Abstract
Background and objectives
Women affected by CKD increasingly choose to get pregnant. Experience with low-protein diets is limited. The aim of this study was to review results obtained from pregnant women with CKD on supplemented vegan–vegetarian low-protein diets.
Design, setting, participants, & measurements
This was a single-arm, open intervention study between 2000–2012 of a low-protein diet in pregnant patients with stages 3–5 CKD or severe proteinuria (>1 g/d in the first trimester or nephrotic at any time). Stages 3–5 CKD patients who were not on low-protein diets for clinical, psychologic, or logistic reasons served as controls. The setting was the Obstetrics-Nephrology Unit dedicated to kidney diseases in pregnancy. The treated group included 24 pregnancies—21 singleton deliveries, 1 twin pregnancy, 1 abortion, and 1 miscarriage. Additionally, there were 21 controls (16 singleton deliveries, 5 miscarriages). The diet was a vegan–vegetarian low-protein diet (0.6–0.8 g/kg per day) with keto-acid supplementation and 1–3 protein-unrestricted meals allowed per week.
Results
Treated patients and controls were comparable at baseline for median age (35 versus 34 years), referral week (7 versus 8), eGFR (59 versus 54 ml/min), and hypertension (43.5% versus 33.3%); median proteinuria was higher in patients on the low-protein diet (1.96 [0.1–6.3] versus 0.3 [0.1–2.0] g/d; P<0.001). No significant differences were observed in singletons with regard to gestational week (34 versus 36) or Caesarean sections (76.2% versus 50%). Kidney function at delivery was not different, but proteinuria was higher in the diet group. Incidence of small for gestational age babies was significantly lower in the diet group (3/21) versus controls (7/16; chi-squared test; P=0.05). Throughout follow-up (6 months to 10 years), hospitalization rates and prevalence of children below the third percentile were similar in both groups.
Conclusion
Vegan–vegetarian supplemented low-protein diets in pregnant women with stages 3–5 CKD may reduce the likelihood of small for gestational age babies without detrimental effects on kidney function or proteinuria in the mother.
Introduction
In the era of epigenetics, the early phases of human development are considered clues for future health and disease. Reports of increased risk for intellectual deficits, behavioral difficulties, and metabolic derangements in small, underdeveloped, or premature babies are gaining relevance (1–9).
However, the clinical applications of these epidemiologic data are controversial because of the different causes of small babies. The literature includes studies on small babies (below 2,500 g at birth), small for gestational age (SGA) babies (birth weight is related to gestational week, sex, and local birth weight references), and fetal growth-restricted babies (in which a previously harmonic growth is impaired by a relevant pathologic event). These definitions partly overlap, but only SGA and growth-restricted babies have a definite pathologic meaning (8–13).
Children born to mothers with CKD are at higher risk of being small and/or premature, and premature children may display increased risk of CKD later in life (14–18). Despite heterogeneous medical literature, the overall risk of delivering small children parallels CKD progression (16–20).
Thus, the success that has been achieved by improving maternal–fetal outcomes in CKD women may result in a paradoxical increase of CKD in their offspring, with important clinical and ethical implications (21–23). In this context, therapeutic interventions in pregnancy are crucial, and the attention is shifting from short- to long-term effects.
Low-protein diets are pivotal for slowing down CKD progression (24–26), and they may be promising tools for counterbalancing hyperfiltration during pregnancy, which very often results in an increase in proteinuria and functional impairment, particularly in patients with severe GFR reduction at baseline.
Indeed, therapeutic approaches that presumably interact with hyperfiltration may allow more babies to reach a later pregnancy stage.
Although high-protein diets are increasingly discouraged during pregnancy, less is known about protein restriction, such as in diets prescribed for CKD (27–30). Our previous study on a small series of patients on supplemented low-protein diets in pregnancy is one of the few available studies (31). Our data were in line with other reports on the advantages of vegetable proteins in reducing kidney disease progression rate in pregnant or lactating animals (31–33).
The aim of the present study was to review our experience with low-protein vegan–vegetarian diets supplemented by amino and keto acids in patients with stages 3–5 CKD or relevant proteinuria during pregnancy. In particular, we focused our analysis on the following outcomes in children: intrauterine growth, clinical problems, and for older children, psychosocial development.
Materials and Methods
Definitions
CKD was defined and staged according to Kidney Disease Outcomes Quality Initiative guidelines (Chronic Kidney Disease Epidemiology Collaboration) and whenever possible, on the basis of preconception data. GFR and proteinuria were assessed by 24-hour urine collections throughout pregnancy (18,34).
Newborns were defined as SGA when birth weight was below the 10th percentile according to Italian birth weight references (35,36). Fetal growth restriction was defined as flattening of the growth curve. Preterm delivery and early preterm delivery were defined as deliveries occurring before 37 and 34 completed weeks of gestational age, respectively. Apgar scores were recorded at 1 and 5 minutes by neonatologists (18,34).
