Abstract
Background and objectives
In ANCA-associated GN, severe renal dysfunction portends a poor prognosis for renal recovery and patient survival. This study evaluated the prognostic factors affecting renal and patient outcomes in patients presenting with severe kidney failure to guide immunosuppressive therapy.
Design, setting, participants, & measurements
This study retrospectively evaluated clinical and histopathologic characteristics of 155 patients who underwent biopsy between October 1985 and February 2011 (median eGFR at presentation, 7.1 ml/min per 1.73 m2; 87% required hemodialysis), all treated with immunosuppressive medications. Three outcomes of interest were measured: patient survival, renal survival, and treatment response (defined as dialysis-free survival without active vasculitis by 4 months after biopsy). Competing risk, Cox, and logistic regression analyses were conducted for each outcome measure.
Results
Within 4 months after biopsy, treatment response was attained in 51% of patients, 35% remained on dialysis, and 14% died. In a competing risk analysis, estimated cumulative incidence rates of ESRD and disease-related mortality were 26% and 17% at 1 year and 32% and 28% at 5 years, respectively. Cyclophosphamide therapy and treatment response by 4 months were independently associated with patient and renal survival, adjusting for the percentage of normal glomeruli, histopathologic chronicity index score, and baseline clinical characteristics. Only 5% of patients still dialysis dependent at 4 months subsequently recovered renal function. Low chronicity index score (odds ratio [OR], 1.16; 95% confidence interval [95% CI], 1.04 to 1.30, per unit decrease) and baseline eGFR>10 ml/min per 1.73 m2 (OR, 2.77; 95% CI, 1.09 to 7.01) were significantly associated with treatment response by 4 months. Among cyclophosphamide-treated patients, the likelihood of treatment response was >14% even with highest chronicity index score and eGFR<10 ml/min per 1.73 m2.
Conclusions
Although low baseline renal function and severe renal scarring are associated with lower treatment response rate, no “futility” threshold could be identified. Conversely, continued immunosuppressive therapy beyond 4 months is unlikely to benefit patients who remain dialysis dependent.
Keywords: ANCA, glomerulonephritis, vasculitis
Introduction
ANCA-associated vasculitis often presents with severe renal failure requiring renal replacement therapy (1–3). Severe renal dysfunction portends a poor prognosis for progression to ESRD and patient survival (4–6) and is associated with increased risk of serious infections from immunosuppression with glucocorticoids and cyclophosphamide (7,8). Whereas immunosuppressive therapy is invariably warranted in the presence of severe extrarenal manifestation of disease (9), implementing such therapy is sometimes questioned when renal involvement is the major clinical manifestation of ANCA vasculitis and the likelihood of improvement is perceived to be low. This study aims to assess the benefit and risk of immunosuppressive therapy for individual patients presenting with severe renal disease. We analyzed the patient and renal survival on the basis of the largest cohort to date. Using multivariate regression analysis, we analyzed the baseline clinical and histologic variables associated with these outcomes and provide individualized estimated likelihoods of recovery of renal function with immunosuppression.
Materials and Methods
Participants and Inclusion Criteria
Study participants were identified from the Glomerular Disease Collaborative Network registry (6,10). After providing informed consent, patients with pauci-immune necrotizing crescentic GN who underwent biopsy between October 1985 and February 2010 and who met the following inclusion criteria were studied: (1) eGFR<15 ml/min per 1.73 m2 according to the Modification of Diet in Renal Disease equation (11), tested immediately before starting dialysis or immunosuppressive therapy; (2) therapy with oral or intravenous glucocorticoids, cyclophosphamide, or other immunosuppressive medications; and (3) follow-up until death or for at least 12 months after the biopsy. Patients with concomitant anti–glomerular basement membrane antibodies or immune complex–mediated disease were excluded.
