Abstract
In vitro tissue engineering of vascular conduits requires a synergy between several external factors, including biochemical supplementation and mechanotranductive stimulation. The goal of this study was to improve adult human vascular smooth muscle cell orientation and elastic matrix synthesis within 3D tubular collagen gel constructs. We used a combination of elastogenic factors (EFs) previously tested in our lab, along with cyclic circumferential strains at low amplitude (2.5%) delivered at a range of frequencies (0.5, 1.5, and 3 Hz). After 21 days of culture, the constructs were analyzed for elastic matrix outcomes, activity of matrix metalloproteinases (MMPs)-2 and −9, cell densities and phenotype, and mechanical properties of constructs. While cell densities remained unaffected by the addition of stretch, contractile phenotypic markers were elevated in all stretched constructs relative to control. Constructs cultured with EFs stretched at 1.5 Hz exhibited the maximum elastin mRNA expression and total matrix elastin (over sixfold vs. the static EFs control). MMP-2 content was comparable in all treatment conditions, but MMP-9 levels were elevated at the higher frequencies (1.5 and 3 Hz). Minimal circumferential orientation was achieved and the mechanical properties remained comparable among the treatment conditions. Overall, constructs treated with EFs and stretched at 1.5 Hz exhibited the most elastogenic outcomes.
Introduction
Elastin, a primary structural protein within elastic arteries, maintains arterial structural stability as they cyclically recoil, and components of elastic fibers also critically regulate vascular cell behavior. Accelerated degradation of elastic matrix, such as that seen in vascular pathologies like abdominal aortic aneurysms, can therefore severely compromise vessel homeostasis. Restoring an intact elastic matrix is essential not only to sustain the structural requirements of the vessel wall, but also to maintain healthy vascular cell function, both of which are equally critical for vascular homeostasis.1,2 A variety of tissue engineering and matrix-regenerative strategies have therefore been extensively investigated to develop biomechanically functional and self-renewing vessel components with the aim of generating viable aortic tissue.3 However, a major challenge has been the insufficient de novo synthesis and assembly of mature elastic matrix structures.4–10 In fact, the few studies that have demonstrated reasonable levels of elastic matrix production have used neonatal or juvenile smooth muscle cells (SMCs) that are inherently elastogenic, unlike adult cells.5,11–13 Success in inducing elastogenesis in adult vascular cells has been limited to demonstrating moderate increases in mRNA expression of tropoelastin.11–13 These limitations impede further progress in engineering elastic vascular tissue replacements for clinical use. Thus, there is a critical need for methods to enhance elastic matrix assembly within these tissue-engineered constructs.
In previous studies conducted in our lab, we demonstrated that stimulation of adult vascular cells toward de novo synthesis of elastic matrix components with elastogenic biomolecules can be achieved even in a 3D collagenous microenvironment that is not particularly conducive to elastogenesis.14 We also showed that cell alignment within rectangular constructs maintained under static uniaxial stretch occurs in the direction of applied strain, positively influencing orientation and alignment of both the pre-existing collagen matrix and the cell-synthesized elastic matrix.14 This mechanical stimulus is however different than what is presented in vivo, where the orientation of the cells and synthesized matrix is tangential to the circumferential strain experienced by artery walls.15 Recent studies have shown that cells, including SMCs, behave differently when they are cultured within tubular constructs relative to when they are cultured within 3D rectangular constructs subjected to static uniaxial tension.16–18 For example, within static constructs like collagen gels, cells and matrix orient in the direction of uniaxial static tension.5,17 On the other hand, cells within tubular constructs subjected to cyclic stretch experience both radial and circumferential forces.19,20 As a result, the cells can be expected to orient in a direction perpendicular to stretch, therefore conforming to a circumferential alignment similar to that seen in vivo.19,20 Thus, we chose to use a tubular collagen gel model that mimics the native blood vessel, with the goal of determining how cyclic distension impacts induction of elastic matrix synthesis and assembly by cells in a collagenous microenvironment.
Three important factors that influence mechanotransductive response by cells, and as a result, cell phenotype and final matrix output, are strain amplitude, frequency, and duration of cyclic stretch.21,22 To date, most studies that applied cyclic stretch regimens have primarily used strain levels of >2.5%, and a majority of them have used 10% strains.16,19,23,24 However, other studies have shown that cellular synthesis and enzymatic activity of the elastolytic protease matrix metalloproteinase-2 (MMP-2) are increased at such high strains.25,26 While MMP-2 plays an important role in matrix turnover and healthy reorganization,25 chronic enzymatic activity can degrade newly synthesized elastic matrix and prevent its maturation and assembly within the scaffolds.4,27 Alternatively, studies have also demonstrated the importance of the frequency of strain perceived by cells. They found that changing the frequency of cyclic strains allows regulation of vascular SMC alignment in an intact actin filament-dependent manner.28,29
The objective of this study was to determine cyclic strain frequencies that stimulate elastic matrix production and organization within a tubular collagen gel. Cyclic strain was applied at frequencies of 0.5, 1.5, and 3 Hz for 21 days of culture. To minimize the detrimental effects of high strain levels, and at the same time promote cell and matrix alignment, we chose to subject SMC-seeded tubular collagen constructs to 2.5% cyclic strain. Elastogenic factors (EFs) were also added to stimulate cellular elastic matrix generation in this study, with the doses (i.e., 0.1 ng/mL transforming growth factor-beta 1 [TGF-β1] and 0.2 μg/mL hyaluronan oligomers [HA-o]) based on results obtained previously.14 In light of results of other studies in our lab that demonstrate that human aortic smooth muscle cells (HASMCs) respond to elastogenic stimulation by EFs,30 we have focused on study of this cell type to provide greater clinical relevance.