Intrauterine growth was assessed by calculating gestational age-adjusted percentiles and Z scores (35,36).
Study Setting and Control Policy
The study was performed at the Maternal-Fetal Medicine Unit of Sant’Anna University Hospital, Turin, Italy. Data were prospectively gathered from January 1, 2000, to December 31, 2012.
The frequency of nephrology and obstetric visits ranged from one time per week to one time per month. Ultrasound biometry was performed every 2–3 weeks for SGA babies or those babies at risk for fetal growth restriction. All visits were personalized as previously described (18,34,37). Cesarean section was performed for fetal indications before or during labor, cases of unfavorable conditions for induction (e.g., prematurity), and lack of response to induction. In all cases, the aim was to delay delivery until 34–36 weeks, depending on fetal–maternal clinical conditions (18,34).
Low-Protein Diet
The diet was an adaptation of the low-protein vegan–vegetarian diet prescribed at our center (38,39). Our diet for nonpregnant patients is vegan, with a protein intake of 0.6 g/kg per day (ideal weight), one to three free meals per week, and keto acid supplementation of 1 pill/8–10 kg ideal BW (IBW) (31,40).
In an empirical attempt to balance the advantages of low-protein diets in CKD and the habit of increasing protein intake in pregnancy, we adjusted the diet in pregnancy to 0.6–0.8 g/kg per day protein based on preconception weight (Supplemental Appendix). The basic diet was vegan supplemented with a mixture of amino and keto acids (commercially available in Italy as Alfa-kappa; each 600-mg tablet contains calcium salts of hydroxyl and keto analogs of isoleucine, leucine, phenylalanine, valine, methionine, and amino acids [i.e., lysine, threonine, histidine, and tyrosine]).
We progressively allowed small quantities of milk and yogurt (100–150 ml/d) for patients who would otherwise have had difficulties reaching the target caloric intake (hence, the definition of vegan–vegetarian). Although our first patients received one tablet of keto acid for 8 kg IBW during the first trimester and up to 1 tablet for 5 kg IBW in the last trimester, we progressively adjusted the diet with a more liberal allowance of vegetable proteins, reducing the supplementation to 1 tablet per 8–10 kg IBW (31). In the absence of reports about specific keto and amino acid mixtures in pregnancy, efforts were made to check for risks linked to protein content and additives. At the time of the present study, no reports on any of these issues had been found or made available by the company (Fresenius Kabi).
On the basis of functional status, level of proteinuria, and patients’ preferences, one to three free meals per week were allowed (unrestricted with regard to both vegetable and animal protein content, although still limiting unsaturated fats and sugars).
Caloric intake was controlled according to the current indications in pregnancy, and it was targeted to about 30 kcal/kg per day considering IBW and the presence/absence of diabetes. In addition to the standard biochemical exams evaluating CKD, we progressively added iron status, B12, 25-OH vitamin D, and electrolytes. Specific supplements were prescribed on the basis of the biochemical results. Erythropoietin was used as required, with a hemoglobin target of 10 g/dl on account of the physiologic hemodilution of pregnancy.
Patient Selection
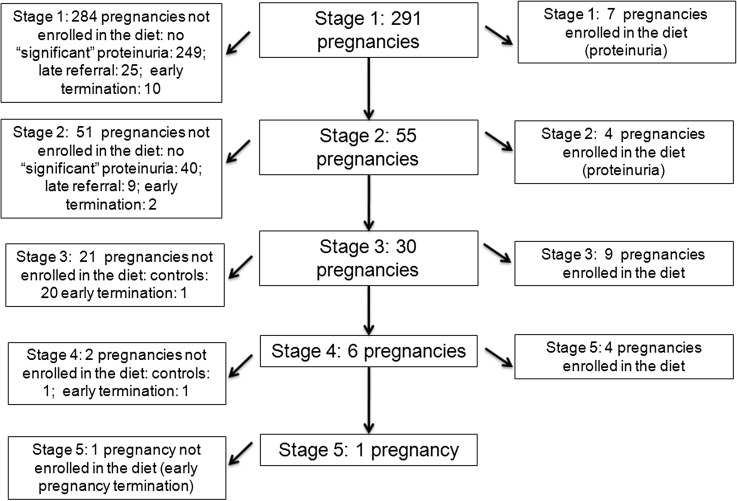
During the study period, 383 pregnancies were referred to us in patients with kidney diseases (stage 1, n=291; stage 2, n=55; stage 3, n=30; stage 4, n=6; stage 5, n=1).
The main indications for this type of diet were pregnancy in patients on a supplemented vegetarian diet, severe CKD (stages 3b–5), severe proteinuria (over 3 g/d or above 1 g/d in the first 20 weeks of gestation), previous nephrotic syndrome, or a combination of any of these elements. Patients referred in the last phases of pregnancy or for whom delivery was expected within less than 4 weeks were not taken into consideration for this approach.
