Abstract
Background: Dysmenorrhea and menstrual migraine may share a common pathogenic pathway. Both appear to be mediated, in part, by an excess of prostaglandin production that occurs during menstruation.
Methods: Data were pooled from two replicate randomized controlled trials of 621 adult menstrual migraineurs with dysmenorrhea who treated migraine with sumatriptan-naproxen or placebo. Along with headache symptoms, nonpain menstrual symptoms (bloating, fatigue, and irritability) and menstrual pain symptoms (abdominal and back pain) were recorded at the time periods of 30 minutes and 1, 2, 4, and 4–24 hours. Relief of menstrual symptoms was compared using a Cochran-Mantel-Haenszel test. Logistic regression was used to determine the odds of a headache response with increasing numbers of moderate to severe dymenorrheic symptoms.
Results: Sumatriptan-naproxen was superior to placebo for relief of tiredness, irritability, and abdominal pain at the time periods of 2, 4, and 4–24 hours (p≤0.023); back pain at the time periods of 4 and 4–24 hours (p≤0.023); and bloating at 4–24 hours endpoint (p=0.01). The odds ratios (ORs) of attaining migraine pain freedom for 2 hours and for sustained 2–24 hours decreased as moderate to severe dysmenorrhea symptoms increased with sumatriptan-naproxen versus placebo.
Conclusions: Treatment with sumatriptan-naproxen may provide relief of menstrual symptoms and migraine in female migraineurs with dysmenorrhea. The presence of moderate to severe dysmenorrhea symptoms is associated with decreased response rates for menstrual migraine, suggesting that the co-occurrence of these disorders may negatively impact the results of migraine-abortive therapy.
Introduction
Menstrual migraine and dysmenorrhea are common menstrually related disorders, affecting millions of women in the United States.1 The prevalence of menstrual migraine is 3% in the general population, but it afflicts 35%–70% of female migraineurs.2–7 Dysmenorrhea occurs in 20%–90% of adolescent girls and in women over the age of 18 years, depending on the criteria used.8–11 Dymenorrheic symptoms tend to decline with age, but “moderate to severe” symptoms still occur in 20% of menstruating women aged 40–45 years.12 In addition, menstrual migraine and dysmenorrhea may share a common pathogenesis that is mediated in part by prostaglandin production that occurs during the perimenstrual time period.13 Therefore, it was theorized that a migraine-abortive medication that contained a nonsteroidal anti-inflammatory drug (NSAID) and a triptan would be particularly effective in the treatment of women experiencing symptoms of both migraine and dysmenorrhea.
In 2008, the US Food and Drug Administration approved a fixed-combination tablet of sumatriptan 85 mg formulated with RT Technology™ and naproxen sodium 500 mg (GlaxoSmithKline, Research Triangle Park, North Carolina) for the acute treatment of migraine attacks with or without aura in adults. This therapy was shown to be more effective than placebo in aborting menstrual migraine associated with dysmenorrhea in two replicate, randomized, double-blind placebo-controlled clinical trials.14 Both studies measured relief of dysmenorrhea symptoms as a secondary endpoint and reported that the relief of nonpain menstrual symptoms (bloating, irritability, and fatigue) was greater in the sumatriptan-naproxen group than in the placebo group but that relief of painful menstrual symptoms (abdominal and back pain) was not. However, the individual studies were not powered to detect differences between treatment groups for the dysmenorrhea symptoms. Therefore, we pooled the data from the two studies.
Menstruating women with dysmenorrhea have decreased pain thresholds throughout the menstrual cycle in a variety of noncontiguous regions of the body (e.g., abdomen, arm) as compared with controls, suggesting that dysmenorrhea may represent a systemic pain disorder.15 A recent functional MRI study demonstrated abnormal cortical processing of experimental stimuli in these patients.15 Giamberardino et al. reported that the presence of dysmenorrheic symptoms was associated with a greater frequency and severity of pain complaints from other conditions, such as irritable bowel syndrome and nephrolithiasis.16 Based on these studies, one might theorize that patients with dysmenorrhea represent a subgroup of patients with more refractory and difficult-to-treat attacks of menstrual migraine. There have been no past studies to determine whether the frequency and/or severity of dysmenorrheic symptoms modulate the response to abortive treatments for menstrual migraine.
This article presents a post hoc analysis of the pooled data from the aforementioned studies. The overall purpose of our study was to explore the interrelationships between dysmenorrheic symptoms and treatment responses to sumatriptan-naproxen. The specific objectives of this analysis were to (1) determine whether sumatriptan-naproxen relieves the concomitant symptoms of dysmenorrhea and menstrual migraine and (2) explore the relationship between the number of moderate to severe dysmenorrhea symptoms and outcome measures for the abortive treatment of migraine headache.
Materials and Methods
The original studies were conducted in the United States from May to November 2006 at 64 centers (48% primary care,37% headache specialists or neurologists, 16% obstetric/gynecologic specialists). Institutional review boards at each center approved the study protocol, and all participants signed a written consent document prior to enrolling in the study.
