Abstract
Background
Marjolin's ulcer is a rare, aggressive condition that arises on chronic skin lesions and diseases. Inthis article, we will report 83 cases of this disease.
Methods
Retrospectively, we retrieved 83 records of patients with cancer arising from chronic skin conditions.Data concerning demography, type of original skin insult, time interval between original lesion and cancer,cancer histology, and lymph node involvement were recorded.
Results
The mean age was 55.30 years (range: 21-90). There were 51 males (61.5%) and 32 females (38.5%).Foot was the most prevalent site of primary skin lesion (49.4%) followed by scalp (15.6%). Original skin insultswere burn (87.9%), osteomyelitis (2.4%), radiation (2.4%), electrical burn (1.2%), surgical scar (2.4%),pemphigus (1.2%), bite (1.2%), and bed sore (1.2%). Histologic diagnosis were well differentiated SCC(38.6%), SCC, differentiation not reported (24.1%), moderately differentiated SCC (13.2%), BCC (9.6%), poorlydifferentiated SCC (6.0%), melanoma (2.4%), verrucous carcinoma (2.4%), MFH (1.2%), mucoepidermoidcarcinoma (1.2%), and leiomyosarcoma (1.2%). Most of the cases occurred more than 20 years after the initialskin insult. There were 6 (7.2%) cases that developed within 1 year (acute Marjolin's Ulcer). Forty three patients(69.3%) had palpable regional lymph nodes.
Conclusion
Data in this series were in confirmation with many other reports. Marjoln's ulcer should be consideredas a significant post-skin injury complication.
Keywords: Marjolin's ulcer, Burn, Chronic skin disease
Introduction
In the 1st century AD Aurelius Cornelius Celsus described cancer in chronic ulcers for the first time (1). In 1828, Jean-Nicolas Marjolin from Paris University wrote a section on ulcers that appeared in Dictionnaire de Medicine, where he described four examples of a type of ulcer under the designation of "ulcère verruqueux". The description, about half a page, did not say that the ulcers were malignant, nor did it associate them with scars or pre-existing chronic ulcers (2). In 1903 John Chalmers DaCosta, professor of surgery at Jefferson Medical College, exemplified two cases of carcinomatous change in chronic varicose ulcers of the leg. He wrote: 'The characterization of this condition as Marjolin's ulcer I think to be proper, because it was first carefully studied and accurately described by professor Marjolin, of Paris, over fifty years ago.' (3). In 1907, Fordyce also used the meaning of Marjolin's ulcer and later, DaCosta extended the definition to include malignancy arising in sinuses as well as malignancy in scars and chronic ulcers (4).
It was Dupuytren in 1839 who first observed that de novo malignancy could arise in chronic wounds by observing this phenomenon in a Belgian man who was treated for a cancer which arose from a burn scar sustained from sulphuric acid (5,6). In 1930, Treves and Pack reported the first, now landmark and classic, review of cancer in burn scars (5).
Today, the term Marjolin’s ulcer is used to describe malignant degeneration in burn scars, chronic venous insufficiency ulcers (7), pressure ulcers (8), vaccination sites, urinary fistulas (6), frostbite (9), snakebites (10), osteomyelitis (11), pilonidal abscesses (12), hydradenitis suppurativa (13), herpes zoster (14), skin graft donor sites (15), dog bites, knife wounds, and gunshot wounds (16).
Although a rare condition, Marjolin's ulcer is an aggressive disease that tends to spread widely in its course. In this article, we present 83 cases of Marjolin's ulcer. This retrospective report is not only one of the large single series of Marjolin's ulcer appeared so far in English literature, but also features some rare occurrences that have been impetus for some authors to publish separate manuscripts as case reports. We have focused our attention on description of patients' demography, type of original skin insult, time interval between original lesion and cancer, cancer histology, and lymph node involvement. We have also performed a literature review on the subject. Issues regarding treatment and survival were not included in this report, as they could make the report too voluminous and therefore, could be best treated in another article.
Methods
Retrospectively, records of the patients who underwent surgery with a clinical diagnosis of Marjolin's ulcer between 1995 and 2012 in Imam khomeini Hospital and Cancer Institute (affiliated to Tehran University of Medical Sciences) were retrieved using the surgical registry. The study protocol was approved by hospital ethics committees. Patients with the pathologic diagnosis of malignancy (Marjolin’s ulcer) were entered into the study and the rest including patients with benign skin lesion or records with other diagnosis except for Majolin’s ulcer were excluded. Based on the contents of the records, each patient's age, gender, original cause of skin lesion and its time of onset, location of the primary skin lesion, presence of palpable regional lymph node(s), and tumor pathology and its grade (differentiation) were recorded. Tumor differentiation was obtained as stated in the pathology report sheets and classified based on a three-grade scale (well differentiated, moderately differentiated, and poorly differentiated). Not all pathology reports contained notes on tumor differentiation.
Statistical Analysis: All data were recorded using a standard data form and then analyzed by SPSS 17. Quantitative values were compared using t-test for independent none, and for categorical data, Chi-squared and Fisher's exact tests were also applied.
