Abstract
Background and objectives
The two largest studies of mammalian target of rapamycin inhibitor treatment of autosomal dominant polycystic kidney disease (ADPKD) demonstrated no clear benefit on the primary endpoint of total kidney volume (TKV) or on eGFR. The present study evaluated two levels of rapamycin on the 12-month change in 125I-iothalamate GFR (iGFR) as the primary endpoint and TKV secondarily.
Design, setting, participants, & measurements
In a 12-month open-label pilot study, 30 adult patients with ADPKD were randomly assigned to low-dose (LD) rapamycin (rapamycin trough blood level, 2–5 ng/ml) (LD group, n=10), standard-dose (STD) rapamycin trough level (>5–8 ng/ml) (STD group, n=10), or standard care (SC group, n=10). They were evaluated with iGFR and noncontrast computed tomography.
Results
Change in iGFR at 12 months was significantly higher in the LD group (7.7±12.5 ml/min per 1.73 m2; n=9) than in the SC group (−11.2±9.1 ml/min per 1.73 m2; n=9) (LD versus SC: P<0.01). Change in iGFR at 12 months in the STD group (1.6±12.1 ml/min per 1.73 m2; n=8) was not significantly greater than that in the SC group (P=0.07), but it was in the combined treatment groups (LD+STD versus SC: P<0.01). Neither eGFR calculated by the CKD-Epidemiology Collaboration equation nor TKV (secondary endpoint) changed significantly from baseline to 12 months in any of the groups. On the basis of results of the mixed model, during the study, patients in the LD group had significantly lower trough blood levels of rapamycin (mean range±SD, 2.40±0.64 to 2.90±1.20 ng/ml) compared with those in the STD group (3.93±2.27 to 5.77±1.06 ng/ml) (P<0.01).
Conclusion
Patients with ADPKD receiving LD rapamycin demonstrated a significant increase in iGFR compared with those receiving standard care, without a significant effect on TKV after 12 months.
Keywords: rapamycin, ADPKD, iothalamate GFR
Introduction
Progressive renal failure in autosomal dominant polycystic kidney disease (ADPKD) (1) has been associated with cystic expansion of total kidney volume (TKV) (2–4) and height-adjusted TKV (5). Cyst development arises from the primary loss-of-function germline mutation (6,7) and “second-hit” somatic mutation (8), and it can be accelerated by a “third hit,” such as ischemia-reperfusion injury (9). Damaging effects of cysts depend primarily on their size and location, and eventually hyperfiltration fails as a compensatory mechanism (10).
Experimental therapies for ADPKD (11,12) include rapamycin, a mammalian target of rapamycin (mTOR) inhibitor (13–25). Prolonged rapamycin treatment inhibits not only mTOR complex 1 (mTORC1, raptor) activity but also mTOR complex 2 (mTORC2, rictor) assembly and Akt/protein kinase B (13,14). mTORC1 affects cell growth and proliferation; mTORC2 affects cell survival, cell polarity, and cytoskeletal organization (13). There is genetic, biochemical, and pharmacologic evidence for excessive mTOR activity promoting cystogenesis in PKD (15).
However, in humans with ADPKD, the mTOR inhibitors rapamycin and everolimus have demonstrated no clear-cut benefit (16–18). After 2 years of everolimus treatment, increasing TKV slowed transiently but renal function worsened (16); 18 months of rapamycin therapy had no effect on TKV but was associated with a trend toward slowing of renal function decline (17); and 6 months of rapamycin treatment had no protective effect on renal function or TKV but was associated with a decrease in the relative change and a trend toward an absolute change in renal cystic volume (18). In human ADPKD studies, GFR was estimated by the Modification of Diet in Renal Disease Study and CKD-Epidemiology Collaboration (CKD-EPI) equations (16,17,26) or measured by plasma clearance of nonradioactive iohexol (18). In this pilot study, our hypothesis was that a more accurate measure of change in GFR using 125I-iothalamate (primary endpoint) might reveal a treatment effect with better tolerated low-dose rapamycin treatment for 12 months.
Materials and Methods
Patients
Thirty patients age 21–66 years with ADPKD (27) and an iGFR≥25 ml/min per 1.73 m2 were enrolled with their informed consent from December 2006 to July 2009 in a 12-month open-label pilot study of rapamycin treatment. Exclusion criteria were diabetes mellitus, hospitalization for acute illness in the previous 2 months, life expectancy <2 years, pregnancy, history of nonadherence or drug or alcohol dependency, serious psychiatric illness, proteinuria>500 mg/24 hours, fasting hypertriglyceridemia>400 mg/dl or LDL cholesterolemia>190 mg/dl despite treatment, thrombocytopenia<100,000 platelets/µl, use of systemic immunosuppressive drugs, or participation in another interventional study. The study was approved by the institutional review board and registered (ClinicalTrials.gov ID: NCT00286156). Patients were randomly assigned in this pilot study to one of three groups: low-dose (LD) rapamycin with trough blood levels (TBLs) of 2–5 ng/ml (LD group; n=10), standard-dose (STD) rapamycin with TBLs>5–8 ng/ml (STD group; n=10), or standard care (SC group; n=10). Rapamycin was provided as a 1-mg Rapamune (Pfizer, Inc., New York, NY) tablet, with doses to achieve the desired blood levels. TBLs were obtained 24 hours after the previous day’s morning dose at weeks 1, 2, 4, and 6 and months 3, 6, 9, and 12. As standard care, fluid intake of 2500–3000 ml/24 hours, primarily water; a low-sodium diet of 2300 mg/24 hours; and caffeine avoidance were recommended to all patients, along with control of hypertension. Adverse events were reviewed every 3 months with an independent physician data safety monitoring officer.