Clinical and Histologic Variables and Outcomes
We extracted demographic and clinical data from clinical source documents of each individual, including age, sex, race, ANCA test, disease phenotype according to the Chapel Hill Consensus Conference nomenclature (12) (microscopic polyangiitis, granulomatosis with polyangiitis, or pauci-immune GN without signs of systemic vasculitis [renal limited disease]), baseline eGFR, pulmonary involvement or hemorrhage, and type and duration of immunosuppressive therapy.
Two pathologists (J.C.J. and A.G.) who were blinded to patient outcomes reviewed the renal biopsy specimens for classification and scoring. Glomerular and tubulointerstitial lesions were scored and analyzed separately and as part of activity index and chronicity index scores. The activity index score is the sum of scores from five designated categories: intracapillary cellular infiltration, intracapillary neutrophil infiltration, glomerular necrosis, glomerular crescent, and interstitial leukocyte infiltration. Each category was scored semi-quantitatively from 0 (none) to 4 (severe) for a maximum of 20 points. For the chronicity index score, four categories—glomerular sclerosis, interstitial fibrosis, crescentic sclerosis, and tubular atrophy—were similarly scored for a maximum of 16 points. Arteriosclerosis was measured in the same manner (range, 0–4). The renal biopsy specimens were also classified according to the European Vasculitis Study Group (EUVAS) schema (13).
We measured three event outcomes: treatment response, death, and ESRD (defined as the need for permanent RRT or transplantation). Treatment response was defined as dialysis independence and eGFR>20 ml/min per 1.73 m2 with no clinical signs of active systemic vasculitis at the end of 4 months after biopsy. Causes of death were categorized into disease- or treatment-related death and death from other causes.
Statistical Analyses
Descriptive statistics were presented as median with interquartile range (IQR) or percentage according to the type of data. During follow-up, death may occur as a disease or treatment-related event before renal response is attained from immunosuppressive treatment. In this setting, death and ESRD are not independent but rather are interrelated, informative outcomes; therefore, death should be dealt with as a competing risk for the precise estimates of renal survival (14). Application of the usual Kaplan–Meier method would produce biased estimates of the incidence of ESRD because it simply censors death. Likewise, mortality from other causes that are not directly related to ANCA vasculitis, such as cancer or death after remission of disease, is also competing with disease-related mortality for the precise risk estimation. To address this problem, we used a nonparametric estimation method for the cumulative incidence function of each outcome in a competing risk setting (15). We compared the outcomes of interest between the treatment response and the no-response groups using the Gray test (16). To evaluate the influence of covariates on each outcome, we performed a competing risk regression analysis (17). Because we considered that the composite outcome of any first-occurring ESRD or death from any cause as important, we also compared and validated these results in Cox proportional hazards models. Logistic regression analysis was used to determine the risk factors associated with treatment response at 4 months.
We assessed baseline characteristics as potential covariates and transformed these into proper function forms to fit the models and meet the inferential goal of finding predictors for the outcome of interest. Age was categorized as quartiles; eGFR was categorized as >10 or ≤10 ml/min per 1.73 m2. The percentage of unaffected glomeruli was categorized as >10% or ≤10%. Myeloperoxidase (MPO)-ANCA (versus proteinase 3 [PR3]-ANCA), use of cyclophosphamide (versus glucocorticoids alone), use of plasmapheresis, and the presence of arteriosclerosis on biopsy were used as binary covariates. Activity and chronicity index scores were used as continuous variables. To screen explanatory variables, univariate analysis for each regression model was conducted with a selection criterion of P<0.1. Thereafter, significant covariates from univariate models were selected by backward elimination using an α level of 0.05. We tested interaction terms between selected covariates using a significance level of P<0.2 in the likelihood ratio test. No significant interaction term was identified among exploratory variables for the treatment response outcome. Estimated probabilities and their confidence intervals for the treatment response were presented on the basis of predictors found in the final model. SAS software, version 9.3.1 (SAS Institute, Inc., Cary, NC), and R software, version 2.15.2 (www.r-project.org), were used for the analysis.
