Abstract
Background: Breastfeeding durations in the United States fall short of public health objectives. We sought to quantify the prevalence and identify risk factors for early, undesired weaning that mothers attribute to physiologic difficulties with breastfeeding.
Methods: We analyzed data from the Infant Feeding Practices Study (IFPS) II, a longitudinal study of US women. We defined disrupted lactation as early, undesired weaning attributed to at least two of the following three problems: breast pain, low milk supply, and difficulty with infant latch. We used logistic regression to estimate the association maternal body mass index (BMI), postpartum depressive symptoms, and disrupted lactation.
Results: Of 4,902 women enrolled in the IFPS II, we analyzed 2,335 women who reported prenatal intention and breastfeeding initiation. The prevalence of disrupted lactation was 12 per 100 women (95% confidence interval [CI] 11, 13) during the first year of life. Women in this group weaned earlier (median 1.2 months, interquartile range [IQR] 0.5–2.8) than women without disrupted lactation (median 7.0 months, IQR 2.8–2.0, p<0.01). In multivariable-adjusted (MV-adj.) models, we found increased odds of disrupted lactation among overweight (odds ratio [OR] 1.6, 95% CI 1.1–2.3) or obese (OR 1.7, 95% CI 1.2–2.6) women, compared with women with a normal pregravid BMI. Maternal depressive symptoms at 2 months, defined as Edinburgh Postnatal Depression Scale ≥13, were also associated with disrupted lactation (MV-adj. OR 1.7, 95% CI 1.1–2.7).
Conclusion: In a longitudinal sample of US women, disrupted lactation affected one in eight mothers who initiated breastfeeding. These findings underscore the need for both improved early breastfeeding support and targeted research to define the underlying pathophysiology and to determine management strategies that will enable more mothers to achieve their breastfeeding goals.
Introduction
Breastfeeding is a significant predictor of health outcomes. For infants, never breastfeeding or early weaning is associated with increased risks of otitis media, diarrhea, lower respiratory tract infection, sudden infant death syndrome, leukemia, and type 1 diabetes.1 Among mothers, never breastfeeding or early weaning is associated with increased risks of breast cancer, ovarian cancer, diabetes, hypertension, and myocardial infarction.2 Based on these associations, all major medical organizations recommend 6 months of exclusive breastfeeding, with continued breastfeeding through the infant's first year and beyond.3
However, breastfeeding rates in the United States fall far short of these recommendations. Although 77% of US mothers initiate breastfeeding, just 16% of mother-infant dyads achieve the recommended 6 months of exclusive breastfeeding.4 Multiple factors impact breastfeeding duration,5–10 and recent public health campaigns have drawn attention to social constraints,11 such as paid maternity leave, attitudes toward nursing in public, and workplace accommodations for mothers of nursing infants. However, successful breastfeeding also depends on the integrated psychology and physiology of mother and child.12 The prevalence of early, undesired weaning that mothers attribute to disrupted physiology is unknown.
Both obesity and depression have been associated with differences in lactation physiology, and these conditions are associated with reduced breastfeeding duration.13–16 Obesity and insulin resistance are associated with differences in prolactin levels,17 onset of lactogensis,18 and the human milk fat layer transcriptome.19 In addition, in animal models, obesity is associated with poor milk production.20 With respect to depression and lactation, women with symptoms of depression and anxiety had lower oxytocin levels during feeding in a recent study,21 and several other neuroendocrine mechanisms may link maternal mood disorders with breastfeeding difficulties.12 Furthermore, in animal models, disruption of oxytocin physiology results in dysregulated stress responses and poor feeding.22 Thus, maternal health conditions may disrupt lactation, leading to early, undesired weaning.
The prevalence of such disrupted lactation is not known. We therefore sought to define the prevalence of early, undesired weaning that mothers attribute to lactation dysfunction, which we defined as difficulties with latch, pain, and milk supply. We used data from the Infant Feeding Practices Study (IFPS) II to estimate the proportion of women who experience disrupted lactation and to estimate associations between demographic characteristics and disrupted lactation. We hypothesized that the prevalence of disrupted lactation would be increased among women with increased maternal body mass index (BMI) or depressive symptoms, independent of sociodemographic confounders.
Methods
The IFPS II has been described in detail elsewhere.26 Briefly, this longitudinal study from the CDC recruited 4,902 women between May 2005 and June 2007 from a nationally distributed panel of more than 500,000 US households. Participants completed questionnaires in the third trimester of pregnancy and through the first 12 months of the child's life. IFPS II participants were more likely to be middle class, employed, and white than nationally representative samples. We included in our analysis all women who (1) reported on the prenatal questionnaire how long they intended to breastfeed and (2) reported that they had ever breastfed on the neonatal questionnaire administered at 1 month postpartum.
Of the 4,902 women enrolled in the IFPS II, 3,452 met birth screener criteria for the parent study, of whom 3,033 completed a qualifying neonatal questionnaire.26 Of these 3,033 participants, 2,403 had reported an intended breastfeeding duration on the prenatal questionnaire, of whom 2,335 initiated breastfeeding. These 2,335 women comprised our study sample.
Assessment of breastfeeding intention and outcome
The IFPS II assessed breastfeeding intention in the prenatal period. Mothers were asked how old they anticipated their infant would be before stopping breastfeeding altogether.
Infant feeding was assessed with monthly questionnaires in the first 7 months, followed by questionnaires at 9, 10, and 12 months. If the dyad had stopped breastfeeding, the mother was asked the infant's age at weaning and whether she had breastfed as long as she wanted to. We defined “early weaning” as discontinuation of breastfeeding earlier than the duration the mother reported in response to the prenatal question “How old do you think your baby will be when you completely stop breastfeeding?” We defined “undesired weaning” as the mother answering no to the question “Did you breastfeed as long as you wanted to?” Upon stopping, mothers were asked to consider a list of reasons for weaning and indicate their importance on a Likert scale. For this analysis, we dichotomized reasons for weaning into two categories of “Not Important” (“Not at all important” or “Not very important”) and “Important” (“Somewhat important” or “Very important”).