Eligible participants included women aged ≥18 years with a history of migraine with or without aura, based on International Headache Society criteria.17 Participants averaged one to six migraine attacks per month in the prior 3 months and typically experienced moderate to severe migraine with an initial mild headache phase. Participants were able to distinguish between a mild migraine headache and a tension-type headache. A 6-month history of menstrual migraine with attacks in at least two of the three perimenstrual periods prior to screening was required. Dysmenorrhea was required at the onset of menstruation in at least 2 of the 3 months prior to screening. Participants were required to be in good health and appropriate candidates for sumatriptan and naproxen treatment consistent with the currently approved regulatory labels for these drugs.18,19
Eligible participants treated their next menstrual migraine attack within 1 hour of onset of migraine with a single fixed-dose tablet of sumatriptan-naproxen sodium (sumatriptan, 85 mg, as the succinate salt, formulated with RT Technology™, and naproxen sodium, 500 mg, GlaxoSmithKline, Research Triangle Park, NC) or placebo in a 1:1 treatment-allocation ratio. Any additional medications, including rescue medications for headache or menstrual symptoms, could be taken 2 hours after ingestion of the first tablet. Sumatriptan-naproxen or alternative rescue medications, including naproxen sodium, sumatriptan succinate, or any medication commonly used by the subject to treat migraine (excluding certain prohibited medications as described in a previous publication) were also permitted.
All participants recorded the following symptoms in a diary at 0, 30 minutes, and 1, 2, 4, and 4–24 hours after administration of study drug or placebo: intensity of headache, photophonia, phonophonia, nausea, and vomiting. Nonpain menstrual symptoms (e.g., bloating, fatigue, and irritability) and menstrual pain symptoms (e.g., abdominal and back pain) were recorded at time periods 30 minutes and 1, 2, 4, and 4–24 hours. Each of these symptoms was rated on a four-point severity scale (0=none, 1=mild, 2=moderate, and 3=severe).
Outcome measures
The dysmenorrhea-related outcome measures were the relief of menstrual symptoms and the sum of pain-intensity differences. The relief of menstrual symptoms was defined as the percentage of participants with a one-point or greater decline in the severity of menstrual symptoms at 1, 2, 4, and 4–24 hours after administration of study medication as compared to the 30-minute baseline. A one-point decline in menstrual symptoms was thought to be clinically relevant, as this represented a 33% decline in menstrual symptoms compared to baseline. The sum of pain intensity differences (SPID) for the menstrual pain symptoms was calculated by subtracting the pain-intensity score of the 30-minute time point from those obtained at 1, 2, and 4–24 hours after administration of study medication in those experiencing the symptom. The sum of these differences for all of the time periods represented the pain-intensity difference for a given participant.
The migraine-related outcome measures were the proportions of study participants who were pain free (severity=0) at 2 hours after administration of study medication and sustained pain free (pain free at 2–24 hours with no return of headache pain or use of rescue medication).
Statistical analyses
The sumatriptan-naproxen and placebo groups were compared with respect to the percentage of participants with postdose menstrual symptoms at 1, 2, 4, and 4–24 hours, using the Cochran-Mantel-Haenszel test. The Wilcoxon rank-sum test was used to compare the treatment groups with respect to SPIDs. Two-sided p values were reported for all comparisons, and p values<0.05 were considered significant in these exploratory analyses.
Prior studies have linked the severity of menstrual symptoms to uterine prostaglandins whose release might also modulate pain response.20 The relationship between pain-free response at 2 hours and the number of moderate to severe menstrual symptoms was examined using logistic regression analysis. The logit (i.e., log odds) of the response probabilities was regressed against the linear predictors treatment, number of moderate to severe dysmenorrhea symptoms at baseline (0, 1, 2, 3, 4, 5, 6), and their interaction. The estimates of the odds ratios (ORs) were obtained by combining appropriate parameters of the logit model. The linearity of placebo-adjusted odds of a response for sumatriptan-naproxen as a function of the number of symptoms was examined using appropriate combinations of the parameter estimates of this model. The logistic regression was implemented using PROC GENMOD in SAS version 9.2 (SAS Institute, Cary, NC). The robustness of the linearity of placebo-adjusted odds of a response was examined by introducing covariates one at a time into the statistical model (e.g., age; race; ethnicity; child-bearing potential; methods of birth control; migraine diagnosis, history, and baseline characteristics; menstrual diagnosis, history, and baseline characteristics; and sleep history and baseline characteristics). These analyses were repeated for the response of sustained pain-free 2–24 hours.
Results
The demographic characteristics of the pooled intention-to-treat population are described in Table 1, and the disposition of participants is reported in Figure 1. A total of 621 participants comprised this population (placebo=319, sumatriptan-naproxen=302). Baseline migraine and menstrual symptoms are reported in Tables 1 and 2.
Table 1.
Demographic Characteristics
| Variable | Placebo (n=319) | Sumatriptan-naproxen (n=302) |
|---|---|---|
| Age, mean (median) | 37 (38) | 36 (37) |
| Race | ||
| White | 289 (91%) | 261 (86%) |
| Other | 30 (9%) | 41 (14%) |
| Birth control methods | ||
| Oral contraceptive | 93 (29%) | 93 (31%) |
| Intrauterine contraceptive device | 7 (2%) | 16 (5%) |
| Depot contraceptive: implants/injectables | 4 (1%) | 4 (1%) |
| Spermicide+physical barrier | 68 (21%) | 54 (18%) |
| Physical barrier: condom/diaphragm | 32 (10%) | 26 (9%) |
| Abstinence | 57 (18%) | 56 (19%) |
| Sterilization of male partner | 51 (16%) | 34 (11%) |
| Other | 22 (7%) | 21 (7%) |
FIG. 1.