The diet was prescribed either at referral or after an initial increase in serum creatinine or proteinuria based on the above-mentioned criteria. Only patients who followed the diet for at least 4 weeks before delivery or pregnancy interruption were included in the present study (Figure 1).
Figure 1.
Flow chart of cases and controls selection criteria. In total, 383 pregnancies were referred in patients with kidney diseases in the study period (stage 1, n=291; stage 2, n=55; stage 3, n=30; stage 4, n=6; stage 5, n=1). Pregnancy terminations within 2 weeks from referral were not considered in the present analysis.
Because the diet was routinely offered to all patients with the above-mentioned characteristics, a strictly equivalent control group was not available. Therefore, our control group consisted of all consecutive subjects with stages 3–5 CKD carrying singletons who did not follow the diet for personal, clinical, or logistic problems, excluding miscarriages or abortions occurring within 2 weeks from referral (or in the first 12 weeks).
Follow-Up Information
Follow-up information for mothers who were still being followed at our center or their nephrology center was obtained during routine clinical visits, whereas for the few patients who were not followed by a nephrology unit, it was obtained by phone interview. The main clinical problems, including hospitalizations, were also recorded. Only two patients in the intervention group and five patients in the control group were lost to long-term follow-up. Follow-up at 3 months was available for all patients who followed the diet and 13 of 16 patients who did not follow the diet.
After delivery, growth analysis was performed according to the international references (41–43).
Statistical Analyses
Descriptive analysis was performed as appropriate. Paired t (pre- and postpregnancy data), t, chi-squared, Fisher’s, and Wilcoxon’s tests were used for comparisons. Significance was set at <0.05. Statistical evaluation was performed using SPSS, version 18.0, for Windows (SPSS, Chicago, IL).
Ethical Issues
Patients were informed that no data regarding the supplemented diet during pregnancy were available, with the exception of a previous report by our group (for the patients who started the diet in the last 3 years) (31). Patients were asked to provide informed consent to the diet. The form stated the limits and goals of the diet and the importance of timely reporting of any side effects possibly linked to the diet.
Neither the mothers nor the children underwent clinical, blood, or urine tests for the sake of the present study, which was purposely limited to recording the most important growth patterns and the main clinical and/or developmental outcomes.
The study was performed according to the principles of the Declaration of Helsinki and approved by the ethics committee of the Ospedale Infantile Regina Margherita Sant’Anna Hospital–Ordine Mauriziano (number 335; protocol 11551/c28.2, 4/3/2011).
Results
Baseline Data
The main baseline data regarding 24 pregnancies in 22 patients who followed our low-protein diet are reported in Table 1. Two patients had two pregnancies, one pregnancy was voluntarily terminated, one patient miscarried, and one pregnancy was a twin pregnancy. Thus, the 24 pregnancies resulted in 23 live-born babies.
Table 1.
Baseline data
| Age (yr) | Referral Week (w) | Kidney Disease | sCr (mg/dl) | EPI–GFR (ml/min per 1.73 m2) | CKD Stage | PtU (g/day) | Total Protein (g/dl) | Albumin (g/dl) | Hypertension at Referral | BMI (Preconception) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dieta | |||||||||||
| 24 Pregnancies | 35 (20–40) | 7 (5-24) | DiabNeph: 8; GN: 9; Kideny graft: 4 | 1.2 (0.6–3.2) | 59 (18–132) | 3 (1–4) | 1.96 (0.1–6.3) | 6.7 (5.1–8.4) | 3.7 (2.3–4.5) | 43.5% | 22 (18.2–31.4) |
| Controlsb | |||||||||||
| 21 Pregnancies | 34 (22–39) | 8 (5–33) | DiabNeph: 1; GN: 3; Kideny graft: 3 | 1.3 (0.8–2.9) | 54 (22–60) | 3 (3–4) | 0.3 (0.1–2) | 6.9 (5.9–8.1) | 3.4 (2.7–5.0) | 33.3% | 20.3 (5.2–26.7) |
| P cases versus controlsc | 0.72 | 0.21 | — | 0.26 | 0.09 | 1 | <0.001 | 0.10 | 0.08 | 0.70 | 0.25 |
Results expressed as median and range. Data at referral: data observed at the first control of follow up in our unit. EPI-GFR, CKD Epidemiology Collaboration equation for estimating GFR from serum creatinine; SLE, systemic lupus erythematous; DiabNeph, diabetic nephropathy; BMI, body mass index; PtU, 24 hour proteinuria; sCr, serum creatinine.
Diet: 24 pregnancies in 22 patients who followed the low-protein supplemented vegan diet, for at least 4 weeks, during pregnancy.
Controls: 21 pregnancies in 19 patients (CKD stages 3 and 4) not on a protein-restricted diet in pregnancy.
Diet versus controls.
