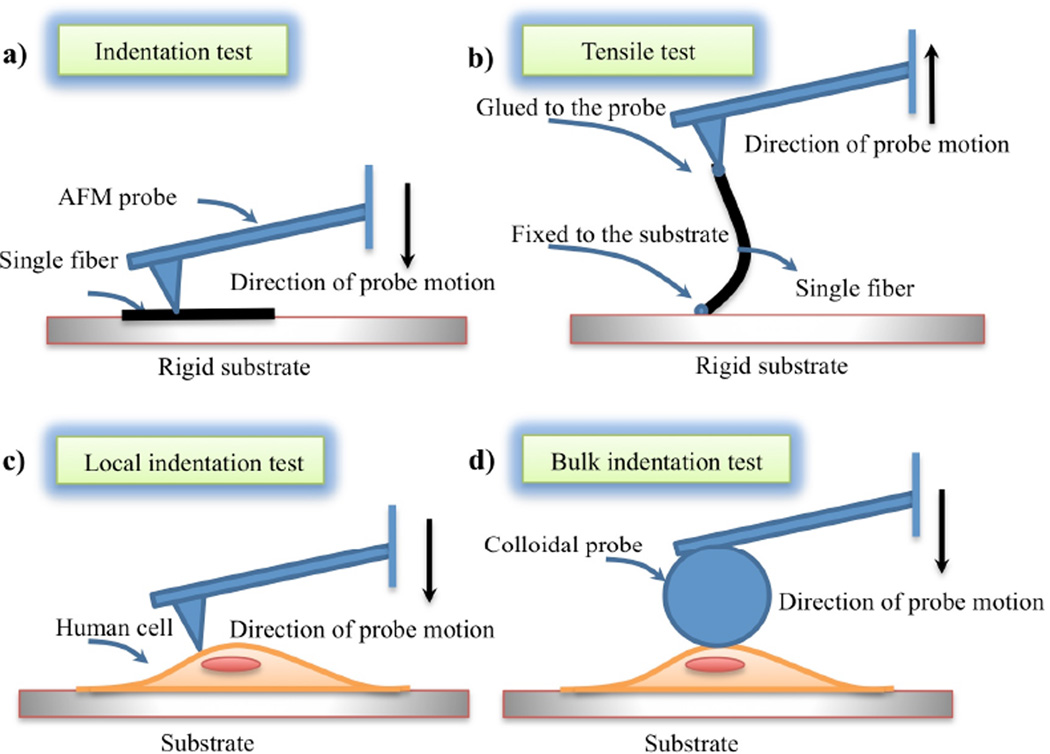
Nano indentation tests can be used to measure the elastic properties of the matrix constituents such as collagen, elastin, and cells. A schematic of AFM based micromechanical tests on fibers and cells is shown in Figure 2. Two AFM techniques, tensile tests [64] and indentation tests [63], can be used to measure the elasticity of one single fiber. In tensile tests, one side of a fiber is glued to the substrate and the other side of the fiber is glued to an AFM probe. Motion of the probe away from the substrate causes the AFM cantilever to bend in proportion to the applied traction force. The force vs displacement data are then used to calculate Young’s elastic modulus. In indentation tests, the sharp AFM tip is pressed onto the surface of a fiber and the resulting force-displacement is used to calculate the elastic indentation modulus. Dynamic properties can be obtained by dynamic displacement and the corresponding forces [65]. The elastic properties of collagen and other ECM constituents reported in the literature are related to those of other organs and may not correspond to the elasticity of vocal fold ECM constituents.
Figure 2.
AFM based tests on fibrils and cells to identify their elastic properties: (a) indentation of single fibril, (b) tensile test on a single fibril, (c) local characterization of cells using sharp tips, and (d) bulk characterization of cells using colloidal probes.