Materials and Methods
Design and principle of operation of bioreactor for dynamic cell culture
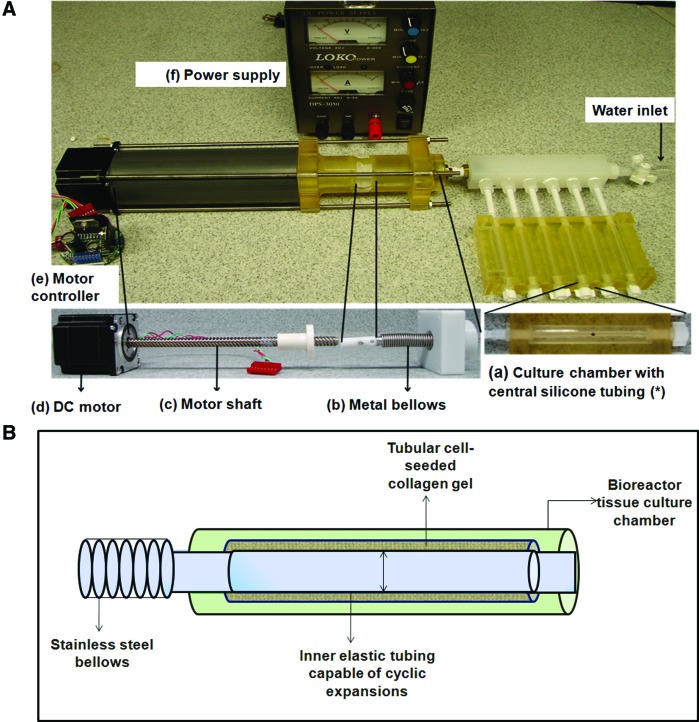
A bioreactor was constructed to deliver cyclic, circumferential strains to tubular collagen gel constructs and the SMCs seeded within it. The setup (Fig. 1A) consisted of (1) tubular tissue culture chambers with centrally placed silicone tubing (around which the collagen gel constructs would compact) connected to (2) compressible metallic bellows distended cyclically by (3) a stepper motor whose motion was controlled by (4) a custom-programmed motor controller. The motor and controller were powered by a standard 3 A power supply. Silicone tubes were used because of the observed low cell and matrix adherence to this type of surface. All bioreactor parts were either custom-fabricated in house, or purchased from Cole Parmer (Vernon Hills, IL) or McMaster Carr (Aurora, OH).
FIG. 1.
Bioreactor design and principle of operation. (A) Bioreactor setup. The tissue culture chamber with an inner silicone tubing (a) is connected via a manifold to the bellows (b), which are in turn coupled to the shaft (c) of the DC motor (d). The forward and backward movement of the shaft of the motor is controlled by the motor controller (e), powered by an external power supply (f). The volume spanning the region within the silicone tubing starting from the tissue culture chamber to the end of the bellow is a closed loop into which sterile water is purged in via a leur lock inlet. (B) Representative figure of the principle of operation. Region between the bellows to the inner silicone tubing in the tissue culture chamber is a closed, air-tight unit, with a constant volume of water, where the bellows and silicone tubing are the only compliant parts. When the bellows contract, the silicone tubing expands, applying circumferential strains to the collagen gel around the tube. Color images available online at www.liebertpub.com/tea
The segment of the bioreactor that was filled with water (from the sealed end of the bellows to the sealed ends of silicone tubes within the culture chamber in Fig. 1B) was a closed, air-tight unit, within which the inner silicone tubes were the only compliant parts. The system therefore worked on the principle that when the bellows were compressed by the motor shaft, the change in volume was experienced only by the elastic silicone tubes and compensated by a resultant change in diameter of the tube. This cyclic change in diameter therefore induced circumferential strains on the SMCs cultured within the collagen gel constructs surrounding the tube. The parameters in the program were set up in order to deliver specific percent strains.
Experimental setup
Five treatment conditions were chosen to systematically investigate the elastogenic effects of EFs under cyclic strains at different frequencies. Three sets of constructs (n=6 biological replicates per case) were cultured with EFs under 2.5% cyclic, circumferential stretch. The strain magnitude was selected to limit the production of the elastolytic MMP-2, which others have shown to be overexpressed at higher strains.25,26 A range of strain frequencies were adopted (i.e., 0.5, 1.5, and 3 Hz), which is a close frequency range centered at the frequency of the human cardiac cycle.15 One set of constructs (n=6 replicates) were cultured with EFs alone under static conditions (stretch controls). Constructs serving as treatment controls (n=6 replicates) were cultured without EFs or applied cyclic strains, and were used for normalization of real-time polymerase chain reaction (RT-PCR), western blotting, and zymography data generated for the constructs in the other cases. All constructs were cultured for 21 days. The strain in the system was validated by taking outer-diameter (OD) measurements of silicone tubes cyclically stretched at the low and the high frequencies used in this study (3 runs for each condition, and 10 cycles/run). The averaged strains were 2.56%±0.08% and 2.54%±0.05% for the 0.5 and 3.0 Hz conditions, respectively.
Fabrication of tubular collagen gel constructs
Collagen gel constructs were prepared as described previously.14 Briefly, adult HASMCs (Cell Applications, San Diego, CA) were trypsinized at passage 4 and mixed with acid-solubilized type I collagen solution (BD Biosciences, Bedford, MA) at a final concentration of 0.5×106 cells/mL and 2 mg/mL collagen. An aliquot of the cell-collagen mixture (5 mL) was then added slowly to each culture chamber such that the mixture uniformly distributed itself around the circumference of the tube. Culture medium (DMEM/F12 containing 20% FBS; Invitrogen, Grand Island, NY) was changed after 24 h. A volume of 5 mL of media can be held in each chamber, which is 1.25 mL/cm of construct. At 48 h, the medium was changed again and fresh medium with the EF doses and dynamic culture conditions were provided. Day 1 of culture for the experiment therefore corresponded to 72 h postseeding. Culture medium was changed every 2 days until harvest after 21 days of treatment. Harvested constructs were transversely cut into four equal parts to be processed further for the different analyses as described later. The constructs were easily removed from the inner tubes, with no sign of adherence to the silicone surface.
Compaction of tissue constructs
All constructs were photographed weekly and their lengths and central widths were measured using Image J software (NIH, Bethesda, MD). At least five measurements for central width and three measurements for the length were made per construct. Construct compaction was measured and reported as the aspect ratio (length:OD) on the day of harvest.
DNA assay for cell quantification
Cell counts within constructs were estimated based on total DNA content as previously described.31 Briefly, construct segments were lyophilized, weighed, and digested in 5 mg/mL proteinase-K (Gibco–Invitrogen, Carlsbad, CA). The digested aliquots were then sonicated, and DNA content was measured using a Hoechst-dye- (Gibco–Invitrogen) based flourometric assay. Cell count was measured based on an estimate of 6 pg DNA/cell,31 and was normalized to dry weights of constructs.