Results
Of 105 recorded patients, 83 had a pathologic diagnosis of malignancy. The mean age of the patients was 55.30 (SD=16.83) years, the youngest 21 and the oldest 90 years old. There were 51 males (61.5%) and 32 females (38.5%). The mean age of males was 50.88 (SD=17.38) years and that of females was 57.18 (SD=15.38) years old (p = 0.058). Table 1 shows the frequency of the sites of the primary (and of course, the site of Marjolin's lesion). Foot was the most prevalent site of primary skin lesion (49.4%) followed by scalp (15.6%).
Table 1. Frequency of primary site of skin lesions.
| Site | Frequency NO. (%) |
| Foot | |
| Right | 15(18.1%) |
| Left | 26(31.3%) |
| Scalp | 13(15.6%) |
| Hand | |
| Right | 5(6.0%) |
| Left | 4(4.8%) |
| Thigh | 4(4.8%) |
| Heel | (4.8%)4 |
| Face | 3(3.6%) |
| Leg | 3(3.6%) |
| Neck | 2(2.4%) |
| Knee | 2(2.4%) |
| Buttock | (1.2%)1 |
| Forearm | 1(1.2%) |
| Total | 83 |
In Table 2 , the conditions leading to the skin lesion upon which Marjolin's ulcer developed are summarized. Burns following contact with hot objects or flame were the most common cause (Fig. 1). Of note, there was Marjolin's ulcer on surgical scar, bite scar, and bed sore.
Table 2. Frequency of different causes of underlying skin lesions.
| Cause | Frequency NO. (%) |
| Burn | 73(87.9%) |
| Osteomyelitis | 2(2.4%) |
| Radiation | 2(2.4%) |
| Electrical | 1(1.2%) |
| Pemphigus | 1(1.2%) |
| Surgical Scar | 2(2.4%) |
| Sting & Bite | 1(1.2%) |
| Bed sore | 1(1.2%) |
Fig.1 .
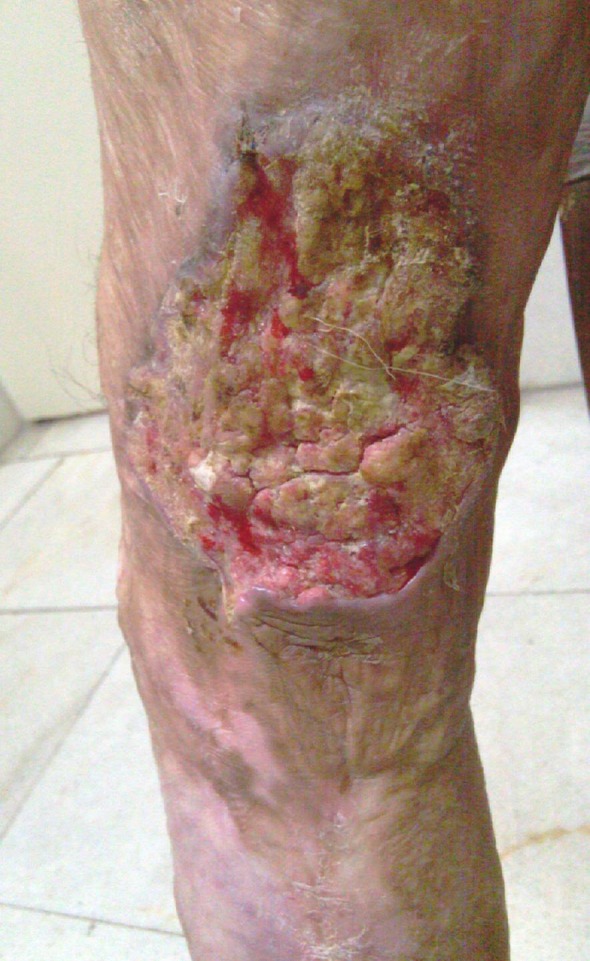
Marjolin's ulcer on a lower limb burn scar
Table 3 depicts prevalence of different time intervals from the time the primary skin lesion was made and the time when the patient sought medical treatment for the newly developed lesion. These data could be obtained in 71 records. Some patients could not remember the exact date of the primary lesion when the lesion developed in a remote time, and mentioned 'infancy' or 'childhood'. We could not make these periods any more precise. The most prevalent time interval was more than 20 years and childhood. Of 83 patients, lymph node status was recorded in 62 patients. Forty three patients (69.3%) had palpable regional lymph nodes.
Table 3. Prevalence of different time intervals between primary lesion and Marjolin's ulcer.
| Time interval |
Frequency NO. (%) |
| Infancy | 4(4.8%) |
| Childhood | 19(22.9%) |
| More than 20 years ago | 21(25.3%) |
| Between 20 and 10 years ago | 10(12.0%) |
| Between 10 and 5 years ago | 5(6.0%) |
| Between 5 and 1 years ago | 6(7.2%) |
| Less than 1 year ago | 6(7.2%) |
Table 4 summarizes histopathologic diagnosis of the lesions. The SCC was the most common diagnosis, and well-differentiated most of the time. Notably, there were cases of melanoma and sarcoma. Our youngest patient was a 21-year-old man who sustained a burn on his right sole when he was 2 years old. Since 8 years prior to surgery, he developed a non-healing wound in the burned scar. Post-surgical pathology report revealed verrucous SCC. Our oldest patient was a 90-year-old man with a burn on his right hand one year prior to surgery. He had positive axillary lymph nodes, and the pathology report indicated a well differentiated SCC.
