Abstract
Formation of bacterial biofilms at solid-liquid interfaces creates numerous problems in biomedical sciences. Conventional sterilization and decontamination methods are not suitable for new and more sophisticated biomaterials. In this paper, the efficiency and effectiveness of gas discharges in inactivation and removal of biofilms on biomaterials were studied. We found that although discharge oxygen, nitrogen and argon all demonstrated excellent antibacterial and antibiofilm activity, gases with distinct chemical/physical properties underwent different mechanisms of action. Discharge oxygen and nitrogen mediated decontamination was associated with strong etching effects, which can cause live bacteria relocation and thus contamination spreading. On the contrary, although discharge argon at low powers maintained excellent antibacterial ability, it had negligible etching effects. Based on these results, an effective decontamination approach using discharge argon was established in which bacteria and biofilms were killed in situ and then removed from contaminated biomaterials. This novel procedure is applicable for a wide range of biomaterials and biomedical devices in an in vivo and clinical setting.
Keywords: Bacteria, Biofilm, Inactivation, Biomaterials, Contamination
1. Introduction
Bacteria prefer to attach to solid surfaces and as a result, this bacterial and substrate interaction leads to the formation of biofilms [Overman 2006]. It is estimated that over 95 % of bacteria existing in nature are found in biofilms. One of the most important features of biofilms is their resistant nature towards antimicrobial treatment. Some bacteria living in biofilms exhibit up to 1,000 times greater resistance to antibiotics than planktonic bacteria [Patel 2005; Stewart 2002; Brooks and Jefferson, 2012]. Therefore, bacterial infections associated with implanted devices and biomaterials are complicated and create potential life-threatening circumstances for patients [Donlan & Costerton 2002; Costerton et al 1999; Vertes et al 2012; Jorge et al 2012].
Biomaterial and biomedical device decontamination or sterilization is essential. However, due to the thermal and chemical instability nature of biomaterials, conventional sterilization and decontamination methods may not be suitable for many of new and sophisticated biomaterials and biomedical devices [Jubyshkina 2011; Sbarra et al. 2009]. High-pressure steam and dry heat cause hydrolysis and deformation of biomaterials and thus cannot be considered for biomaterials and devices that are sensitive to heat and water [Belkoff & Haut 1992]. Gamma irradiation is known to break chemical bonds and change the chemical make-up of the biomaterials, as well as cause a decrease in both tensile strength and modulus, rendering this type of treatment detrimental to both the biocompatibility and efficiency of biomaterials [Olde Damink 1995]. Although ethylene oxide gas infiltration can be applied to all biomaterials and biomedical devices [Centola et al 2001], ethylene oxide treated items must undergo aeration in order to remove the residual ethylene oxide, resulting in lengthy cycle time, high cost, and potential hazards to both patients and staff [Mendes 2007]. More importantly, like all other chemicals and antibacterial agents, ethylene oxide proves to be ineffective against bacteria in biofilms [Mendes 2007]. The failure of sterilization may result in surgical replacement of contaminated devices along with prolonged antibiotic therapy, long periods of hospitalization, and morbidity.
The antibacterial ability of gas discharge plasma has been extensively studied [Opretzka et al. 2007; Morris et al. 2009]. One of the attractive features of discharge gas is the ability to achieve enhanced gas phase chemistry without the need for elevated gas temperatures. Therefore, plasma has short treatment time along with limited effects on biomaterials. In addition, the excellent ability of discharge gases to inactivate biofilms was confirmed in recent publications [Traba & Liang 2011; Joaquin et al. 2009]. These properties make plasma very attractive for their potential applications in biomaterials sterilization and decontamination [De Geyter & Morent 2012]. However, our recent study revealed that plasma mediated decontamination involved two mechanisms: 1) killing adhered bacteria and biofilms and 2) removing them from contaminated surfaces through etching [Traba & Liang 2011]. Because bacteria can be released from attached surfaces before they are completely killed, the etching ability of plasma represents an undesired effect, which may cause the relocation of live bacteria to the uncontaminated areas, resulting in contamination spreading and sterilization failure.
In this paper, we found that argon discharge at low discharge powers (<80 watts) maintained excellent antibacterial and anti-biofilm activity but had muted etching ability. A very efficient and effective decontamination approach by killing bacteria and biofilms in situ and then removing them from contaminated surfaces were established and tested on sophisticated biomaterials with varied properties and nanostructures.
2. Materials and methods
2.1. Bacterial strains and medium
Staphylococcus aureus (penicillin resistant, ATCC 29213), a good biofilm forming S. aureus strain was purchased from the American Type Culture Collection (ATCC, Manassas, VA). Tryptic soy broth supplemented with 0.2% glucose (TSBG) was purchased from Sigma (St Louis, MO).
2.2. Reagents and solutions
A LIVE/DEAD staining kit was purchased from Invitrogen Life Technologies (Carlsbad, CA) for the staining of bacteria within biofilms. Also, 5% methylthiazolyldiphenyl-tetrazolium bromide, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), in phosphate buffered saline (PBS), crystal violet (CV), dimethylsulfoxide (DMSO), and other reagents were all purchased from the Sigma Chemical Laboratory (St Louis, MO).
2.3. Growth of biofilms on different materials
For each experiment, an isolated single bacterial colony was picked from an agar plate, transferred to 10–15 ml of TSBG medium and then incubated under orbital agitation (100–150 rpm) at 37 °C for 18–24 h. This overnight culture of S. aureus was diluted in TSBG to 2×106 cells ml−1 and then inoculated on surfaces of different materials including 8-well glass chambers, polyethylene terephthalate films, polystyrene 6-well plates, silicon wafers, and polycaprolactone (PCL) coated micro-fibers. S. aureus biofilms of 15~20 μm in thickness were formed on all tested materials within 24 h. At the end of incubation, the formed biofilms were washed with PBS in order to remove planktonic and loosely attached bacteria. These biofilms had typical biofilm structures and developed antibiotic resistance as demonstrated in previous study [Traba & Liang 2011].