The size of a vocal fold fibroblast is several tens of micrometers. Nanoindentation is a well-established technique to measure the local elastic properties of the cells [66,67] (Figure 2c). The bulk properties of the cells are required for micromechanical models. Micro-indentation or compression of fibroblast cells using colloidal probes can be used for characterizing bulk properties [68] (Figure 2d). Although these measurements give initial preliminary data on vocal fold fibroblast elasticity, adaptation of fibroblast elasticity to the substrate stiffness [69] limits the use of such data for micromechanical models. Vocal fold fibroblasts can be cultured in different constituent concentrations (i.e., elastin, hyaluronic acid, and fibronectin) to be similar to in vivo conditions. The corresponding elastic properties can be used as input data for micromechanical models.
With the concentration, constituent organization and elastic properties of the substrate or ECM known, the magnitude of mechanical stress can be predicted at the cell level. At this point, biological data of the cellular response to the mechanical stress can be collected and modeled. For the ABMs to take into account the cellular response to mechanical force, relevant mechano-transduction pathways should be identified and programmed into the ABMs.
Vocal Fold Cell Mechanotransduction
The understanding of the exact mechanism of sensing mechanical force and converting it to biochemical signals remains a challenge in molecular cell biology [70]. The ECM-integrin-cytoskeleton pathway is one of the most studied signaling pathways in fibroblast cell lines for other parts of the body. Integrins, cytoskeleton, G proteins, receptor tyrosine kinase (RTKs), mitogen activated protein kinase (MAPKs), and stretch-activated ion channels are other well-understood mechnotransduction pathways [70,71]. However, none of the aforesaid channels have been studied in vocal fold fibroblasts to date.
The biochemical activity of cells and their response to mechanical stimulation requires the identification of mechanostransducer molecules and corresponding pathways leading to the expression of genes and proteins that alter the metabolites of the cells. Mechanotransduction can be studied using isolated cells with specific platforms [72] combined with live cell imaging [73]. The imposed mechanical stress can be computed from micromechanical models. Cellular responses to some mechanical forces such as tensile, shear and compression have been widely studied for mechano-sensitive cells such as fibroblasts and chondrocytes [70]. Most of these mechanobiological studies, however, have been limited to focus on oversimplified types of force, homogeneous substrates, and static conditions. It would be more beneficial to study the response of vocal fold fibroblasts to combined mechanical stresses as similar to the in vivo microenvironment. In order to achieve this goal, an engineered vocal fold platform that can deliver isolated or combined mechanical stimulations and also allow time-lapse fluorescent imaging for studying live cellular response should be designed.
Conclusion
Although biomechanical forces clearly affect the physiology and pathology of the vocal folds, current knowledge of the mechanical forces regulating these processes at the cellular and molecular level is insufficient. Research on the vocal fold mechanobiology is warranted. We propose a systems-based approach using integrated physical, biological and computational method to capture the complex dynamics of vocal fold mechanobiology for the eventual multi-scale biosimulation, spanning from molecules to the eventual voice physiology.
Acknowledgements
The study was supported in part by the National Institutes of Health grants R03DC012112-01 to N.Y.K. Li and R01-DC-005788 to L. Mongeau.