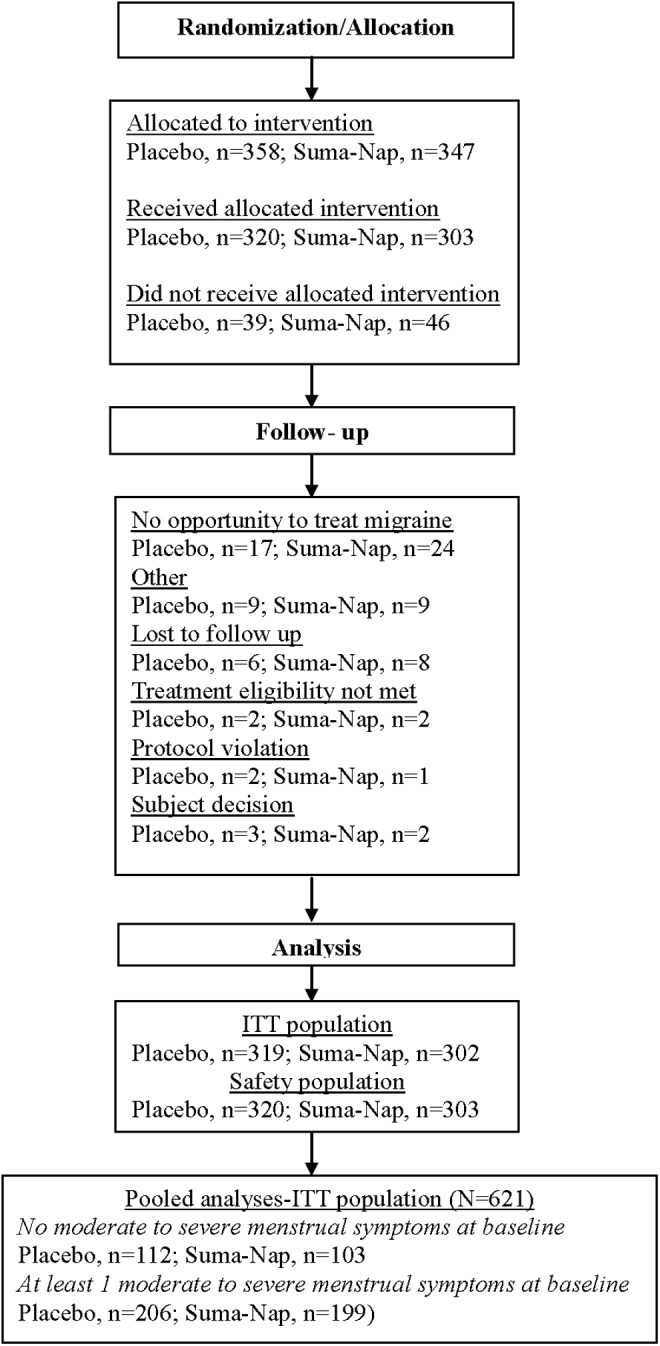
Disposition of participants in the pooled analysis.
Table 2.
Migraine and Menstrual Migraine History
| Variable | Placebo (n=319) | Sumatriptan-naproxen (n=302) |
|---|---|---|
| Migraine history | ||
| Monthly mean number (median) migraine attacks | 3 (3) | 3 (3) |
| Daily mean number (median) with headache per month in past year | 6 (5) | 6 (5) |
| Presence of aura | 58/313 (19%) | 63/296 (21%) |
| Migraine pain severity at dosing (baseline) | ||
| Mild | 282 (89%) | 271 (90%) |
| Moderate | 28 (9%) | 20 (7%) |
| Severe | 7 (2%) | 10 (3%) |
| Reproductive history | ||
| Age onset menstrual migraine | 24 (22) | 24 (22) |
| Mean (median) age onset dysmenorrhea, years | 17 (15) | 17 (14) |
| Dysmenorrhea diagnosis | ||
| Primary | 268 (84%) | 256 (85%) |
| Secondary | 8 (3%) | 5 (2%) |
| Diagnosis of endometriosis or fibroids | 30 (9%) | 32 (11%) |
| Treatment for dysmenorrhea | ||
| Nothing | 60 (19%) | 65 (22%) |
| Nonpharmacological | 10 (13%) | 6 (2%) |
| Over-the-counter medication | 228 (71%) | 208 (69%) |
| Prescription medication | 21 (7%) | 23 (8%) |
| Pregnant at least once during life | 213 (67%) | 212 (70%) |
Relief of menstrual symptoms
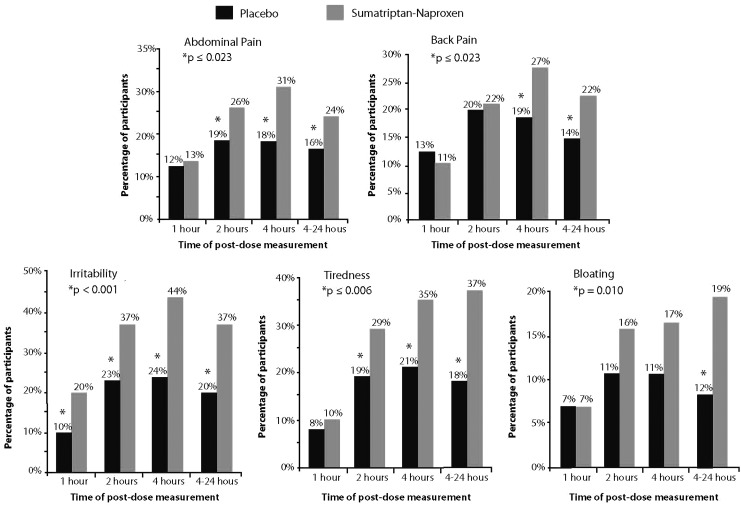
Relief of each individual menstrual symptom is depicted in Figure 2. Sumatriptan-naproxen was superior to placebo for relief of tiredness, irritability, and abdominal pain, at 2, 4, and 4–24 hours postdose (p≤0.023). However, sumatriptan-naproxen was significantly greater for back pain at the endpoints 4 and 4–24 hours (p≤0.023); bloating, only at 4–24 hours postdose (p=0.01).