2.4. Biofilm assays
Widely used CV staining method in combination with colony-forming unit (CFU) counting and the MTT based viability assay was used to assess biofilm susceptibility to discharge gases. Unlike CV staining, which is used for staining bacterial cells (both live and dead) and other macromolecules such as polysaccharides, DNA, and proteins in biofilm extracellular matrix, CFU counting and the MTT assay were designed for live bacteria by measuring the overall metabolic activity of bacterial cells in biofilms. Thus, CV staining was used for the quantification of biofilms (total biomass of biofilm) while CFU counting and the MTT assay was utilized to evaluate viability of bacteria in biofilms.
In CV staining, biofilms were stained with 0.1% (w/v) CV for 10 min. The excess dye was removed by thoroughly rinsing the plate with water. CV dye associated with biofilms was then extracted by 33% glacial acetic acid and quantified using a microplate reader by measuring solution absorbance values at 570 nm.
In the MTT assay, biofilms were incubated with MTT at 37 °C for 10 min. After washing, the purple formazan formed inside the bacterial cells was dissolved by SDS and then measured using a microplate reader by setting the detecting and reference wavelengths at 570 nm and 630 nm, respectively [Traba & Liang 2011; Kharidia & Liang, 2012].
In the CFU counting assay, preformed biofilms in 6-well plates were washed to remove planktonic and loosely bound bacteria. Three milliliters of PBS was added to each well and biofilms were subjected to sonication treatment for 10 min to release bacteria. Resulting bacterial suspension was diluted 10,000 times. An aliquot (100 μl) of bacterial solution was applied on agar plates to perform CFU counting.
2.5. Generation of gas discharge plasma
Discharges were generated using Plasma Prep III device (SPI Supplies, AC 110 watts) with a frequency of 13.56 MHz as described previously [Traba & Liang 2011]. Bottled gases of oxygen, nitrogen and argon were purchased from Praxair (Keasbey, NJ) and were prepared by Cryogenic Air separation which led to a purity of >99.9%. Preformed biofilm samples were placed at the top, middle, and bottom positions in the chamber, which were 3 (Top), 6 (Middle), and 8 (Bottom) cm away from the gas inlet and not grounded. The system was first evacuated to 40 Pa at a fixed gas flow rate of 6.8 × 104 cm3 h−1. During the treatment process, discharge powers were controlled in the range between 0 and 100 W to generated plasma. The chamber pressure was maintained at 460 mtorr with a temperature of about 40 – 50°C. Due to manufacturing settings the plasma chamber is vacuumed and therefore isolated from the environment, resulting in no or very minimal plasma chamber contamination.
2.6. Staining of live and dead bacteria in biofilms
Live and dead bacterial distributions in biofilms were studied by confocal laser scanning microscopy using a LIVE/DEAD staining kit as described previously [Kharidia & Liang, 2012]. Biofilms grown on LabTek 8-well cover-glass chambers were washed with PBS to remove planktonic bacteria and TSBG medium. After that LIVE/DEAD dyes in water were added and incubated for 15 min at room temperature. Stained live (green) and dead (red) bacteria in biofilms were visualized by confocal fluorescence microscopy according to the protocol provided by the manufacture.
2.7. Biofilm re-growth experiments
One-day old S. aureus biofilms were treated by gas discharge plasma for the indicated minutes. Bacteria re-growth was conducted under different experimental conditions: 1) plasma treated biofilms were fed with fresh TSBG media; 2) plasma treated biofilms were subjected to sonication treatment with a Branson 5510 Ultrasonic Cleaner purchased from Thomas Scientific. This treatment was conducted at 37 °C for 10 min to release bacteria from biofilms. After that, biofilms were fed with fresh TSBG media containing 10% glucose, in an attempt to stimulate biofilm growth. All re-growth experiments were done at 37 °C for the indicated time. Bacterial growth was measured for 32 h by monitoring the supernatant absorbance change at 570 nm.
2.8. Preparation of PCL aligned microfibers on silicon
PCL aligned microfibers on silicon wafers were prepared using an aluminum rotating disk collector with a 10 mm flat edge [Valmikinathan et al. 2009]. The linear rate of the rotating disk at the edge was set at 9.5 m/s. Briefly, 15% PCL (150 mg, average molecular weight of-80,000) was dissolved in 4 ml hexafluoroisopropanol (HFIP) at room temperature. For the electrospinning process, 1.0 ml of 15% PCL solution was poured into a 5.0 ml BD plastic syringe with a 20 gauge flat-tip for generating fibers. The needle was connected to a high-voltage power supply at 15 kV to charge the polymer solution. A distance from the needle tip toward the ground rotating disk collector was fixed at 55 mm. A syringe pump was placed perpendicularly to the collector and the flow rate of syringe pump was fixed at 0.25 ml/hr. The fibers were spun onto 15-by-15 mm silicon wafer attached on the edge of the rotating disk using a piece of double-sided tape. After a 20-min electrospinning process, the scaffolds were removed and the aligned fibers were folded to the other side to prevent the fibers detached.
2.9. Tissue cell cultures
Tissue cells (Human fetal osteoblast cell HfOB 1.19, Human lung adenocarcinoma epithelial cell A549, and mouse embryonic fibroblast cells NIH/3T3) in antibiotic-free complete medium were seeded on the surfaces of silicon wafers and cultured at 37 °C for 6.0 h. Before the assay, cells were washed with PBS three times and stained with the LIVE/DEAD staining kit for 15 min. Cell images were recorded using a Zeiss LSM510 Confocal Microscope. The excitation wavelength was fixed at 488 nm and the emission wavelengths were set at 505–530 nm (for the live cells) and 560 nm (for the dead cells).
2.10. Atomic force microscopy
AFM measurements of treated biofilms were performed in air at room temperature using a NSCRIPTOR dip pen nanolithography system, Nanoink. The instrument was operated in the ac (tapping) mode using P-MAN-SICC-0 AFM cantilevers (Pacific Nanotechnology, Inc.) with a nominal force constant of 40 N/m.
3. Results and Discussion
3.1. Implications of etching in antibacterial and anti-biofilm activity of discharge gases
It is known that oxygen, argon and nitrogen all have different ionization energies. Oxygen has relatively low ionization energy (13.6 K kJ/mol) in comparison with nitrogen (14.2 K kJ/mol) and argon (15.8 K kJ/mol). Therefore, unlike the noble gas argon, in which a significant amount of the electron energy is used for atom/molecule ionization, discharge oxygen consumes a significant portion of the electron energy for their rotational, vibrational excitation, and atom dissociation. For this reason, discharge argon under the same experimental conditions has a higher density of charged particles in comparison with oxygen and nitrogen, which have a higher concentration of radicals.