RT-PCR for mRNA expression of SMC phenotypic markers and matrix proteins
RNA was isolated from snap-frozen segments using an RNeasy kit (Qiagen, Valencia, CA), as per the instructions provided in the kit manual. RNA content was measured using a Ribo-green assay kit (Invitrogen), and 120 ng of total RNA was reverse transcribed into cDNA using iScript cDNA synthesis kit (BioRad, Hercules, CA). Expression of genes for the contractile SMC markers,32 such as α-smooth muscle actin (ACTA2) and caldesmon, and osteopontin (an activated SMC marker)33; the elastic fiber component proteins,34 such as elastin, fibrillin-1, fibulin-5, and the elastin crosslinking enzyme lysyl oxidase (LOX)35; and also the elastolytic proteases MMPs-2 and −936 was analyzed using the comparative threshold method (Ct) with 18s as the normalizing gene.37 Primers for all genes were either purchased as optimized primer sets from Real Time Primers (Elkins Park, PA), or designed using the NIH software PerlPrimer® (Table 1).38
Table 1.
Forward and Reverse Sequences of Primers Designed and Purchased from Applied Biosystems
| Gene | Primer sequence (5′–3′) | Size (bp) | Accession No. | |
|---|---|---|---|---|
| FBLN1 | Forward | GCTCCAGATCCATACAACAC | 145 | NG_008805.2 |
| Reverse | ACACCTTCCTCCATTGAGAC | |||
| COL1A1 | Forward | AAGGGACACAGAGGTTTCAG | 189 | NG_007400.1 |
| Reverse | TAGCACCATCATTTCCACGA | |||
| CALD1 | Forward | CCCAAACCTTCTGACTTGAG | 162 | NG_029186.1 |
| Reverse | CGAATTAGCCCTCTACAACTG | |||
| OPN1 | Forward | TGTGCCATACCAGTTAAACAG | 147 | NG_030362.1 |
| Reverse | ACTTACTTGGAAGGGTCTGTG | |||
FBLN1, fibrillin-1; COL1A1, collagen-1; CALD1, caldesmon; OPN1, osteopontin-1.
Fastin assay for quantification of matrix elastin
Matrix elastin content was measured in the form of the less-crosslinked alkali-soluble elastin and the highly crosslinked alkali-insoluble elastin using a commercially available Fastin assay kit (Accurate Scientific and Chemical Corporation, Westbury, NY). Lyophilized construct segments were weighed and digested in 0.1 N NaOH at 98°C for 1 h. Following this, the digestate was centrifuged, the supernatant was collected separately for analysis of alkali-soluble matrix elastin, and the pellet containing the alkali-insoluble elastin fraction was solubilized in 0.25 M oxalic acid (98°C for 1 h) to convert it into the soluble α-elastin form that is measurable using the Fastin kit. The solubilized sample was concentrated using 10 kDa cut-off membranes (Millipore, Billerica, MA). Matrix elastin content measured as alkali-soluble and -insoluble elastin was normalized to dry weights of constructs.
Western blotting for cellular and matrix proteins
Matrix and cellular proteins of lyophilized construct segments were homogenized and solubilized in radio-immunoprecipitation assay (RIPA) lysis buffer (Invitrogen) along with a protease inhibitor cocktail (Thermo Scientific, Rockford, IL). The digested samples were then centrifuged, and supernatants were collected for further analysis. A bicinchoninic acid (BCA) assay was performed to measure total protein concentration in each sample using a commercially available kit (Thermo Scientific). Western blotting was performed to confirm continued expression of phenotypic markers of contractile SMCs, such as α-SMA and smooth muscle-22-α (SM22α) (early stage markers),32 myosin heavy chain (MHC; a late-stage marker),39 and calponin (a mid-stage marker),40 by cells cultured under the different conditions described previously. Details of antibodies used are provided in Supplementary Table S1. Western blotting was also performed to semiquantitatively compare the synthesis of elastolytic MMPs-2 and −9. Western blotting was attempted for elastic matrix assembly proteins, such as LOX, fibrillin-1, and fibulins-4 and −5, although they were poorly detectable at the protein concentrations tested (i.e., 5 μg). Volumes equivalent to 5 μg protein were loaded under reduced conditions onto 4%–12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels for LOX, fibulin, α-SMA, SM22, calponin, and caldesmon, with MES running buffer, and 10% SDS-PAGE gels for the MMPs, fibrillin-1, and MHC with MOPS running buffer, along with a prestained molecular weight ladder (either 3.5–250 kDa for 4%–12% gels, or 10–190 kDa for 10% gels). The gels were then transferred onto nitrocellulose membranes using an iBlot® Transfer system. Blots were blocked for 1 h using a commercially available blocking solution (Li-Cor, Lincoln, NE), incubated overnight with primary antibodies against the respective proteins, and then incubated for 1 h with secondary antibody solutions (Li-Cor). Blots were imaged using an Odyssey® Imaging System (Li-Cor). β-Actin was used as a normalizing protein for all blots. All primary antibodies were purchased from Abcam (Cambridge, MA), except LOX, fibrillin-1, and fibulin-5 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and MMP-9 (Millipore, Billerica, MA), and all western blotting supplies were purchased from Invitrogen, unless otherwise mentioned. More specific information about the antibodies is included within Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/tea).
Protein band intensities were measured using Image-J (NIH) software in relative density units (RDUs), normalized to the corresponding β-actin bands in the same blots and reported as a fold change in normalized RDU values relative to treatment control samples. Samples from three biological replicates were analyzed for each protein, except for the SMC markers (n=1 blot each), for which semiquantitative analysis of band intensities was not performed.
Gelatin zymography for detection of enzyme activities of MMPs-2 and −9
Gelatin zymography was performed on the RIPA-buffer-lysed samples. Volume equivalents of 6 μg of protein were loaded into each lane of a 10% gelatin zymogram (Invitrogen), alongside a prestained protein ladder (Invitrogen), and purified protein standards of MMPs-2 and −9 (Anaspec, Fremont, CA). The gels were developed and destained to estimate the activity of MMPs-2 and −9 proportional to the extent of gelatin-digestion bands in the gels. Band intensities were quantified (n=3/case) and reported as outlined in the previous section.
Visualization of elastic matrix
Histology and immunolabeling were performed to visualize the distribution and ultrastructure of elastic matrix generated within the control and test collagen gel constructs. For histology and immunolabeling, one segment from each experimental case was fixed in 4% w/v paraformaldehyde and embedded in paraffin. For immunolabeling, sections (10-μm thick) were deparaffinized and epitope-retrieved in 10 mM citrate buffer. Sections were then blocked in 5% v/v goat serum and immunolabeled for elastin (1:100 primary, overnight, 4°C) and fibrillin-1 (1:50 primary, overnight, 4°C), with Alexa Flour®-633 (Invitrogen) secondary antibody (1:1000, 1 h at room temperature). The labeled sections were mounted in Vectashield with DAPI (Vector Laboratories, Inc., Burlingame, CA) to stain for nuclei, cover-slipped, and visualized. Both cross-sections and longitudinal sections of the constructs were labeled and imaged. For histological visualization of elastic matrix, paraffin sections (30-μm thick, cross and longitudinal) were stained using a commercially available VVG-based staining kit (ScyTek Laboratories, Inc., Cache, UT), according to the manufacturer's instructions.