Two patients were already on the diet before pregnancy, whereas in the other cases, the diet was prescribed during pregnancy. The main indications for the diet were almost evenly divided between severe CKD and proteinuria. Seven patients (eight pregnancies) were affected by type 1 diabetes (Table 1).
For the control group with 21 pregnancies, the main reasons for not prescribing the diet during pregnancy were stable kidney function (seven patients) and previous eating disorders (three patients). Five miscarriages were recorded, thus resulting in 16 live-born children for the analysis (Table 1).
Despite the nonrandom selection of cases, the two cohorts were comparable for age, referral week, kidney function, prevalence of hypertension, and body mass index, whereas because of the selection policy, proteinuria was significantly higher in the low-protein diet patients (P<0.001).
Pregnancy Outcomes: Kidney Function and Proteinuria
All patients in the intervention group were able to follow the diet throughout pregnancy. No diet or supplementation side effects were reported. On the basis of dietary recall, all patients followed the diet with good compliance; the main drawbacks were monotony and the need to account for the double goal of low-protein diet and low weight gain (maximum=10–12 kg).
As expected in a CKD population, the antihypertensive drug requirement increased in both groups (Table 2). Hypertension was the main reason for delivery in 6 of 22 cases in the diet group and 2 of 16 cases in the control group (Table 3).
Table 2.
Main data at and after delivery
| Gestational Age (wk) | sCr (mg/dl) | GFR (ml/min per 1.73 m2) | CKD Stage | PtU (g/d) | Total Protein (g/dl) | Albumin (g/dl) | Weight Gain | Antihypertensives at Delivery | sCr 3 mo | GFR (ml/min per 1.73 m2) 3 mo | PtU (g/d) 3 mo | Serum Albumin (g/dl) 3 mo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Casesa | |||||||||||||
| 22 Pregnancies | 34 (28–38) | 1.51 (0.5–5) | 45.0 (11–140) | 3 (1–5) | 3.4 (0.8–17.3) | 5.4 (4.2–6.7) | 2.8 (1.8–3.6) | 10 (4–16) | 21 /22 | 1.8 (0.7–4.5) | 42.5 (10–125) | 2.0 (0.3–4.4) | 3.2 (2.2–4.0) |
| Controlsb | |||||||||||||
| 16 Pregnancies | 36 (28–38) | 1.3 (0.7–4.2) | 49.5 (14–101) | 3 (1–5) | 1.2 (0.2–7.2) | 6.2 (5.1–7.5) | 3.2 (2.5–4.5) | 10 (2–19) | 16/16 | 1.4 (0.9–5.2) | 53.5 (11–85) | 0.7 (0.1–2.5) | 3.7 (3.1–4.2) |
| P value (diet versus controls)c | 0.10 | 0.87 | 0.96 | 0.60 | 0.001 | 0.01 | 0.01 | 0.98 | 1 | 0.64 | 0.75 | <0.001 | 0.004 |
Results expressed as median and range. PtU, 24 hour proteinuria; sCr, serum creatinine.
Diet: data in 22 pregnancies who delivered, and who followed the low-protein supplemented vegan diet for at least 4 weeks (21 singletons, 1 twin pregnancy).
Controls: data at and after delivery in the 16 pregnancies who delivered, and who followed a nonrestricted diet in pregnancy (all singletons).
Singleton deliveries.
Table 3.
Main pregnancy outcomes and intrauterine growth
| Cases | Gestational Age (wk) | Type of Delivery | Main Indication for Delivery | Sex | Weight (g) | SGA % | Apgar (1–5 min) | NICU | Neonatal Problems |
|---|---|---|---|---|---|---|---|---|---|
| 22 Singletonsa | 34 (28–38) | CS: 16/21 76.2% | Increase in BP and/or PtU: 9 SLE relapses: 2 PROM: 2 IUGR: 1 | M: 9 F: 14 | 1975 (935–3330) | 3/21 singletons 14% | 1 min: 8 (4–9) 5 min: 9 (7–9) | 14/21 singletons 67% | RDS: 11 PDA: 2 Jaundice: 16 |
| 16 Singletonsb | 36 (28–38) | CS: 8/16 50% | Increase in BP and/or PtU: 4 IUGR: 1 | M: 9 F: 7 | 2345 (750–3330) | 7 singletons (44%) | 1 min: 8 (5–9) 5 min: 9 (8–9) | 5/16 31.2% | RDS: 1 PDA: 2 Jaundice: 9 |
| P value (diet versus controls)c | 0.10 | 0.19 | — | 1 | 0.32 | 0.05 | 1 | 0.07 | — |
Centiles according with InES Charts . Results expressed as median and range. CS, Caesarean section; CTG, cardio-tocography; PROM, premature rupture of membranes; M, male; F, female; RDS, respiratory distress syndrome; PDA, patent ductus arteriosus; NICU, Neonatal Intensive Care Unit; Centile (Par), centile according to the Parazzini Italian charts.