Definition of disrupted lactation
The goal of our study was to estimate the proportion of women who were unable to sustain their intended duration of breastfeeding owing to physiologic problems with lactation. To identify such women within the constraints of a secondary analysis, we analyzed self-reported physiologic reasons for early undesired weaning. Prior studies have reported that pain, low milk supply, and difficulty with infant latch were the most commonly cited reasons for early weaning.27 We therefore defined disrupted lactation as early, undesired weaning in the setting of a mother's reporting at least two of these three problems as important reasons for stopping breastfeeding. We used at least two of these reasons as our threshold in order to define a population that reported multiple difficulties with lactation physiology.
Assessment of breastfeeding experience and support
At the time of weaning, mothers rated their feelings about their experience of having breastfed their baby on a Likert scale from “1 - Very unfavorable” to “5 - Very favorable” and their likelihood of breastfeeding again if they had another child (“1 - Not at all likely” to “5 - Very likely”). Answers were dichotomized as “Favorable” (4 or 5) or “Unfavorable” (1, 2, or 3) and as “Likely” (4 or 5) or “Unlikely” (1, 2, or 3). On the neonatal questionnaire, mothers were asked whether they had received breastfeeding help from a health professional and whether that help had solved the problems or made them better, using a Likert scale from “No, not at all (1)” to “Yes, very much (5).”
Assessment of maternal BMI and mood
On the prenatal questionnaire, mothers reported their weight immediately before pregnancy and their height. We used these values to calculate pregravid BMI, kg/m2, which we categorized as underweight (BMI <18.5 kg/m2), normal (BMI 18.5–<25 kg/m2), overweight (BMI 25–<30 kg/m2), or obese (BMI ≥30 kg/m2). Maternal mood was assessed on the 2-month postnatal questionnaire using the Edinburgh Postnatal Depression Scale (EPDS). This 10-item, well-validated questionnaire measures depression and anxiety symptoms during the perinatal period.28 We classified women with an EPDS ≥13 as having depressive symptoms. This threshold has a sensitivity of 75% and specificity of 84% for major depression among postnatal women.28
Analysis
We classified women into five groups: (1) early, undesired weaning; (2) early, desired weaning; (3) expected, undesired weaning; (4) expected, desired weaning; and (5) breastfed ≥12 months. Women with disrupted lactation comprised a subset of women with early, undesired weaning. We used breastfeeding for ≥12 months as our referent category because the American Academy of Pediatrics recommends at least 1 year of breastfeeding; thus, women in this group have succeeded according to a consensus public health recommendation.3 We further conducted a sensitivity analysis to determine how classifying women who breastfed ≥12 months into the four weaning categories would affect the prevalence of early, undesired weaning.
Loss to follow-up occurred over the 15 months that women participated in the IFPS II. Of the 2,335 women who were included in our analysis, 1,414 completed the 12-month questionnaire. We used multiple imputation to approximate a complete set of covariates for all women who completed the prenatal and neonatal questionnaires. We implemented multiple imputation using PROC MI in SAS (SAS Institute, Cary, NC) for variables used in the analysis, including demographic variables, maternal BMI, EPDS score at 2 months, breastfeeding duration, pain and problems with early breastfeeding, and reasons for weaning. Ten imputations were generated for evaluation. We used these imputed data sets to estimate the prevalence of disrupted lactation in our study population and to measure associations with risk factors for this outcome. We used the methods described in Rubin29 to construct confidence intervals (CIs) for prevalence. Using our imputed data set, we calculated means and standard deviations or counts and frequencies, as appropriate, for IFPS II participants.
We used PROC LIFETEST in SAS (SAS Institute, Cary, NC) to estimate Kaplan- Meier survival curves. The average across the 10 survival curve estimates was used to generate a summary survival curve to compare durations of breastfeeding among these five groups. R-2.14 was used to graph the survival curves. The average duration of breastfeeding between the groups (excluding the group breastfeeding at the last study questionnaire) was compared using the imputed data with SAS procedures MIXED and MIANALYZE (SAS Institute, Cary, NC).
We compared durations of breastfeeding between women who did or did not meet criteria for disrupted lactation. We also compared help received from health professionals, maternal feelings about the breastfeeding experience, and likelihood of breastfeeding a future child for women who did or did not meet criteria for disrupted lactation using SAS procedures LOGISTIC (SAS Institute, Cary, NC) and MIANALYZE.
Several maternal and infant risk factors have been associated with early discontinuation of breastfeeding owing to physiologic difficulties, including maternal obesity13,17 and postpartum depression.15,30 We modeled associations between these factors and both disrupted lactation and early, undesired weaning, using LOGISTIC and MIANALYZE. We present both unadjusted models and models adjusting for maternal age, parity, education, race/ethnicity, marital status, and Women, Infants, and Children (WIC) participation. All statistical analyses were conducted using SAS.V9.3.
The IFPS II was approved by the Research Involving Human Subjects Committee of the US Food and Drug Administration (FDA). This secondary analysis was reviewed by the University of North Carolina Office of Human Research Ethics and found not to require institutional review board (IRB) approval.
Results
The 2,335 IFPS II participants who both reported an intended duration of breastfeeding and initiated breastfeeding comprised our study population. Participants were predominantly married (75.6%), white (81.4%), and multiparous (68.0%), and 38.8% had completed a college degree. About a third of participants participated in postnatal WIC (37.0%) (Table 1).
Table 1.