Mechanical testing of constructs
Tensile properties of the tubular collagen constructs were analyzed on 0.8-cm-wide ring sections of the constructs (n=6) as outlined by Isenberg and Tranquillo19 and Bashur et al.41 Small ring sections (3–4 rings of 2–3-mm width) flanking each construct segment were imaged using an upright Olympus histology microscope (Pittsburgh, PA) and their initial diameter and thickness were measured using the Olympus DP2 Imaging Software®. Tensile testing was performed using an Instron BioPuls 5988 system (Norwood, MA) with a 10-N load cell, and in a 37°C phosphate-buffered saline bath. The ring specimens were held in place via two stainless steel hooks, which were placed between the metal grips of the instrument. The initial runs performed on test samples indicated that the constructs began to show plastic deformation and failure at very low loads, that is, <1 N. Therefore, the constructs were not subjected to preconditioning as other studies have done.40 Load-to-failure tests were performed at a rate of 10 mm/min. The gauge length (L) was calculated when the construct was truly in tension. This was calculated by dividing the circumference of the construct by 2, and then subtracting the circumference of the stainless steel hooks (half of the top and the bottom, equating to the full circumference of one hook). A simplified equation is shown below, which includes the radius (r):
 |
The thickness was considered to be twice the thickness of the construct, since the stresses were experienced by both sides of the ring. Tensile modulus and yield properties were determined from the engineering stress and strain values. Modulus was calculated as the linear slope of the stress–strain curve, that is, the section spanning a 20%–30% strain range with the highest modulus. A 10%–15% (i.e., 0.32-mm displacement) offset was used to calculate the yield point to determine the stress–strain values.
Statistical analysis
All quantitative results were analyzed from n=6 biological replicate constructs per condition and analytical measurements were made in triplicates, unless otherwise indicated. Results were reported as mean±standard deviation. Statistics was performed with SPSS software using a one-way ANOVA and Fisher LSD multiple comparisons, and results were deemed significant for p-values≤0.05.
Results
Compaction of tissue constructs and cell quantification
The degree of contraction (OD) and the aspect ratios (length:OD) of constructs did not differ significantly across treatment groups and in general exhibited values of ∼11 (Supplementary Fig. S1). A DNA assay performed on tubular collagen constructs indicated that constructs cultured under the different sets of conditions did not exhibit any statistically significant differences in their cell proliferation ratios (i.e., cell number at 21 days/cell number at 1 day) (Fig. 2). The cell densities appear to be less than the seeding density (i.e., 25,000 cells/mg collagen), but this is largely due to the inefficient seeding with many of the cells washed away during the first media change.
FIG. 2.
Effect of the addition of elastogenic factors (EFs) and dynamic conditioning on cell density after 21 days of treatment. No significant differences were observed between different treatment conditions.
Analysis of SMC phenotypic markers
RT-PCR analysis of SMC contractile (ACTA2 and caldesmon) and activated (osteopontin) phenotypic markers was performed to assess how the presence of the EFs and concomitant application of cyclic stretch to the constructs at the different frequencies altered the HASMC phenotype (Fig. 3A). Addition of EFs did not affect expression of the gene (ACTA2) for the early stage contractile marker α-SMA by HASMCs within statically cultured constructs. A 2.2±0.4-fold increase in ACTA2 expression relative to treatment controls (p=0.004) and an increase relative to static constructs cultured with EFs (p=0.02) were observed in the EF-treated constructs conditioned at 0.5 Hz. The fold increases in ACTA2 expression were further increased to 4.1±0.99-fold in constructs treated with EFs and conditioned at 1.5 Hz, relative to treatment controls (p<0.001). However, further increasing the stretch frequency to 3 Hz in the presence of EFs diminished cellular ACTA2 expression to levels observed in static constructs with EFs (1.9±0.2-fold vs. no EF, static constructs; p=0.06). ACTA2 expression was significantly higher in constructs with EFs under 1.5 Hz relative to all treatment conditions (p<0.001). Expression of caldesmon mRNA in statically cultured constructs was unchanged upon exposure to the EFs. However, caldesmon gene expression was increased in EF-treated constructs stretched at frequencies of 0.5 and 1.5 Hz versus no EF, static controls (p<0.001), and versus static constructs cultured with EFs (p≤0.003). Caldesmon gene expression in EF-treated constructs stretched at 3 Hz was again lower than that observed at lower stretch frequencies, and was similar to static constructs with or without EFs (1.1±0.3 relative to no EF, static control, p>0.95).
FIG. 3.

Effect of the addition of EFs and cyclic stretch on smooth muscle cell (SMC) phenotype. (A) Fold change in mRNA expression of SMC phenotypic markers from the static EF control. Frequency-dependent regulation of gene expression of ACTA2, caldesmon, and osteopontin was observed. “*” Represents significant difference from control, “#” from static EFs, “+” from EFs+3 Hz, and “$” relative to every other condition, for p≤0.05. (B) Representative western blots of contractile SMC phenotypic markers, along with normalizing protein β-actin. Contractile marker band intensities appeared to be higher in the three stretched constructs relative to static constructs. Color images available online at www.liebertpub.com/tea
Expression of the osteopontin gene was unchanged upon culturing static constructs with the EFs and upon subjecting EF-treated constructs to low-frequency (0.5 and 1.5 Hz) stretch. On the other hand, osteopontin gene expression was elevated when EF-treated constructs were cultured under 3 Hz cyclic stretch conditions (p<0.001, relative to no EF, static controls). This increase was also statistically significant compared with all other treatment conditions (p<0.001).
Western blotting confirmed that contractile SMC phenotypic marker proteins (Fig. 3B) were expressed within constructs subjected to the different treatments. Similar to the gene expression trends, band intensities of α-SMA, calponin, and SM22 proteins appeared higher in all constructs that were dynamically conditioned. There were no differences in the band intensities corresponding to static constructs cultured without and with the EFs.