Results
Cohort Description and Outcomes
A total of 155 patients with pauci-immune necrotizing and crescentic GN were included in the study. Table 1 shows the baseline characteristics of our cohort. Eighty-seven percent received hemodialysis, and the median eGFR at presentation was 7.1 ml/min per 1.73 m2. Fifty-two percent of patients were diagnosed with microscopic polyangiitis, 29% had renal limited disease, and 19% had granulomatosis with polyangiitis; 56% were positive for MPO-ANCA and 44% for PR3-ANCA. Pulmonary involvement was present in 46% of patients, and 28% had alveolar hemorrhage and received therapeutic plasmapheresis. A total of 135 patients (87%) were treated with oral or intravenous cyclophosphamide in addition to high-dose glucocorticoids (intravenous methylprednisolone followed by daily oral prednisone), while 20 patients (13%) received high-dose glucocorticoids alone. The renal biopsy specimens of 153 patients were reviewed. The median number of glomeruli examined per biopsy specimen was 16 (IQR, 11–26). The pattern of glomerular injury according to the EUVAS schema was categorized as sclerotic in 32% of cases, focal in 6%, crescentic in 43%, and mixed class in 6%. The median activity index score was 7 (IQR, 5–9), and the median chronicity index score was 6 (IQR, 4–9). Eighty-eight percent of patients had at least mild arteriosclerosis.
Table 1.
Baseline characteristics of whole cohort
| Characteristic | Data (n=155) |
|---|---|
| Clinical characteristics | |
| Age (yr) | 67 (53, 74) |
| Men, n (%) | 87 (56) |
| White, n (%) | 136 (88) |
| Diagnosis, n (%) | |
| MPA | 81 (52) |
| GPA | 30 (20) |
| RL | 44 (28) |
| ANCA type, n (%) | |
| MPO-ANCA | 87 (56) |
| PR3-ANCA | 68 (44) |
| Lung involvement, n (%) | 71 (46) |
| eGFR (ml/min per 1.73 m2) | 7 (5, 9) |
| Cyclophosphamide + glucocorticoids, n (%) | 135 (87) |
| Cyclophosphamide regimen, n (%) | |
| Intravenous pulse, n (%) | 68 (50) |
| Oral | 47 (35) |
| Both | 20 (15) |
| Exposure time to cyclophosphamide (mo)a | 5 (3, 10) |
| Glucocorticoids alone, n (%) | 20 (13) |
| Plasmapheresis, n (%) | 43 (28) |
| Histologic characteristics | |
| Glomeruli examined (n) | 16 (11, 26) |
| Globally sclerotic glomeruli (%) | 21 (7, 42) |
| Normal glomeruli (%) | 7 (0, 22) |
| Activity index scoreb (0–24) | 7 (5, 9) |
| Chronicity index scorec (0–16) | 6 (4, 9) |
| Arteriosclerosis mild or greater, n (%) | 135 (88) |
Unless otherwise noted, data are expressed as median (25th, 75th percentiles) or n (%). MPA, microscopic polyangiitis; GPA, granulomatous polyangiitis; RL, renal limited; MPO, myeloperoxidase; PR3, proteinase 3.
Oral cyclophosphamides were prescribed at 2 mg/kg in daily dosage and intravenous formulas were 750–1000 mg per pulse.
Sum of semiquantitative scores of five items (each scored from 0 to 4 points): intracapillary cellular infiltration, intracapillary neutrophil infiltration, glomerular necrosis, glomerular crescent, and interstitial leukocyte infiltration
Sum of semiquantitative scores of four items (each scored from 0 to 4 points): glomerular sclerosis, interstitial fibrosis, crescentic sclerosis, and tubular atrophy.