Demographic Characteristics for Actual and Imputed Data for Women Who Intended to Breastfeed and Had Ever Breastfed (n= 2,335)
| Complete case | Imputed data | |||||
|---|---|---|---|---|---|---|
| n (%) | Missing outcome | Disrupted lactation | Undisrupted lactation | Disrupted lactation | Undisrupted lactation | |
| Postnatal WIC | ||||||
| No | 1,471 (63.0) | 366 (24.9) | 107 (9.7) | 998 (90.3) | 145 (9.8) | 1,327 (90.2) |
| Yes | 864 (37.0) | 248 (28.7) | 101 (16.4) | 515 (83.6) | 136 (15.7) | 728 (84.3) |
| Household size | ||||||
| 1–2 | 642 (27.5) | 140 (21.8) | 77 (15.3) | 425 (84.7) | 97 (15.1) | 545 (84.9) |
| 3–4 | 1,339 (57.3) | 368 (27.5) | 103 (10.6) | 868 (89.4) | 141 (10.5) | 1,198 (89.5) |
| 5 or more | 354 (15.2) | 106 (29.9) | 28 (11.3) | 220 (88.7) | 42 (11.9) | 312 (88.1) |
| Marital status | ||||||
| Married | 1,766 (75.6) | 439 (24.9) | 137 (10.3) | 1,190 (89.7) | 197 (10.5) | 1,668 (89.5) |
| Not married | 420 (18.0) | 117 (27.9) | 55 (18.2) | 248 (81.8) | 84 (17.8) | 387 (82.2) |
| Missing | 149 (6.4) | 58 (38.9) | 16 (17.6) | 75 (82.4) | 0 (0.0) | 0 (0.0) |
| Race or ethnicity | ||||||
| White | 1,901 (81.4) | 476 (25.0) | 170 (11.9) | 1,255 (88.1) | 234 (11.9) | 1,725 (88.1) |
| African American | 103 (4.4) | 35 (34.0) | 6 (8.8) | 62 (91.2) | 10 (9.3) | 97 (90.7) |
| Hispanic | 149 (6.4) | 45 (30.2) | 15 (14.4) | 89 (85.6) | 21 (14.1) | 128 (85.9) |
| Other | 119 (5.1) | 40 (33.6) | 10 (12.7) | 69 (87.3) | 16 (13.2) | 105 (86.8) |
| Missing | 63 (2.7) | 18 (28.6) | 7 (15.6) | 38 (84.4) | 0 (0.0) | 0 (0.0) |
| Maternal age | ||||||
| 18 to younger than 24 | 390 (16.7) | 131 (33.6) | 61 (23.6) | 198 (76.4) | 81 (20.7) | 310 (79.3) |
| 24 to younger than 28 | 589 (25.2) | 162 (27.5) | 51 (11.9) | 376 (88.1) | 72 (12.2) | 518 (87.8) |
| 28 to younger than 32 | 632 (27.1) | 155 (24.5) | 50 (10.5) | 427 (89.5) | 66 (10.5) | 567 (89.5) |
| 32 or older | 720 (30.8) | 163 (22.6) | 46 (8.3) | 511 (91.7) | 61 (8.5) | 660 (91.5) |
| Missing | 4 (0.2) | 3 (75.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| Parity | ||||||
| >1 | 1,588 (68.0) | 426 (26.8) | 105 (9.0) | 1,057 (91.0) | 152 (9.4) | 1,463 (90.6) |
| 1 | 694 (29.7) | 171 (24.6) | 97 (18.5) | 426 (81.5) | 129 (17.8) | 592 (82.2) |
| Missing | 53 (2.3) | 17 (32.1) | 6 (16.7) | 30 (83.3) | 0 (0.0) | 0 (0.0) |
| Education | ||||||
| Less than high school | 46 (2.0) | 16 (34.8) | 6 (20.0) | 24 (80.0) | 10 (19.8) | 41 (80.2) |
| High school | 327 (14.0) | 92 (28.1) | 36 (15.3) | 199 (84.7) | 54 (14.8) | 312 (85.2) |
| 1–3 years of college | 897 (38.4) | 267 (29.8) | 93 (14.8) | 537 (85.2) | 140 (14.5) | 825 (85.5) |
| College or postgraduate | 906 (38.8) | 176 (19.4) | 54 (7.4) | 676 (92.6) | 76 (8.0) | 876 (92.0) |
| Missing | 159 (6.8) | 63 (39.6) | 19 (19.8) | 77 (80.2) | 0 (0.0) | 0 (0.0) |
| Employment | ||||||
| Employed full time | 705 (30.2) | 157 (22.3) | 69 (12.6) | 479 (87.4) | 103 (12.7) | 707 (87.3) |
| Employed part time | 264 (11.3) | 61 (23.1) | 24 (11.8) | 179 (88.2) | 37 (12.7) | 258 (87.3) |
| Full-time homemaker | 719 (30.8) | 196 (27.3) | 45 (8.6) | 478 (91.4) | 81 (9.8) | 750 (90.2) |
| Other | 329 (14.1) | 92 (28.0) | 34 (14.3) | 203 (85.7) | 59 (14.8) | 340 (85.2) |
| Missing | 318 (13.6) | 108 (34.0) | 36 (17.1) | 174 (82.9) | 0 (0.0) | 0 (0.0) |
| Occupation | ||||||
| Not employed | 828 (35.5) | 232 (28.0) | 55 (9.2) | 541 (90.8) | 101 (10.3) | 884 (89.7) |
| Professional specialty | 329 (14.1) | 62 (18.8) | 23 (8.6) | 244 (91.4) | 41 (9.8) | 377 (90.2) |
| Managerial | 144 (6.2) | 25 (17.4) | 16 (13.4) | 103 (86.6) | 25 (13.4) | 162 (86.6) |
| Administrative support | 208 (8.9) | 55 (26.4) | 28 (18.3) | 125 (81.7) | 52 (18.2) | 232 (81.8) |
| Sales | 93 (4.0) | 27 (29.0) | 8 (12.1) | 58 (87.9) | 15 (12.7) | 102 (87.3) |
| Technical | 239 (10.2) | 61 (25.5) | 22 (12.4) | 156 (87.6) | 47 (13.5) | 297 (86.5) |
| Missing | 494 (21.2) | 152 (30.8) | 56 (16.4) | 286 (83.6) | 0 (0.0) | 0 (0.0) |
Data from the Infant Feeding Practices Study II. Imputed counts are rounded for display purposes.