Diet: babies whose mothers followed the low-protein supplemented vegan diet for at least 4 weeks in pregnancy (23 babies, 22 pregnancies, 21 singletons, 2 twins).
Controls: 16 babies whose mothers followed a nonrestricted diet in pregnancy (16 singletons).
Singleton deliveries.
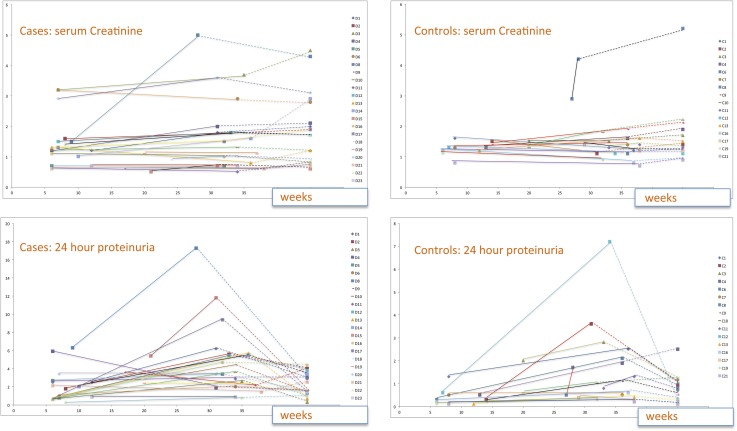
In the intervention group, one patient doubled serum creatinine levels during pregnancy, and one patient increased by one CKD stage (Table 2). Serum creatinine increased significantly in the diet group during pregnancy (P=0.02); the increase is not merely explained by the steep rise in a single case (Figure 2).
Figure 2.
Serum creatinine and proteinuria at referral, delivery, and 3 months after delivery. First point, referral; second point, delivery; third point and dotted lines, 3 months after delivery. C, control; D, diet.
Proteinuria increased significantly in both groups (diet: P<0.01; controls: P=0.001) (Figure 2, Table 2). Despite the increase in proteinuria, serum albumin and total proteins were only moderately decreased. Proteinuria decreased after pregnancy in all but two cases.
No significant differences were observed between the diet and control groups in week of delivery and kidney function, whereas proteinuria remained higher and total proteins and albumin remained lower in the diet group (P=0.01) (Table 2).
Pregnancy Outcomes: Intrauterine Growth.
The prevalence of SGA babies was lower for mothers who followed the diet: three singletons and one twin in the diet group versus seven singletons in the nondiet group (P=0.05 in singletons).
Of note, 8 of 10 SGA singletons were preterm (Table 3).
Cesarean section was performed preterm for maternal reasons in 10 of 22 patients in the diet group and 5 of 16 patients in the control group. It was performed for fetal reasons in 6 of 22 cases in the diet group and 4 of 16 cases in the control group (P=1). The need for hospitalization in the neonatal intensive care unit was higher in the diet group; the difference did not reach statistical significance and was presumably linked to the lower, albeit nonsignificantly lower, gestational age.
Follow-Up Data
The main data regarding long-term follow-up of the children (6 months to 10 years) are reported in Table 4. No socialization or schooling problems were reported. Prevalence of hospitalization was similar in both groups (4 of 19 children in the diet group and 4 of 15 children in the control group), and the prevalence of children below the third percentile was also similar (height, weight, or both: 4 of 19 children in the diet group versus 2 of 15 children in the control group).
Table 4.
Long-term follow-up of the children at least 6 months of age (17 children born to mothers on the diet and 12 children born to mothers on unrestricted diets)
| Age | Children with Height and/or Weight Measurements <3rd Centile According to Cacciari-cdc | Hospitalizations | |
|---|---|---|---|
| Children from on-diet mothersa | |||
| 17 | 2 years (3 months-10 years) | 4/17 available cases | Hospitalized at least once: 4/17 available cases (intestinal infection; pneumonia; orthopaedic surgery -2 cases) |
| Children from control mothersb | |||
| 12 | 2 years (3 months – 9 years) | 2/12 available cases | Hospitalized at least once: 4/12 available cases (patent Botallus; cyst excision; allergic reaction; malaria) |
| P value (diet versus controls)c | — | 0.33 | 0.27 |
Children born to mothers who followed the low-protein supplemented vegan diet for at least 4 weeks in pregnancy. Updates available in 17 children aged>3 months.
Children born to from mothers who followed a nonrestricted diet in pregnancy. Updates available in 12 children aged>3 months.
Singletons.
Discussion
The interaction between placental development, renal hyperfiltration, increase in proteinuria, and development of pregnancy-related hypertensive disorders is still not fully understood (16–20). Because of their teratogenicity, the need to discontinue drugs that act on the renin-angiotensin system complicates the management of proteinuric patients (44,45).