Figure 1 summarizes the outcome of patients during the first year of follow-up. Most outcome events occurred within the first 4 months after biopsy. Seventy-nine patients (51%) responded to the immunosuppressive treatment and came off dialysis after a median duration of 4.4 weeks (range, 1–39 weeks). At the end of 4 months, 55 patients (35%) remained dialysis dependent; 3 of these came off dialysis later (at 5, 9, and 10 months). Among patients who responded by 4 months, most maintained stable renal function through the first year of follow-up. Only 2 patients returned to dialysis dependence: 1 patient at 5 months after response due to disease relapse and the other at 7 months due to CKD progression.
Figure 1.
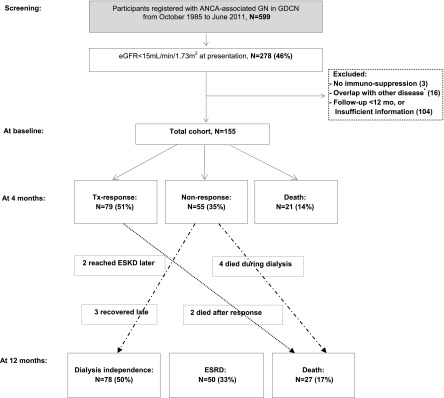
Clinical outcomes: the treatment response beyond 4 months after biopsy is uncommon. Treatment response was defined as dialysis independence, with eGFR>20 ml/min per 1.73 m2, and without clinical sign of active vasculitis. Six of 16 were positive with anti–glomerular basement membrane antibody, and 6 had combined immune complex–mediated GN; the remaining 4 patients had membranoproliferative GN, cryoglobulin-associated necrotizing crescentic GN, fibrillary GN, and Takayasu vasculitis, respectively. ANCA-GN, ANCA-associated GN; GDCN, Glomerular Disease Collaborative Network; Tx, treatment.
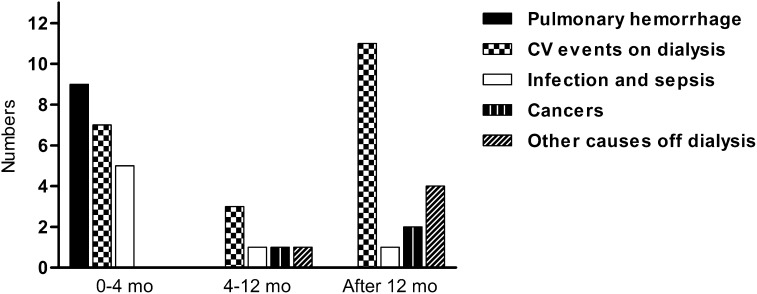
The most common causes of death during the first 4 months were pulmonary hemorrhage (n=9), cardiovascular events on dialysis (n=7), and infection (n=5) (Figure 2). Among patients who had an initial response to treatment, 2 died between 4 and 12 months (1 of leukemia at 8 months and the other of unknown cause in remission of the disease at 5 months). Eighteen patients died more than 12 months after diagnosis: 11 due to cardiovascular events during long-term dialysis remote to the acute period, 2 due to cancer at 28 and 145 months, and 1 due to pneumonia during disease relapse after 23 months. Four dialysis-independent patients died of other causes without active vasculitis at 21, 48, 71, and 175 months.
Figure 2.
Cause of death according to time periods after diagnosis. Horizontal axis labels show time periods after biopsy. Other causes of dialysis represent death in dialysis-independent patients without signs of active vasculitis. CV event, cardiovascular events defined as acute coronary syndromes, strokes, or sudden cardiac deaths.