FIG. 2.
Menstrual symptom relief at 1, 2, 4, and 4–24 hours posttreatment.
The SPIDs were also greater in the sumatriptan-naproxen group than in the control group for the composite outcome measures of menstrual pain (p=0.005) and nonpain menstrual symptoms (p<0.001). SPIDs of the individual menstrual symptoms were likewise significantly higher for the following individual menstrual symptoms: abdominal pain (p<0.001), irritability (p<0.001), fatigue (p<0.001), and bloating (p=0.018). Back pain did not have a significantly different SPID compared with placebo (p=0.08)
Effect of symptoms on treatment response
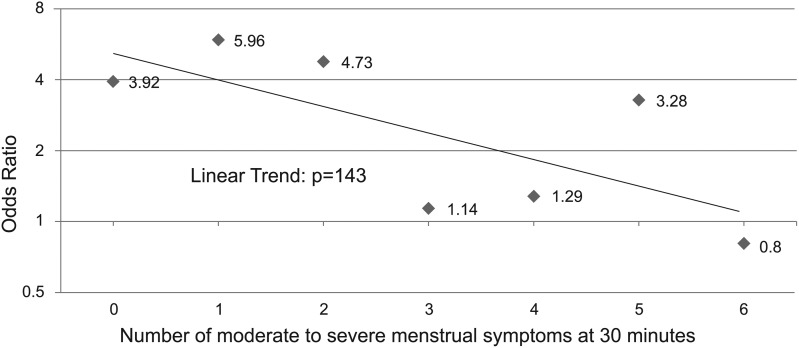
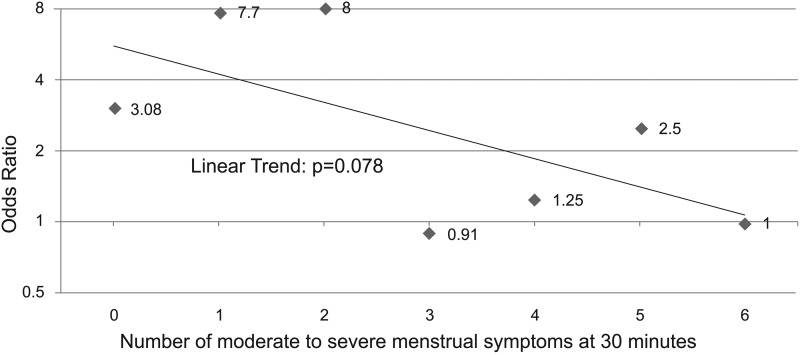
The ORs of attaining 2-hour pain freedom and 2–24 hours sustained pain freedom for migraine decreased as the number of moderate to severe dysmenorrhea symptoms increased in those receiving the sumatriptan-naproxen combination compared with placebo (Table 3). Note that the ORs for a treatment response decline abruptly and also lose statistical significance when patients experience three or more moderate to severe dysmenorrhea symptoms. The OR for 2-hour pain freedom ranged from 3.92 to 5.96 in patients with zero to two dysmenorrhea symptoms and 1.14 to 3.28 in those with three or more symptoms. Likewise, the OR for sustained pain freedom ranged from 3.08 to 8.0 in those with zero to two menstrual symptoms and 0.91 to 2.5 in those with three or more symptoms.
Table 3.
Distribution of Pain-Free Responses: 2 Hours and Sustained 2–24 Hours
| 2 hours | 2–24 hours | ||||||
|---|---|---|---|---|---|---|---|
| Number of menstrual symptoms at 30 minutes | Treatment group | No | Yes | Odds ratio | No | Yes | Odds ratio |
| 0 | Placebo | 76 | 36 | 3.92a | 88 | 24 | 3.08a |
| Suma-Nap | 36 | 67 | 56 | 47 | |||
| 1 | Placebo | 62 | 10 | 5.96a | 66 | 6 | 7.7a |
| Suma-Nap | 26 | 25 | 30 | 21 | |||
| 2 | Placebo | 36 | 7 | 4.73a | 40 | 3 | 8.0a |
| Suma-Nap | 25 | 23 | 30 | 18 | |||
| 3 | Placebo | 31 | 10 | 1.14 | 36 | 6 | 0.91 |
| Suma-Nap | 28 | 10 | 33 | 5 | |||
| 4 | Placebo | 18 | 4 | 1.29 | 20 | 2 | 1.25 |
| Suma-Nap | 28 | 8 | 32 | 4 | |||
| 5 | Placebo | 15 | 4 | 3.28 | 15 | 4 | 2.5 |
| Suma-Nap | 8 | 7 | 9 | 6 | |||
| 6 | Placebo | 8 | 1 | 0.8 | 9 | 0 | 1 |
| Suma-Nap | 10 | 1 | 11 | 0 | |||
p value<0.05
Suma-Nap, sumatriptan-naproxen.