Laboratory and Radiographic Methods
GFR was measured at baseline and 6 and 12 months using 125I-iothalamate and urinary clearances (coefficient of variation, 9.2%) (28). eGFR was calculated by the CKD-EPI equation (26) in the same patients who had iGFR data at those time points. Rapamycin blood levels were measured by liquid chromatography-mass spectrometry (29). All computed tomography (CT) scans were obtained at baseline and 6 and 12 months, without intravenous or oral contrast, on a multidetector CT scanner (Siemens Medical Solutions, Forchheim, Germany) using submillimeter collimation. Images were reconstructed at 3 mm and then 1-cm slice thickness and interval for volume measurements. The liver and renal margins in each contiguous 1-cm-thick slice were traced by hand by a single CT technologist using commercially available software that automatically calculates the area within each tracing. The organ volume was calculated by summing the product of the area measurements for each slice multiplied by the thickness of each slice (1 cm). All measurements were reviewed by an experienced radiologist (B.R.H.). This method has been validated for calculating liver volumes in living donor liver transplantation (30).
Statistical Analyses
The Fisher exact test was used to test the association between study groups and categorical variables. Statistical comparisons of baseline levels and changes in study measures between baseline and follow-up were tested with between-patient ANOVA and t tests. Pairwise group comparisons were conducted with Tukey adjustment for multiple testing. Mixed models were also used for evaluating changes of study measures over time. For mixed models, maximum likelihood was used to estimate effects with missing levels; for ANOVA models, observations with missing values were excluded. The covariance structures for mixed models were selected on the basis of the smallest Akaike information criteria based on iteratively testing common covariance structures. Study measures used for ANOVA and mixed models were tested for normality assumptions according to the Shapiro–Wilk tests, and there was no evidence of departures from normality. A type I error probability <0.05 was considered to represent statistical significance throughout the study.
Results
Baseline demographic characteristics and risks for rapid progression of ADPKD did not significantly differ among the three groups (Table 1). The mean (±SD) age of patients in this study was 49.3±12.0 years. Change in mean arterial blood pressure (MAP) over time was not significantly different by study group based on the mixed model (P=0.34) (Supplemental Table 1). However, the STD rapamycin group did have higher overall MAP levels than both the LD rapamycin group (P=0.03) and the SC group (P=0.01). MAP levels did not change significantly over time by group (P=0.34) or time overall (P=0.26). Significant separation of rapamycin TBLs was achieved between the LD and the STD groups. On the basis of results of the mixed model, during the study the LD group had significantly lower rapamycin TBLs (mean range, 2.40±0.64 to 2.90±1.20 ng/ml) than those in the STD group (mean range, 3.93±2.27 to 5.77±1.06 ng/ml) (P<0.01) (Supplemental Table 2). Of the 136 rapamycin TBLs expected for the 17 rapamycin-treated patients completing the study, 118 (86.8%) were obtained (Supplemental Table 2).
Table 1.
Baseline characteristics and risk factors of the three groups of patients with autosomal dominant polycystic kidney disease
| Variable | Low-Dose Rapamycin (n=10) | Standard-Dose Rapamycin (n=10) | Standard Care (n=10) | P Valuea |
|---|---|---|---|---|
| Mean age±SD (yr) | 44.9±8.6 | 53.2±15.0 | 49.4±11.0 | 0.31 |
| Whites (n) | 10b | 10 | 9 | >0.99 |
| Men (n) | 5 | 5 | 7 | 0.72 |
| Hypertension (BP≥140/90 mmHg before age 35 yr) (31) (n) | 3 | 3 | 5 | 0.71 |
| Family history of ESRD from ADPKD before age 56 yr (6) (n) | 8 | 4 | 7 | 0.25 |
| Initial iGFR 25–59 ml/min per 1.73 m2 (n) | 4 | 3 | 2 | 0.88 |
| Initial TKV>1500 ml (3) (n) | 7 | 6 | 6 | >0.99 |
| Initial height-adjusted TKV≥600 ml/m (5) (n) | 8 | 6 | 6 | 0.70 |
ADPKD, autosomal dominant polycystic kidney disease; iGFR, 125I-iothalamate GFR; TKV, total kidney volume.