WIC, Women, Infants, and Children.
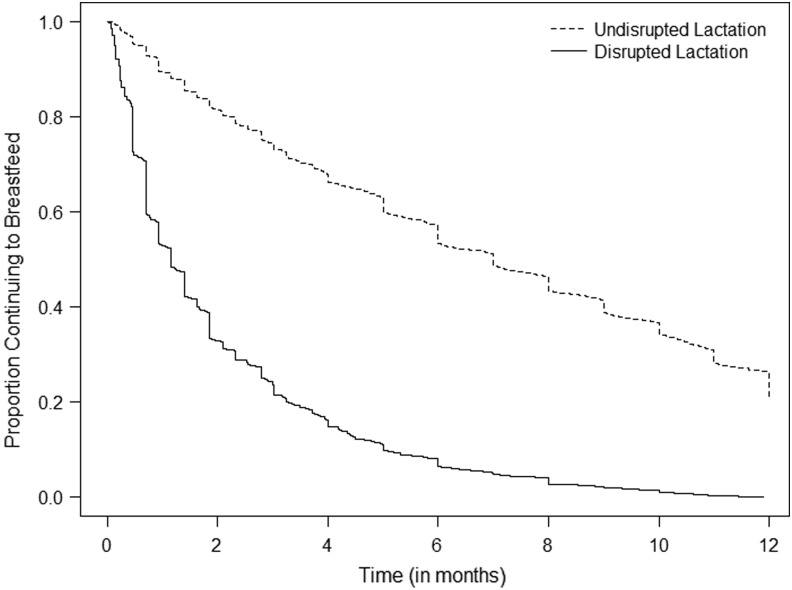
In our complete case analysis, 12.1 women per 100 (208/1,721) met criteria for disrupted lactation. This result was similar to the proportion in our imputed data set (12/100, 95% CI 11, 13). The median duration of breastfeeding among women who met criteria for disrupted lactation was 1.2 months (quartile [Q]1, Q3: 0.5, 2.8 months), compared with 7.0 months (Q1, Q3: 2.8, 12.0) among mothers who did not meet criteria for disrupted lactation (Fig. 1, Table 2). Compared with women who did not meet criteria for disrupted lactation, those who did were more likely to be young, Hispanic, unmarried, and nulliparous; to not have a college degree; to receive postnatal WIC; to live in a household with one to two people; to be employed; and to have a nonprofessional occupation (Table 1).
FIG. 1.
Proportion of women continuing to breastfeed over the child's first year, by disrupted lactation status.
Table 2.
Prevalence of Weaning Categories and of Disrupted Lactation
| na | Prevalenceb(95% CI)a | Intended breastfeeding duration (months), median (Q1, Q3)c | Achieved breastfeeding duration (months), median (Q1, Q3)a | |
|---|---|---|---|---|
| Disrupted lactation | 280 | 12 (11,13) | 7.0 (6.0,12.0) | 1.2 (0.5,2.8) |
| Undisrupted lactation | 2,055 | 88 (87,89) | 10.0 (6.0,12.0) | 7.0 (2.8,12.0) |
| Early, undesired | 1,055 | 45.2 (43.6,46.8) | 9.0 (6.0,12.0) | 2.7 (0.9,5.4) |
| Early, desired | 484 | 20.7 (19.4,22.0) | 9.1 (6.0,12.0) | 5.0 (2.2,8.5) |
| Expected, undesired | 86 | 3.7 (3.1,4.3) | 6.0 (3.1,8.2) | 8.0 (5.6,10.0) |
| Expected, desired | 277 | 11.8 (10.8,12.9) | 6.1 (6.0,11.0) | 10.0 (7.0,12.0) |
| Breastfed ≥12 months | 434 | 18.6 (17.4,29.8) | 12 (12,18) | >12d |
Estimates, based on multiple imputation.
Prevalence estimate per 100 breastfeeding women and 95% CI, using multiple imputation for missing outcome data.
Reported intention.
Unknown values (known to be greater than 12 months); cannot impute, owing to lack of true values.
CI, confidence interval; Q, quartile.
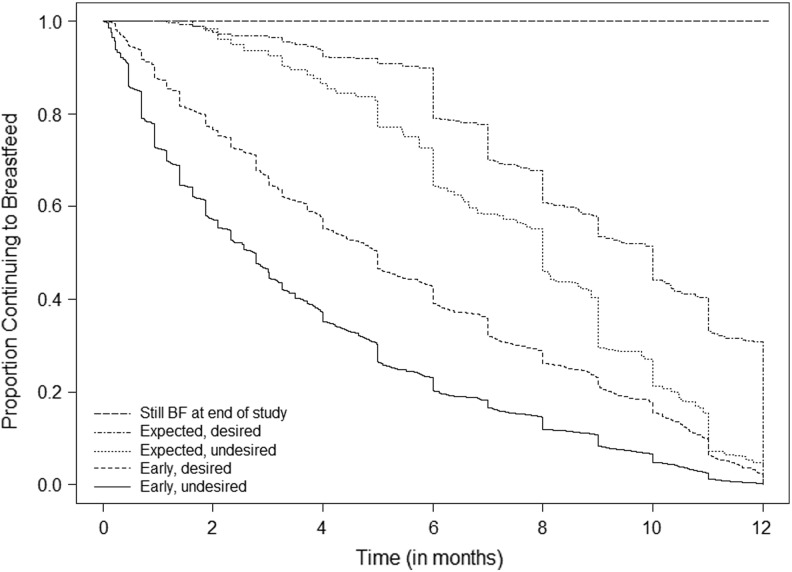
Forty-five per 100 study participants (95% CI 44, 47) reported early, undesired weaning, with a median breastfeeding duration of 2.7 months (Q1, Q3: 0.9, 5.4 months). Median duration of breastfeeding differed for women with expected vs. early and desired vs. undesired weaning (Fig. 2, Table 2, p<0.0001). Women with early weaning intended to breastfeed longer than women with expected weaning. The median intended duration among women who breastfed ≥12 months was 12 months (interquartile range [IQR] 12, 18).