References
- 1.Zhang K, Siegmund T, Chan RW. A two-layer composite model of the vocal fold lamina propria for fundamental frequency regulation. J AcoustSoc Am. 2007;122:1090–1101. doi: 10.1121/1.2749460. [DOI] [PubMed] [Google Scholar]
- 2.Gunter HE. A mechanical model of vocal-fold collision with high spatial and temporal resolution. J AcoustSoc Am. 2003;113:994–1000. doi: 10.1121/1.1534100. [DOI] [PubMed] [Google Scholar]
- 3.Gunter HE. Modeling mechanical stresses as a factor in the etiology of benign vocal fold lesions. J Biomech. 2004;37:1119–1124. doi: 10.1016/j.jbiomech.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Gunter HE, Howe RD, Zeitels SM, Kobler JB, Hillman RE. Measurement of vocal fold collision forces during phonation: methods and preliminary data. J Speech Lang Hear Res. 2005;48:567–576. doi: 10.1044/1092-4388(2005/039). [DOI] [PubMed] [Google Scholar]
- 5.Jiang JJ, Diaz CE, Hanson DG. Finite element modeling of vocal fold vibration in normal phonation and hyperfunctional dysphonia: implications for the pathogenesis of vocal nodules. Ann OtolRhinolLaryngol. 1998;107:603–610. doi: 10.1177/000348949810700711. [DOI] [PubMed] [Google Scholar]
- 6.Gray SD. Cellular Physiology of the Vocal Folds. In: Rosen CA, Murry T, editors. The Otolaryngologic Clinics of North America. Philadelphia: WB Saunders Company; 2000. [DOI] [PubMed] [Google Scholar]
- 7.Gray SD, Hirano M, Sato K. Molecular and Cellular Structure of Vocal Fold Tissue. In: Titze IR, editor. Vocal Fold Physiology. San Diego: Singular Publishing Group Inc; 1993. [Google Scholar]
- 8.Gray SD, Pignatari SS, Harding P. Morphologic ultrastructure of anchoring fibers in normal vocal fold basement membrane zone. J Voice. 1994;8:48–52. doi: 10.1016/s0892-1997(05)80318-2. [DOI] [PubMed] [Google Scholar]
- 9.Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann OtolRhinolLaryngol. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- 10.Hirano M. Structure and vibratory behavior of the vocal folds. In: Sawashima M, Fankin SC, editors. Dynamic Aspects of Speech Production. Tokyo: University of Tokyo Press; 1977. pp. 13–30. [Google Scholar]
- 11.Hirano M. Structure of the vocal fold in normal and disease states anatomical and physical studies. In: Rockville MD, editor. Assessement of Vocal Pathology. USA: American Speech-Language-Hearing Association; 1981. [Google Scholar]
- 12.Hirano M, Kurita S, Nakashima T. The structure of the vocal folds. In: Stevens KN, Hirano M, editors. Vocal Fold Physiology. Tokyo: University of Tokyo Press; 1981. [Google Scholar]
- 13.Gray SD. Basement membrane zone injury in vocal nodules. In: Gauffin J, Hammarberg B, editors. Vocal fold physiology: acoustic, perceptual, and physiological aspects of voice mechanics. San Diego: Singular Publishing Group Inc; 1989. pp. 21–27. [Google Scholar]
- 14.Boseley ME, Hartnick CJ. Development of the human true vocal fold: depth of cell layers and quantifying cell types within the lamina propria. Ann OtolRhinolLaryngol. 2006;115:784–788. doi: 10.1177/000348940611501012. [DOI] [PubMed] [Google Scholar]
- 15.Hammond TH, Gray SD, Butler J, Zhou R, Hammond E. A study of age and gender related elastin distribution changes in human vocal folds. Otolaryngol Head Neck Surg. 1998;119:314–322. doi: 10.1016/S0194-5998(98)70071-3. [DOI] [PubMed] [Google Scholar]
- 16.Hammond TH, Gray SD, Butler JE. Age- and gender-related collagen distribution in human vocal folds. Ann OtolRhinolLaryngol. 2000;109(10 Pt 1):913–920. doi: 10.1177/000348940010901004. [DOI] [PubMed] [Google Scholar]