The linear trend analyses demonstrated a pattern of declining ORs of a treatment response to sumatriptan-naproxen with increasing numbers of moderate to severe menstrual symptoms, but the trend did not reach statistical significance (p values of 0.143 for 2-hour pain freedom and 0.078 for sustained pain freedom [Figs. 3 and 4]). Covariate analyses showed that the linear trend in the odds of 2-hour pain-free and sustained pain-free responses versus the count of number of menstrual symptoms at baseline was unaffected by any of the covariates examined.
FIG. 3.
Odds ratios for pain-free response versus number of moderate to severe menstrual symptoms at 30 minutes: sumatriptan-naproxen versus placebo.
FIG. 4.
Odds ratios for sustained pain-free response versus number of moderate to severe menstrual symptoms at 30 minutes: sumatriptan-naproxen versus placebo.
Adverse events
The adverse events of the two individual studies have been fully described in a previous publication.14 No serious adverse events reported in either study and no new or unexpected adverse events were reported. Adverse events considered by the investigator to be related to the study drug occurred at a rate of less than 1% in study 1. The drug-related adverse events that occurred more frequently than placebo in study 2 were dizziness (5% vs. 2%), nausea (4% vs. 1%), dry mouth (2% vs. 1%, and paresthesia (2% vs. 0).
Discussion
The results of this exploratory pooled analysis provide evidence that sumatriptan-naproxen, a combination product containing both an NSAID and a triptan, relieves most menstrual symptoms within 4 hours after treatment in female migraineurs experiencing dysmenorrhea comorbid with a migraine attack. Participants treated with sumatriptan-naproxen experienced statistically significant and clinically relevant relief of the individual menstrual symptoms of tiredness, irritability, abdominal pain, fatigue, and back pain up to 24 hours after treatment. Sustained relief from bloating was observed (4 to 24 hours) but not at the individual time points.
Sumatriptan-naproxen was also significantly more effective than placebo in the overall SPID both for the composite endpoints of menstrual pain (abdominal and back pain) and nonpain menstrual (irritability, fatigue, and bloating) symptoms and for all the individual menstrual symptoms except back pain. There was a trend toward a higher SPID for back pain in the sumatriptan-naproxen group, but it did not meet statistical significance (p=0.08). These results suggest that sumatriptan-naproxen is more effective than placebo in relieving menstrual symptoms present during a migraine over a 1- to 4-hour time period after administration.
To our knowledge, this is the first study to report that a migraine-abortive medication could relieve the neuropsychological symptoms of fatigue and irritability, which are associated with both migraine and dysmenorrhea. Past diary studies have demonstrated that fatigue and irritability occur during the prodrome of a migraine (e.g., time period immediately preceding a migraine) in 42%–73% and 31%–42% of attacks, respectively.21–23 Likewise, fatigue and irritability have been reported to occur in 72% and 51% of those with dysmenorrhea.24 Fatigue and irritability are also common symptoms of premenstrual stress disorder that co-occurs with dysmenorrhea in 53% of patients.25 The fact that neuropsychological symptoms diminish after treatment with a migraine-abortive medication could suggest that these symptoms might occur as a direct result of the migraine itself or from the dysmenorrhea encountered in these patients.
The two studies described here represent a unique and previously unstudied large population of women who experience menstrual migraine attacks with concomitant symptoms of dysmenorrhea. This exploratory pooled analysis provides evidence that sumatriptan-naproxen may provide additional benefits above and beyond those related to the treatment of migraine, namely, the relief of dysmenorrheic symptoms when they occur together with migraine in female migraineurs. These analyses cannot determine whether the reduction of dysmenorrheic symptoms in the sumatriptan-naproxen group is best attributed to one of the sumatriptan-naproxen components—sumatriptan or naproxen—alone or to both in combination. However, given that NSAIDs have been shown in multiple studies to decrease symptoms of dysmenorrhea, it is likely that the naproxen component had the greater effect on dysmenorrheic symptoms in this study.26,27
Although one might expect that the naproxen component of this product would relieve dysmenorrheic symptoms, we believe that it is necessary to prove this in a clinical trial, as its pharmacologic properties (in combination with sumatriptan) differ from that of other naproxen products. In fact, the maximum concentration of naproxen is reached in 6 hours in the sumatriptan-naproxen combination compared to 1 hour in pills containing naproxen alone.28 Despite such a delayed release of naproxen, it is interesting to note that many of the dysmenorrheic symptoms were relieved 2–4 hours after treatment with sumatriptan-naproxen.