FIG. 2.
Proportion of women continuing to breastfeed over the child's first year of life, by weaning status.
In our sensitivity analysis, we found that among women who had breastfed ≥12 months, 57.6% (250/434) had exceeded their prenatal intention and so met criteria for expected weaning. We found that 19 women had breastfed for ≥12 months and weaned prior to completing the last study questionnaire. Among these women, 84.2% (16/19) reported desired weaning, and 15.8% (3/19) reported undesired weaning. We used these probabilities to estimate the prevalence of desired vs. undesired weaning among women who were still breastfeeding at the last study questionnaire. If we assumed that all women who were breastfeeding at the last questionnaire went on to wean prior to meeting their intended duration, the prevalence of early, undesired weaning in our population would be 46.4%. If all women who were still breastfeeding achieved their intended duration, the prevalence of early, undesired weaning would be 45.2%.
Women with disrupted lactation were more likely than women without disrupted lactation to get help with breastfeeding from a health professional (63.5% vs. 54.9%, p=0.004) but were less likely to report that the assistance they received was helpful (26.1% vs. 55% “Yes, very much”) in response to “The breastfeeding help solved the problem/made it better,” p=< 0.001). Women who met criteria for disrupted lactation were also less likely to report favorable feelings about having breastfed (58.4% vs. 87.9% favorable, p=< 0.001), and fewer women with disrupted lactation reported that they were likely to breastfeed again if they had another child (78.9% vs. 92.2% likely to breastfeed again, p=< 0.001).
We then measured the association between postpartum depressive symptoms, defined as EPDS score ≥13 at 2 months postpartum, and both disrupted lactation and early, undesired weaning (Table 3). Among women with depressive symptoms at 2 months (EPDS ≥13), 19 per 100 women (95% CI 18–21) met criteria for disrupted lactation, compared with 11 per 100 women (95% CI 10–12) with EPDS <13 (adjusted odds ratio [OR] 1.7, 95% CI 1.1–2.7). Moreover, among women with depressive symptoms at 2 months, 56 per 100 women (95% CI 55–58) reported early, undesired weaning, compared with 44 per 100 women (95% CI 42–46) without depressive symptoms. In both unadjusted multinomial logistic regression models and multivariable-adjusted models, depression symptoms at 2 months were associated with an increased odds of early, undesired weaning compared with the referent category, breastfeeding ≥12 months.
Table 3.
Association of Maternal BMI and Depressive Symptoms with Disrupted Lactation and with Early, Undesired Weaning
| Disrupted lactation | Early, undesired weaning | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total n | n | Prevalencea(95% CI) | Unadjusted ORb(95% CI) | MVc-adjusted ORb(95% CI) | n | Prevalencea(95% CI) | Unadjusted ORd(95% CI) | MVc-adjusted ORd (95% CI) | |
| Total | 2,335 | 280 | 12.0 (11.0,13.0) | 1,055 | 45.2 (43.6,46.8) | ||||
| EPDS <13 | 2,114 | 238 | 11.3 (10.3,12.2) | 1.00 (ref.) | 1.00 (ref.) | 931 | 44.0 (42.5,45.6) | 1.00 (ref.) | 1.00 (ref.) |
| EPDS ≥13 | 221 | 43 | 19.3 (18.0,20.5) | 1.88 (1.21,2.91) | 1.73 (1.09,2.70) | 124 | 56.2 (54.6,57.7) | 1.86 (1.21,2.88) | 1.72 (1.10,2.68) |
| Underweight BMI | 60 | 8 | 12.9 (11.9,14.0) | 1.44 (0.42,2.00) | 1.31 (0.38,4.51) | 26 | 43.2 (41.7,44.8) | 1.32 (0.50,3.48) | 1.20 (0.41,3.49) |
| Normal BMI | 831 | 75 | 9.0 (8.1,9.9) | 1.00 (ref.) | 1.00 (ref.) | 326 | 39.2 (37.7,40.1) | 1.00 (ref.) | 1.00 (ref.) |
| Overweight BMI | 742 | 97 | 13.0 (12.0,14.1) | 1.52 (1.06,2.17) | 1.58 (1.09,2.28) | 348 | 46.9 (45.3,48.4) | 1.23 (0.91,1.66) | 1.26 (0.92,1.73) |
| Obese BMI | 702 | 101 | 14.4 (13.3,15.5) | 1.71 (1.15,2.54) | 1.73 (1.16,2.60) | 356 | 50.7 (49.1,52.2) | 1.58 (1.16,2.16) | 1.53 (1.10,2.12) |
Data taken from the Infant Feeding Practices Survey II. Prevalence estimate and 95% CI per 100 women, using multiple imputation for missing data.
OR from logistic regression for disrupted lactation vs. referent group that did not meet criteria for disrupted lactation (n=2,055).
MV-adjusted models include WIC status, marital status, race/ethnicity, maternal age, parity, and education.
OR from multinomial regression for early, undesired weaning vs. referent group with breastfeeding ≥12 months (n=434).