- 17.Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001;124:607–614. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- 18.Gray SD, Titze IR, Chan R, Hammond TH. Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope. 1999;109:845–854. doi: 10.1097/00005537-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Thibeault SL, Bless DM, Gray SD. Interstitial protein alterations in rabbit vocal fold with scar. J Voice. 2003;17:377–383. doi: 10.1067/s0892-1997(03)00064-x. [DOI] [PubMed] [Google Scholar]
- 20.Patel NR, Bole M, Chen C, Hardin CC, Kho AT, et al. Cell Elasticity Determines Macrophage Function. PLoS One. 2012;7:e41024. doi: 10.1371/journal.pone.0041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrosio F, Wolf SL, Delitto A, Fitzgerald GK, Badylak SF, et al. The emerging relationship between regenerative medicine and physical therapeutics. PhysTher. 2010;90:1807–1814. doi: 10.2522/ptj.20100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dado D, Sagi M, Levenberg S, Zemel A. Mechanical control of stem cell differentiation. Regen Med. 2012;7:101–116. doi: 10.2217/rme.11.99. [DOI] [PubMed] [Google Scholar]
- 23.Maul TM, Chew DW, Nieponice A, Vorp DA. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2011;10:939–953. doi: 10.1007/s10237-010-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen DM, Chen CS. Mechanical control of stem cell differentiation. Cambridge (MA): StemBook; 2008. [PubMed] [Google Scholar]
- 25.Hanson SE, Kim J, Johnson BH, Bradley B, Breunig MJ, et al. Characterization of mesenchymal stem cells from human vocal fold fibroblasts. Laryngoscope. 2010;120:546–551. doi: 10.1002/lary.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano M, Kurita S, Nakashima T. Growth, development and aging of human vocal folds. In: Bless DM, Abbs JH, editors. Vocal Fold Physiology. San Diego: College-Hill Press; 1983. pp. 22–43. [Google Scholar]
- 27.Ishii K, Yamashita K, Akita M, Hirose H. Age-related development of the arrangement of connective tissue fibers in the lamina propria of the human vocal fold. Ann OtolRhinolLaryngol. 2000;109:1055–1064. doi: 10.1177/000348940010901112. [DOI] [PubMed] [Google Scholar]
- 28.Sato K, Hirano M, Nakashima T. Fine structure of the human newborn and infant vocal fold mucosae. Ann OtolRhinolLaryngol. 2001;110(5 Pt 1):417–424. doi: 10.1177/000348940111000505. [DOI] [PubMed] [Google Scholar]
- 29.Hartnick CJ, Rehbar R, Prasad V. Development and maturation of the pediatric human vocal fold lamina propria. Laryngoscope. 2005;115:4–15. doi: 10.1097/01.mlg.0000150685.54893.e9. [DOI] [PubMed] [Google Scholar]
- 30.Sato K, Umeno H, Nakashima T, Nonaka S, Harabuchi Y. Histopathologic Investigations of the Unphonated Human Child Vocal Fold Mucosa. J Voice. 2012;26:37–43. doi: 10.1016/j.jvoice.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Sato K, Sakamoto K, Nakashima T. Expression and distribution of CD44 and hyaluronic acid in human vocal fold mucosa. Ann OtolRhinolLaryngol. 2006;115:741–748. doi: 10.1177/000348940611501005. [DOI] [PubMed] [Google Scholar]
- 32.Sato K, Nakashima T, Nonaka S, Harabuchi Y. Histopathologic investigations of the unphonated human vocal fold mucosa. Acta Otolaryngol. 2008;128:694–701. doi: 10.1080/00016480701675643. [DOI] [PubMed] [Google Scholar]
- 33.Sato K, Umeno H, Ono T, Nakashima T. Histopathologic study of human vocal fold mucosa unphonated over a decade. Acta Otolaryngol. 2011;131:1319–1325. doi: 10.3109/00016489.2011.615067. [DOI] [PubMed] [Google Scholar]