Evidence suggests that both menstrual migraine and dysmenorrhea share a common pathogenesis that could in part be related to prostaglandin release during the perimenstrual time period. First, both disorders occur at the same time period of the menstrual cycle. Second, serum and/or uterine levels of prostaglandins are increased during both disorders.29–34 Prostaglandins are released from the uterus as a result of declining serum levels of progesterone at the time of menses. The uterine release of PGF2α has been positively correlated with the severity of symptoms of dysmenorrhea.35 Nattero et al. found that plasma levels of PGE2 were increased during an attack of menstrual migraine when compared to an interictal time period between attacks and controls during different time periods of the menstrual cycle.36 Third, medications that inhibit prostaglandin synthesis, such as NSAIDs, have been demonstrated to treat both menstrual migraine and dysmenorrhea in past studies.37–42 Finally, results of a pilot substudy within these two studies showed that salivary levels of prostaglandins increased during the menstrual migraine attacks associated with dysmenorrhea; after treatment with sumatriptan-naproxen, salivary levels of prostaglandins were not elevated at 2 and 4 hours.43 Collectively, these data suggest that prostaglandins modulate symptoms of both menstrual migraine and dysmenorrhea.
This exploratory analysis also answered the second clinical question: Is there a relationship between the number of baseline moderate to severe menstrual symptoms and migraine headache pain? Based on the exploratory data, women migraneurs with a greater number of moderate to severe symptoms of dysmenorrhea had lower 2-hour pain-free response rates and lower sustained pain-free 2- to 24-hour response rates for migraine pain than did those without these symptoms. This difference was most pronounced when patients experienced three or more moderate to severe dysmenorrheic symptoms. In support of these data, migraneurs with endometriosis have been shown to have more frequent and disabling headaches.44 These data suggest that women with both menstrual migraine and frequent moderate to severe dysmenorrheic symptoms represent a subgroup of migraineurs who are more refractory to abortive migraine medications, at least when the two conditions are comorbid. Moderate to severe symptoms of dysmenorrhea may be a marker for greater synthesis and/or release of prostaglandins that may render attacks of menstrual migraine more difficult to treat. It would be interesting to know whether migraines that occur in this subgroup outside of menses are similarly refractory.
The limitations of the data-collection methodology should be noted. First, the menstrual symptoms were first recorded 30 minutes after administration of the study medication. Therefore, a true baseline for dysmenorrhea symptoms was not obtained for this study. This omission could have led to decreased response rates within the sumatriptan-naproxen group if the study medication relieved the menstrual symptoms at 30 minutes. Second, rescue medications for headache were administered to 53%–69% of the placebo groups and 31%–37% of the sumatriptan-naproxen groups in the two trials. We cannot rule out the possibility that the use of rescue medications may have influenced the results of some of our outcome measures, particularly those recorded 2 or more hours after initial administration of study medication. Third, 89% of our study population was Caucasian, and the mean age was 36. Our results may not generalize to populations that do not share similar demographics to our population. Fourth, our results may apply only to persons with dysmenorrhea who are not receiving oral contraceptives, as only 19%–21% of our participants were taking these medications. Oral contraceptives inhibit ovulation, decrease the thickness of the endometrium, and reduce prostaglandin production within the uterus. Therefore, we cannot rule out the possibility that the treatment response to sumatriptan-naproxen might vary in those receiving oral contraceptives. Fifth, the linearity of placebo-adjusted odds of a headache response for sumatriptan-naproxen as a function of the number of moderate to severe dysmenorrhea symptoms did not achieve statistical significance. This is likely the result of small numbers of participants with 5 or 6 moderate to severe symptoms (e.g., <20 in each group).
Conclusions
The results of these exploratory analyses suggest that early treatment with a combination product containing both an NSAID and a triptan (i.e., sumatriptan-naproxen) may provide relief from symptoms of migraine and dysmenorrhea, as well as some of the neuropsychological symptoms related to these disorders. A triptan formulated with an NSAID may be advantageous in migraneurs with dysmenorrhea because many of these menstrual symptoms are thought to arise as the result of uterine prostaglandin release. Furthermore, the data suggest a relationship between the number of baseline moderate to severe menstrual symptoms and modulation of the primary outcome (migraine headache pain); the relative efficacy of sumatriptan-naproxen compared with placebo tended to decrease with the number of dysmenorrhea symptoms at baseline in this study.
Acknowledgments
We thank Kim Poinsett-Holmes, PharmD (Poinsett Publications, Fuqay-Varina, North Carolina), for her editorial assistance with the submitted manuscript; Jonathan White, MS (GlaxoSmithKline, Research Triangle Park, North Carolina), for contribution to study design and statistical support; and Roger Cady, MD (Headache Care Center, Springfield, MO) and Merle Diamond, MD (Diamond Headache Clinic, Chicago, IL), for their contributions to study design and overall data interpretation.
This study was funded by GlaxoSmithKline Clinical Trial Numbers TRX105850 and TRX105852; NCT00329459 and NCT00329355.
Author Disclosure Statement
GlaxoSmithKline was the sponsor of (and supported) both studies.
Vincent T. Martin has received research grants from Merck & Co. and GlaxoSmithKline. He has been a consultant for GlaxoSmithKline, Nautilus, Allergan, Zogenix, MAPP, Merck, Endo, and Pfizer. He has served on the speakers' bureau for GlaxoSmithKline, Endo, and Merck.
Jeanne Ballard has no conflicts of interest.
At the time of the study, Lisa K. Mannix had received research grants from Alexza, Allergan, Endo, GlaxoSmithKline, MAP, Merck & Co., Ortho-McNeil Neurologics, Pozen, and Torrey Pines. She has been a consultant for Allergan, Endo, GlaxoSmithKline, MAP, Merck & Co., and Ortho-McNeil Neurologics. She has served on the speakers' bureau for Endo, GlaxoSmithKline, Merck & Co., and Ortho-McNeill Neurologics. She has served on the advisory boards of Allergan, Endo, GlaxoSmithKline, MAP, Merck & Co., and Ortho McNeil Neurologics.
