Abstract
The burden of cognitive impairment appears to increase with progressive renal disease, such that the prevalence of dementia among those starting dialysis, or those already established on dialysis, is high. The appropriateness of dialysis initiation in this population has been questioned, and current Renal Physician Association guidelines suggest forgoing dialysis in individuals who have dementia and lack awareness of self and environment. Patients are, however, also entitled to equal rights and respect, equal access to health care services, and an opportunity to engage in shared decision-making processes, particularly if there is concern over reversibility of disease. This article discusses, on the basis of principles of beneficence and nonmaleficence, the arguments in favor of and against dialysis use, and the process of determining an appropriate care plan. Factors discussed include the current societal trend toward a technological imperative, premature fatalism, survival benefits, and the implications of providing care to patients who are unable to express their tolerance for symptoms associated with the treatment or lack of treatment.
Introduction
Patients approaching or undergoing long-term maintenance hemodialysis or peritoneal dialysis are at high risk of cognitive impairment and/or dementia. Marked cognitive impairment at the time of dialysis initiation may increase the symptom burden and the risks associated with dialysis initiation. This may have the adverse effect of altering the fine balance between treatment beneficence and nonmaleficence to the point where dialysis initiation may cause more harm than benefit. The 2010 Renal Physician Association guideline recommends that patients with advanced dementia be advised against initiation of dialysis, particularly when they are unable to cooperate with the dialysis process. Factors such as the decreased ability to communicate symptoms during dialysis or to comprehend diet and medication restrictions, or the increased burden associated with travel to and from dialysis centers or postdialysis fatigue, should be included in discussions with caregivers and substitute decision-makers during the shared decision-making process.
Case History
Mrs. T.C. was a 72-year-old widow referred to the predialysis assessment clinic for evaluation and management of advanced kidney disease secondary to hypertension and vascular disease. She was a wealthy woman who lived in her own home supported by two dedicated live-in caregivers. She was also well supported by her son and daughter-in-law, who lived nearby and visited daily. Several years earlier, she had designated her son as her health care power of attorney.
Mrs. T.C.’s medical history also included osteoporosis, diagnosed after she had sustained a hip fracture, and well-controlled hypothyroidism. Laboratory tests at the time of referral revealed iron deficiency anemia, easily controlled hyperphosphatemia, and an estimated GFR of 16 ml/min. She was pleasant and always came to the clinic in a wheelchair escorted by family and a caregiver. She often came early and sat laughing and conversing with her family, the staff, and other patients in the waiting room. At each of three visits over a period of 6 months, she was found to be disorientated to time, place, and person. Her family reported that she had for some time been forgetful but that this had deteriorated significantly in recent months. She also began to require assistance with transferring, and her mobility was limited, even with the aid of a walker. The caregivers reported giving some assistance with bathing and grooming activities, although she remained continent of both urine and stool. She enjoyed reading and playing the piano and remained able to use the telephone and to eat her meals if her food was left close by. The caregivers reported no paranoid symptoms, aggressiveness, or hallucinations. Screening tests (using the Montreal Cognitive Assessment) confirmed impaired cognition, although further formal testing was not done. Reversible causes of progressive cognitive decline had been excluded, and it was felt she had mixed vascular and Alzheimer dementia of moderate severity.
At the third visit, after the renal team completed their assessment, the family and patient were counseled on both dialytic and nondialytic care pathways. A few weeks after her third visit, Mrs. T.C. was brought to the hospital with a fever, shortness of breath, and a decreased level of alertness. She was diagnosed with community-acquired pneumonia and antibiotics were initiated. Her creatinine began to climb, and it was felt that Mrs. T.C.’s renal function had deteriorated to a point that necessitated dialysis. The social worker questioned whether it was ethical to initiate dialysis therapy, knowing that recent data showed increased mortality with dialysis in patients who had premorbid functional dependence and dementia.
Cognitive impairment has been shown, in multiple studies, to be more common in patients with estimated GFRs<60 ml/min (CKD) than in those with normal renal function (1–10). Progressive cognitive decline appears to progress faster in patients with CKD than in those without (probably because of the high burden of vascular disease), such that by the time patients are established on dialysis, >30% have severe cognitive impairment consistent with dementia (8,10,11). However, for multiple reasons, cognitive impairment and dementia are often under-recognized and underdocumented in medical charts (12,13). In the case above, a patient with progressive cognitive loss is seen in a multidisciplinary renal clinic several months or years before the need for dialysis initiation. The patient’s cognitive decline is appreciated by the staff and caregivers, resulting in an opportunity for advance care planning. In this article we describe some of the medical, social, ethical, and legal issues around the use of dialysis therapy in those with moderate to severe dementia.