Based on ANOVA for continuous variables and Fisher exact test for categorical variables.
Number in each group.
According to ANOVA results for the difference between 12-month and baseline levels, the primary endpoint of change in iGFR was significantly higher in the LD group (7.7±12.5 ml/min per 1.73 m2) but not in the STD group (1.6±12.1 ml/min per 1.73 m2) compared with the SC group (−11.2±9.1 ml/min per 1.73 m2) (LD versus SC: P<0.01; STD versus SC: P=0.07; LD versus STD: P=0.52) (Table 2, Figure 1). The medians (25th, 75th percentiles) for change in iGFR were as follows: LD group, 2.0 (−1.0, 18.0) ml/min per 1.73 m2, respectively; STD group, 2.5 (−7.0, 6.5) ml/min per 1.73 m2; and SC group, −11.0 (−18.0, −1.0) ml/min per 1.73 m2 (Figure 1). The combined treatment groups also had significantly improved iGFR at 12 months (LD+STD versus SC: P<0.01) (Table 2). At 6 months, the change in iGFR was also significantly higher in the LD group than in the SC group (P=0.04) and in the LD+STD versus SC groups (P=0.01), but not in the STD versus SC groups (P=0.09) or the LD versus STD groups (P=0.94) (Table 2). In a mixed model with autoregressive covariance structure, the qualitative findings were consistent with a significant change in iGFR by study group (P for time×group interaction<0.01). Pairwise comparisons between groups using the mixed model results indicated that after accounting for multiple testing, the change in iGFR was statistically significant between the LD and SC groups (P<0.01) but not significant between the LD and STD (P=0.23) or STD and SC (P=0.05) groups. In secondary analyses, change in estimated GFR by the CKD-EPI equation at the same time points in these same patients did not show any significant change (Table 2). These results were also consistent in mixed models indicating no group, group×time, or overall time effect.
Table 2.
125I-Iothalamate glomerular filtration rate, CKD-Epidemiology Collaboration eGFR, and absolute changes during 12 months of rapamycin treatment or standard care
| GFR | Low-Dose Rapamycin (n=10) (ml/min per 1.73 m2) | Standard-Dose Rapamycin (n=10) (ml/min per 1.73 m2) | Standard Care (n=10) (ml/min per 1.73 m2) | P Value |
|---|---|---|---|---|
| iGFR | ||||
| Baseline iGFR (n=30) | 66.2±28.6 (n=10) | 65.0±28.0 (n=10) | 69.0±23.1 (n=10) | 0.94a |
| Baseline iGFR (n=26 with 12-mo data) | 70.3±27.0 (n=9) | 72.8±25.7 (n=8) | 73.1±20.3 (n=9) | 0.97a |
| 6-mo iGFR | 71.3±34.3 | 71.8±31.3 | 59.4±16.2 | |
| iGFR change (n=29) | 5.1±11.4 (n=10) | 3.1±8.3 (n=9) | −9.6±16.6 (n=10) | 0.03a |
| 12-mo iGFR | 78.0±35.0 | 74.4±34.4 | 61.9±15.6 | |
| iGFR change (n=26) | 7.7±12.5 (n=9) | 1.6±12.1 (n=8) | −11.2±9.1 (n=9) | <0.01a |
| CKD-EPI eGFR | ||||
| Baseline eGFR (n=30) | 70.4±33.0 (n=10) | 65.1±33.5 (n=10) | 69.9±18.8 (n=10) | 0.90b |
| Baseline eGFR (n=26 with 12-mo data) | 75.6±30.4 (n=9) | 74.6±30.6 (n=8) | 70.9±19.7 (n=9) | 0.93b |
| 6-mo eGFR | 68.8±33.1 | 72.0±34.0 | 69.6±22.9 | |
| eGFR change (n=29) | −1.7±4.8 (n=10) | 2.6±12.7 (n=9) | −0.4±7.2 (n=10) | 0.56b |
| 12-mo eGFR | 72.4±32.4 | 77.2±31.6 | 71.3±24.4 | |
| eGFR change (n=26) | −3.2±8.1 (n=9) | 2.6±14.3 (n=8) | 0.4±11.6 (n=9) | 0.58b |
iGFR change at 6 months: low dose (LD) versus standard care (SC): P=0.04; STD versus SC: P=0.09; LD versus standard dose (STD): P=0.94; LD+STD versus SC: P=0.01. iGFR change at 12 months: LD versus SC: P<0.01; STD versus SC: P=0.07; LD versus STD: P=0.52; LD+STD versus SC: P<0.01. Median (25th, 75th percentiles) for change in iGFR at 12 months: LD, 2.0 (−1.0, 18.0) ml/min per 1.73 m2, respectively; STD, 2.5 (−7.0, 6.5) ml/min per 1.73 m2, respectively; and SC, −11.0 (−18.0, −1.0) ml/min per 1.73 m2, respectively. Median (25th, 75th percentiles) for change in CKD-EPI eGFR at 12 months: LD, −1.8 (−7.7, −0.7) ml/min per 1.73 m2; STD, 3.5 (−2.0, 9.4) ml/min per 1.73 m2; and SC, −1.3 (−7.5, 7.3) ml/min per 1.73 m2. Values expressed with a plus/minus sign are the mean±SD. CKD-EPI, CKD-Epidemiology Collaboration.