BMI, body mass index; EPDS, Edinburgh Postnatal Depression Scale; MV, multivariable; OR, odds ratio.
We similarly modeled the association between maternal pregravid BMI and both disrupted lactation and undesired, early weaning (Table 3). The prevalence of disrupted lactation was lowest among normal BMI women (9/100 women, 95% CI 8, 10), with higher prevalence among overweight (13/100 women, 95% CI 12, 14) and obese women (14/100, 95% CI 13, 16). These associations persisted with adjustment for sociodemographic confounders (adjusted OR overweight vs. normal weight: 1.6, 95% CI 1.1, 2.3; obese vs. normal weight: 1.7, 95% CI 1.2, 2.6). Undesired, early weaning was also more prevalent among women with a pregravid BMI that was underweight (43/100 women, 95% CI 42, 45), overweight (47/100 women, 95% CI 45, 48), or obese (51/100 women, 95% CI 49, 52), compared with women who were normal weight prior to pregnancy (39/100 women, 95% CI 38, 41). In multinomial logistic regression models adjusting for demographic variables, the odds of early, undesired weaning vs. our referent category of breastfeeding ≥12 months were increased for women with obese vs. normal pregravid BMI (adjusted OR 1.5, 95% CI 1.1, 2.1).
In models mutually adjusting for depression symptoms, BMI, and sociodemographic confounders, both maternal depression symptoms and BMI remained independently associated with disrupted lactation (data not shown).
Discussion
In a longitudinal cohort study of US women, we found that nearly half of mothers reported early, undesired weaning. One in 8 mothers (12/100 women, 95% CI 11, 13) reported early, undesired weaning attributed to difficulties with latch, pain, and milk supply, a constellation of symptoms that we used to define “disrupted lactation.” We found higher prevalence of disrupted lactation among young, unmarried, nonprofessional women without a college degree. Both obesity and maternal depression symptoms were associated with increased odds of disrupted lactation, independent of sociodemographic confounders. These associations suggest that both socioeconomic constraints and psychobiological mechanisms may contribute to a woman's capacity to achieve her breastfeeding intentions.
Our results confirm and extend earlier work regarding the prevalence and risk factors for lactation difficulties. Neifert et al. followed 319 primiparous women who were motivated to breastfeed.31 When women with prior breast surgery were excluded, 13.1% had insufficient milk production. In a secondary analysis of participants in Project Viva,32 67 of 495 mothers (13.5%) reported early introduction of formula or weaning at less than 3 months due to problems with milk production.
We found that maternal obesity was associated with early, undesired weaning, consistent with observational studies reporting lower initiation, delayed lactogenesis, and reduced duration of breastfeeding among women who are overweight or obese.13,14,18 Overweight BMI has also been associated with differences in prolactin response to suckling,17 and insulin resistance is associated with both low milk supply and differences in the milk fat layer transcriptome,19 suggesting that biological as well as sociocultural factors may affect breastfeeding success among overweight women. We also found higher rates of perceived lactation dysfunction among women with postpartum-depression symptoms. Postpartum depression is associated with reduced breastfeeding duration,15,16,33–36 and neuroendocrine mechanisms may underlie this association.12,21 Moreover, reduced maternal sensitivity37 in the setting of depression may also contribute to breastfeeding difficulties.
The Surgeon General's Call to Action asserts that most lactation problems can be solved with access to appropriate care.11 We found that two-thirds of women with disrupted lactation sought help from a health professional, but only one in four reported that the assistance they received solved the problems or made it better. This lack of resolution may be due to true physiologic dysfunction, or it may reflect the paucity of evidence-based recommendations for lactation management and the uneven quality of lactation training for health professionals in the United States.38 Increasing access to high-quality lactation care may improve breastfeeding outcomes.
Strengths of our study include prospective assessment of breastfeeding intention to define early weaning, our large sample size, and our use of multiple imputation to reduce bias due to differential loss to follow-up. However, our findings must be interpreted in the context of the study design. We measured the prevalence of early, undesired weaning in the context of perceived lactation dysfunction. Multiple barriers to breastfeeding, such as poor maternity care practices,10,39 uneven training for health professionals,38,40 lack of access to postpartum support41 and maternity leave,5 and return-to-work requirements,42 can affect whether a woman is able to achieve her breastfeeding goals. The prevalence of disrupted lactation “in a perfect world” is therefore likely to be lower than 12%. On the other hand, the IFPS II sample was largely Caucasian, educated, and well off. Given the direct association between low socioeconomic status and breastfeeding difficulties in our sample and in other studies,43 our analysis may underestimate the prevalence of disrupted lactation in the general population.
Our use of self-report measures is both a strength and a limitation of our study. Use of self-reported data provides a framework for assessing disrupted lactation in epidemiologic studies. Moreover, this approach quantifies the proportion of women who attribute their early, undesired weaning to physiologic dysfunction. However, we were not able validate self-report against clinical assessment. Perception of insufficient milk supply is common,44 and such perception is correlated with low parenting self-efficacy.45 Of note, a recent study found that parenting magazines targeting low-income women were more likely to focus on difficulties with breastfeeding than was a magazine targeting high-income women.46 Thus, self-report of problems with breastfeeding may reflect societal constraints that systematically undermine women's confidence in their ability to breastfeed, and disrupted lactation may reflect both socioeconomic disadvantage and psychobiologic dysfunction. To determine the true prevalence of early, unplanned weaning due to lactation dysfunction would require a prospective, longitudinal study that included clinical assessment of each mother-infant dyad. This analysis is intended to lay the groundwork for such prospective studies.