Nonmaleficence versus Beneficence
The decision to initiate dialysis is not a simple one for any patient, but for the individual with dementia, the stakes are even higher. Not only are the benefits of dialysis increasingly limited (12–15), particularly if patients also have progressive functional dependence, but individuals with dementia are often unable to express their tolerance for symptoms associated with the treatment (or lack of treatment) and do not have the capacity to understand and appreciate what is involved in the dialysis process. This leaves those closest to them with the difficult task of making surrogate decisions.
As with any other medical therapy, the purported goal of dialysis is to enhance or support the health of individuals who are being treated. This goal represents the ethical principle of “beneficence.” In contrast, the principle of “nonmaleficence” is commonly represented by the expression “first, do no harm” and reflects the reality that every medical intervention brings with it some degree of risk or adversity. In an ideal situation, care is individualized (16) and beneficence is balanced against nonmaleficence. For many patients, the main benefit of dialysis is prolonged survival and a reduction in symptoms related to uremia and fluid overload. The potential harms include access-related complications and a high burden of pain and nonspecific symptoms (17,18) (Table 1). As with all life-sustaining therapies, dialysis is medically indicated only if its benefits outweigh the burdens.
Table 1.
Potential medical, emotional, and social harms related to dialysis
| Medical |
| Iatrogenic infections |
| Complications of catheter insertion |
| Possible acceleration of progressive renal failure |
| Worsening of pre-existing vascular disease and dementia |
| Functional decline in vulnerable or frail individuals |
| Pain with needling of arteriovenous fistula during dialysis |
| Discomfort with prolonged immobilization |
| Dialysis-related muscle cramps, headache, and fatigue |
| Chronic low back pain from persistent abdominal distention in patients undergoing peritoneal dialysis |
| Feeling of unwellness |
| Emotional and social |
| Time and cost associated with commuting to hospital and dialysis centers |
| Limited employment opportunities |
| Social isolation (especially if required to move to be closer to a dialysis center) |
| Leisure travel restrictions |
| Unnecessary medicalization of death |
Increasingly in Western culture, a high value is placed on the individual’s autonomy, and as such, the patient is seen as the best judge of what medical decision is most appropriate for himself or herself. The decision to initiate dialysis should be influenced by the patient’s own values, culture, religion, and social circumstances, and this philosophy is echoed in the guideline published by the Renal Physicians Association (RPA) (19). In this guideline the RPA emphasizes the need for establishing a strong therapeutic relationship and open communication to facilitate shared decision-making. When properly instituted, shared decision-making involves a collaborative approach between the patient and the physician, with information flowing bi-directionally. Physicians use medical evidence to inform, while the patient integrates this into his or her own value system, allowing both parties to help define the best care plan.
The “Technological Imperative”
In recent decades, advances in electronics, including smartphones and other technology, have changed the way society functions and communicates. Health care is no different. Advances in interventional technology, replacement therapies, and diagnostic methods have altered expectations, and families and patients increasingly associate good-quality medical care with the use of highly sophisticated technology (20–22). Increasingly, machines are seen to be the “medical decision-makers” or “medical care providers,” and in doing so, the value of the interaction between patient and physician or nurse is lessened. In its extreme, the “technological imperative” (a term coined in 1968 by Victor Fuchs [23]) argues that dialysis is being overused in patients who are unlikely to benefit from the treatment because of societal expectations and pressures (Figure 1). Some of the beliefs that may be contributing to these practices are discussed below.
Figure 1.
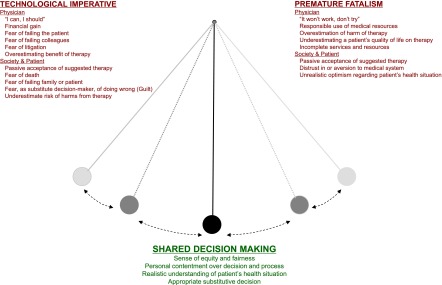
Potential for a pendulum-like swing from an overreliance on technology to the other extreme of underuse of technology due to a prematurely fatalistic outlook.