Overall P value based on ANOVA; pairwise comparisons adjusted for multiple testing (Tukey).
Overall P value based on ANOVA.
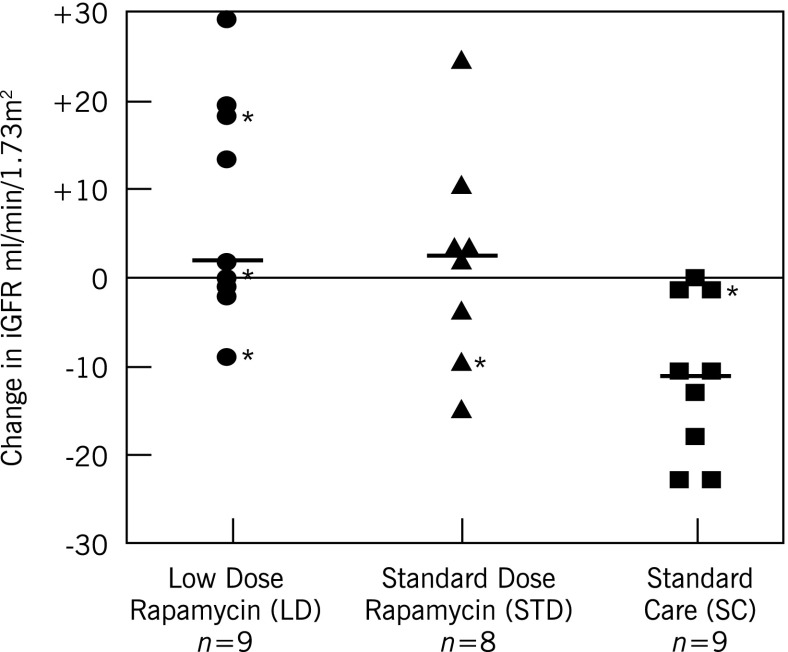
Figure 1.
Individual absolute 125I-iothalamate GFR (iGFR) changes at 12 months with low-dose (LD) or standard-dose (STD) rapamycin or without rapamycin. iGFR change at 12 months (mean±SD): LD, 7.7±12.5; STD, 1.6±12.1; and standard care (SC), −11.2±9.1 ml/min per 1.73 m2. LD versus SC: P<0.01; STD versus SC: P=0.07; LD+STD versus SC: P<0.01; LD versus STD: P=0.52. P values based on Tukey-adjusted t tests for multiple comparisons. Median (—) and 25th and 75th percentile changes in iGFR: LD, 2.0 (−1.0, 18.0) ml/min per 1.73 m2, respectively; STD, (2.5 (−7.0, 6.5) ml/min per 1.73 m2, respectively; and SC, −11.0 (−18.0, −1.0) ml/min per 1.73 m2, respectively. Twenty-one of these 26 patients had an initial iGFR≥60 ml/min per 1.73 m2 and 5 (*) did not.
TKV had not significantly changed at 12 months with LD or STD rapamycin (Table 3, Supplemental Figure 1). These results were consistent in the mixed model using measures at baseline and 6 and 12 months. There was no difference in overall TKV levels (P=0.54) or group×time interaction (P=0.20), but there was a significant increase over time (P<0.01). Change in total liver volume did not significantly differ in the LD or STD group (Supplemental Table 3). There were no significant effects of group, time, or time×group interaction on total liver volume in the mixed model.
Table 3.