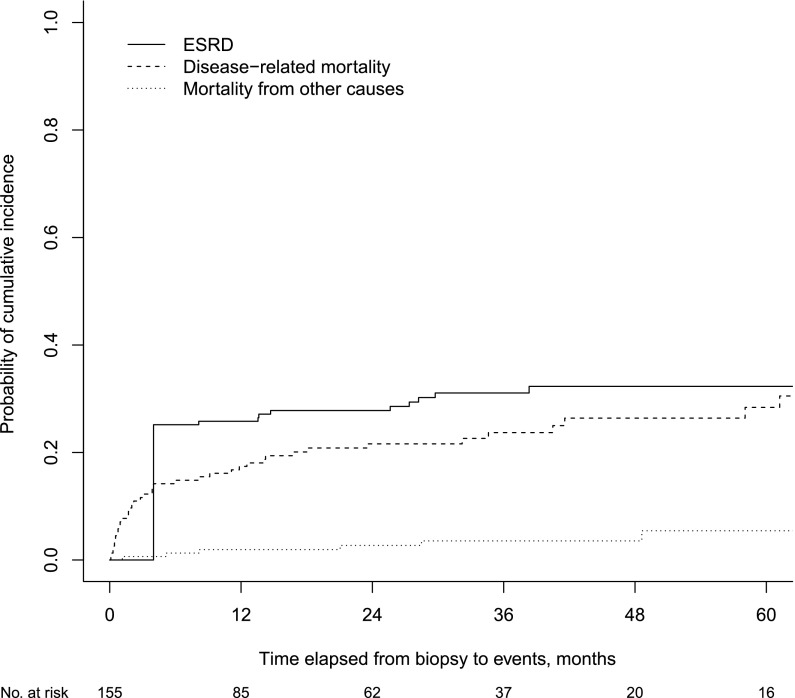
Figure 3 illustrates the cumulative incidence functions of the outcomes of interest with competing risks of the entire cohort. The median time to any event was 1.5 years (IQR, 4 months to 7 years). Estimated cumulative incidence rates of ESRD and disease-related mortality for the entire cohort were 26% (95% confidence interval [95% CI], 19% to 33%) and 17% (95% CI, 11% to 23%) at 1 year after diagnosis and 32% (95% CI, 25% to 40%) and 28% (95% CI, 20% to 37%) at 5 years, respectively (Supplemental Appendix Table 1, Figure 1). Supplemental Appendix Figure 1 shows a comparison of cumulative incidence functions between the treatment response group and no‐response group.
Figure 3.
Probability of cumulative incidence of ESRD, disease-related death, or death from other cause for the entire cohort. The bold line shows the cumulative incidence of ESRD, after adjusting for competing risk, mortality from disease-related causes, and others. Patients who required dialysis at presentation and never recovered renal function were considered to be at ESRD after 3 months. Mortality from other causes represents those unrelated to ANCA disease and/or ESRD.
Risk Factors for Long-Term Outcomes
Table 2 shows the results of competing risk regression and Cox regression models. By univariate analysis, dialysis independence was associated with a baseline eGFR>10 ml/min per 1.73 m2, cyclophosphamide use, treatment response within 4 months as a time-varying variable, lower chronicity index score, normal glomeruli>10%, and EUVAS biopsy sclerotic class (versus focal class) (P<0.1). After adjustment for these variables, multivariate analysis with competing risk regression model indicated that treatment response within 4 months was independently associated with long-term renal survival.
Table 2.