Table 4. Frequency of histopathologic diagnoses.
| Pathologic diagnosis | Frequency NO. (%) |
| Well differentiated SCC | 32 (38.6%) |
| SCC, differentiation not reported | 20(24.1%) |
| Moderately Differentiated SCC | 11(13.2%) |
| BCC | 8(9.6%) |
| Poorly Differentiatd SCC | 5(6.0%) |
| Melanoma | 2(2.4%) |
| Verrucous carcinoma | 2(2.4%) |
| MFH | 1(1.2%) |
| Mucoepidermoid carcinoma | 1(1.2%) |
| Leiomyosarcoma | 1(1.2%) |
Interval between the time of original injury and the subsequent malignancy was less than 1 year in 6 patients and between 1 and 5 years in another 6 patients. Table 5 shows data specifically for these none. In Table 6 , data concerning unusual forms of Marjolin's ulcer, as well as poorly differentiated SCC are summarized.
Table 5. Characteristics of patients with "acute onset" Marjolin Ulcer.
| Group | Age | Gender | Causes of underlying skin lesions | Pathology |
| Less than 1 year |
71.5 (90) |
Male: 5 (83.3%) Female: 1(16.7%) |
Burn: 5 (83.3%) Pemphigus: 1 (16.7%) |
Well diff SCC: 2 (33.3%) Poor diff SCC: 1 (16.7%) BCC: 1 (16.7%) SCC, diff not reported: 1 (16.7%) Leiomyosarcoma: 1 (16.7%) |
| Between 1 and 5 year. |
62 (45-71) |
Male:4 (66.7%) Female: 2(33.3%) |
Burn: 5 (83.3%) Sting: 1 (16.7%) |
Well diff SCC: 1 (16.7%) Mod diff SCC: 1 (16.7%) BCC: 1 (16.7%) Scc, diff not reported: 1 (16.7%) |
Table 6. Data on less common Marjolin's ulcer histologies.
| Group (number of cases) | Mean Age | Gender | Causes of underlying skin lesions |
| BCC (8) | 62.75 |
Male: 2 (25%) Female: 6 (75%) |
Burn: 6 (75%) Radiation: 2 (25%) |
| Melanoma (2) | 65.5 |
Male: 1 (50%) Female: 1 (50%) |
Burn: 2 |
| MFH (1) | 81 | Female:1 | Burn: 1 |
| Leimyosarcoma (1) | 71 | Male: 1 | Burn: 1 |
| Mucoepidermoid carcinoma (1) | 28 | Male:1 | Burn: 1 |
| Poorly diff SCC (5) | 59.6 | Male: 5 |
Burn: 4 (80%) Osteomyelitis: 1 (20%) |
Discussion
In a classic report by Treves and Pack, more than 2,000 patients with skin cancer were evaluated; and it was revealed that 2% of all squamous cell carcinomas and 0.3% of basal cell carcinomas resulted from burn scar conversion. The SCC was the most common histology followed by the BCC (5,17). Similar to findings in our report, in a series by Esther et al (18), the male to female ratio was 3:1, with the average age of patients being over 50 years. Reports by Gül and Kiliç (19) and Lefebvre et al (20) also indicated similar ratios.
Malignant changes in burn scars can be observed at any age. In literature, the average age at diagnosis ranged between 53 and 56 years. The SCC originating in burn scars manifests 20–40 years after the original burn (17,21). Lefebvre et al found the mean age as 65.14 in their study (20). In another study, the mean age was found as 53.5 for acute burn carcinoma and 56 years for chronic burn carcinoma (22). Perhaps the most comprehensive review of burn scar neoplasms so farwas conducted by Kowal-Vern and Criswekll (23), who reviewed 412 cases from 146 articles between 1923 and 2004. In their article, the mean ages at tumor diagnosis was 50 and 20 years for the mean age at the time of burn injury, and the latency period was 31 years, with a 2:1 male:female distribution. While the SCC and sarcoma were equally distributed, but BCC and other neoplasms were more frequent in males, and melanoma was more frequent in females.
Fleming et al (24) state that over 90% of Marjolin’s ulcers degenerate into malignancies of epidermoid origin, such as SCCs, basal cell carcinomas and malignant melanomas. Most burn scar carcinomas are SCC, whereas most arising in areas of radiation dermatitis are basal cell carcinoma (1). The most common histological type of malignancy originated from chronic wounds is the squamous cell carcinoma, and the second-most common one is the basal cell carcinoma. Other types of malignancies such as malignant melanoma, liposarcoma, osteosarcoma, adenocarcinoma and fibrosarcoma can also be seen, though very rare (25,26). In Kowal-Vern and Criswell report (23), 71% (293 cases) of the tumors were SCC, 12% (48 cases) BCC, 6% (23 cases) melanoma, 2% (6 cases) SCC–BCC, 1% (5 cases) SCC–melanoma, 5% (21 cases) sarcoma, and 4% (16 cases) other neoplasms. Of the 21 sarcomas, malignant fibrous histiocytoma (MFH) (8 cases), fibrosarcoma (3 cases), liposarcoma (2 cases), dermatofibrosarcoma protuberans (2 cases) were the most common types.