Elastic matrix synthesis
RT-PCR was performed to evaluate the effects of EFs and cyclic strains applied at different frequencies on expression of genes encoding for tropoelastin and other proteins involved in elastic matrix assembly, such as fibrillin-1, fibulin-5, and LOX (Fig. 4A). Culture of static constructs with EFs significantly increased elastin gene expression to 4.8±0.4-fold (p<0.001), relative to static constructs cultured with no EFs. Constructs cultured with EFs and stretched at 0.5 Hz showed a 3.1±0.5-fold increase in elastin gene expression relative to controls (p=0.001), which was lower than the increases seen in static constructs cultured with EFs (p=0.003). Increasing the frequency of applied cyclic stretch to 1.5 Hz significantly increased elastin gene expression in EF-treated constructs further to 6.7±1.5-fold relative to controls (p≤0.002 relative to all treatment conditions). However, at even greater stretch frequency (3 Hz), elastin gene expression remained at levels seen in static, EF-untreated control constructs.
FIG. 4.
Effect of the addition of EFs and varying cyclic strain frequency on elastic matrix outcomes. (A) Fold change in mRNA expression of genes encoding elastic matrix proteins elastin, fibrillin-1, fibulin-5, and lysyl oxidase (LOX). (B) Matrix elastin content was measured in terms of alkali-soluble and -insoluble elastin and normalized to mg dry weight of constructs. Significant differences represented by “*” from control, “#” from static EFs, “+” from EFs+3 Hz, “$” relative to every other treatment condition, and “@” between conditions indicated, for p≤0.05.
A 2.0±0.6-fold increase in fibrillin-1 gene expression was observed in static constructs cultured with EFs over static, no-EF control constructs (p=0.007). Expression of fibrillin-1 mRNA in the constructs cultured with EFs was in general higher in the cyclically stretched constructs relative to the control, but was only statistically different for constructs stretched at 1.5 Hz (1.8±0.5-fold; p=0.045). However, there were no statistical differences in level of fibrillin-1 gene expression between any of the four EF-treated construct groups. Supplementation of statically cultured constructs with EFs or concomitant application of cyclic stretch at all tested stretch frequencies had no significant effect on fibulin-5 mRNA expression, which in all conditions remained unchanged from controls.
Treatment-specific differences in expression of LOX mRNA showed trends similar to that for tropoelastin mRNA. Culturing static constructs with EFs caused a 2.8±1.1-fold increase in LOX gene expression relative to controls (p=0.005). Application of strains at 0.5 and 3 Hz to EF-treated constructs did not significantly alter LOX gene expression relative to controls. However at the intermediate 1.5 Hz, LOX gene expression was increased by 2.7±1.2-fold (p=0.01) versus control. The LOX expression at the 1.5 Hz frequency was also significantly higher than expression levels in constructs stretched at 3 Hz (p<0.02).
Matrix elastin content was quantified as the sum of the amounts of the alkali-soluble and more highly crosslinked alkali-insoluble elastic matrix fractions. As seen in Figure 4B, addition of EFs modestly increased alkali-soluble matrix elastin content in static cultures, though it was not statistically different from the controls. However, application of cyclic strains in the presence of EFs did improve alkali-soluble matrix elastin output in a frequency-dependent manner, with increases seen at 0.5 and 1.5 Hz, although it was only in the latter case that the increase was statistically significant (fivefold increase relative to controls and threefold relative to static constructs with EFs, p<0.001). Application of stretch at a higher 3 Hz frequency, however, resulted in decreased production of alkali-soluble matrix elastin relative to controls, although the change was not significantly different. It is important to note that the differences in alkali-soluble elastin content between treatment groups were not statistically significant except for the constructs treated with EFs at 1.5 Hz stretch where elastic matrix amounts were significantly greater than in the other cases (p<0.001). Insoluble matrix elastin production was increased in statically maintained constructs cultured with EFs relative to static, no-EF controls (3.9±0.6 μg/mg vs. 2.2±0.4 μg/mg; p=0.026). However, EF-treated constructs that were concurrently subjected to cyclic stretch contained alkali-insoluble elastic matrix amounts that were not different from the controls.
MMP-2 production and activity
RT-PCR, western blotting, and zymography were performed to analyze treatment-specific expression, synthesis, and activity of elastolytic enzymes synthesized by SMCs (i.e., MMPs-2 and −9). As seen in Figure 5A, relative to the controls, mRNA expression of MMP-2 remained unchanged within constructs treated with EFs under static conditions and additionally subjected to cyclic stretch applied at 0.5 and 1.5 Hz, relative to controls. However, when EF-treated constructs were stretched at 3 Hz, a 1.9±0.3-fold increase in MMP-2 mRNA expression was observed relative to controls, which was significantly higher than the expression at all other treatment conditions (p≤0.001). As seen in Figure 5B and C, zymography and western blotting performed to estimate total protein content of zymogen and active forms of MMP-2 did not reveal any significant differences among the treatment conditions, both static and dynamic.
FIG. 5.
Effect of the addition of EFs and the change in cyclic stretch frequency on matrix metalloproteinase-2 (MMP-2) synthesis and activity. (A) Fold change in mRNA expression relative to control. Shown are representative (B) zymogram and (C) western blots showing MMP-2 band intensities at both active and zymogen forms. Semiquantitative analysis of the change in MMP-2 enzyme activity relative to control for western blot is also included. Color images available online at www.liebertpub.com/tea
MMP-9 production and activity
Expression of MMP-9 mRNA was uniformly low in all constructs, and their Ct values were at or below the detection limits of the instrument at the cDNA concentrations tested. Similarly, bands corresponding to either active or inactive forms of the protease were too low to be detected by zymography at the protein concentrations tested (5 μg/case). However, western blotting analysis of MMP-9 (Fig. 6A, B) showed increase in band intensities of both active and zymogen forms of MMP-9 in EF-treated constructs subjected to cyclic stretch at frequencies of 1.5 and 3 Hz. Relative to control, 1.6±0.5-fold increase in both active and inactive forms of MMP-9 was seen under 1.5 Hz (p<0.05). With the application of stretch at 3 Hz, increase of 1.7±0.5-fold for MMP-9 zymogen and 1.4±0.1-fold for active MMP-9 was seen relative to the controls (p<0.04). This fold increase in MMP-9 synthesis observed for the 1.5 and 3 Hz stretch conditions was significantly higher than the static+EFs condition and EFs+0.5 Hz stretch conditions as well (p<0.04).
FIG. 6.