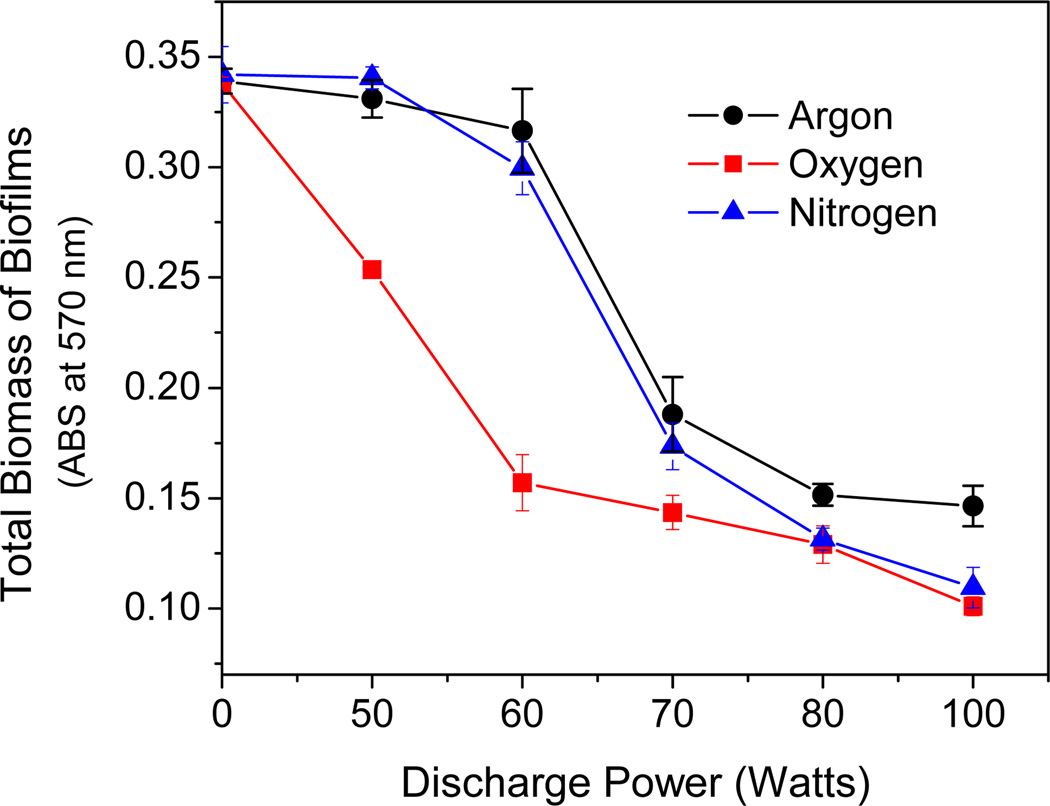
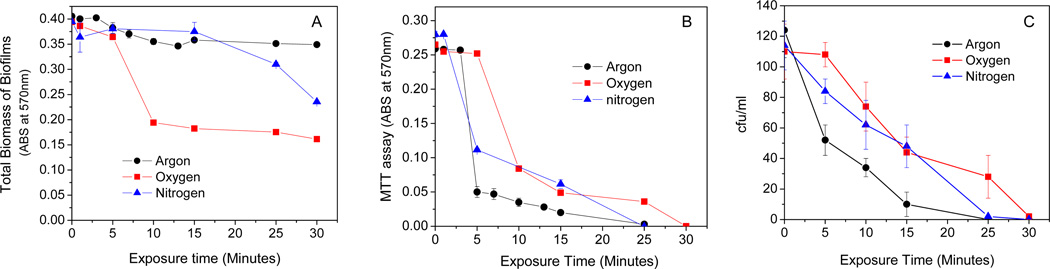
We compared the antibacterial and etching activity of discharge oxygen, nitrogen, and argon at varied discharge powers. As shown in Fig. 1, discharge argon and nitrogen behaved differently from discharge oxygen at low discharge powers and showed negligible biofilm etching ability in the range of 50–60 watts. When the discharge powers were fixed, exposure time positively affected the etching activity of discharge oxygen and discharge nitrogen. Discharge nitrogen induced biomass loss had long induction times and significant biomass loss occurred after 20 min exposure. However, discharge argon at 60 watts did not show noticeable etching activity even after prolonged time (>30 min) exposure (Fig. 2A). Surprisingly, despite the huge etching activity differences among the three tested gases, they all maintained strong antibacterial activity even at low discharge powers (Fig. 2B & Fig. 2C). Obviously, discharge gas-mediated biofilm deactivation undergoes different mechanisms. The antibacterial and etching activities of discharge argon might be governed by different reactive species.
Figure 1.
Discharge powers affected etching activities (CV staining) of discharge argon, nitrogen, and oxygen as tested on 1-day old S. aureus biofilms. Exposure time: 25 min for argon and nitrogen, and 30 min for oxygen. Data represents the mean of at least three biofilm samples.
Figure 2.
A) Exposure time affected etching (CV staining) activity of low power (60 watts) discharge gases as tested on 1-day old S. aureus biofilms; B) & C) Exposure time affected antibacterial (B, MTT assay; C, CFU counting) activity of low power (60 watts) discharge gases as tested on 1-day old S. aureus biofilms. Data represents the mean of at least three biofilm samples.
It should be pointed out that biofilms must be exposed to discharge oxygen, nitrogen, and argon at 60 watts for 30, 25, and 25 min, respectively, in order to achieve effective biofilm inactivation. Significant amounts of bacteria were killed by discharge argon after 5 min exposure, even when biomass loss was not yet observed in the treated biofilms. When the overall contribution of the plasma etching effect (Fig. 2A) to the measured antibacterial activity (Fig. 2B & Fig. 2C) is considered, discharge argon possesses the most antibacterial ability and is much more potent than discharge nitrogen and oxygen.