Total kidney volume with absolute and percentage change during 12 months of rapamycin treatment or standard care
| Variable | Low-Dose Rapamycin (n=10) | Standard-Dose Rapamycin (n=10) | Standard Care (n=10) | P Valuea |
|---|---|---|---|---|
| Baseline TKV (ml) (n=30) | 2479.3±1965.7 (n=10) | 1718.5±932.7 (n=10) | 2072.7±1184.4 (n=10) | 0.50 |
| Baseline TKV (ml) (n=26 with 12-mo data) | 1919.1±903.6 (n=9) | 1454.1±801.5 (n=8) | 1907.1±1126.8 (n=9) | 0.54 |
| 6-mo TKV (ml) | 1983.4±913.1 | 1743.8±1114.0 | 2140.9±1239.2 | |
| TKV change (ml) | 64.3±77.5 | 85.2±205.5 | 68.2±104.9 | 0.94 |
| TKV change (%) (n=29) | 3.5±4.1 (n=10) | 2.6±7.5 (n=9) | 3.3±3.5 (n=10) | 0.93 |
| 12-mo TKV (ml) | 2115.8±1035.0 | 1537.0±864.3 | 2059.78±1236.0 | |
| TKV change (ml) | 197.7±201.2 | 82.9±111.3 | 152.7±129.4 | 0.33 |
| TKV change (%) (n=26) | 9.3±7.0 (n=9) | 4.8±5.3 (n=8) | 7.8±4.8 (n=9) | 0.29 |
Mixed model results indicated no significant differences in overall group levels (P=0.54) and changes over time (group×time P=0.20), but a significant increase over time (P<0.01). Median (25th, 75th percentiles) for change in total kidney volume at 12 months: low dose, 143.0 (126.0, 201.0) ml, respectively; standard dose, 51.5 (30.5, 81.5) ml, respectively; and standard care, 129.0 (52.0, 189.0) ml, respectively. Values expressed with a plus/minus sign are the mean±SD.
P value based on ANOVA.
Except for an increased platelet count at 12 months, the groups did not significantly differ for hemoglobin; fasting LDL cholesterol or triglycerides; serum magnesium (Supplemental Table 4); or use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, or triglyceride-lowering medication (fibric acid derivatives or omega-3) (Supplemental Table 5). Diuretics (hydrochlorothiazide in five patients and torsemide in one) were used significantly more frequently in the STD group (six patients) than in the LD group (no patients) for treatment of calcium oxalate renal stones (three patients), hypertension (two patients), and edema (one patient) (Supplemental Table 5).
Three patients withdrew from the study: one in the LD group (because of pulmonary embolus) and two in the STD group (one because of nephrotic-range proteinuria and one because of pneumonia). One patient in the SC group, who did not withdraw, missed his 12-month visit. Rapamycin was used for <300 days in two LD recipients (one with pulmonary embolus who withdrew and one who underwent pilonidal cyst surgery and had oral ulcerations), and in five STD recipients (one with nephrotic-range proteinuria who withdrew, one with pneumonia who withdrew, one with stomatitis and intense anxiety, one who had elective plastic surgery, and one with vision change). Adverse events did not significantly differ among the groups except for oral ulcerations (Table 4). No episode of AKI occurred.
Table 4.
Adverse events during 12 months of rapamycin treatment
| Variable | Low-Dose Rapamycin (n=10) | Standard-Dose Rapamycin (n=10) | Standard Care (n=10) | P Valuea |
|---|---|---|---|---|
| Serious adverse events (see text) | 1 | 2 | 1 | >0.99 |
| Oral ulcerations | 2 | 6 | 0 | 0.01b |
| Nonserious infections | 2 | 6 | 5 | 0.27 |
| Edema | 0 | 1 | 2 | 0.75 |
| Gastrointestinal symptoms | 1 | 3 | 5 | 0.20 |
| Dermatitis | 2 | 0 | 0 | 0.31 |
| Miscellaneous/other | 2 | 7 | 7 | 0.05 |
| Total adverse events | 10 | 25 | 20 | 0.09 |
Based on Poisson model for count data.
Adjusted pairwise comparisons: low dose (LD) versus standard care (SC): P=0.47; standard dose (STD) versus SC: P=0.01; LD versus STD: P=0.17; LD+STD versus SC: P=0.03.
Discussion
The renal effects of inactivating mutations of PKD1 in the Han:SPRD rat (19–22) and in the orpk-rescue and bpk murine models (23,24) were mitigated by the mTOR inhibitor rapamycin, which significantly reduced cyst formation and preserved renal function. However, rapamycin doses exceeded those safe for humans (19–24). In a recent animal study of both low- and high-dose rapamycin, a clinically acceptable blood level of 3 ng/ml was achieved, but doses of 10 mg/kg were required (25). In a seminal study in Han:SPRD rats (19), rapamycin “did not completely prevent progression of cystic disease” but “markedly retarded renal functional loss,” an evaluation indicating its more measurable effect on renal function than on cystic expansion in this animal model (20).
In the present study, LD rapamycin was associated with a significant increase in GFR measured by 125I-iothalamate without a significant change in TKV, and without concordance for the GFR effect when estimated by the CKD-EPI equation. These findings raise several questions.
First, why was there a significant increase in iGFR and no significant slowing of TKV increase? It is now known that for every renal cyst detectable at a threshold diameter of 0.9 mm by T2-weighted magnetic resonance imaging (MRI) there are histologically about 62 smaller cysts (10). Consequently, size might decrease in thousands of exquisitely small cysts, calculated to average about 35,800 in two kidneys (10), which could improve GFR with minimal or no detectable slowing of an increase in TKV. For example, on the basis of the MRI threshold of 0.9 mm, if all the nonvisible cysts were 0.8 mm in diameter and half were eliminated by a particular treatment, the reduction in TKV would be <5 ml (1/2×35,800×4/3×3.1416×0.403/1000) (10). On the basis of a starting TKV of 1500 ml, an expected 5.3% annual TKV increase (3) of approximately 80 ml would still be 75 ml (i.e., an 80-ml expected TKV increase at 1 year is diminished by only 5 ml despite a treatment effect). The hypothesized functional improvement from changes in such minute cysts would depend on the location of the cysts (renal cortex: less compression of the vasculature, tubules, and glomeruli; medullary collecting ducts: decreased obstruction of multiple upstream nephrons) (10).