- 34.Swanson ER, Ohno T, Abdollahian D, Garrett CG, Rousseau B. Effects of raised-intensity phonation on inflammatory mediator gene expression in normal rabbit vocal fold. Otolaryngol Head and neck surg. 2010;143:567–572. doi: 10.1016/j.otohns.2010.04.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rousseau B, Ge P, French LC, Zealear DL, Thibeault SL, et al. Experimentally induced phonation increases matrix metalloproteinase-1 gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. 2008;138:62–68. doi: 10.1016/j.otohns.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdolini K, Rosen CA, Branski RC, Hebda PA. Shifts in biochemical markers associated with wound healing in laryngeal secretions following phonotrauma: A preliminary study. Ann Otol Rhinol Laryngol. 2003;112:1021–1025. doi: 10.1177/000348940311201205. [DOI] [PubMed] [Google Scholar]
- 37.Courey MS, Shohet JA, Scott MA, Ossoff RH. Immunohistochemical characterization of benign laryngeal lesions. Ann Otol Rhinol Laryngol. 1996;195:525–531. doi: 10.1177/000348949610500706. [DOI] [PubMed] [Google Scholar]
- 38.Berry DA, Verdolini K, Montequin DW, Hess MM, Chan RW, et al. A quantitative output-cost ratio in voice production. J Speech Lang Hear Res. 2001;44:29–37. doi: 10.1044/1092-4388(2001/003). [DOI] [PubMed] [Google Scholar]
- 39.Peterson KL, Verdolini-Marston K, Barkmeier JM, Hoffman HT. Comparison of aerodynamic and electroglottographic parameters in evaluating clinically relevant voicing patterns. Ann Otol Rhinol Laryngol. 1994;103:335–346. doi: 10.1177/000348949410300501. [DOI] [PubMed] [Google Scholar]
- 40.Verdolini K, Druker DG, Palmer PM, Samawi H. Laryngeal adduction in resonant voice. J Voice. 1998;12:315–327. doi: 10.1016/s0892-1997(98)80021-0. [DOI] [PubMed] [Google Scholar]
- 41.Branski RC, Perera P, Verdolini K, Rosen CA, Hebda PA, et al. Dynamic biomechanical strain inhibits IL-1beta-induced inflammation in vocal fold fibroblasts. J Voice. 2007;21:651–660. doi: 10.1016/j.jvoice.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdolini Abbott K, Li NY, Branski RC, Rosen CA, Grillo E, et al. Vocal exercise may attenuate acute vocal fold inflammation. J Voice. 2012;26:814 e1–814 e13. doi: 10.1016/j.jvoice.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 44.Dinarello CA. Proinflammatory Cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 45.Guirao X, Lowry SF. Biologic control of injury and inflammation: much more than too little or too late. World J Surg. 1996;20:437–446. doi: 10.1007/s002689900069. [DOI] [PubMed] [Google Scholar]
- 46.Titze IR, Hitchcock RW, Broadhead K, Webb K, Li W, et al. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37:1521–1529. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Webb K, Hitchcock RW, Smeal RM, Li W, Gray SD, et al. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. J Biomech. 2006;39:1136–1144. doi: 10.1016/j.jbiomech.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 48.Kutty JK, Webb K. Vibration stimulates vocal mucosa-like matrix expression by hydrogel-encapsulated fibroblasts. J Tissue Eng Regen Med. 2010;4:62–72. doi: 10.1002/term.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolchok JC, Brokopp C, Underwood CJ, Tresco PA. The effect of bioreactor induced vibrational stimulation on extracellular matrix production from human derived fibroblasts. Biomaterials. 2009;30:327–335. doi: 10.1016/j.biomaterials.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 50.Gaston J, Quinchia Rios B, Bartlett R, Berchtold C, Thibeault SL. The response of vocal fold fibroblasts and mesenchymal stromal cells to vibration. PLoS ONE. 2012;7:e30965. doi: 10.1371/journal.pone.0030965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farran AJ, Teller SS, Jia F, Clifton RJ, Duncan RL, et al. Design and characterization of a dynamic vibrational culture system. J Tissue Eng Regen Med. 2011;7:213–225. doi: 10.1002/term.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li NY, Verdolini K, Clermont G, Mi Q, Rubinstein EN, et al. A patient-specific in silico model of inflammation and healing tested in acute vocal fold injury. PLoS One. 2008;3:e2789. doi: 10.1371/journal.pone.0002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li NY, Vodovotz Y, Kim KH, Mi Q, Hebda PA. Biosimulation of acute phonotrauma: An extended model. Laryngoscope. 2011;121:2418–2428. doi: 10.1002/lary.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li NYK. Biosimulation of vocal fold inflammation and healing, in Communication Science and Disorders. University of Pittsburgh; Pittsburgh: 2009. [Google Scholar]