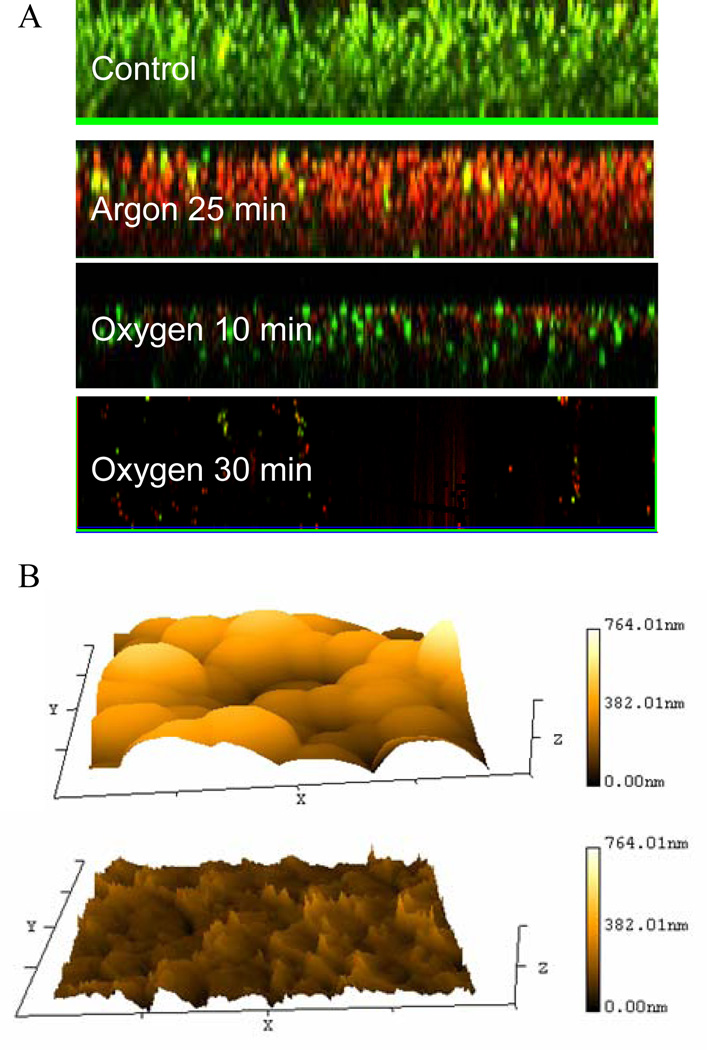
Details on gas discharge mediated biofilm inactivation were visualized with a Live/Dead staining assay (Fig. 3A). Low-power discharge argon hardly affected the integrity of biofilms, while completely inactivating bacteria within the biofilm. Both the architecture and the thickness of biofilms remained relatively unchanged during the entire exposure period to discharge argon (25 min). On the contrary, biofilms were gradually etched by discharge oxygen, beginning from the top of biofilms. Bacteria killing and biofilm removal happened simultaneously during discharge oxygen treatments as reported before [Traba & Liang 2011]. Typical morphologies of discharge gas treated biofilms were provided in Fig. 3B. The etching effect of discharge gas was confirmed by the significantly reduced biofilms thickness in treated samples as indicated by AFM images.
Figure 3.
A) Z-Stack confocal microscopy images of 1-day old S. aureus biofilms stained using Live/Dead staining kit. Discharge power: 60 watts. The numbers in images indicated the exposure time; B) Typical AFM images of S. aureus biofilms before (Top) and after (bottom) discharge oxygen (60 watts) treatment for 30 min.
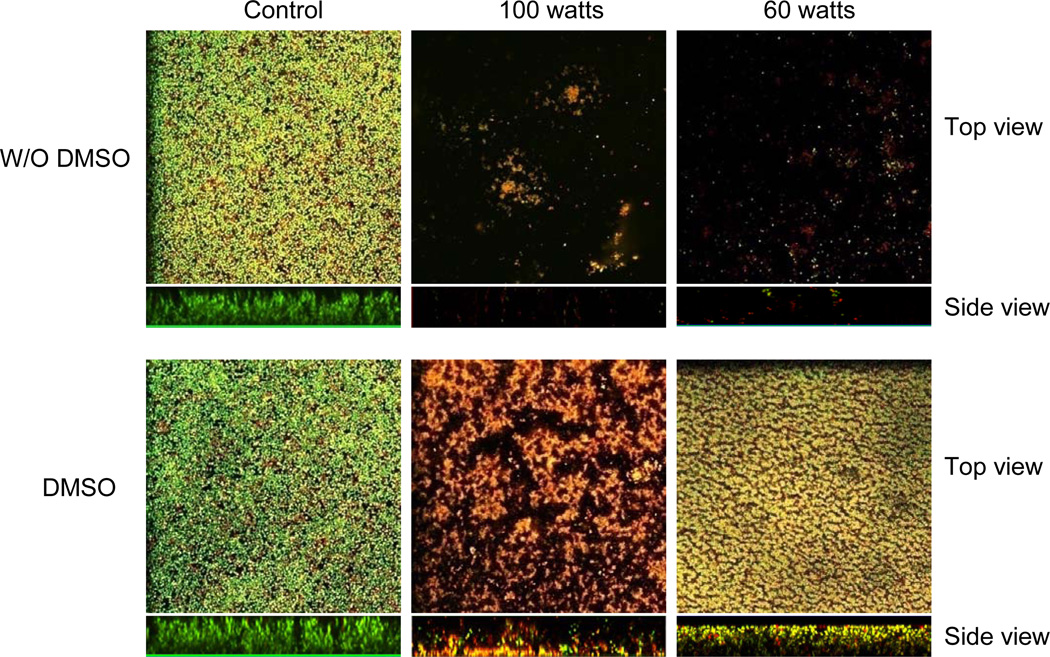
It has been well established that discharge gases under conventional experimental conditions contain ionized atoms and molecules, free radical species, combination of ions, electrons, along with excited atoms that can emit UV radiation [De Geyter & Morent 2012]. However, the exact species that are generated have not yet been determined. Nonetheless, all of these reactive species generated in plasma discharge gas including UV radiation have been determined to be the active species responsible for both bacterial cell damage and death [Boudam et al. 2006; Philip et al. 2002]. In order to further understand the acting mechanism of discharge gases, we examined the role of free radicals in biofilm inactivation. We found that when biofilms were soaked in DMSO, a proven free radical scavenger before exposure to discharge oxygen generated at low-powers (60 watts), the etching ability of discharge oxygen was drastically reduced while the antibacterial activity of oxygen plasma was hardly affected (Fig. 4). Bacteria embedded in biofilms were killed (red color) or severely damaged (yellow color) by discharge oxygen in the presence of DMSO. Another free radical scavenger, L-ascorbic acid, was used to replace DMSO in these experiments. The same phenomenon was observed in L-ascorbic acid treated biofilms (data not shown). Results from these studies suggest that free radicals are main specie responsible for the etching effect and are less important for the antibacterial activity of discharge oxygen. Since the antibacterial and etching ability of discharge gases have never been considered separately, the antibacterial/anti-biofilm activity of discharge gases reported in all previous studies represents combined contributions from bacterial killing and removal.