Another pathway that could affect early cyst formation and initially have little measurable effect on TKV involves cyst-promoting and cystogenic cells (32,33). In a polycystic mouse model, alternatively activated macrophages home to cystic areas and promote cyst growth (32). Rapamycin decreases macrophage colony-stimulating factor–dependent macrophage proliferation in a dose-dependent manner (34) and can inhibit macrophage chemotaxis and phagocytosis (35), but alternatively activated macrophages have not been studied specifically. However, CD133+ progenitor cells from human polycystic kidneys, but not from normal kidneys, form cysts in vitro and in severe combined immunodeficiency mice (33). Rapamycin inhibits CD133+ proliferation in vitro and suppresses CD133+ progenitor cell–induced cystogenesis (33). Although speculative, these findings raise the possibility that rapamycin might suppress select cell populations, with GFR improving before any detectable effect on TKV or “intermediate volume” (36).
Although the most frequently used method for measuring TKV is MRI (3–5,7,10,16,17,27,37), CT with iodinated contrast is also used in ADPKD (2,4,18,36). Direct comparisons between noncontrast CT and MRI in ADPKD show a TKV difference within 3.1% below to 3.6% above true TKV (mean of MRI and CT) (Irazabal MV, personal communication, November 3, 2012).
Other factors that could diminish the effect of rapamycin on TKV include patients with limited rapamycin exposure (see Results), rapamycin not reaching target renal tubular epithelial cells (38), only low levels of mTOR activity being present in 65%–70% of ADPKD cysts (39), polycystic kidneys reaching a point of “irreversible damage” unresponsive to therapy (40), male sex accelerating the rate of renal function decline in ADPKD (41) and exhibiting a diminished response to mTOR inhibitors when the eGFR is <60 ml/min per 1.73 m2 (42).
Second, why was the change in iGFR not seen when GFR was estimated by the CKD-EPI equation? Although GFR estimated by the CKD-EPI equation is “somewhat more precise and accurate than the Modification of Diet in Renal Disease equation, especially at higher GFRs,” iGFR remains the standard against which CKD-EPI is evaluated (26). The CKD-EPI equation “does not overcome the limitations of serum creatinine as an endogenous filtration marker” (26). Nonradiolabeled iothalamate (37) and nonradiolabeled iohexal plasma disappearance have had similar results (18).
Third, what type of ADPKD population do our patients represent? On the basis of their older age and other risk factors for progression (Table 1), which include a greater baseline TKV (Table 3, Supplemental Figure 1), our patients had a higher risk for progressive renal failure based on the ADPKD patient profiles described in the Consortium for Radiologic Imaging Studies (3).
Fourth, why did low, but not standard, doses of rapamycin show a positive iGFR effect? LD rapamycin had fewer adverse effects than STD and permitted more patients to complete ≥300 days of treatment: eight in the LD group versus five in the STD group. The number of rapamycin-treated patients with data at 12 months was nine in the LD group and eight in the STD group. Taken together, these results reflect a longer duration of therapy and a larger number of 12-month results in LD. In a PKD1-mutant mouse model (PKD1nlnl), LD rapamycin started early at 3 weeks (blood levels approximately 3 ng/ml) did have a significant cyst reduction effect after 5 weeks of treatment and trended toward an effect after 13 weeks; no renal function data were presented (25).
Three serious adverse events occurred in the rapamycin groups. In the LD group, a 46-year-old woman with a massive right kidney, TKV of 7521 ml, and iGFR of 29 ml/min per 1.73 m2 developed bilateral pulmonary emboli from which she recovered uneventfully with prompt anticoagulation. Rapamycin was discontinued. Large right polycystic kidneys have been associated with thromboembolic disease (43), and ADPKD is the greatest risk for pulmonary emboli after renal transplantation (44). Rapamycin has been associated with thromboembolic disease (45), although that has been challenged (46). In the STD group, a 56-year-old man with an initial iGFR of 36 ml/min per 1.73 m2 and 24-hour urine protein of 290 mg developed nephrotic-range proteinuria at 6 months. Rapamycin was discontinued. An open renal biopsy showed FSGS. Primary glomerulopathies, most commonly FSGS, can occur with ADPKD in the absence of rapamycin (47). Rapamycin has been reported to decrease proteinuria in some steroid-resistant patients with FSGS (48). However, de novo FSGS has been reported with rapamycin levels >12.8 ng/ml in kidney transplant recipients (49). In the STD group, a 55-year-old woman with decreased visual acuity and newly identified severe sleep apnea was diagnosed by a neuro-ophthalmologist as having mild retinal cone dysfunction after 6 months of rapamycin. Although the neuro-ophthalmologist did not find any association with rapamycin, rapamycin was discontinued while she continued in the study. A second examination showed definite improvement. This entity has not been reported with rapamycin.