Effect of the change in cyclic strain frequency and addition of EFs on MMP-9 protein content. (A) Representative western blot of MMP-9 showing active and zymogen forms of the enzyme. (B) Semiquantitative analysis of fold change in relative density unit (RDU) of band intensities of both forms of the protein relative to control. Significant differences are represented by “*” from control, “#” from static EFs, and “+” from EFs+0.5 Hz, for p≤0.05. Color images available online at www.liebertpub.com/tea
Matrix ultrastructure
Cross-sections and longitudinal sections of all treatment conditions were labeled for elastin and fibrillin-1 proteins. As seen in Figures 7–9, cells and fibers were found to be oriented more in the longitudinal than in the circumferential direction. Application of stretch was found to improve orientation in the circumferential direction in the regions closer to the silicone tubing, and progressively decreased in regions further away from it. However, in the longitudinal direction, as seen both in VVG-stained images (Fig. 7) and the IF images (Fig. 8), mature elastic fibers were found in all the cases. Importantly, the distribution of these fibers is not uniform across the width or circumference of the constructs, as they appear to be denser in some regions more than others. This is not unexpected due to the viscous losses within hydrogels. Immunolabeling of elastin and fibrillin-1 (Figs. 8 and 9) showed that these matrix proteins have similar fibrous structures.
FIG. 7.

Effects of EF treatment and variation of strain frequency on matrix assembly and orientation. Representative images of 30-μm-thick longitudinal sections (scale bar=50 μm, 20× magnification) and cross-sections (scale bar=100 μm, 10× magnification) of all constructs stained with modified VVG. Elastic fibers, stained dark purple, within cyclically strained constructs appear oriented more toward the longitudinal direction, than in the circumferential direction. Circumferential alignment can be seen closer to the lumen (L) within strained constructs, which does not appear to have uniformly translated across the thickness of the constructs. Color images available online at www.liebertpub.com/tea
FIG. 8.
Effects of EF treatment and cyclic strain frequency variation on synthesis and assembly of elastin. Representative images of 10-μm-thick longitudinal (scale bar=50 μm, 20× magnification) and cross-sections (scale bar=100 μm, 10× magnification) of constructs immunolabeled for elastin (red) and nuclei (blue). Longer fibers of elastin are seen in the longitudinal direction than circumferentially. White arrows indicate longitudinal direction of constructs. L, lumen. Color images available online at www.liebertpub.com/tea
FIG. 9.

Effect of EF treatment and varying frequency of stretch on fibrillin assembly and orientation. Representative fluorescent images of 10-μm-thick longitudinal sections of constructs immunolabeled for fibrillin (red) and nuclei (DAPI). White arrows indicate longitudinal direction of constructs. L, lumen. Scale bar=50 μm, 20× magnification. Color images available online at www.liebertpub.com/tea
Mechanical properties of constructs
Ring specimens of the compacted gels were tested to correlate ECM composition and organization to the resulting tensile properties on the conduits. Stress–strain curves for the samples exhibited an initial toe region while the rings were straightening, prior to a region of increased slope when the rings were in tension (Fig. 10A). Variation between conditions was found after the yield region. The stresses continued to increase after yield for the static controls, something that was not observed for the conditions with cyclic conditioning. These graphs represent the sample for each condition with the median modulus and yield stress. Few significant differences in tensile modulus with treatment conditions were observed (Fig. 10B). The tensile modulus for constructs cultured at 1.5 Hz (1.9±1.1 kPa) was not statistically different than those under static conditions. The constructs cultured with 3 Hz frequency had a lower modulus than the no-EF, static controls (p=0.048). Differences in gel contraction between samples within individual conditions contributed to the large error bars.
FIG. 10.
Effect of EF treatment and varying frequency of stretch on construct tensile properties. (A) Representative stress–strain curves for each of the experimental conditions. Construct tensile property results including (B) yield stress, (C) yield strain, and (D) modulus. Significant differences are represented by “*” from control, “#” from static EFs, and “+” from EFs+3.0 Hz, for p≤0.05. Color images available online at www.liebertpub.com/tea
As seen in Figure 10B, yield stress was found to exhibit similar trends as the modulus. Yield stress was significantly lower for all constructs with dynamic conditioning compared with static controls (p<0.037). No statistical differences were found between constructs with different strain frequencies. Yield strains were the lowest for constructs with EFs at 0.5 Hz, and significantly lower than for no-EF static, EF static, and EFs at 3 Hz (p=0.037, 0.002, and 0.009, respectively). Unlike modulus and yield stress, EFs at 3 Hz had a significantly higher yield strain than the EFs at 1.5 Hz condition (p=0.024).
Discussion
Among the various hemodynamic forces encountered in the vasculature, cyclic circumferential strains most critically influence vascular SMCs.15 Cyclic strains serve to regulate integrin-mediated, mechanotransductive effects on vascular SMC phenotypic state, matrix synthesis, and orientation.21,22 The cellular response to these strains is dependent on several parameters (e.g., strain amplitude, frequency, and duration of cyclic stretch).21,22 The impact of strain amplitude on SMCs and their matrix production has been previously reported in several studies.16,19,23,24 Less is known about the impact of strain frequency, especially on elastic matrix generation by SMCs cultured with EFs. Thus, in this study we systematically varied the cyclic stretch frequency to determine its impact on SMC proliferation, phenotype, and ECM production.
The addition of EFs by themselves impacted SMC elastogenesis. Similar to our previous study with rat SMCs,42 this study with human SMCs did not show any differences in cell densities after 21 days of treatment with a low dose of EFs (i.e., 0.1 ng/mL TGF-β1 and 0.2 μg/mL HA-o). While we did find evidence of the elastogenic benefit of EFs relative to static, no-EF controls, it was not as dramatic as we had previously observed in the static model. This can be attributed to possible limitations to in-diffusion and bioavailability of exogenous EFs to cells within the 3D constructs. While higher doses of the factors, and of TGF-β in particular, have been shown to attenuate SMC proliferation,42 even the low dose used in this study did not impact this. At the tested doses, the EFs also increased tropoelastin gene expression more than fourfold for static cultures, although the increases in alkali-soluble elastic matrix content were not statistically significant. This result was surprising since our previous study with rectangular collagen constructs showed that the EFs significantly enhance matrix elastin deposition, particularly the alkali-soluble matrix elastin.42 These differences could be due to the fact that human SMCs are induced to a lesser extent by EFs than rat SMCs.30 This result supports the need to provide additional stimuli, such as cyclic stretch, to further improve elastic matrix generation by human SMCs.
The cell densities in this study were comparable for both static and dynamic cultures. This limited impact of cyclic stretch on cell densities is in accordance with other studies that applied 2.5%–10% strains at frequencies of 0.5–1 Hz to collagen gel scaffolds.19,24 However, a study by Kim et al. noted increased cell proliferation at 7% strains (frequency 0.050–0.25 Hz) for SMCs in poly(glycolic acid) (PGA) scaffolds.16 It is likely that collagen gels, which exhibit very high viscous losses with strain, will impact the transfer of applied forces to SMCs.19 However, even though the strain levels applied in this study did not produce a change in cell density, we have shown that they significantly impact other aspects of SMC phenotype in a cyclic-stretch-frequency-dependent manner.