Low-protein diets may represent a very interesting therapeutic tool to contrast pregnancy-related hyperfiltration and reduce functional stress on remnant nephrons (46). However, experience with low-protein diets in pregnancy is limited. Studies in vegans underline the safety of this diet, provided that vitamin intake (mainly B12 and iron levels) and nutritional status are kept under control (47–49). The few available animal studies, performed on rats with genetic kidney diseases fed a soya-enriched diet, suggest an advantage for the offspring (32,33).
In this context, we planned our study, and the aim was to analyze the development of children born to mothers affected by advanced or intensely proteinuric CKD who followed a moderately protein-restricted vegan–vegetarian diet in pregnancy. In an attempt to determine the effects of the diet relative to the effects of global care, we identified a control group of patients who were not on a diet for clinical and/or logistic reasons (late referral, stable disease, eating disorders, and cultural–logistic barriers) followed in the same unit.
The main result was a significantly lower incidence of SGA singleton babies in children born to mothers on the diet: 3 of 21 singletons in the diet group versus 7 of 16 singletons in the control group (P=0.05). The overall incidence was in line with the literature, which reports SGA babies in the 10%–45% range in CKD (50,51).
In agreement with the absence of detrimental effects on children's growth, the growth data at 3 months to 10 years were likewise supportive, with low hospitalization rates and normal psychosocial development.
Within the limits of a nonrandomized study, which was considered not ethically sound in pregnant women, the results reassure us of the lack of negative long-term effects on children's growth both in utero and in the later follow-up. Indeed, despite higher levels of proteinuria in the intervention group, pregnancy and fetal outcomes did not differ and were actually even better for intrauterine growth, which likely means a positive effect of the diet (Tables 1–4).
This larger series confirms our previous data, which targeted maternal outcomes (31). None of the patients started dialysis during or within the first 3 months after pregnancy, whereas only one diabetic patient in the intervention group doubled her serum creatinine levels. The neonatal death that was recorded (one preterm boy twin affected by great vessel transposition who died on day 6 of life from cerebral hemorrhage after cardiac surgery) was presumably linked to diabetes, because it had been detected before the development of nephrotic syndrome and before the start of the diet.
Our pilot study has weakness and strengths. The first limitation is the low number of cases with high heterogeneity in CKD stage and underlying disease. This limit is particularly important in the analysis of the long-term follow-up of the children. Nevertheless, our study population represents, to the best of our knowledge, the largest series ever reported of moderate protein restriction in pregnant CKD patients.
The second limitation is the lack of randomization and a perfectly matched control group. This bias reflects an ethical choice to allow patients to choose their own diets during pregnancy, an issue that is shared with long-term diet treatments (46). It is important to point out that the control cohort, which mostly included stage 3 CKD patients, may present two opposite biases: a positive selection, because some patients had milder disease (only one patient had diabetic nephropathy, and baseline proteinuria was significantly lower), and a negative selection because of late referral or cultural and language barriers, often reflecting a lower sociocultural status, which is itself linked with less favorable outcomes. Nevertheless, neither GFR nor any of the other baseline data differed between groups. In contrast, proteinuria was higher in the diet group, further underlining the importance of results obtained in a subset of cases with an a priori poorer prognosis.
As a result of these limitations, our analysis supports the lack of severe side effects of the diet, but it is not powered enough to show a specific advantage in terms of progression of renal impairment.
Finally, hyperfiltration is a complex issue in pregnancy, and only a dedicated study, with pre- and postassessment of GFR after various meals, could formally show a direct effect of the diet on this parameter. This suggestion may set the ground for additional pathophysiologic studies.
The main strength of this study is its novelty, because it focuses on fetal and child growth as well as identification, within the limits mentioned above, of a comparable control group, at least for the decisions on the timing of delivery.
Only larger multicenter studies and long-term follow-up may solve the issues of efficacy and long-term advantages. Also, they may identify uncommon side effects or drawbacks of protein-restricted vegan–vegetarian diets in pregnant women affected by advanced CKD, proteinuria, or other kidney diseases.
Pregnancy is a great challenge for CKD patients but a major determinant of quality of life. In the context of the limited literature data, our pilot study may support a supplemented vegan–vegetarian diet as a safe option for pregnant CKD patients, because it does not interfere with intrauterine growth and may actually even facilitate it; furthermore, it has no negative impact on child growth. Our promising results, which were obtained in a field where treatment options are very limited, should be the basis for additional studies on this crucial, and so far, neglected issue.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06690613/-/DCSupplemental.