The Substitute Decision-Maker: “Fear of Doing Wrong”
Patients with dementia are often deemed incapable of making complex medical decisions and their next of kin or designated substitute decision-maker (SDM) is assigned the task of deciding on their behalf. Without explicit advance directives, it is difficult for SDMs to interpret what a patient would want in terms of dialysis. They fear misrepresenting the patients’ best interest or the guilt of doing too little. Several studies support the trend toward a technological imperative. These studies show that SDMs choose more aggressive medical options than patients (24). In this context, this would imply that patients with dementia are less likely to receive the care they would have chosen for themselves had they been able.
The Medical Advocate: “Fear of Doing Wrong”
Health care providers also display an attitudinal shift toward the technological imperative (22,25). This is seen in the recent RPA guideline recommending that when the balance of benefits to burdens is uncertain, a trial of dialysis be offered (recommendation 7) (19). Clinicians often feel conflicted in their role as decision-makers, particularly because candidates for palliative renal care have varied comorbidity and functional profiles (26). Under these circumstances the use of a dialysis trial is an extension of biomedicalization (22) to nephrology. Clinicians may want to avoid “letting down” their colleagues, who may have invested much in the care of the patient (22); fear litigation or formal complaints; or worry that they did not try hard enough. They may perceive a short trial of dialysis as most appropriate but may place less emphasis on the potential adverse consequences (Table 1), not least the potential to promote the loss of residual renal function and therefore hasten death after dialysis withdrawal (27,28).
Passive Acceptance
Modern medicine has shifted from a paternalistic approach to a collaborative approach. A large number of patients may still be largely influenced by, or prefer, an explicit physician recommendation. This finding is particularly true among older patients and those coping with chronic, nonmalignant conditions (29). In one study of dialysis patients, 52% of patients attributed their decision to initiate dialysis to the fact that their physicians had wished it, compared with only 35% who felt the decision was based on their own preferences (30). Nephrologists therefore should be aware that when faced with a decisional stalemate, a “trial of dialysis” may be mistaken as an overt recommendation and may disproportionately influence patients and families to accept dialysis. A sobering statistic from the same previous study of dialysis patients found that 60.7% of the patients voiced some regret over their decision to start renal replacement therapy.
Premature Fatalism
Having recognized the societal shift toward a technological imperative, recent guidelines and editorials advise against dialysis therapy under several conditions (31–34). The RPA guideline recommends that dialysis should be withheld or withdrawn in patients with irreversible, profound neurologic impairment such that they lack signs of thought, sensation, purposeful behavior, and awareness of self and environment (19,35). They also recommend that nephrologists consider forgoing dialysis in patients with high functional dependence, malnutrition, or multiple complex comorbidity (31–34) based on the limited survival and relatively poor quality-of-life gains (35). One reactionary consequence may be a pendulum-like swing, from an overreliance on technology to the other extreme of underuse of technology (Figure 1) due to a prematurely fatalistic outlook.
Does the Higher Risk of Death Justify Not Starting Dialysis?
A documented diagnosis of dementia in patients undergoing dialysis is associated with a 1.5- to 2-fold increase in mortality compared with individuals with no dementia, based on data from the US Renal Data System (13) and the Dialysis Outcomes and Practice Patterns Study (12). Similar associations are seen between dementia and earlier stages of CKD (15). Although both dialysis studies (12,13) were limited because they defined patients as having dementia only on the basis of clinical documentation in the medical chart (dementia rates in these studies were estimated at 0.6% and 4%, respectively, in contrast to an estimated 37% in a formal study of cognitive function in hemodialysis patients [10]]), the persistence of the relationship across all stages of CKD strengthens the association.
The ethical principles concerning whether a treatment is warranted in a specific or vulnerable population (such as those with dementia would be) if there is a known increased mortality risk are complex and beyond the scope of this article. However, they are worth a brief introduction. Egalitarian distributive justice theorists argue that basic medical treatments should be equally distributed because social class, wealth, illness, and health are random chance events (36). However, a policy where health care were to be distributed equally as needed could potentially overburden current medical systems; a framework to fairly allocate limited resources must exist. Alternatively, one may direct resources to those most likely to benefit society (utilitarian distributive justice). The latter may, however, lead to discrimination favoring those with higher premorbid function, a consequence that utilitarians would argue is “unfortunate … but not unfair (37).” Within nephrology, egalitarian distributive justice is commonly practiced: diabetic patients initiating dialysis have an increase in the relative risk of death similar to that reported for dementia, reflecting the equality (or egalitarian distributive justice) between diabetic and nondiabetic patients (38). With this in mind, it is important to ensure that the reason for considering forgoing dialysis is not the increased mortality but rather that the dialysis treatment will cause more burden than benefit.