Our analysis of risk factors for disrupted lactation is also limited by constraints of the IFPS II study. Maternal height and weight were obtained by self-report, and underreporting of pregravid BMI may have led to misclassification, potential biasing our results. Moreover, in the IFPS II, perinatal mood was assessed at 2 months postpartum, when 75% of women with disrupted lactation had already weaned. We therefore cannot determine whether preexisting mood symptoms contributed to breastfeeding problems or whether breastfeeding problems contributed to depression symptoms. However, given that one in five women with depression symptoms met criteria for disrupted lactation, health professionals who care for mothers and infants should be prepared to assess and manage both breastfeeding difficulties and perinatal mood symptoms.
Conclusions
Our results suggest that one in eight women experience early, undesired weaning that they attribute to difficulties with the physiology of breastfeeding. These findings challenge assertions that every mother can breastfeed. As Marianne Neifert has written: “The bold claims made about the infallibility of lactation are not cited about any other physiologic processes. A health care professional would never tell a diabetic woman that ‘every pancreas can make insulin’ or insist to a devastated infertility patient that ‘every woman can get pregnant.’ The fact is that lactation, like all physiologic functions, sometimes fails because of various medical causes” (p. 278).47
Neither the International Classification of Diseases (ICD)-9 nor the ICD-10 provides a diagnosis code for early, undesired weaning attributed to lactation dysfunction. Agalactia, derived from Latin and Greek roots to signify “absence of milk,” is subdivided in ICD-10 into primary complete (O92.3), partial (O92.4), and secondary, elective, or therapeutic agalactia (O92.5), with “Failure of lactation” as a synonym. In this article, we use the term “disrupted lactation” to describe early, undesired weaning attributed to lactation dysfunction. However, this term may not capture the impact of disrupted lactation on a woman's postpartum experience. In our clinical work with breastfeeding mothers, we regularly encounter women who have taken extraordinary measures to breastfeed. Women visit multiple specialists, ingest countless herbal preparations, and endure every-hour pumping regimens, supplemental nursing systems, and topical ointments in an effort to establish a normal breastfeeding relationship. For these mothers, disrupted lactation constitutes a “lactastrophe.” When we have shared that word with scores of struggling mothers we have cared for, they have uniformly endorsed it as a fitting description of their experience. We therefore propose “lactastrophe” as a descriptor for emotional distress in the setting of disrupted lactation.
Our study's design did not allow us to disentangle sociodemographic factors from biological determinants of early, undesired weaning attributed to physiologic problems. However, the prevalence of disrupted lactation in a contemporary setting underscores the need to increase access to high-quality lactation support. In addition, research is needed to determine underlying causes, compare the efficacy and effectiveness of prevention and treatment strategies, and disseminate best practices among those who care for mother-infant dyads. Such efforts may ultimately enable a larger proportion of women to achieve their infant-feeding goals.
Acknowledgments
We thank Noah Green for editorial contributions to this manuscript.
This article was supported by funding from NIH HD073220, 5K12HD050113 (to Alison M. Stuebe), T32ES007018 (to Bethany J. Horton), K01DA019949-01A1 (to Karen Grewen), K23MH085165 (to Samantha Meltzer-Brody), and UL1RR025747 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ip S, Chung M, Raman G, et al. . Breastfeeding and maternal and infant health outcomes in developed countries. Evid Report/Technol Assess (Full Rep) 2007:153:1–186 [PMC free article] [PubMed] [Google Scholar]
- 2.Stuebe AM, Schwarz EB. The risks and benefits of infant feeding practices for women and their children. J Perinatol 2010;30:155–162 [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–e841 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Breastfeeding among U.S. children born 2000–2010, CDC National Immunization Survey; Available at: http://www.cdc.gov/breastfeeding/data/NIS_data/index.htm (accessed March15, 2012 [Google Scholar]
- 5.Ogbuanu C, Glover S, Probst J, Liu J, Hussey J. The effect of maternity leave length and time of return to work on breastfeeding. Pediatrics 2011;127:e1414–e1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott JA, Binns CW, Oddy WH, Graham KI. Predictors of breastfeeding duration: Evidence from a cohort study. Pediatrics 2006;117:e646–e655 [DOI] [PubMed] [Google Scholar]
- 7.Baxter J, Cooklin AR, Smith J. Which mothers wean their babies prematurely from full breastfeeding? An Australian cohort study. Acta Paediatr 2009;98:1274–1277 [DOI] [PubMed] [Google Scholar]
- 8.Fein SB, Mandal B, Roe BE. Success of strategies for combining employment and breastfeeding. Pediatrics 2008;122:S56–S62 [DOI] [PubMed] [Google Scholar]
- 9.Witt AM, Smith S, Mason MJ, Flocke SA. Integrating routine lactation consultant support into a pediatric practice. Breastfeeding Med 2012;7:38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiGirolamo AM, Grummer-Strawn LM, Fein SB. Effect of maternity-care practices on breastfeeding. Pediatrics 2008;122:S43–S49 [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. The Surgeon General's call to action to support breastfeeding. Washington, DC: U.S. Department of Health and Human Services, Office of the Surgeon General, 2011 [Google Scholar]
- 12.Stuebe AM, Grewen K, Pedersen CA, Propper C, Meltzer-Brody S. Failed lactation and perinatal depression: Common problems with shared neuroendocrine mechanisms? J Womens Health (Larchmt) 2012;21:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker JL, Michaelsen KF, Sorensen TI, Rasmussen KM. High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. Am J Clin Nutr 2007;86:404–411 [DOI] [PubMed] [Google Scholar]