In our study, mRNA expression of the two contractile phenotypic markers tested (i.e., ACTA2 and caldesmon) increased systematically with increasing strain frequencies from 0.5 to 1.5 Hz, while osteopontin, a marker of an activated SMC phenotype, was not impacted by strain frequencies up to 1.5 Hz. Cyclic stretch of a variety of strain frequencies is also known to improve the expression of various contractile phenotypic markers of SMCs, such as α-SMA, caldesmon, and MHC.18 However, at a 3 Hz frequency, the levels of the contractile markers dropped significantly, while osteopontin gene expression was elevated at this higher frequency. The fact that all frequencies of cyclic strain did not downregulate osteopontin expression appears to contrasts with a study by Nikolovski et al. where this gene was downregulated by the addition of cyclic stretch at 7% strain and 1 Hz frequency.43 The impact of frequency in their study is not however directly comparable with ours, partially because a preprepared collagen sponge was used that would likely result in differences in force transduction to the cells. Overall, these studies suggest that cyclic strain can have beneficial or negative effects depending on the particular experimental system. Osteopontin expression in SMCs has been linked to injury and cell damage,44 and its upregulation is a concern for engineered vascular constructs. Since our TGF-β1 dose is 100-fold lower than the concentration that is known to induce osteogenic differentiation of SMCs,45 the osteopontin upregulation is likely due to cellular damage from the high, 3 Hz strain frequency.
It is known that cyclic stretch can either stimulate cells or lead to cell damage and apoptosis depending on the level of stimuli.43,46 Most commonly this shift has been observed in response to excessively high strain magnitudes (e.g., 10%).46 However, it is possible that high strain frequencies can also lead to cell damage. Any impact of strain frequency on vascular SMCs would modulate the cells through integrin receptors.28 While articles show that this signaling response is strain frequency dependent,28,29 the exact mechanism of frequency sensitivity in SMCs is not known. Since the phenotype of SMCs changed with cyclic strain frequency in our study, we also expected to observe differences in the cellular production of ECM.
A frequency-dependent change in both the tropoelastin mRNA expression and soluble matrix elastin generation was observed with cyclic stretch, similar to the results for contractile markers. The most elastogenic strain frequency was 1.5 Hz, while the higher 3 Hz frequency that promoted osteopontin expression also reduced elastogenesis. Overall, these results suggest that cyclic strain has an increasingly positive impact on SMC elastogenesis with increasing frequency up to the point where cell damage appears to occur. However, in this study, other proteins critical to elastic fiber formation (e.g., fibrillin-1 and LOX) exhibited less mRNA upregulation, and low levels of generated protein, for all strain frequencies. These proteins are important since fibrillin-1 is the main component of the microfibrillar scaffolds on which tropoelastin units coacervate34 and LOX is a Cu2+-dependent enzyme that crosslinks the coacervated tropoelastin molecules.34,47 Low levels of these other elastic matrix components could potentially have contributed to limited amount of the stable, highly crosslinked alkali-insoluble elastin found in this study.
The less-crosslinked elastic matrix found in tissue-engineered constructs is very susceptible to degradation by proteases. Thus, we analyzed the presence of elastic-matrix-degrading gelatinases, MMPs-2 and −9, which are known to be secreted by SMCs. We found that MMP-2 gene expression was only significantly upregulated when strain was applied with a 3 Hz frequency, consistent with an environment with more cellular stress.26 However, the total MMP-2 protein content and enzyme activity were both comparable among all treatment conditions. This finding is in contrast to that observed by other groups, wherein both gene expression and enzyme activity of MMP-2 have been reported to increase under cyclic stretch relative to static controls.25,26 The reason for this difference is not known, but it could be attributed to the use of low strains (i.e., 2.5%) in our studies, compared with the 10% strains used in other studies. On the other hand, moderate increase in MMP-9 protein content, both zymogen and active forms, was seen in constructs stretched with the higher frequencies of 1.5 and 3 Hz. An increase in proteases and matrix remodeling is expected with mechanical stretch, but overall, the results from this study indicate that the only strain frequency where overexpression of MMPs is potentially a significant concern is the 3 Hz frequency condition. At this frequency, the high levels of MMPs could potentially impact elastic matrix quantity, maturity, and ultrastructure.
The matrix ultrastructure and orientation of cells in the scaffolds were contrary to what we had hypothesized. In particular, the cells and elastic fibers appear to be oriented longitudinally instead of circumferentially, even in the static conditions. The application of appropriate frequencies of circumferential cyclic stretch has the potential to change this alignment to the circumferential direction, as it has been reported that maximum cell alignment is usually obtained in frequencies between 0.5 and 1.25 Hz.28,29 Surprisingly, our study showed only limited impact of cyclic stretch on cell and collagen fibril realignment. It is possible that levels of mechanical stimulus required to induce cell orientation and to increase the expression of SMC contractile markers (i.e., something that we did observe) are different. It is also possible that higher strain magnitudes might be needed to induce the required cell circumferential orientation. Finally, we cannot rule out the possibility that the actual applied strains in our bioreactor may have decreased over time due to water permeability of silicone tubing and an osmotic pressure gradient.
Consistent with the cell alignment results in this study, limited impact of cyclic stretch frequency on tensile properties of the resulting conduit was observed. However, there was a very low modulus and yield stress for all conditions. These tensile properties are significantly lower than what we had observed in our unpublished study with static rectangular strips. One possible reason for the low tensile properties in this study is the lower SMC seeding density, and more cells may be needed for the initial seeding when producing tissue-engineered vascular constructs.
Conclusion
In this study we have demonstrated a positive influence of the application of dynamic stimulation along with EFs up to 1.5 Hz frequency. Both increases in mRNA expression of genes encoding for proteins involved in elastic matrix assembly as well as increase in alkali-soluble matrix elastin content were found to be highest in the constructs treated with EFs and stretched at frequency of 1.5 Hz. While the application of lower strain amplitude helped in preventing an increase in MMP-2 levels, it could have also contributed to the lack of a high degree of circumferential orientation. Alkali-insoluble matrix elastin also remained unchanged with the addition of dynamic stimulation. Together, these factors also likely resulted in comparable mechanical properties between stretched and static constructs. While this study provides evidence of the positive influence of combining circumferential stretch with biochemical stimulation, further studies are warranted to target synthesis of mature elastin and cellular orientation specifically.