Michael P. Diamond has received grants and/or conducted research for NIH, GlaxoSmithKline, Bioasante, Novartis, Boeringher Ingelheim, and Interlace Medical. He has served as a consultant to EMD, Serono, Auxogyn, Halt Medical, BioRegen, Neomend, Genzyme, ZSX Medical, Sanofi-Aventis, and BioRegen. He receives educational support from Ferring. He owns stock in Synthermed and Neomend. He is on the Board of Directors of the American Society of Reproductive Medicine.
Shelly E. Lener, Frederick J. Derosier, Susan McDonald, and Alok Krishen were GlaxoSmithKline employees during the conduct and reporting of these studies.
Data for this study were presented, in part, at the American Academy of Neurology 60th annual meeting, April 12–19, 2008, in Chicago, IL.
References
- 1.Wang L, Wang X, Wang W, et al. . Stress and dysmenorrhea: A population based prospective study. Occup Environ Med 2004;61:1021–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granella F, Sances G, Zanferrari C, Costa A, Martignoni E, Manzoni GC. Migraine without aura and reproductive life events: A clinical epidemiological study in 1300 women. Headache 1993;33:385–389 [DOI] [PubMed] [Google Scholar]
- 3.Granella F, Sances G, Pucci E, Nappi RE, Ghiotto N, Napp G. Migraine with aura and reproductive life events: A case control study. Cephalalgia 2000;20:701–707 [DOI] [PubMed] [Google Scholar]
- 4.Cupini LM, Matteis M, Troisi E, Calabresi P, Bernardi G, Silvestrini M. Sex-hormone-related events in migrainous females. A clinical comparative study between migraine with aura and migraine without aura. Cephalalgia 1995;15:140–144 [DOI] [PubMed] [Google Scholar]
- 5.Mattsson P. Hormonal factors in migraine: A population-based study of women aged 40 to 74 years. Headache 2003;43:27–35 [DOI] [PubMed] [Google Scholar]
- 6.Koseoglu E, Nacar M, Talaslioglu A, Cetinkaya F. Epidemiological and clinical characteristics of migraine and tension type headache in 1146 females in Kayseri, Turkey. Cephalalgia 2003;23:381–388 [DOI] [PubMed] [Google Scholar]
- 7.Couturier EG, Bomhof MA, Neven AK, van Duijn NP. Menstrual migraine in a representative Dutch population sample: Prevalence, disability and treatment. Cephalalgia 2003;23:302–308 [DOI] [PubMed] [Google Scholar]
- 8.Davis AR, Westhoff CL. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J Pediatr Adolesc Gynecol 2001;14:3–8 [DOI] [PubMed] [Google Scholar]
- 9.Unsal A, Ayranci U, Tozun M, Arsian G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci 2010;115:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol 1996;87:55–58 [DOI] [PubMed] [Google Scholar]
- 11.Sundell G, Milsom I, Andersch B. Factors influencing the prevalence and severity of dysmenorrhea in young women. Br J Obstet Gynaecol 1990;97:588–594 [DOI] [PubMed] [Google Scholar]
- 12.Weissman AM, Hartz AJ, Hansen MD, Johnson SR. The natural history of primary dysmenorrhea. BJOG 2004;111:345–352 [DOI] [PubMed] [Google Scholar]
- 13.Mannix LK. Menstrual-related pain conditions: Dysmenorrhea and migraine. J Womens Health (Larchmt) 2008;17:879–891 [DOI] [PubMed] [Google Scholar]
- 14.Mannix LK, Martin VT, Cady RK, et al. . Combination treatment for menstrual migraine and dysmenorrhea using sumatriptan-naproxen: Two randomized controlled trials. Obstet Gynecol 2009;114:106–113 [DOI] [PubMed] [Google Scholar]
- 15.Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhea is associated with central changes in otherwise healthy women. Pain 2011;152:1966–1975 [DOI] [PubMed] [Google Scholar]
- 16.Giamberardino MA, Costantini R, Affaitati G, et al. . Viscero-visceral hyperalgesia: Characterization in different clinical models. Pain 2010;151:307–322 [DOI] [PubMed] [Google Scholar]
- 17.Headache classification subcommittee of the International Headache Society The international classification of headache disorders, 2nd edition. Cephalalgia 2004; 24Suppl 1:9–160 [DOI] [PubMed] [Google Scholar]
- 18.Naprosyn complete prescribing information. Nutley, NJ: Roche Laboratories Inc., 2006 [Google Scholar]
- 19.Treximet® Complete prescribing information. Research Triangle Park, NC: GlaxoSmithKline Inc., 2008 [Google Scholar]
- 20.Dawood MY. Primary dysmenorrhea: Advances in pathogenesis and management. Obstet Gynecol 2006;108:428–441 [DOI] [PubMed] [Google Scholar]
- 21.Giffin NJ, Ruggiero L, Lipton RB, et al. . Premonitory symptoms in migraine: An electronic diary study. Neurology 2003;60:935–940 [DOI] [PubMed] [Google Scholar]