Figure 4.
Free radical scavenger dimethylsulfoxide, DMSO, affected antibacterial and etching activity of discharge oxygen. One-day old S. aureus biofilms, treated with or without DMSO before they were exposed to discharge oxygen for 25 min. At the end of experiments, live and dead bacteria in biofilms were stained green or red, respectively, by using Live/Dead staining kit and visualized under confocal microscopy.
3.2. Antibacterial and anti-biofilm ability of low power discharge argon
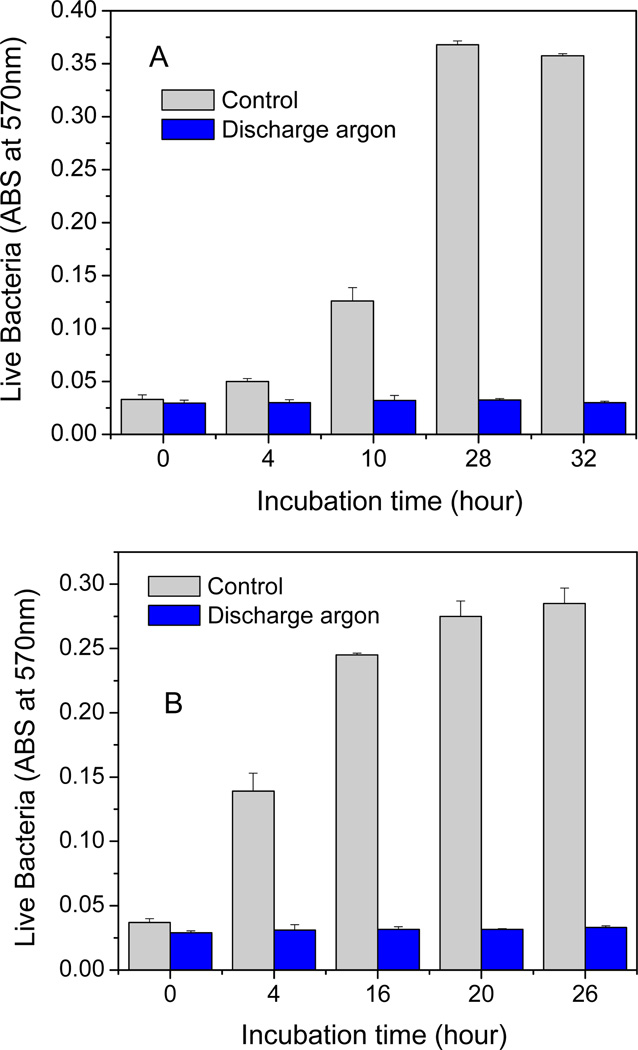
In order to further evaluate the antibacterial ability of low power discharge argon, a well designed re-growth assay was performed on treated biofilms (Fig 5a). Although untreated biofilms experienced a rapid growth in the first 24 h after the medium change, no bacterial or biofilm re-growth was observed in low power discharge argon treated biofilms even after 24 h of incubation.
Figure 5.
Bacterial re-growth. One-day old S. aureus biofilms were treated by low power (60 watts) discharge argon for 25 min. Re-growth was conducted under different experimental conditions: A) plasma treated biofilms were fed with fresh TSBG media; B) plasma treated biofilms were sonicated for 10 min and then fed with fresh TSBG media containing 10% glucose. All re-growth experiments were done at 37 °C for the indicated time. Bacteria re-growth from untreated (control) and plasma treated biofilms were monitored by measuring culture medium absorbance changes at 570 nm. Data represents the mean of at least three biofilm samples.
It should be noted that although re-growth experiments indicate no live bacteria in low-power discharge argon treated biofilms, a very few amount of “live” (green color) bacteria was visualized throughout the treated biofilm, even after optimal exposure times (Fig. 3A). It is known that small colony variants (SCV) of bacteria can enter a viable but nonculturable (VBNC) state as a response to the environmental stresses involving nutrient, temperature, and osmotic conditions changes [Dhiaf et al. 2008, Proctor et al. 2006]. In order to examine if the “live” bacteria in low-power discharge argon treated biofilms were indeed VBNC bacteria, we fed discharge argon treated biofilms grown in 6-well plates with TSGB medium containing high concentration (10%) of glucose. Such high concentrations of glucose were utilized in an attempt to stimulate bacterial growth [Allison et al. 2011]. After 10 min of sonication, which was required to release bacteria from biofilms into the culture medium, the plates were placed back into the incubator and cultured at 37 °C overnight. Again, we did not see bacteria regrowth in biofilms treated with low power discharge argon (Fig. 5b).