Cultures that encouraged chronic exposure to thermal skin injuries had historically been associated with skin neoplasms: the Kangri burn cancer in India, the Kairo of the Japanese and the Kang cancer of north-west China, the British reports of erythema ab igne, and the Algerian charcoal heaters (5,27-30). These tumors are characteristically slow growing initially, but very aggressive following local surgical therapy. Experiment performed by Arons et al on rats (31) suggested a pattern of malignant change in trauma starting with acanthosis, going through stages of basal cell hyperplasia and pseudo-epitheliomatous hyperplasia with atypical basal cell changes, and finally ending with epidermoid carcinoma. The healed burn injury, especially if the skin healed by secondary intention is at risk for continued injury during the course of daily activities because it no longer has the accoutrements of skin such as a normal dermis, nerves, vessels, and adnexa, but is rather a less elastic covering, more easily injured, ripped, and ulcerated compared to normal skin (23).
In order to explain rarity of neoplasm other than SCC and BCC in burn scar, Fleming and Rezek suggested, perhaps those connective tissue tumors occur much less frequently than epidermal malignancies because the deeper tissue is subjected to less trauma and undergoes less tissue regeneration than the superficial, more vulnerable, epidermis (24).
In Treves and Pack report (5), tissue toxins released by the burn eschar through autolysis and poor vascularization of scar tissue that predisposes it to ulceration, acting as a protective barrier against metastases were proposed to explain emergence of cancer in burn scar. Since then, various other factors have been implicated: immunological factors, cocarcinogens (32), and miscellaneous factors such as irritation, poor lymphatic regeneration, antibodies, DNA mutations and local toxins (33). Immunologic theory merits further elaboration. Bostwick et al (34) suggested that cancers that develop within chronic scars may do so within an immunologically privileged site. Dense scars surrounding these lesions may obliterate afferent lymphatics, preventing antigens specific for the tumor from being recognized by usual immune-surveillance mechanisms.
Although the precise pathogenesis of burn scar carcinoma remains unknown, it is mostly believed that malignant degeneration is caused by repeated ulcers and healings. Poor lymphatic regeneration, undernourished state of the burn scar, thickness, and degree of contraction in scars are the other factors that predispose burns to develop carcinoma (17,21,22,35)
Recent advances at the molecular level have shown that burn scar with the SCC condition have mutation in Fas gene which controls apoptosis hence it, may contribute to neoplastic proliferations in burn scars (36,37). A ‘double insult’ or two hit concept has also been put forward. It suggests that the burn or injury, while not in itself carcinogenic, may cause the wound tissue to be more susceptible to other carcinogens such as ultraviolet light or radiation (38).
Encompassing all burn scars, prevailing theories today depict the burn as an immunologically privileged site, with the scar hindering natural immune-surveillance. Crawley emphasized that patients with a lymphocytic infiltrate around the tumor were more likely to survive since they had mounted an immunologic attack on the tumor and limited the spread (39). Heredity may play a role as evidenced by recent findings that HLA DR4 could be associated with the development of cancer (40). Hayashi et al (41) and Harland et al (42) independently reported on the abnormalities in the p53 gene in patients with burn scar carcinoma. In Kowal-Vern and Criswell review (23), the major risk factors for the development of post-burn neoplasms have been healing by secondary intention, non-healing wounds, and fragile scars that ulcerated and were easily traumatized.
The pattern of initial skin insult may not be uniform among different nations. For example, in a report by Asuquo et al from Nigeria (43), chronic traumatic ulcer was the leading problem, found in 83.3% of patients, followed by burns. The latency period (18.5 years) was somewhat less than the previously reports (range of 20–50 years); and the histologic diagnosis in all the cases were squamous cell carcinoma. In another report (44) from Nigeria, Marjolin’s ulcers represent up to 30% of primary skin cancers, and the predisposing lesion has not been burns. The histologic condition was squamous cell carcinoma in all of the cases.
Marjolin’s ulcer tends to be more aggressive than other types of skin cancer, and has a higher regional metastasis and fatality rate. Although most SCCs have a metastasis rate of 0.5–3.0%, those originating from a burn scar have a metastasis rate averaging approximately 30% (45,46). The most significant prognostic factor predicting recurrence is tumor graded histologically, with grade II (moderately differentiated SCC) having a propensity for rapid spread to lymph nodes. Grade I was less likely to spread and was slower if it did spread (47).
In Kewal-Vern and Criswell report (23), regional lymph node involvement was seen in 22% of the cases. This was much lower than rate of lymph node involvement in our study.
In a report by Copcu et al (48) 31 cases of Marjolin's ulcer were reviewed, with the majority of ulcers located on the extremities (18 of 31; 58%). Distant metastases were not detected. However, enlarged lymph nodes were found in six (19%) patients, three of whom had a tumor on the leg. The other three patients had a tumor on the hand and axillary lymph node enlargement.