The present study has several limitations. The number of patients was small (n=30); 26 completed the study. Because of the small sample size, the lack of statistical significance should not be interpreted as absence of treatment effect. Three of the four patients who did not have 12-month data were in the rapamycin groups (one in the LD group and two in the STD group). Although there was significant separation between TBLs in the LD and STD groups, it was more difficult to maintain STD in the desired TBL range of ≥5–8 ng/ml because of adverse events. Rapamycin medication was open-label without placebo control. The study duration was only 12 months.
In conclusion, LD rapamycin was associated with improved short-term (6 and 12 months) renal function measured by change in iGFR without significant change in TKV or eGFR. Although the sequence for progression of ADPKD involves numerous microcysts expanding into macrocysts that measurably increase TKV and decrease GFR, the sequence for response to certain therapies might first be a reduction in microcysts, then initial improvement in GFR, and finally diminution of macrocystic disease and slowing of the increase in TKV. These findings and their underlying hypotheses might be useful considerations in larger studies of rapamycin or other ADPKD treatments.
Disclosures
B.H. reports having received grant funding from Siemens Healthcare for research in CT dose reduction; he has no personal financial disclosures.
Acknowledgments
The authors wish to acknowledge Mr. Henry Rolin for performing the iGFR studies, Ms. Lindsay Oppman for assistance in the CT studies, Ms. Nikki Williams for manuscript preparation, Dr. David Barnes for serving as the data safety monitoring officer, and Dr. Vincent Dennis for encouragement in undertaking this study.
This investigator-initiated research study was supported by a $250,000 research grant from Wyeth Pharmaceuticals, now Pfizer/Wyeth. No one from either of those companies has had any role whatsoever in the study design, data handling or evaluation, or any aspect of preparing or writing this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02650313/-/DCSupplemental.
See related editorial, “Novel Treatments of Autosomal Dominant Polycystic Kidney Disease,” on pages 831–836.
References
- 1.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 2.King BF, Reed JE, Bergstralh EJ, Sheedy PF, 2nd, Torres VE: Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 11: 1505–1511, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP, CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bae KT, Grantham JJ: Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 96–106, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barua M, Cil O, Paterson AD, Wang K, He N, Dicks E, Parfrey P, Pei Y: Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 20: 1833–1838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP, CRISP Consortium : Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Qian F, Watnick TJ, Onuchic LF, Germino GG: The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 87: 979–987, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, Zhou J: Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 18: 2523–2531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grantham JJ, Mulamalla S, Grantham CJ, Wallace DP, Cook LT, Wetzel LH, Fields TA, Bae KT: Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol 7: 1087–1093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belibi FA, Edelstein CL: Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs 19: 315–328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76: 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberthal W, Levine JS: Mammalian target of rapamycin and the kidney. I. The signaling pathway. Am J Physiol Renal Physiol 303: F1–F10, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM: Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Lieberthal W, Levine JS: Mammalian target of rapamycin and the kidney. II. Pathophysiology and therapeutic implications. Am J Physiol Renal Physiol 303: F180–F191, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt K-U: Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, Ondei P, Rubis N, Diadei O, Gherardi G, Prandini S, Panozo A, Bravo RF, Carminati S, De Leon FR, Gaspari F, Cortinovis M, Motterlini N, Ene-Iordache B, Remuzzi A, Remuzzi G: Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol 21: 1031–1040, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao Y, Kim J, Schrier RW, Edelstein CL: Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wüthrich RP: Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 21: 598–604, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Zhang T, Wang L, Xiong X, Mao Z, Wang L, Mei C: Mycophenolate mofetil versus rapamycin in Han:SPRD rats with polycystic kidney disease. Biol Res 42: 437–444, 2009 [PubMed] [Google Scholar]