- 14.Amir L, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth 2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennis C-L, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: A qualitative systematic review. Pediatrics 2009;123:e736–e751 [DOI] [PubMed] [Google Scholar]
- 16.Paul IM, Downs DS, Schaefer EW, Beiler JS, Weisman CS. Postpartum anxiety and maternal-infant health outcomes. Pediatrics 2013;131:e1218–e1224 [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 2004;113:e465–e471 [DOI] [PubMed] [Google Scholar]
- 18.Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr 2010;92:574–584 [DOI] [PubMed] [Google Scholar]
- 19.Lemay DG, Ballard OA, Hughes MA, Morrow AL, Horseman ND, Nommsen-Rivers LA. RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS ONE 2013;8:e67531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen KM. Effects of under- and overnutrition on lactation in laboratory rats. J Nutr 1998;128 (2 Suppl):390S–393S [DOI] [PubMed] [Google Scholar]
- 21.Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J Womens Health (Larchmt) 2013;22:352–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lonstein JS, Stern JM. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal, and aggressive behaviors in rats. Brain Res 1998;804:21–35 [DOI] [PubMed] [Google Scholar]
- 23.Feldman R, Zagoory-Sharon O, Weisman O, et al. . Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry 2012;72:175–181 [DOI] [PubMed] [Google Scholar]
- 24.Pang WW, Hartmann PE. Initiation of human lactation: Secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia 2007;12:211–221 [DOI] [PubMed] [Google Scholar]
- 25.Flaherman VJ, Hicks KG, Cabana MD, Lee KA. Maternal experience of interactions with providers among mothers with milk supply concern. Clin Pediatr (Phila) 2012;51:778–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: Study methods. Pediatrics. 2008;122:S28–S35 [DOI] [PubMed] [Google Scholar]
- 27.Ahluwalia IB, Morrow B, Hsia J. Why do women stop breastfeeding? Findings from the Pregnancy Risk Assessment and Monitoring System. Pediatrics 2005;116:1408–1412 [DOI] [PubMed] [Google Scholar]
- 28.Cox JL, Chapman G, Murray D, Jones P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J Affect Disord 1996;39:185–189 [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley, 1987 [Google Scholar]
- 30.Watkins S, Meltzer-Brody S, Zolnoun D, Stuebe A. Early breastfeeding experiences and postpartum depression. Obstet Gynecol 2011;118:214–221 [DOI] [PubMed] [Google Scholar]
- 31.Neifert M, DeMarzo S, Seacat J, Young D, Leff M, Orleans M. The influence of breast surgery, breast appearance, and pregnancy-induced breast changes on lactation sufficiency as measured by infant weight gain. Birth 1990;17:31–38 [DOI] [PubMed] [Google Scholar]
- 32.Stuebe AM, Mantzoros C, Kleinman K, et al. . Duration of lactation and maternal adipokines at 3 years postpartum. Diabetes 2011;60:1277–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taveras EM, Capra AM, Braveman PA, Jensvold NG, Escobar GJ, Lieu TA. Clinician support and psychosocial risk factors associated with breastfeeding discontinuation. Pediatrics 2003;112:108–115 [DOI] [PubMed] [Google Scholar]
- 34.Chaudron LH, Klein MH, Remington P, Palta M, Allen C, Essex MJ. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol 2001;22:103–112 [DOI] [PubMed] [Google Scholar]
- 35.Dennis CL. The breastfeeding self-efficacy scale: Psychometric assessment of the short form. J Obstet Gynecol Neonatal Nurs 2003;32:734–744 [DOI] [PubMed] [Google Scholar]
- 36.Hahn-Holbrook J, Haselton MG, Dunkel Schetter C, Glynn LM. Does breastfeeding offer protection against maternal depressive symptomatology? A prospective study from pregnancy to 2 years after birth. Arch Womens Ment Health 2013;16:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry 2009;48:919–927 [DOI] [PubMed] [Google Scholar]
- 38.Feldman-Winter LB, Schanler RJ, O'Connor KG, Lawrence RA. Pediatricians and the promotion and support of breastfeeding. Arch Pediatr Adolesc 2008;162:1142–1149 [DOI] [PubMed] [Google Scholar]
- 39.Perrine C, Shealy KR, Scanlon KS, Grummer-Strawn LM, Galuska D, Cohen JH. Vital Signs: Hospital Practices to Support Breastfeeding—United States, 2007 and 2009. MMWR 2011;60:1020–1025 [PubMed] [Google Scholar]
- 40.Feldman-Winter L, Barone L, Milcarek B, et al. . Residency curriculum improves breastfeeding care. Pediatrics 2010;126:289–297 [DOI] [PubMed] [Google Scholar]
- 41.Renfrew MJ, McCormick FM, Wade A, Quinn B, Dowswell T. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev 2012;5:CD001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haider SJ, Jacknowitz A, Schoeni RF. Welfare work requirements and child well-being: Evidence from the effects on breast-feeding. Demography 2003;40:479–497 [DOI] [PubMed] [Google Scholar]
- 43.Jones JR, Kogan MD, Singh GK, Dee DL, Grummer-Strawn LM. Factors associated with exclusive breastfeeding in the United States. Pediatrics 2011;128:1117–1125 [DOI] [PubMed] [Google Scholar]
- 44.McCann MF, Baydar N, Williams RL. Breastfeeding attitudes and reported problems in a national sample of WIC participants. J Hum Lact 2007;23:314–324 [DOI] [PubMed] [Google Scholar]
- 45.McCarter-Spaulding DE, Kearney MH. Parenting self-efficacy and perception of insufficient breast milk. J Obstet Gynecol Neonatal Nurs 2001;30:515–522 [DOI] [PubMed] [Google Scholar]
- 46.Duckett ND. Rethinking the importance of social class: How mass market magazines portray infant feeding. In: Smith PH, Hausman BL, Labbok MH, eds. Beyond health, beyond choice: Breastfeeding constraints and realities. New Brunswick, NJ: Rutgers University Press; 2012:236–245 [Google Scholar]
- 47.Neifert MR. Prevention of breastfeeding tragedies. Pediatr Clin North Am 2001;48:273–297 [DOI] [PubMed] [Google Scholar]