Anatomic location seemed to play a role in the metastatic potential of the tumor. Those lesions of the lower extremities are at a higher risk than other locations. Metastatic rates have been reported to be 30% in some studies (49).It is important to note that Marjolin’s ulcers have been shown to behave differently, depending on their location. Novick et al (1), in a review of 46 patients from M.D. Anderson Hospital, reported a metastatic rate from lower extremity lesions that was twice as high as rates in any other part of the body.
The SCC is frequently seen at the head/neck localization, whereas those arising on burn scars is mostly localized on lower extremities (20). Most lesions of Marjolin’s ulcer occur on the extremities (60%), especially flexion creases, where blood supply is decreased and trauma is increased, with the head and face occurring less frequently (30%) and those on the trunk the least frequent at 10% (5,32). This pattern of distribution was also observed among our patients. In Kewal-Vern and Criswell report (23), of 396 cases, the lower extremities were most frequently affected 132 (33%), followed by the head (face, scalp, neck areas) 118 (30%), upper extremities 73 (19%). Of interest, BCC was not seen on the lower extremities.
In a recent study by Gül et al (19), 20 of 36 SCCs developing on burn scars were localized on lower extremities (55.55%), with the head/neck occurring less frequently (22.22%) and those upper extremities (11.11%) and trunk (11.11%) the least frequent. Also, carcinomas on lower extremities were more likely to metastasize. (Figure 2)
Fig. 2 .
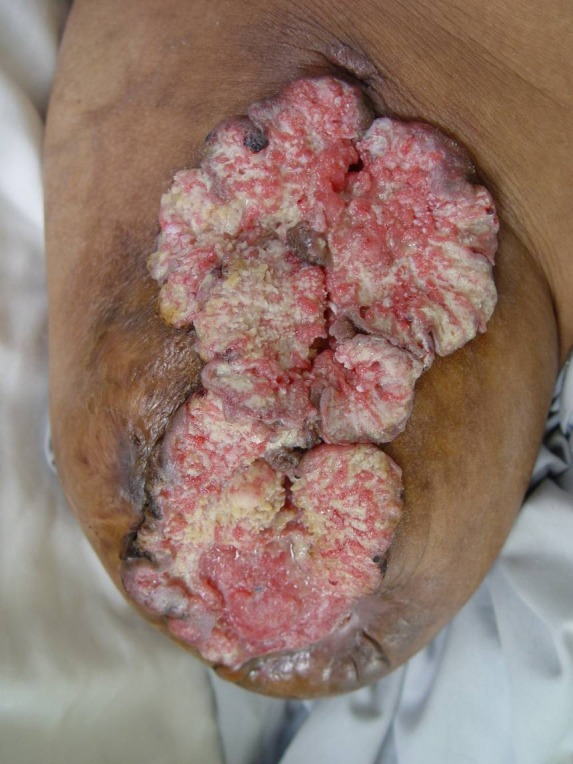
Marjolin's ulcer located on the stump of a knee amputation
Development of malignancy tends to be slow. Multiple authors have reported case series with an average lag period between injury and onset of cancer between 19 and 36 years. Average age at the time of tumor onset is the fifth or sixth decade of life (1,10,18,21,38,48,50,51).
Treves and Pack (5) reported that malignant degeneration within a burn scar can very rarely be acute, occurring within 12 months, or more commonly chronic, occurring after 12 months. When acute, it is more often a basal cell carcinoma and associated with more superficial burn scars. When acute malignant degeneration to squamous cell carcinoma does occur, it more frequently presents in the older age group, over 50, with keratotic skin. Cases with acute onset have been reported to emerge within weeks (15,52).
A case of well-differentiated SCC has been reported to appear in a 14-year-old boy 6 weeks postburn (52). Regarding the age of the patient and of the burn scar, in 1952, Lawrence (53) hypothesized that a patient’s age at the time of the burn is inversely proportional to the interval to formation of cancer. In Kewal-Vern and Criswell report (23), that while the majority of the patients were burned in childhood, those who developed BCC had their burn injury when they were significantly older (43-21 years) compared to patients with the other tumor types (20-19 years). The neoplastic latency period was significantly shorter for patients with BCC (19-22 years) compared to those with SCC (32-18 years) and sarcoma (35-18 years) none. In their review, only 19 (4.6%) of the tumors had a latency period of less than 1 year. Younger patients (40-19 years old) were more likely to have a latency period of less than 1 year compared to patients (50-18 years old) with a latency period greater than 1 year.
Fibrosarcoma is the most common type of sarcoma in the scar, perhaps because precursor cells of fibrosarcoma (fibroblasts) exist in abundance in the skin scar tissues. In addition, it is suggested that the histogenesis of both fibrosarcoma and malignant fibrous histiocytoma which are the most common two burn scar sarcoma types, is the same; they originate from undifferentiated mesenchymal cells (54-56). In burn scar sarcomas, the mean time between the burn injury and diagnosis of the tumor was 35.7 years with a range 3-71 years, comparable with the average latent period of burn scar carcinomas (53,57,58). Unlike burn scar carcinomas, acute onset of a malignancy was not reported in burn scar sarcomas. The earliest onset of post burn sarcoma was 3 years (33).
Malignant fibrous histiocytoma (MFH) is the most common soft tissue sarcoma (59). Although there is controversy regarding the exact number of reported MFH arising in burn scar, this entity is definitely a rare occurrence. Yücel et al (60) in 2000 reported two cases of MFH developing in burn scar, indicating that there were only 3 cases reported before hand.