- 55.Li NY, Vodovotz Y, Kim KH, Mi Q, Hebda PA, et al. Biosimulation of Acute Phonotrauma: an Extended Model. Laryngoscope. 2011;121:2418–2428. doi: 10.1002/lary.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li NY, Vodovotz Y, Hebda PA, Abbott KV. Biosimulation of inflammation and healing in surgically injured vocal folds. Ann Otol Rhinol Laryngol. 2010;119:412–423. doi: 10.1177/000348941011900609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verdolini Abbott K, Li NY, Branski RC, Rosen CA, Grillo E, et al. Vocal exercise may attenuate acute vocal fold inflammation. Voice. 2012;26:e1–e13. doi: 10.1016/j.jvoice.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toumi H, F’Guyer S, Best TM. The role of neutrophils in injury and repair following muscle stretch. J Anat. 2006;208:459–470. doi: 10.1111/j.1469-7580.2006.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev. 2005;11:64–85. [PubMed] [Google Scholar]
- 60.Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41:457–465. [PMC free article] [PubMed] [Google Scholar]
- 61.Miri AK, Tripathy U, Mongeau L, Wiseman PW. Nonlinear laser scanning microscopy of human vocal folds. Laryngoscope. 2012;122:356–363. doi: 10.1002/lary.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kollmannsberger P, Fabry B. Linear and nonlinear rheology of living cells. 2011:75–97. [Google Scholar]
- 63.Marco PEW, Laurent B, Michael AH, Patrick M. Mechanical properties of collagen fibrils. Biophysical Journal. 2007;93:1255–1263. doi: 10.1529/biophysj.106.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van der RJA, Van der WKO, Bennink ML, Dijkstra PJ, Feijen J. Micromechanical testing of individual collagen fibrils. Macromol Biosci. 2006;6:699–702. doi: 10.1002/mabi.200600063. [DOI] [PubMed] [Google Scholar]
- 65.Kazemirad S, Heris HK, Mongeau L. Experimental methods for the characterization of the frequency-dependent viscoelastic properties of soft materials. J Acoust Soc Am. 2013;133 doi: 10.1121/1.4798668. article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sirghi L, Ponti J, Broggi F, Rossi F. Probing elasticity and adhesion of live cells by atomic force microscopy indentation. Eur Biophys J. 2008;37:935–945. doi: 10.1007/s00249-008-0311-2. [DOI] [PubMed] [Google Scholar]
- 67.Touhami A, Nysten B, Dufrêne YF. Nanoscale mapping of the elasticity of microbial cells by atomic force microscopy. Langmuir. 2003;19:4539–4543. [Google Scholar]
- 68.Valentin L, Tiffany Z, Huan YC, Fu TL, Gang YL. Cell mechanics using atomic force microscopy-based single-cell compression. Langmuir. 2006;22:8151–8155. doi: 10.1021/la060561p. [DOI] [PubMed] [Google Scholar]
- 69.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5:1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- 71.Ingber DE. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 72.Kim DH, Wong PK, Park J, Levchenko A, Sun Y. Microengineered platforms for cell mechanobiology. Annu Rev Biomed Eng. 2009;11:203–233. doi: 10.1146/annurev-bioeng-061008-124915. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Shyy JY, Chien S. Fluorescence proteins, live-cell imaging, and mechanobiology: Seeing is believing. Annu Rev Biomed Eng. 2008;10:1–38. doi: 10.1146/annurev.bioeng.010308.161731. [DOI] [PubMed] [Google Scholar]