Should Dialysis Be Limited to Those Who Will Have a Good Quality of Life?
It is possible that in patients with dementia, the balance between benefits and harms of dialysis may shift toward the latter. In the illustrative case, our patient appears to seek out social interaction in the waiting room by coming early and chatting with both staff and strangers. She appears to have a quality of life (QoL) that is appreciated by family, caregivers, and the health care team, and therefore many team members or family members may suggest dialysis would be appropriate. When deciding on the “value” of a treatment, we often implicitly use information about our current and expected future QoL and incorporate the anticipated decrement in QoL resulting from dialysis and renal disease into this decision-making process. But QoL can fluctuate unpredictably over the span of a lifetime, and in the case presented it is unclear what the current QoL is and what decrease in QoL would be tolerated. Will dietary and fluid restrictions lead to feelings of hostility and disrespect? Could the highly regulated hemodialysis schedule negatively affect QoL? There are no data to answer these questions. Patients with dementia are excluded from questionnaire studies by virtue of their dementia, but it is known that QoL scores from all patients undergoing dialysis are disappointingly low. Cross-sectional studies, however, suggest that older patients have better-preserved multidimensional QoL on dialysis than younger patients (39).
Are Surrogate or Substitutive Measures of QoL Appropriate?
Unlike family members, who interpret gestures, tones, and movements, physicians use objective measures, such as independence, social interaction, and physical limitations, to evaluate QoL, resulting almost invariably in an underestimation of the benefits (25,40). As a result, we often turn to the family or SDM to help advise the best treatment option. To do so, patients and families must understand what the medical symptoms mean and distinguish between the medical options presented. The SDMs are expected to represent the patients’ views and not their own (a concept known as “substituted judgement”). However, rarely can they do so well enough to evaluate whether death is a preferable health state (22,25). Additionally, SDMs do not want the responsibility for such decision-making and may delay or “dodge” making a choice (41). Consequently, SDM’s choices are dissimilar to those of the patient in one third of cases (42). Interestingly, one study showed that the use of advance directives did not improve the accuracy even if previously discussed with the SDM (42). Accuracy was lowest in making decisions for patients with dementia (58%; 95% confidence interval, 52% to 64%) but better in deciding about dialysis initiation (67%; 95% confidence interval, 59% to 74%) (42).
Should Dialysis Be Withheld from the Patient with Dementia Who May Compromise the Quality of Their Own Care?
Behavioral and psychiatric symptoms are commonly seen in dementia, with an estimated two thirds of patients having some behavioral abnormality (wandering, agitation, sexual inappropriateness, paranoia, or delusions) (43–45). Of patients with a diagnosis of dementia, those with a vascular cause (commonly seen in dialysis patients) are more likely to present with agitation and aggression (43). Dialysis may predispose to acute delirium through dialysis disequilibrium, rapid changes in fluid balance, BP, fluctuations, vascular access declotting, or catheter-related bacteremia. In the hemodialysis suite, agitated behaviors can place the patient and those around him or her at a high risk of harm, particularly because the patient’s agitation can result in disconnection of needles or lines during the treatment, which may lead to significant blood loss or transmission of infection through bodily fluids. Clinical strategies, such as subscapular placement of a dialysis catheter, may decrease the incidence of accidental disconnection but does not prevent the agitation or reduce the distress experienced by the patient, the family, and others observing the behaviors. The use of physical or chemical restraints (e.g., mittens, sedation) may further alter the balance between beneficence and nonmaleficence, particularly because harm may come not only to the patient but also to others in the dialysis unit. Although the use of restraints under some circumstances may be ethically justifiable (45), physical restraints are known to be associated with increased mortality and may be interpreted as brutality (46,47). Under these circumstances it is appropriate (and recommended) that one consider withholding or withdrawing dialysis in these individuals (RPA recommendation 6) (19,35). However, collaboration and input from palliative care consultants and geriatricians would help to ensure that symptoms that may be contributing to agitation are optimally managed both environmentally and pharmacologically and should be strongly considered in these circumstances.