In general, cutaneous leiomyosarcoma is a rare disease. The origin of primary cutaneous leiomyosarcoma is from the erector muscles of hair and muscles of sweat glands (61).
To the best of our knowledge, only 1 case of leiomyosarcoma has been reported in the literature. The patient was a 22-year-old male complaining of non-healing scalp ulcer in the pre-existing burn scar. This scar was caused by a burn injury when he was 2 years old (57).
Conclusion
Marjolin's ulcer, once related only to burn scar and known to be of limited histologic variety, is now better identified and its association with virtually any chronic, and even acute or subacute, skin condition and astonishing histologic diversity better understood. We presented a relatively large series of patients with Marjolin' ulcer with significant range of both underlying skin conditions and histology, as well as latent periods. For the sake of brevity, we did not include discussion on some rare features like surgical and bite site as the underlying skin derangement, as well as some histologies like verrucous carcinoma. This study also suffers some limitations. Due to its retrospective nature, some data could not be accurately recorded (latent periods), and some data were not available (metastasis). Vigilance on the part of caring physicians in combination with close surveillance and patient education may help identify emergence of Marjolin's Ulcer as early as possible to provide the best possible treatment for this aggressive, violent disease.
References
- 1.Novick M, Gard DA, Hardy SB. Burn scar carcinoma: a review and analysis of 46 cases. J Trauma. 1977;17:809–17. [PubMed] [Google Scholar]
- 2.Cruickshank AH, Gaskell E. Jean-Nicolas Marjolin: Destined To Be Forgotten? Med Hist. 1963;7:383–4. doi: 10.1017/s0025727300028854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Da Costa JC. Carcinomatous Changes in an Area of Chronic Ulceration, or Marjolin's Ulcer. Ann Surg. 1903;37(4):496–502. [PMC free article] [PubMed] [Google Scholar]
- 4.Fordyce JA. Malignant diseases in scars and ulcers- Marjolin's ulcer In: Keen WW, ed Surgery, its Principles and Practice. Philadelphia, USA: W B Saunders. 1911;2:631–2. [Google Scholar]
- 5.Treves N, Pack GT. The development of cancer in burns scars. Surg Gynecol Obstet. 1930;51:749–82. [Google Scholar]
- 6.Steffen C. Marjolin’s ulcer: Report of two cases and evidence that Marjolin did not describe cancer arising in scars of burns. Am J Dermatopathol. 1984;6(2):187–93. [PubMed] [Google Scholar]
- 7.Olewiler SD. Marjolin's ulcer due to venous stasis. Cutis. 1995;56(3):168–70. [PubMed] [Google Scholar]
- 8.Grotting JC, Bunkis J, Vasconez LO. Pressure sore carcinoma. Ann Plast Surg. 1987;18(6):527–32. doi: 10.1097/00000637-198706000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Ryan RF, Litwin MS, Krementz ET. A new concept in the management of Marjolin's ulcers. Ann Surg. 1981;193(5):598–605. doi: 10.1097/00000658-198105000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith J, Mello LF, Nogueira Neto NC, Meohas W, Pinto LW, Campos VA. et al. Malignancy in chronic ulcers and scars of the leg (Marjolin's ulcer): a study of 21 patients. Skeletal Radiol. 2001;30(6):331–7. doi: 10.1007/s002560100355. [DOI] [PubMed] [Google Scholar]
- 11.Bowers RF, Young JM. Carcinoma arising in scars, osteomyelitis, and fistulae. Arch Surg. 1960;80:564–70. doi: 10.1001/archsurg.1960.01290210032006. [DOI] [PubMed] [Google Scholar]
- 12.Lerner HJ, Dietrick G. Squamous cell carcinoma of the pilonidal sinus: Report of a case and review of the literature. J Surg Oncol. 1979;11(2):177–83. doi: 10.1002/jso.2930110212. [DOI] [PubMed] [Google Scholar]
- 13.Lin MT, Breiner M, Fredricks S. Marjolin’s ulcer occurring in hidradenitis suppurativa. Plast Reconstr Surg. 1999;103(5):1541–3. doi: 10.1097/00006534-199904050-00046. [DOI] [PubMed] [Google Scholar]
- 14.Mirshra D, Raji MA. Squamous cell carcinoma occurring at site of prior herpes zoster of the scalp: Case report of Marjolin ulcer. J Am Geriatr Soc. 2004;52(7):1221–2. doi: 10.1111/j.1532-5415.2004.52327_7.x. [DOI] [PubMed] [Google Scholar]
- 15.Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising in a skin graft donor site. J Trauma. 1987;27(6):681–3. doi: 10.1097/00005373-198706000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Barr LH, Menard JW. Marjolin’s ulcer: The LSU experience. Cancer. 1983;52(1):173–5. doi: 10.1002/1097-0142(19830701)52:1<173::aid-cncr2820520131>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Phillips TJ, Salman SM, Bhawan J, Rogers GS. Burn scar carcinomaDiagnosis and management. Dermatol Surg. 1998;24(5):561–5. [PubMed] [Google Scholar]