- 22.Quintela E, Castillo J, Munoz P, Pascual J. Premonitory and resolution symptoms in migraine: A prospective study in 100 unselected patients. Cephalalgia 2006;26:1051–1060 [DOI] [PubMed] [Google Scholar]
- 23.Schoonman GG, Evers DJ, Terwindt GM, van Dijk JG, Ferrari MD. The prevalence of premonitory symptoms in migraine: A questionnaire study in 461 patients. Cephalalgia 2006;26:1209–1213 [DOI] [PubMed] [Google Scholar]
- 24.Chen HM, Chen CH. Related factors and consequences of menstrual distress in adolescent girls with dysmenorrhea. Kaohsiung J Med Sci 2005;21:121–127 [DOI] [PubMed] [Google Scholar]
- 25.Derman O, Kanbur NO, Tokur TE, Kutluk T. Premenstrual syndrome and associated symptoms in adolescent girls. Eur J Obstet Gynecol Reprod Biol 2004;116:201–206 [DOI] [PubMed] [Google Scholar]
- 26.Daniels SE, Torri S, Desjardins PJ. Valdecoxib for treatment of primary dysmenorrhea. A randomized, double-blind comparison with placebo and naproxen. J Gen Intern Med 2005;20:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang WY, Li Wan Po A. Efficacy of minor analgesics in primary dysmenorrhea: A systematic review. Br J Obstet Gynaecol 1998;105:780–789 [DOI] [PubMed] [Google Scholar]
- 28.Kori S, Littlefield D, Taylor D, et al. . Pharmacokinetics of a single-tablet formulation of sumatriptan RT Technology™ and naproxen sodium [abstract]. Cephalalgia 2005;25:933.. Abstract. [Google Scholar]
- 29.Goadsby PJ. The pharmacology of headache. Prog Neurobiol 2000;62:509–525 [DOI] [PubMed] [Google Scholar]
- 30.Sarchielli P, Alberti A, Vaianella L, et al. . Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache 2004;44:961–968 [DOI] [PubMed] [Google Scholar]
- 31.Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 2000;20:907–918 [DOI] [PubMed] [Google Scholar]
- 32.Nattero G, Allais G, De Lorenzo C, et al. . Menstrual migraine: New biochemical and psychological aspects. Headache 1988;28:103–107 [DOI] [PubMed] [Google Scholar]
- 33.Benedetto C. Eicosanoids in primary dysmenorrhea, endometriosis and menstrual migraine. Gynecol Endocrinol 1989;3:71–94 [DOI] [PubMed] [Google Scholar]
- 34.Jaobbour HN, Sales KJ, Smith OP, Battersby S, Boddy SC. Prostaglandin receptors are mediators of vascular function in endometrial pathologies. Mol Cell Endocrinol 2006;252:191–200 [DOI] [PubMed] [Google Scholar]
- 35.Chan WY, Dawood MY. Prostaglandin levels in menstrual fluid of nondysmenorrheic and of dysmenorrheic subjects with and without oral contraceptive or ibuprofen therapy. Adv Prostaglandin Thromboxane Res 1980;8:1443–1447 [PubMed] [Google Scholar]
- 36.Nattero G, Allais G, De Lorenzo C, et al. . Relevance of prostaglandins in true menstrual migraine. Headache 1989;29:232–237 [DOI] [PubMed] [Google Scholar]
- 37.Daniels S, Robbins J, West CR, Nemeth MA. Celecoxib in the treatment of primary dysmenorrhea: Results from two randomized, double-blind, active- and placebo-controlled crossover studies. Clin Ther 2009;31:1192–1208 [DOI] [PubMed] [Google Scholar]
- 38.Chantler I, Mitchell D, Fuller A. Diclofenac potassium attenuates dysmenorrhea and restores exercise performance in women with primary dysmenorrhea. J Pain 2009;10:191–200 [DOI] [PubMed] [Google Scholar]
- 39.Suthisisang C, Poolsup N, Kittikulsuth W, Pudchakan P, Wiwatpanich P. Efficacy of low-dose ibuprofen in acute migraine treatment: Systematic review and meta-analysis. Ann Pharmacother 2007;41:1782–1791 [DOI] [PubMed] [Google Scholar]
- 40.Saper J, Dahlof C, So Y, et al. . Rofecoxib in the acute treatment of migraine: A randomized controlled clinical trial. Headache 2006;46:264–275 [DOI] [PubMed] [Google Scholar]
- 41.Suthisisang CC, Poolsup N, Suksomboon N, Lertpipopmetha V, Tepwitukgid B. Meta-analysis of the efficacy and safety of naproxen sodium in the acute treatment of migraine. Headache 2010;50:808–818 [DOI] [PubMed] [Google Scholar]
- 42.Loo CY, Tan HJ, Teh HS, Raymond AA. Randomised, open label, controlled trial of celecoxib in the treatment of acute migraine. Singapore Med J 2007;48:834–839 [PubMed] [Google Scholar]
- 43.Durham PL, Vause CV, Derosier F, McDonald S, Cady R, Martin V. Changes in salivary prostaglandin levels during menstrual migraine with associated dysmenorrhea. Headache 2010;50:844–851 [DOI] [PubMed] [Google Scholar]
- 44.Tietjen GE, Bushnell CD, Herial NA, Utley C, White L, Hafeez F. Endometriosis is associated with prevalence of comorbid conditions in migraine. Headache 2007;47:1069–1078 [DOI] [PubMed] [Google Scholar]