References
- 1.Bollati V, Baccarelli A: Environmental epigenetics. Heredity (Edinb) 105: 105–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perera F, Herbstman J: Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol 31: 363–373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilera O, Fernández AF, Muñoz A, Fraga MF: Epigenetics and environment: A complex relationship. J Appl Physiol (1985) 109: 243–251, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Leung A, Schones DE, Natarajan R: Using epigenetic mechanisms to understand the impact of common disease causing alleles. Curr Opin Immunol 24: 558–563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joss-Moore LA, Lane RH: The developmental origins of adult disease. Curr Opin Pediatr 21: 230–234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jirtle RL, Skinner MK: Environmental epigenomics and disease susceptibility. Nat Rev Genet 8: 253–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivorra C, García-Vicent C, Chaves FJ, Monleón D, Morales JM, Lurbe E: Metabolomic profiling in blood from umbilical cords of low birth weight newborns. J Transl Med 10: 142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boney CM, Verma A, Tucker R, Vohr BR: Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115: e290–e296, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Zhang ZX, George LK, Wang ZS, Fan ZJ, Xu T, Zhou XL, Han SM, Wen HB, Zeng Y: Birth measurements, family history, and environmental factors associated with later-life hypertensive status. Am J Hypertens 25: 464–471, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mu M, Wang SF, Sheng J, Zhao Y, Li HZ, Hu CL, Tao FB: Birth weight and subsequent blood pressure: A meta-analysis. Arch Cardiovasc Dis 105: 99–113, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A: Birth weight and subsequent risk of type 2 diabetes: A meta-analysis. Am J Epidemiol 165: 849–857, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lee PA, Chernausek SD, Hokken-Koelega AC, Czernichow P, International Small for Gestational Age Advisory Board : International Small for Gestational Age Advisory Board consensus development conference statement: Management of short children born small for gestational age, April 24–October 1, 2001. Pediatrics 111[6 Pt 1]: 1253–1261, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Relton CL, Davey Smith G: Epigenetic epidemiology of common complex disease: Prospects for prediction, prevention, and treatment. PLoS Med 7: e1000356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacchetta J, Harambat J, Dubourg L, Guy B, Liutkus A, Canterino I, Kassaï B, Putet G, Cochat P: Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int 76: 445–452, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE: Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7: 17–25, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Williams D, Davison J: Chronic kidney disease in pregnancy. BMJ 336: 211–215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MJ, Lehnerz SD, Hebert JR, Parikh CR: Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis 43: 415–423, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Piccoli GB, Attini R, Vasario E, Conijn A, Biolcati M, D'Amico F, Consiglio V, Bontempo S, Todros T: Pregnancy and chronic kidney disease: A challenge in all CKD stages. Clin J Am Soc Nephrol 5: 844–855, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imbasciati E, Gregorini G, Cabiddu G, Gammaro L, Ambroso G, Del Giudice A, Ravani P: Pregnancy in CKD stages 3 to 5: Fetal and maternal outcomes. Am J Kidney Dis 49: 753–762, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Maynard SE, Thadhani R: Pregnancy and the kidney. J Am Soc Nephrol 20: 14–22, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Abou-Jaoude P, Dubourg L, Bessenay L, Pinçon A, Jolivot A, Guebre-Egziabher F, Cochat P, Bacchetta J: What about the renal function during childhood of children born from dialysed mothers? Nephrol Dial Transplant 27: 2365–2369, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Blowey DL, Warady BA: Outcome of infants born to women with chronic kidney disease. Adv Chronic Kidney Dis 14: 199–205, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Greene AC, Tripaldi M, Chiarelli F, McKiernan P, Morris A, Newton R, Greene S, Hvidøre Study Group for Childhood Diabetes : Cross-cultural differences in the management of children and adolescents with diabetes. Horm Res 57[Suppl 1]: 75–77, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Bergström J: Discovery and rediscovery of low protein diet. Clin Nephrol 21: 29–35, 1984 [PubMed] [Google Scholar]
- 25.Mitch WE: Beneficial responses to modified diets in treating patients with chronic kidney disease. Kidney Int 94[Suppl 4]: S133–S135, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Fouque D, Laville M: Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev 3: CD001892, 2009 [DOI] [PubMed] [Google Scholar]
- 27.National Collaborating Centre for Women’s and Children’s Health : Antenatal Care: Routine Care for the Healthy Pregnant Woman. Clinical Guideline, RCOG Press, London, 2008 [PubMed] [Google Scholar]