Patients on dialysis are expected to participate in self-care tasks, such as fluid and diet restrictions, the appropriate use of calcium binders, monitoring of fistula or graft function (if relevant), and use of specialized bathing methods for hemodialysis or peritoneal dialysis catheters. The argument that patients with dementia cannot participate in self-care and therefore compromise their own dialysis therapy is countered by the widespread nonadherence observed in the absence of dementia (e.g., dialysis patients who routinely miss sessions are not prohibited from treatment). However, the inability to report symptoms such as shortness of breath, fatigue, cramping, or dizziness may decrease the overall “beneficence” from dialysis and potentially tip the balance toward withholding therapy. (Interestingly, babies cannot participate in symptom-driven care because they too are unable to communicate symptoms, but they would never be denied dialysis on this basis. Perhaps this perceived double-standard can be explained by bioethicist Daniels’ theory that health care’s moral importance lies in its ability to protect a person’s range of future opportunities [48].)
Avoiding the Potential for Inequity
Equity is an important societal principle that may be compromised were a philosophy that promotes nondialytic care ahead of dialysis in all individuals with severe dementia to be instituted. This compromise arises from the relationship between intelligence, socioeconomic status, and the method used to determine severe dementia such that there is potential to inadvertently disadvantage those in lower socioeconomic classes. Dementia is defined as the presence of cognitive impairment severe enough to interfere with normal daily activities. It is therefore a diagnosis that is not related to the type or degree of injury to the brain but rather depends on the effect the injury has on “normal” activity. Thus, dementia as a medical diagnosis is ill-defined. It does not refer to the disease burden but rather to the capacity of the individual to manage in their environment. Previously highly functioning individuals are more likely to know how to keep notes or make lists and can consequently mitigate the effects of their cognitive loss despite a significant impairment. These individuals will therefore be less likely to be diagnosed with dementia than individuals with similar cognitive capabilities who, for example, have had less education and are less able to use these sorts of strategies as well.
Implementing a Philosophy of “Best Respect”
The RPA shared decision-making guideline offers some practical solutions to the debate (19). They emphasize educating SDMs about all treatment options, while ensuring the patient-specific prognosis, benefits, and harms are discussed (recommendations 2 and 3). They suggest that decision-makers respect previous wishes to forgo dialysis (recommendation 5) and that teams use a formal process for stepwise negotiation in the event opinions are conflicting (recommendation 8).
In many circumstances, the SDM may not feel ready to “let go,” may feel there was insufficient opportunity to discuss dying or illness with the patient, or may feel his or her personal beliefs resulted in a different interpretation of the loved one’s previously expressed views. When uncertain of what the patient would have wanted, the SDM is often asked to decide according to “best interests,” a vague concept aimed at maximizing a patient’s well-being. Martyn, using the ethical debate around the use of artificial feeding, addresses such conflict by proposing that we use the principle of “best respect” (49). This concept is an extension of current collaborative decision-making processes that incorporates previous tolerances and experiences. He suggests that key decision-makers start with a basic level of respect for the individual in their own setting (e.g., their home), regardless of the environment or capacity. From this they build an understanding of the individual through discussion around the circumstances where the patient had experienced positive or negative feelings (e.g., respect, pleasure, humiliation, or disrespect). Past tolerances for ill health, symptoms, and treatments should be voiced and incorporated into the understanding of the person’s likely response. Unique to dialysis, the discussions need to include information that highlights how the treatment encompasses not just the therapy sessions but also other components: having to be punctual for dialysis; dietary and fluid restrictions; and the higher likelihood of symptoms such as pain, postdialysis fatigue, and muscle cramps associated with impaired patient-physician communication. Through these discussions, the family and health care team can build an understanding of the benefits and harms specific to the individual patient, and by doing so may help guide decision-making (49).