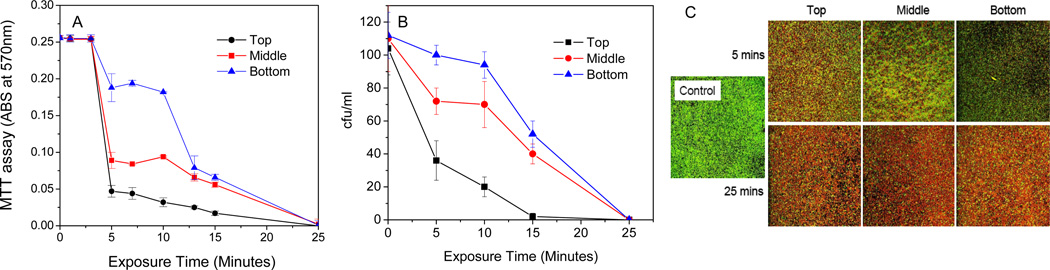
Further studies revealed that the reactive species corresponding to the antibacterial activity of discharge argon seemed to be short-lived. Moving biofilm samples away from the gas inlet had a negative impact on the antibacterial activity of low power discharge argon (Fig. 6). However, despite significantly delayed bacteria killing in the biofilm samples placed furthest away from the gas inlet, complete inactivation could be achieved after long exposure times.
Figure 6.
Spatial arrangement of biofilm samples with respect to gas outlet and location inside plasma chamber affected antibacterial activity of low power (60 watts) discharge argon. Live S. aureus in biofilms as measured using MTT assay (A) and CFU counting (B), respectively; C) Confocal microscopy images of S. aureus biofilms stained using Live/Dead staining kit. Biofilm samples were placed 3 cm (Top), 6 cm (Middle), 8 cm (Bottom) away from the gas inlet of plasma chamber. Images from the top and bottom were biofilms treated by low power discharge (60 watts) argon for 5 min and 25 min, respectively. Data represents the mean of at least three biofilm samples.
3.3. An effective two-step decontamination approach using discharge argon
The etching activity of discharge gases may represent an undesired effect for biomedical applications such as biomedical device sterilization, decontamination, wound infection treatments, and tissue debridement. Since some adherent bacteria including bacteria in biofilms can be removed before they were killed, it may result in bacterial relocation and thus contamination spreading. Decoupled etching and antibacterial activity (Fig. 2) found in low power discharge argon is very unique and makes this particular plasma more suitable for some biomedical applications.
Based on above results, we tested a two-step sterilization approach using discharge argon as demonstrated in Fig. 7. In the first step, contaminated biomaterials were exposed to discharge argon at 60 watts to completely kill adherent bacteria as well as bacteria within biofilms. Due to negligible etching activity of discharge argon at this power, all attached bacteria were killed in situ and thus bacterial relocation along with contamination spreading was prevented. In the second step, dead bacteria as well as biofilm debris was effectively removed from the biomaterials by utilizing the etching effect of discharge argon in our favor by simply increasing discharge powers to over 80 watts. We had compared bacterial adhesion and biofilm formation on the original and regenerated (biofilm contained surfaces after treatment with plasma) surfaces. We did not see the preference of bacteria to these re-generated surfaces. On the contrary, bacteria from an outside source grew slower on regenerated than on the control surface (data not shown).
Figure 7.
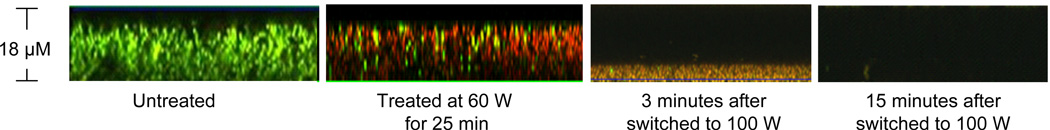
Testing the two-step sterilization approach of discharge argon on S. aureus biofilms formed on PET films. Biofilm samples taken at different time points were stained with Live/Dead kit and visualized using confocal microscopy.
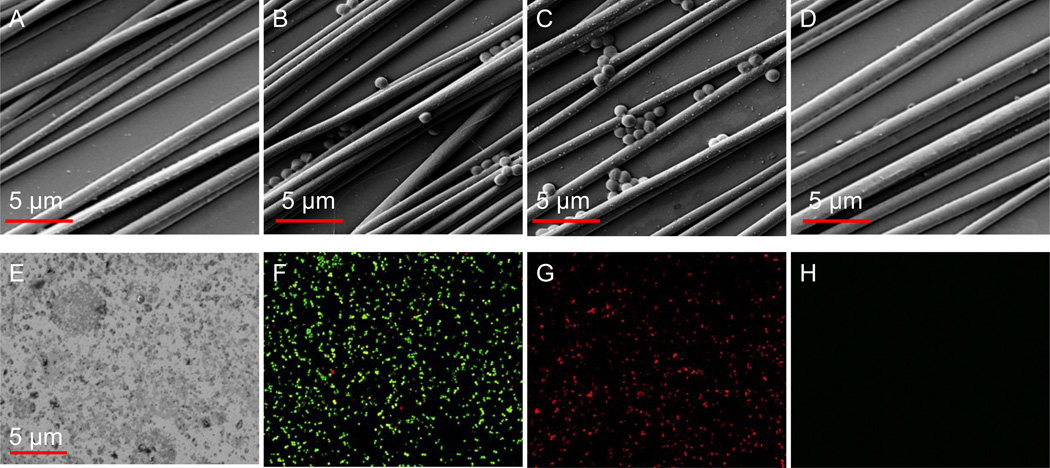
The effectiveness and efficiency of this two-step approach was further tested on surfaces covered with PCL aligned microfibers (Fig. 8). Such surfaces are essential for assisting in bone and neuron cell growth and have a wide range of biomedical applications [Abidian et al. 2009]. However, the conventional autoclaving method completely destroyed surface micro-structures (Fig. 8E) and thus was not suitable for such sophisticated biomaterials. The etching effects of plasma may cause the spreading of live bacteria into macro-structures, leading to decontamination failure. With this two-step sterilization approach as demonstrated in Fig. 7, bacteria adhered to these sophisticated surfaces were killed in situ (Fig. 8C & 8G) and then completely removed (Fig. 8D & 8H) from the surfaces. It should be stated that despite the close diameter/size, solid and uniform PCL fibers are different from bacteria with fragile lipid bilayer of cell membranes. Therefore, only bacteria were damaged and removed but the micro-structures (Fig. 8D) were fully maintained in the resulting bacteria-free surfaces, resulting in bacteria-free surfaces (Fig. 8H).
Figure 8.