- 28.Yakoob MY, Menezes EV, Soomro T, Haws RA, Darmstadt GL, Bhutta ZA: Reducing stillbirths: Behavioural and nutritional interventions before and during pregnancy. BMC Pregnancy Childbirth 9[Suppl 1]: S3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Symonds ME, Stephenson T, Gardner DS, Budge H: Long-term effects of nutritional programming of the embryo and fetus: Mechanisms and critical windows. Reprod Fertil Dev 19: 53–63, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Oster M, Murani E, Metges CC, Ponsuksili S, Wimmers K: A high protein diet during pregnancy affects hepatic gene expression of energy sensing pathways along ontogenesis in a porcine model. PLoS One 6: e21691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piccoli GB, Attini R, Vasario E, Gaglioti P, Piccoli E, Consiglio V, Deagostini C, Oberto M, Todros T: Vegetarian supplemented low-protein diets. A safe option for pregnant CKD patients: Report of 12 pregnancies in 11 patients. Nephrol Dial Transplant 26: 196–205, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Cahill LE, Peng CY, Bankovic-Calic N, Sankaran D, Ogborn MR, Aukema HM: Dietary soya protein during pregnancy and lactation in rats with hereditary kidney disease attenuates disease progression in offspring. Br J Nutr 97: 77–84, 2007 [DOI] [PubMed] [Google Scholar]
- 33.McMullen S: Soya protein during pregnancy—an opportunity to attenuate the progression of chronic kidney disease? Br J Nutr 97: 4–5, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Piccoli GB, Fassio F, Attini R, Parisi S, Biolcati M, Ferraresi M, Pagano A, Daidola G, Deagostini MC, Gaglioti P, Todros T: Pregnancy in CKD: Whom should we follow and why? Nephrol Dial Transplant 27[Suppl 3]: iii111–iii118, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Parazzini F, Cortinovis I, Bortolus R, Fedele L: Standards of birth weight in Italy. Ann Ostet Ginecol Med Perinat 112: 203–246, 1991 [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention, National Center for Health Statistics. Z-Score Data Files. Available at: http://www.cdc.gov/growthcharts/zscore.htm Accessed June 6, 2013
- 37.Piccoli GB, Gaglioti P, Attini R, Parisi S, Bossotti C, Olearo E, Oberto M, Ferraresi M, Rolfo A, Versino E, Biolcati M, Todros T: Pre-eclampsia or chronic kidney disease? The flow hypothesis. Nephrol Dial Transplant 28: 1199–1206, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Barsotti G, Morelli E, Cupisti A, Meola M, Dani L, Giovannetti S: A low-nitrogen low-phosphorus Vegan diet for patients with chronic renal failure. Nephron 74: 390–394, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Barsotti G, Guiducci A, Ciardella F, Giovannetti S: Effects on renal function of a low-nitrogen diet supplemented with essential amino acids and ketoanalogues and of hemodialysis and free protein supply in patients with chronic renal failure. Nephron 27: 113–117, 1981 [DOI] [PubMed] [Google Scholar]
- 40.Piccoli GB, Ferraresi M, Deagostini MC, Vigotti FN, Consiglio V, Scognamiglio S, Moro I, Clari R, Fassio F, Biolcati M, Porpiglia F: Vegetarian low-protein diets supplemented with keto analogues: A niche for the few or an option for many? Nephrol Dial Transplant 28: 2295–2305, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Bertino E, Di Nicola P, Varalda A, Occhi L, Giuliani F, Coscia A: Neonatal growth charts. J Matern Fetal Neonatal Med 25[Suppl 1]: 67–69, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, Cerutti F, Gargantini L, Greggio N, Tonini G, Cicognani A: Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest 29: 581–593, 2006 [DOI] [PubMed] [Google Scholar]
- 43.The World Health Organization. The WHO Child Growth Standards. Available at: http://www.who.int/childgrowth/standards/en/ Accessed June 6, 2013
- 44.Vasilakis-Scaramozza C, Aschengrau A, Cabral HJ, Jick SS: Antihypertensive drugs and the risk of congenital anomalies. Pharmacotherapy 33: 476–482, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Polifka JE: Is there an embryopathy associated with first-trimester exposure to angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists? A critical review of the evidence. Birth Defects Res A Clin Mol Teratol 94: 576–598, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Mitch WE, Remuzzi G: Diets for patients with chronic kidney disease, still worth prescribing. J Am Soc Nephrol 15: 234–237, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Pawlak R, Parrott SJ, Raj S, Cullum-Dugan D, Lucus D: How prevalent is vitamin B(12) deficiency among vegetarians? Nutr Rev 71: 110–117, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Koletzko B, Bauer CP, Bung P, Cremer M, Flothkötter M, Hellmers C, Kersting M, Krawinkel M, Przyrembel H, Rasenack R, Schäfer T, Vetter K, Wahn U, Weißenborn A, Wöckel A: Nutrition in pregnancy—practice recommendations of the Network “Healthy Start—Young Family Network.” Dtsch Med Wochenschr 137: 1366–1372, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Craig WJ, Mangels AR, American Dietetic Association : Position of the American Dietetic Association: Vegetarian diets. J Am Diet Assoc 109: 1266–1282, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Nevis IF, Reitsma A, Dominic A, McDonald S, Thabane L, Akl EA, Hladunewich M, Akbari A, Joseph G, Sia W, Iansavichus AV, Garg AX: Pregnancy outcomes in women with chronic kidney disease: A systematic review. Clin J Am Soc Nephrol 6: 2587–2598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piccoli GB, Conijn A, Attini R, Biolcati M, Bossotti C, Consiglio V, Deagostini MC, Todros T: Pregnancy in chronic kidney disease: Need for a common language. J Nephrol 24: 282–299, 2011 [DOI] [PubMed] [Google Scholar]