- 18.Esther R, Lamps L, Schwartz H. Marjolin ulcers: secondary carcinomas in chronic wounds. J South Orthop Assoc. 1999;8:181–7. [PubMed] [Google Scholar]
- 19.Gül U, Kiliç A. Squamous Cell Carcinoma Developing on Burn Scar. Ann Plast Surg. 2006;56(4):406–8. doi: 10.1097/01.sap.0000200734.74303.d5. [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre PH, Rouge D, Chavoin Costagliola M. Scar carcinomas: report of fourteen cases. Ann Chir Plast Esthet. 1991;36:330–335. [PubMed] [Google Scholar]
- 21.Türegün M, Nişanci M, Güler M. Burn scar carcinoma with longer lag period arising in previously grafted area. Burns. 1997;23:496–497. doi: 10.1016/s0305-4179(97)00041-7. [DOI] [PubMed] [Google Scholar]
- 22.Spring PM, Myers JN, El-Naggar AK. et al. Malignant melanoma arising within a burn scar. Ann Otol Rhinol Laryngeol. 2001;110:369–376. doi: 10.1177/000348940111000414. [DOI] [PubMed] [Google Scholar]
- 23.Kowal-Vern A, Criswell B.k. Burn scar neoplasms: A literature review and statistical analysis. Burns. 2005;31(4):403–13. doi: 10.1016/j.burns.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Fleming MD, Hunt JL, Purdue GF, Sandstad J. Marjolin’s ulcer: a review and reevaluation of a difficult problem. J Burn Care Rehabil. 1990;11:460–9. [PubMed] [Google Scholar]
- 25.Alconchel MD, Olivares C, Alvarez R. Squamous cell carcinoma, malignant melanoma and malignant fibrous histiocytoma arising in burn scars. Br J Dermatol. 1997;137:793–8. [PubMed] [Google Scholar]
- 26.Muhlemann MF, Griths RW, Briggs JC. Malignant melanoma and squamous cell carcinoma in a burn scar. Br J Plast Surg. 1982;35:474–7. doi: 10.1016/0007-1226(82)90048-0. [DOI] [PubMed] [Google Scholar]
- 27.Neve EF. Kangri-burn cancer. Br Med J. 1923;2:1255–6. doi: 10.1136/bmj.2.3287.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suryanarayan CR. Kangri cancer in Kashmir valley: preliminary study. J Surg Oncol. 1973;5:327–33. doi: 10.1002/jso.2930050409. [DOI] [PubMed] [Google Scholar]
- 29.Laycock HT. The ‘‘Kang cancer’’ of north-west China. Br Med J. 1948;1:982. doi: 10.1136/bmj.1.4559.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterkin GAG. Malignant change in erythema ab igne. Br Med J. 1955;2:1599–602. doi: 10.1136/bmj.2.4956.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arons MS, Rodin AE, Lynch JB, Lewis SR, Blocker TG. Scar tissue carcinoma: IIAn experimental study with special reference to burn scar carcinoma. Ann Surg. 1966;163(3):445–60. doi: 10.1097/00000658-196603000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konigova R, Rychterova V. Marjolin’s ulcer. Acta Chir Plast. 2000;42:91–4. [PubMed] [Google Scholar]
- 33.Celikoz B, Demiriz M, Selmanpakoglu N. A shorter lag period of mesenchymal malignancy on Marjolin’s ulcer. Burns. 1997;23:72–4. doi: 10.1016/s0305-4179(96)00026-5. [DOI] [PubMed] [Google Scholar]
- 34.Bostwick J, Pendergrast WJ, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66, 9. [PubMed] [Google Scholar]
- 35.Kikuchi H, Nishida T, Kurokawa M, Setoyama M, Kisanuki A. Three cases of malignant melanoma arising on burn scars. J Dermatol. 2003;30(8):617–24. doi: 10.1111/j.1346-8138.2003.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Shin MS, Kim HS. Somatic mutations of Fas (Apo-1/CD95) gene in cutaneous cell carcinomas arising from a burn scar. J Invest Dermatol. 1999;114:122–6. doi: 10.1046/j.1523-1747.2000.00819.x. [DOI] [PubMed] [Google Scholar]
- 37.Baliarsing AK. Will Fas gene help to diagnose burn scar squamous cell carcinoma? Plast Reconst Surg. 2000;106:203. doi: 10.1097/00006534-200108000-00053. [DOI] [PubMed] [Google Scholar]
- 38.Hahn SB, Kim DJ, Jeon CH. Clinical study of Marjolin’s ulcer. Yonsei Med J. 1990;31:234–41. doi: 10.3349/ymj.1990.31.3.234. [DOI] [PubMed] [Google Scholar]
- 39.Crawley WA, Dellon AL, Ryan JJ. Does Host Response determine the prognosis in scar carcinoma? Plast Reconstr Surg. 1978;62:407–14. doi: 10.1097/00006534-197809000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Czarnecki D, Nicholson I, Tait B, Nash C. HLA DR4 is associated with the development of multiple basal cell carcinomas and malignant melanoma. Dermatolgica. 1993;187:16–8. doi: 10.1159/000247190. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi M, Tamura G, Kato N, Ansai S, Kondo S, Motoyama T. Genetic analysis of cutaneous squamous cell carcinomas arising from different areas. Path Inter. 2003;53:602–7. doi: 10.1046/j.1440-1827.2003.01523.x. [DOI] [PubMed] [Google Scholar]