Case Epilogue
Because of the early involvement of the predialysis assessment clinic, a multidisciplinary team was able to take time to appreciate Mrs. T.C.’s values and philosophies. Guided by this information, the team was also able to personalize the information provided to the patient and family about the diagnosis and treatment options (recommendation 2), taking into account the patient’s current situation and condition (recommendation 3) (16). In our case, our patient had previously expressed a desire to try any treatment that would preserve life. She had herself experienced persecution during the Second World War and had as a result developed an attitude that accepted suffering and hardship. With the family it was decided she would proceed with dialysis but that were she to demonstrate any behaviors that placed her or others at harm (e.g., agitation) or were she to become unable to live at home with her two caregivers (e.g., requiring ventilation, suctioning, artificial feeding), dialysis would be discontinued. Over the following 10 months she continued to attend thrice-weekly dialysis. She remained stable and continued to enjoy playing the piano and participating in family reunions until she experienced a stroke. Several days after the stroke, when it was clear the deficits would interfere with her ability to eat, the family approached us and requested withdrawal of dialysis care. She was transitioned to a comfort care pathway to limit symptoms from secretions and pain (recommendations 4 and 9), and she died 5 days later.
Disclosures
S.V.J. has participated as a speaker for Amgen on one occasion in the past 2 years.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kurella M, Chertow GM, Luan J, Yaffe K: Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 52: 1863–1869, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, Satterfield S, Ayonayon H, Yaffe K: Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 16: 2127–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Murray AM, Pederson SL, Tupper DE, Hochhalter AK, Miller WA, Li Q, Zaun D, Collins AJ, Kane R, Foley RN: Acute variation in cognitive function in hemodialysis patients: A cohort study with repeated measures. Am J Kidney Dis 50: 270–278, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Bartolomei K, Scott T, Price LL, Griffith JL, Rosenberg I, Levey AS, Folstein MF, Sarnak MJ: Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis 53: 438–447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McQuillan R, Jassal SV: Neuropsychiatric complications of chronic kidney disease. Nat Rev Nephrol 6: 471–479, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW, Sehgal AR, Cohen D, Anderson C, Appel L, Desalvo K, Ojo A, Seliger S, Robinson N, Makos G, Go AS; Chronic Renal Insufficiency Cohort Investigators: Chronic kidney disease and cognitive function in older adults: Findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 58: 338–345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurella Tamura M, Xie D, Yaffe K, Cohen DL, Teal V, Kasner SE, Messé SR, Sehgal AR, Kusek J, DeSalvo KB, Cornish-Zirker D, Cohan J, Seliger SL, Chertow GM, Go AS: Vascular risk factors and cognitive impairment in chronic kidney disease: The Chronic Renal Insufficiency Cohort (CRIC) study. Clin J Am Soc Nephrol 6: 248–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto Y, Ohara T, Nagakane Y, Tanaka E, Morii F, Koizumi T, Akiguchi I: Chronic kidney disease, 24-h blood pressure and small vessel diseases are independently associated with cognitive impairment in lacunar infarct patients. Hypertens Res 34: 1276–1282, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Seliger SL, Weiner DE: Cognitive impairment in dialysis patients: Focus on the blood vessels? Am J Kidney Dis 61: 187–190, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL: Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kurella M, Yaffe K, Shlipak MG, Wenger NK, Chertow GM: Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis 45: 66–76, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kurella M, Mapes DL, Port FK, Chertow GM: Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 21: 2543–2548, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Rakowski DA, Caillard S, Agodoa LY, Abbott KC: Dementia as a predictor of mortality in dialysis patients. Clin J Am Soc Nephrol 1: 1000–1005, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP: Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis 56: 693–703, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Raphael KL, Wei G, Greene T, Baird BC, Beddhu S: Cognitive function and the risk of death in chronic kidney disease. Am J Nephrol 35: 49–57, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowling CB, O’Hare AM: Managing older adults with CKD: Individualized versus disease-based approaches. Am J Kidney Dis 59: 293–302, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murtagh FE, Addington-Hall J, Higginson IJ: The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Bourbonnais FF, Tousignant KF: The pain experience of patients on maintenance hemodialysis. Nephrol Nurs J 39: 13–19, quiz 20, 2012 [PubMed] [Google Scholar]
- 19.Renal Physicians Association: Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis Clinical Practice Guideline, 2nd Ed., Rockville, MD, Renal Physicians Association, 2010 [Google Scholar]