Application of the two-step sterilization approach for the decontamination of biomaterials with nano-patterned surfaces. A) surface of silica wafer coated with PCL nano-fibers; B & F) S. aureus contaminated surfaces; C & G) S. aureus contaminated surfaces treated with discharge argon generated at low power (60 watts) for 8 min; D & H) S. aureus contaminated surfaces treated with discharge argon generated at high power (100 watts) for 6 min; E) the same S. aureus contaminated surfaces treated with the conventional autoclaving method. Nano-structures on the surface were completely destroyed. A-E are SEM images. F-G are confocal microscopy images acquired using Live/Dead kit.
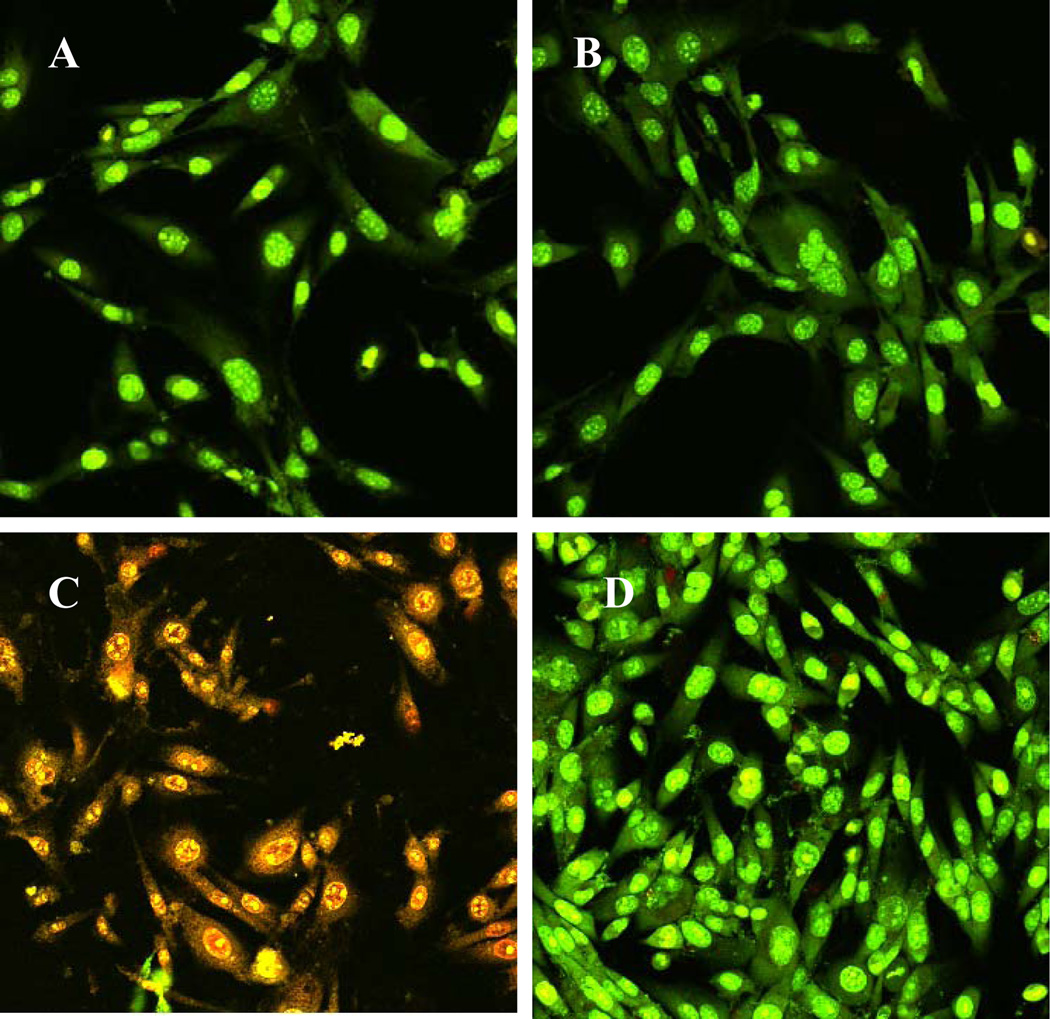
We further tested tissue cell growth on regenerated surfaces from bacteria contaminated silicon wafers. As shown in Fig. 9C, toxins released from surface attached S. aureus caused immediate tissue cell death, and almost all tissue cells were strained red by Live/Dead kit after 6 h incubation. However, tissue cells grew normal and healthy (green color) on regenerated surfaces (Fig. 9D), confirming the decontamination effectiveness of this two-step approach using discharge argon. The same results were also obtained from tissue cell culture using other cell lines including human fetal osteoblast cell HfOB 1.19 and human lung adenocarcinoma epithelial cell A549 (data not shown). Since low power discharge argon was much more gentle than the currently accepted and standardized plasma sterilization (RF powers of >20k), this two-step approach had no or negligible effects on the biocompatibility of biomaterials as we expected.
Figure 9.
Confocal microscopy images of NIH/3T3 cells on A) uncontaminated and untreated surface; B) uncontaminated and plasma treated surfaces; C) contaminated with S. aureus bacteria with no plasma treatment; and D) contaminated with S. aureus bacteria with plasma treatment (Argon discharge gas at 60 watts for 25 min and then 100 watts for 6 minutes. S. aureus suspension (2×103/ml) was loaded onto each surface and incubated at room temperature for 2 h. At the end of the incubation time, each surface was washed in order to remove loosely adhered bacteria. Then NIH/3T3 cells in antibiotic free medium were seeded and incubated at 37 °C for an additional 6 hrs. At the end of incubation, cells were stained using Live/Dead kit.
The etching effect makes discharge argon the most effective biofilm sterilization method in terms of bacteria inactivation and biofilm removal efficiency. However, like all other sterilization methods, trace amounts of biomacromolecules such as DNA, polysaccharides, lipids, and other extracellular matrix components were still detected on plasma treated surfaces (data not shown). This is extremely true in the case of mature biofilms (Fig. 3B bottom). Although, tissue cells grew normal on regenerated surfaces through short period of culture (Fig. 9D), possible endotoxin effects of these residuals along with the biocompatibility of plasma-regenerated surfaces need to be further studied. Data from related studies will be published in a followed paper.