- 42.Harland DL, RobinsonWA RobinsonWA, FranklinWA FranklinWA. Deletion of the p53 gene in a patient with aggressive burn scar carcinoma. J Trauma. 1997;42:104–7. doi: 10.1097/00005373-199701000-00018. [DOI] [PubMed] [Google Scholar]
- 43. Asuquo M, Ugare G, Ebughe G, Jibril P. Marjolin’s ulcer: the importance of surgical management of chronic cutaneous ulcers. International Journal of Dermatology 2007; 46(Suppl. 2): 29 –32. [DOI] [PubMed]
- 44.Onah II, Olaitan PB, Ogbonnaya IS, Onuigbo WI. Marjolin’s ucler at a Nigerian hospital (1993–2003) J Plast Reconstr Aesthet Surg. 2006;59(5):565–6. doi: 10.1016/j.bjps.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Moller R, Reymann F, Hou-Jensen K. Metastases in dermatological patients with squamous cell carcinoma. Arch Dermatol. 1979;115:703–5. doi: 10.1001/archderm.1979.04010060011017. [DOI] [PubMed] [Google Scholar]
- 46.Dvorak HF. Dvorak HFTumors; wounds that do not healSimilarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–7. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 47.Lifeso RM, Bull CA. Squamous cell carcinoma of the extremities. Cancer. 1985;55:2862–7. doi: 10.1002/1097-0142(19850615)55:12<2862::aid-cncr2820551226>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 48.Copcu E, Aktas A, Sişman N, Oztan Y. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28(2):138–41. doi: 10.1046/j.1365-2230.2003.01210.x. [DOI] [PubMed] [Google Scholar]
- 49.Sabin SR, Goldstein G, Rosenthal HG, Haynes KK. Aggressive Squamous Cell Carcinoma Originating as a Marjolin’s Ulcer. Dermatol Surg. 2004;30:229–230. doi: 10.1111/j.1524-4725.2004.30072.x. [DOI] [PubMed] [Google Scholar]
- 50.Ozek C, Celik N, Bilkay U, Akalin T, Erdem O, Cagdas A. Marjolin’s ulcer of the scalp: Report of 5 cases and review of the literature. J Burn Care Rehabil. 2001;22(1):65–9. doi: 10.1097/00004630-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Liebau J, Pallua N. Scar carcinoma as a late complication after burns. Langenbecks Arch Chir. 1995;380(3):158–61. doi: 10.1007/BF00207722. [DOI] [PubMed] [Google Scholar]
- 52.Love RL, Breidahl AF. Acute squamous cell carcinoma arising within a recent burn scar in a 14 year old boy. Plast Recon Surg. 2000;106:1069–71. doi: 10.1097/00006534-200010000-00017. [DOI] [PubMed] [Google Scholar]
- 53.Lawrence EA. Carcinoma arising in the scar of thermal burns: with special reference to the influence of the age at burn on the length of the induction period. Surg Gynecol Obstet. 1952;92:579—88. [PubMed] [Google Scholar]
- 54.Ozyazgan I, Kontaş O. Burn scar sarcoma. Burns. 1999;25(5):455–8. doi: 10.1016/s0305-4179(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 55.Fu YS, Gabbiani G. Malignant soft tissue tumors of probable histiocytic origin. Cancer. 1975;35:176. doi: 10.1002/1097-0142(197501)35:1<176::aid-cncr2820350123>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 56.Taxy JB, Battifora H. Malignant fibrous histiocytoma. Cancer. 1977;40:254. doi: 10.1002/1097-0142(197707)40:1<254::aid-cncr2820400138>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 57.Can Z, Yilmaz S, Riza A, Apaydin EI, Kuzu I. Sarcoma developing in a burn scar: case report and review of the literature. Burns. 1998;24(1):68–71. doi: 10.1016/s0305-4179(97)00091-0. [DOI] [PubMed] [Google Scholar]
- 58.Giblin T, Pickrell K, Pitss W, and Armstrong D. Malignant degeneration in burn scars. Ann Surg. 1965;162:291. doi: 10.1097/00000658-196508000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rööser B, Willén H, Gustafson P, Alvegård TA, Rydholm A. Malignant fibrous histiocytoma of soft tissue: a population- based epidemiologic and prognpstic study of 137 patients. Cancer. 1991;67(2):499–505. doi: 10.1002/1097-0142(19910115)67:2<499::aid-cncr2820670230>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 60.Yücel A, Yazar S, Demirkesen C, Durak H, Dervişoğlu S, Altintaş M. An unusual long-term complication of burn injury: malignant fibbrous histiocytoma developed in chronic burn scar. Burns. 2000;26(3):305–10. doi: 10.1016/s0305-4179(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 61.Chow J, Sabet LM, Clark BL, Coire CI. Cutaneous leiomyosarcoma: case reports and review of the literature. Ann Plast Surg. 1987;18(4):319–22. doi: 10.1097/00000637-198704000-00009. [DOI] [PubMed] [Google Scholar]