- 20.Barger-Lux MJ, Heaney RP: For better and worse: The technological imperative in health care. Soc Sci Med 22: 1313–1320, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Katz MH, Grady D, Redberg RF: Undertreatment improves, but overtreatment does not. JAMA Intern Med 173: 93, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Kaufman SR, Shim JK, Russ AJ: Revisiting the biomedicalization of aging: Clinical trends and ethical challenges. Gerontologist 44: 731–738, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs VR: The growing demand for medical care. N Engl J Med 279: 190–195, 1968 [DOI] [PubMed] [Google Scholar]
- 24.Meeker MA, Jezewski MA: Family decision making at end of life. Palliat Support Care 3: 131–142, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kaufman SR, Shim JK, Russ AJ: Old age, life extension, and the character of medical choice. J Gerontol B Psychol Sci Soc Sci 61: S175–S184, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke G, Harrison K, Holland A, Kuhn I, Barclay S: How are treatment decisions made about artificial nutrition for individuals at risk of lacking capacity? A systematic literature review. PLoS ONE 8: e61475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carson RC, Juszczak M, Davenport A, Burns A: Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 4: 1611–1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussain JA, Mooney A, Russon L: Comparison of survival analysis and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med 27: 829–839, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Say R, Murtagh M, Thomson R: Patients’ preference for involvement in medical decision making: A narrative review. Patient Educ Couns 60: 102–114, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Davison SN: End-of-life care preferences and needs: Perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 195–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schell JO, Da Silva-Gane M, Germain MJ: Recent insights into life expectancy with and without dialysis. Curr Opin Nephrol Hypertens 22: 185–192, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Murtagh FE, Cohen LM, Germain MJ: The “no dialysis” option. Adv Chronic Kidney Dis 18: 443–449, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Germain MJ, Davison SN, Moss AH: When enough is enough: The nephrologist’s responsibility in ordering dialysis treatments. Am J Kidney Dis 58: 135–143, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Moss AH: To dialyze or not: The patient with metastatic cancer and AKI in the intensive care unit. Clin J Am Soc Nephrol 7: 1507–1512, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Moss AH: Revised dialysis clinical practice guideline promotes more informed decision-making. Clin J Am Soc Nephrol 5: 2380–2383, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Beauchamp TL, Childress JF: Justice. In: The Principles of Biomedical Ethics, 5th Ed., edited by Beauchamp TL, Childress JF, Oxford, United Kingdom, Oxford University Press, 2001, pp 225–239
- 37.Engelhardt HT, Wildes KW: The four principles of health care ethics and post-modernity: why a libertarian interpretation is unavoidable. In: Principles of Health Care Ethics, edited by Gillon R, New York, John Wiley & Sons, 1994, pp 135–147 [Google Scholar]
- 38.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium: Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unruh ML, Newman AB, Larive B, Dew MA, Miskulin DC, Greene T, Beddhu S, Rocco MV, Kusek JW, Meyer KB; Hemodialysis Study Group: The influence of age on changes in health-related quality of life over three years in a cohort undergoing hemodialysis. J Am Geriatr Soc 56: 1608–1617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppola KM, Ditto PH, Danks JH, Smucker WD: Accuracy of primary care and hospital-based physicians’ predictions of elderly outpatients’ treatment preferences with and without advance directives. Arch Intern Med 161: 431–440, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Lynn J, Arkes HR, Stevens M, Cohn F, Koenig B, Fox E, Dawson NV, Phillips RS, Hamel MB, Tsevat J; Study to Understand Prognoses and Preferences and Risks of Treatment: Rethinking fundamental assumptions: SUPPORT’s implications for future reform. J Am Geriatr Soc 48[Suppl]: S214–S221, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Shalowitz DI, Garrett-Mayer E, Wendler D: The accuracy of surrogate decision makers: A systematic review. Arch Intern Med 166: 493–497, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC: Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. Am J Psychiatry 157: 708–714, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Lawlor B: Managing behavioural and psychological symptoms in dementia. Br J Psychiatry 181: 463–465, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Behrman S, Dunn M: Physical restraint of medical inpatients: Unravelling the red tape. Clin Ethics 5: 16–21, 2010 [Google Scholar]
- 46.Berzlanovich AM, Schöpfer J, Keil W: Deaths due to physical restraint. Dtsch Arztebl Int 109: 27–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miles SH, Irvine P: Deaths caused by physical restraints. Gerontologist 32: 762–766, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Daniels N: Justice, health, and healthcare. Am J Bioeth 1: 2–16, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Martyn SR: Substituted judgment, best interests, and the need for best respect. Camb Q Healthc Ethics 3: 195–208, 1994 [DOI] [PubMed] [Google Scholar]