Acknowledgements
This work was supported by NIH grant AI078176 and AI079608. Mr. Traba is a recipient of the Stanley Fellowship. Mr. Chen is a recipient of the Innovation and Entrepreneurship Doctoral Fellowship. PCL aligned microfibers samples were kindly prepared by Mr. Wei Chang in Prof. Xiaojun Yu’s lab at Stevens Tech.
Abbreviations
- MTT
methylthiazolyldiphenyl-tetrazolium bromide, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- CV
crystal violet
- TSBG
tryptic soy broth supplemented with 0.2 % glucose
- c.f.u
colony forming unit
- DMSO
dimethylsulfoxide
- PBS
phosphate buffered saline
References
- Abidian MR, Ludwig KA, Marzullo TC, Martin DC, Kipke DR. Interfacing conducting polymer nanotubes with the central nervous system: chronic neural recording using poly(3,4-ethylenedioxythiophene) nanotubes. Adv Mater. 2009;21:3764–3770. doi: 10.1002/adma.200900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkoff SM, Haut RC. Microstructurally based model analysis of gamma-irradiated tendon allografts. J Orthop Res. 1992;10:461–46. doi: 10.1002/jor.1100100320. [DOI] [PubMed] [Google Scholar]
- Boudam M, Moisan M, Saoudi B, Popovici C, Gherardi N, Massines F. Bacterial spore inactivation by atmospheric-pressure plasmas in the presence or absence of uv photons as obtained with the same gas mixture. J Phys D: Appl Phys. 2006;39:3494–3507. [Google Scholar]
- Brooks JL, Jefferson KK. Staphylococcal biofilms: quest for the magic bullet. Adv Appl Microbiol. 2012;81:63–87. doi: 10.1016/B978-0-12-394382-8.00002-2. [DOI] [PubMed] [Google Scholar]
- Centola DT, Ayoub KI, Lao NT, Lu HTC, Page BFJ. Variables affecting simulated use determination of residual ethylene oxide in medical devices. J AOAC Int. 2011;84:512–518. [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- De Geyter BN, Morent R. Non-thermal Plasma Sterilization of Living and Nonliving Surfaces. Annu Rev Biomed Eng. 2012;14:255–274. doi: 10.1146/annurev-bioeng-071811-150110. [DOI] [PubMed] [Google Scholar]
- Dhiaf A, Bakhrouf A, Witzel KP. Resuscitation of eleven-year VBNC Citrobacter. J Water Health. 2008;6:565–568. doi: 10.2166/wh.2008.131. [DOI] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. ReV. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joaquin J, Kwan C, Abramzon N, Vandervoort K, Brelles-Marino G. Is gas Discharge plasma a new solution to the old problem of biofilm inactivation? Microbiology. 2009;155:724–732. doi: 10.1099/mic.0.021501-0. [DOI] [PubMed] [Google Scholar]
- Jorge P, Lourenço A, Pereira MO. New trends in peptide-based anti-biofilm strategies: a review of recent achievements and bioinformatic approaches. Biofouling. 2012;28:1033–1061. doi: 10.1080/08927014.2012.728210. [DOI] [PubMed] [Google Scholar]
- Jubyshkina G, Zupancic B, Stukelj M, Groselj D, Marion L. The Influence of Different Sterilization Techniques on the Time-Dependent Behavior of Polyamides. J Biomaterial and Nanobiotechnology. 2011;2:361–368. [Google Scholar]
- Kharidia R, Liang JF. The activity of a small lytic peptide PTP-7 on Staphylococcus aureus biofilms. J Microbiol. 2011;49:663–668. doi: 10.1007/s12275-011-1013-5. [DOI] [PubMed] [Google Scholar]
- Mendes GC, Brandão TR, Silva CL. Ethylene oxide sterilization of medical devices: a review. Am J Infect Control. 2007;35:574–581. doi: 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Olde Damink HL, Dijkstra PJ, Van Luyn MJ, Van Wachem PB, Nieuwenhuis P, Feijen J. Influence of ethylene oxide gas treatment on the in vitro degradation behavior of dermal sheep collagen. J Biomed Mater Res. 1995;29:149–155. doi: 10.1002/jbm.820290203. [DOI] [PubMed] [Google Scholar]
- Opretzka J, Benedikt J, Awakowicz P, Wunderlich J, von Keudell A. The role of chemical sputtering during plasma sterilization of bacillus atrophaeus. J Phys D: Appl Phys. 2007;40:2826–2830. [Google Scholar]
- Overman PR. Biofilm: A new view of plaque. J. Contempt Dent Pract. 2006;1:1–11. [PubMed] [Google Scholar]
- Patel R. Biofilms and antimicrobial resistance. Clin Orthop Relat Res. 2006;437:41–47. doi: 10.1097/01.blo.0000175714.68624.74. [DOI] [PubMed] [Google Scholar]
- Philip N, Saoudi B, Crevier MC, Moisan M, Barbeau J, Pelletier J. The respective roles of UV photons and oxygen atoms in plasma sterilization at reduced gas pressure: The case of N2-O2 mixtures. IEEE Trans. Plasma Sci. 2002;30(4):1429–1436. [Google Scholar]
- Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4(4):295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- Sbarra MS, Arciola CR, Di Poto A, Saino E, Rohde H, Speziale P, Visai L. The photodynamic effect of tetra-substituted N-methyl-pyridyl-porphine combined with the action of vancomycin or host defense mechanisms disrupts Staphylococcus epidermidis biofilms. Int J Artif Organs. 2009;32(9):574–583. doi: 10.1177/039139880903200906. [DOI] [PubMed] [Google Scholar]
- Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- Traba C, Liang JF. Susceptibility of Staphylococcus aureus biofilms to reactive discharge gases. Biofouling. 2011;27:763–772. doi: 10.1080/08927014.2011.602188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmikinathan CM, Wang J, Smiriglio S, Golwala NG, Yu X. Magnetically induced protein gradients on electrospun nanofibers. Comb Chem High Throughput Screen. 2009;12(7):656–663. doi: 10.2174/138620709788923683. [DOI] [PubMed] [Google Scholar]
- Vertes A, Hitchins V, Phillips SK. Analytical Challenges of Microbial Biofilms on Medical Devices. Anal Chem. 2012;84:3858–3866. doi: 10.1021/ac2029997. [DOI] [PubMed] [Google Scholar]