- 22.Zafar I, Belibi FA, He Z, Edelstein CL: Long-term rapamycin therapy in the Han:SPRD rat model of polycystic kidney disease (PKD). Nephrol Dial Transplant 24: 2349–2353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shillingford JM, Piontek KB, Germino GG, Weimbs T: Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol 21: 489–497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novalic Z, van der Wal AM, Leonhard WN, Koehl G, Breuning MH, Geissler EK, de Heer E, Peters DJ: Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. J Am Soc Nephrol 23: 842–853, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman AB, Torres VE, Perrone RD, Steinman TI, Bae KT, Miller JP, Miskulin DC, Rahbari Oskoui F, Masoumi A, Hogan MC, Winklhofer FT, Braun W, Thompson PA, Meyers CM, Kelleher C, Schrier RW: The HALT polycystic kidney disease trials: Design and implementation. Clin J Am Soc Nephrol 5: 102–109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolin HA, Hall PM: Evaluation of glomerular filtration rate and renal plasma flow. In: The Principles and Practice of Nephrology, edited by Klahr S, Jacobson HR, Striker GE, Philadelphia: Dekker, 1989, p 158 [Google Scholar]
- 29.Wang S, Miller A: A rapid liquid chromatography-tandem mass spectrometry analysis of whole blood sirolimus using turbulent flow technology for online extraction. Clin Chem Lab Med 46: 1631–1634, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Kamel IR, Kruskal JB, Warmbrand GS, Goldberg SN, Pomfret EA, Raptopoulos V: Accuracy of volumetric measurements after virtual right hepatectomy in potential donors undergoing living adult liver transplantation. AJR Am J Roentgenol 176: 483–487, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Johnson AM, Gabow PA: Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol 8: 1560–1567, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG: Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalhosa R, Deambrosis I, Carrera P, Pasquino C, Rigo F, Ferrari M, Lasaponara F, Ranghino A, Biancone L, Segoloni G, Bussolati B, Camussi G: Cystogenic potential of CD133+ progenitor cells of human polycystic kidneys. J Pathol 225: 129–141, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Comalada M, Valledor AF, Sanchez-Tilló E, Umbert I, Xaus J, Celada A: Macrophage colony-stimulating factor-dependent macrophage proliferation is mediated through a calcineurin-independent but immunophilin-dependent mechanism that mediates the activation of external regulated kinases. Eur J Immunol 33: 3091–3100, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Fox R, Nhan TQ, Law GL, Morris DR, Liles WC, Schwartz SM: PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J 26: 505–515, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caroli A, Antiga L, Conti S, Sonzogni A, Fasolini G, Ondei P, Perico N, Remuzzi G, Remuzzi A: Intermediate volume on computed tomography imaging defines a fibrotic compartment that predicts glomerular filtration rate decline in autosomal dominant polycystic kidney disease patients. Am J Pathol 179: 619–627, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rule AD, Torres VE, Chapman AB, Grantham JJ, Guay-Woodford LM, Bae KT, Klahr S, Bennett WM, Meyers CM, Thompson PA, Miller JP, CRISP Consortium : Comparison of methods for determining renal function decline in early autosomal dominant polycystic kidney disease: The consortium of radiologic imaging studies of polycystic kidney disease cohort. J Am Soc Nephrol 17: 854–862, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Canaud G, Knebelmann B, Harris PC, Vrtovsnik F, Correas JM, Pallet N, Heyer CM, Letavernier E, Bienaimé F, Thervet E, Martinez F, Terzi F, Legendre C: Therapeutic mTOR inhibition in autosomal dominant polycystic kidney disease: What is the appropriate serum level? Am J Transplant 10: 1701–1706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, Watnick T, Henske EP: The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 18: 151–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watnick T, Germino GG: mTOR inhibitors in polycystic kidney disease. [Editorial] N Engl J Med 363: 879–881, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Klahr S, Breyer JA, Beck GJ, Dennis VW, Hartman JA, Roth D, Steinman TI, Wang SR, Yamamoto ME, Modification of Diet in Renal Disease Study Group : Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. J Am Soc Nephrol 5: 2037–2047, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Walz G, Budde K, Eckardt K-U: mTOR inhibitor and autosomal dominant polycystic kidney disease. N Engl J Med 364: 287–288, 2011 [DOI] [PubMed] [Google Scholar]
- 43.O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF: Compression of the inferior vena cava by right renal cysts: An unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol Letters to the editor 49: 332-334, 1998 [PubMed] [Google Scholar]
- 44.Tveit DP, Hypolite I, Bucci J, Hshieh P, Cruess D, Agodoa LYC, Welch PG, Abbott KC: Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 14: 361–368, 2001 [PubMed] [Google Scholar]
- 45.Wyeth Pharmaceutical Data on File Nov 9, 2007
- 46.Langer RM, Kahan BD: Sirolimus does not increase the risk for postoperative thromboembolic events among renal transplant recipients. Transplantation 76: 318–323, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Hiura T, Yamazaki H, Saeki T, Kawabe S, Ueno M, Nishi S, Miyamura S, Gejyo F: Nephrotic syndrome and IgA nephropathy in polycystic kidney disease. Clin Exp Nephrol 10: 136–139, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Tumlin JA, Miller D, Near M, Selvaraj S, Hennigar R, Guasch A: A prospective, open-label trial of sirolimus in the treatment of focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 1: 109–116, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Letavernier E, Bruneval P, Mandet C, Duong Van Huyen JP, Péraldi M-N, Helal I, Noël L-H, Legendre C: High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol 2: 326–333, 2007 [DOI] [PubMed] [Google Scholar]