Supplementary Material
Acknowledgments
This study was supported by NIH Grants from the National Heart, Lung, and Blood Institute (HL092051-01A1 and HL092051-01S) and the National Institute of Biomedical Imaging and Bioengineering (EB006078-01A1) awarded to A.R.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kielty C.M.Elastic fibres in health and disease. Expert Rev Mol Med 8,1, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Li D.Y., Brooke B., Davis E.C., Mecham R.P., Sorensen L.K., Boak B.B., Eichwald E., and Keating M.T.Elastin is an essential determinant of arterial morphogenesis. Nature 393,276, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell S.L., and Niklason L.E.Requirements for growing tissue-engineered vascular grafts. Cardiovasc Pathol 12,59, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Patel A., Fine B., Sandig M., and Mequanint K.Elastin biosynthesis: the missing link in tissue-engineered blood vessels. Cardiovasc Res 71,40, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Long J.L., and Tranquillo R.T.Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol 22,339, 2003 [DOI] [PubMed] [Google Scholar]
- 6.L'Heureux N., Paquet S., Labbe R., Germain L., and Auger F.A.A completely biological tissue-engineered human blood vessel. FASEB J 12,47, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Kim B.S., and Mooney D.J.Engineering smooth muscle tissue with a predefined structure. J Biomed Mater Res 41,322, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Shaikh F.M., Callanan A., Kavanagh E.G., Burke P.E., Grace P.A., and McGloughlin T.M.Fibrin: a natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs 188,333, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Swartz D.D., Russell J.A., and Andreadis S.T.Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol 288,H1451, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Zhang X., Baughman C.B., and Kaplan D.L.In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials 29,2217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramamurthi A., and Vesely I.Evaluation of the matrix-synthesis potential of crosslinked hyaluronan gels for tissue engineering of aortic heart valves. Biomaterials 26,999, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Gao J., Crapo P., Nerem R., and Wang Y.Co-expression of elastin and collagen leads to highly compliant engineered blood vessels. J Biomed Mater Res A 85,1120, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Liu J.Y., Swartz D.D., Peng H.F., Gugino S.F., Russell J.A., and Andreadis S.T.Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res 75,618, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Venkataraman L., and Ramamurthi A.Induced elastic matrix deposition within three-dimensional collagen scaffolds. Tissue Eng Part A 17,2879, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagenseil J.E., and Mecham R.P.Vascular extracellular matrix and arterial mechanics. Physiol Rev 89,957, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim B.S., Nikolovski J., Bonadio J., and Mooney D.J.Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol 17,979, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Shi Y., and Vesely I.Characterization of statically loaded tissue-engineered mitral valve chordae tendineae. J Biomed Mater Res A 69,26, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Stegemann J.P., and Nerem R.M.Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp Cell Res 283,146, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Isenberg B.C., and Tranquillo R.T.Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng 31,937, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Seliktar D., Black R.A., Vito R.P., and Nerem R.M.Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng 28,351, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Gupta V., and Grande-Allen K.J.Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res 72,375, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Haga J.H., Li Y.S., and Chien S.Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech 40,947, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Kona S., Chellamuthu P., Xu H., Hills S.R., and Nguyen K.T.Effects of cyclic strain and growth factors on vascular smooth muscle cell responses. Open Biomed Eng J 3,28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schutte S.C., Chen Z., Brockbank K.G., and Nerem R.M.Cyclic strain improves strength and function of a collagen-based tissue-engineered vascular media. Tissue Eng Part A 16,3149, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Seliktar D., Nerem R.M., and Galis Z.S.The role of matrix metalloproteinase-2 in the remodeling of cell-seeded vascular constructs subjected to cyclic strain. Ann Biomed Eng 29,923, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Grote K., Flach I., Luchtefeld M., Akin E., Holland S.M., Drexler H., and Schieffer B.Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92,e80, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Galis Z.S., and Khatri J.J.Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90,251, 2002 [PubMed] [Google Scholar]
- 28.Liu B., Qu M.J., Qin K.R., Li H., Li Z.K., Shen B.R., and Jiang Z.L.Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro. Biophys J 94,1497, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu M.J., Liu B., Wang H.Q., Yan Z.Q., Shen B.R., and Jiang Z.L.Frequency-dependent phenotype modulation of vascular smooth muscle cells under cyclic mechanical strain. J Vasc Res 44,345, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Gacchina C., Brothers T., and Ramamurthi A.Evaluating smooth muscle cells from CaCl2-induced rat aortal expansions as a surrogate culture model for study of elastogenic induction of human aneurysmal cells. Tissue Eng Part A 17,1945, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labarca C., and Paigen K.A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 102,344, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Rensen S.S., Doevendans P.A., and van Eys G.J.Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15,100, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gadeau A.P., Campan M., Millet D., Candresse T., and Desgranges C.Osteopontin overexpression is associated with arterial smooth muscle cell proliferation in vitro. Arterioscler Thromb 13,120, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Wagenseil J.E., and Mecham R.P.New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 81,229, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Kagan H.M., and Li W.Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 88,660, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Keeling W.B., Armstrong P.A., Stone P.A., Bandyk D.F., and Shames M.L.An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc Endovascular Surg 39,457, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Marshall O.J.PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20,2471, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Miano J.M., Cserjesi P., Ligon K.L., Periasamy M., and Olson E.N.Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res 75,803, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Hughes S., and Chan-Ling T.Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci 45,2795, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Bashur C.A., Eagleton M.J., and Ramamurthi A.Impact of electrospun conduit fiber diameter and enclosing pouch pore size on vascular constructs grown within rat peritoneal cavities. Tissue Eng Part A 19,809, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkataraman L., and Ramamurthi A.Induced elastic matrix deposition within three-dimensional collagen scaffolds. Tissue Eng Part A 17,2879, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikolovski J., Kim B.S., and Mooney D.J.Cyclic strain inhibits switching of smooth muscle cells to an osteoblast-like phenotype. FASEB J 17,455, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Moses S., Franzen A., Lovdahl C., and Hultgardh-Nilsson A.Injury-induced osteopontin gene expression in rat arterial smooth muscle cells is dependent on mitogen-activated protein kinases ERK1/ERK2. Arch Biochem Biophys 396,133, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Simionescu A., Philips K., and Vyavahare N.Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun 334,524, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Wernig F., Mayr M., and Xu Q.Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by beta1-integrin signaling pathways. Hypertension 41,903, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Narayanan A.S., Page R.C., Kuzan F., and Cooper C.G.Elastin cross-linking in vitro. Studies on factors influencing the formation of desmosines by lysyl oxidase action on tropoelastin. Biochem J 173,857, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.