Risk factors associated with ESRD, disease-related mortality, and the composite outcome of ESRD or death
| Variable | ESRD: CRRa | Disease-Related Mortality: CRRb | Composite Outcome: Coxc | |||
|---|---|---|---|---|---|---|
| SHR (95% CI) | P Value | SHR (95% CI) | P Value | HR (95% CI) | P Value | |
| Univariate model | ||||||
| Age, quartiles | ||||||
| 54–64 yr (versus <54 yr) | 1.24 (0.73 to 2.11) | 0.42 | 4.06 (1.14 to 14.4) | 0.03 | 1.35 (0.74 to 2.44) | 0.33 |
| 65–74 yr (versus <54 yr) | 1.16 (0.67 to 2.00) | 0.59 | 6.01 (1.73 to 20.8) | 0.01 | 1.27 (0.71 to 2.27) | 0.42 |
| ≥75 yr (versus <54 yr) | 1.42 (0.81 to 2.46) | 0.22 | 6.07 (1.72 to 21.5) | 0.01 | 1.67 (0.92 to 3.03) | 0.09 |
| MPO-ANCA (versus PR3) | 1.29 (0.72 to 2.31) | 0.40 | 1.21 (0.65 to 2.25) | 0.54 | 1.21 (0.80 to 1.82) | 0.37 |
| eGFR>10 ml/min per 1.73 m2 (versus ≤10) | 0.25 (0.08 to 0.81) | 0.02 | 1.03 (0.49 to 2.16) | 0.94 | 0.54 (0.29 to 0.99) | 0.04 |
| Lung involvement (versus none) | 1.23 (0.70 to 2.19) | 0.47 | 1.34 (0.70 to 2.56) | 0.38 | 1.42 (0.94 to 2.17) | 0.10 |
| Cyclophosphamide (versus no use) | 0.39 (0.20 to 0.79) | 0.01 | 0.28 (0.13 to 0.60) | 0.01 | 0.35 (0.21 to 0.58) | 0.01 |
| Plasmapheresis (versus no use) | 0.90 (0.47 to 1.72) | 0.74 | 0.86 (0.43 to 1.75) | 0.68 | 0.92 (0.58 to 1.46) | 0.73 |
| Treatment response (versus no response) | 0.06 (0.02 to 0.15) | <0.001 | 0.09 (0.04 to 0.20) | <0.001 | 0.10 (0.06 to 0.16) | <0.001 |
| Normal glomeruli>10% (versus ≤10%) | 0.59 (0.32 to 1.08) | 0.09 | 0.52 (0.27 to 1.01) | 0.05 | 0.65 (0.43 to 0.99) | 0.04 |
| Arteriosclerosis (versus none) | 0.93 (0.42 to 2.08) | 0.86 | 6.33 (0.87 to 46.1) | 0.07 | 1.72 (0.83 to 3.55) | 0.15 |
| Activity index score (unit decrease) | 0.96 (0.88 to 1.04) | 0.33 | 0.99 (0.89 to 1.09) | 0.81 | 0.98 (0.93 to 1.05) | 0.60 |
| Chronicity index score (unit decrease) | 0.91 (0.84 to 0.99) | 0.03 | 0.93 (0.85 to 1.02) | 0.14 | 0.93 (0.88 to 0.99) | 0.02 |
| EUVAS classification (13) | ||||||
| Focal (versus sclerotic) | 0.49 (0.26 to 1.02) | 0.08 | 0.53 (0.06 to 4.38) | 0.55 | 0.44 (0.15 to 1.29) | 0.14 |
| Mixed (versus sclerotic) | 0.50 (0.20 to 1.29) | 0.16 | 1.95 (0.81 to 4.69) | 0.14 | 0.83 (0.49 to 1.40) | 0.49 |
| Crescentic (versus sclerotic) | 0.69 (0.32 to 1.18) | 0.22 | 1.45 (0.58 to 3.66) | 0.43 | 0.67 (0.38 to 1.18) | 0.22 |
| Multivariate model | ||||||
| Age | ||||||
| 65–74 yr (versus <54 yr) | 5.06 (1.38 to 18.5) | 0.01 | ||||
| ≥75 yr (versus <54 yr) | 4.38 (1.13 to 16.9) | 0.03 | ||||
| Treatment response | 0.06 (0.02 to 0.14) | <0.001 | 0.09 (0.04 to 0.22) | <0.001 | 0.09 (0.02 to 0.15) | <0.001 |
| Cyclophosphamide use | 0.43 (0.19 to 0.9) | 0.0 | 0.39 (0.21 to 0.73) | 0.01 | ||
Activity index score ranges from 0 to 20; chronicity index score ranges from 0 to 16. Treatment response is time-varying variable changing status after 4 months. CRR, competing risk regression model; Cox, Cox proportional hazard model; SHR, subdistribution hazard ratio; 95% CI, 95% confidence interval; HR, hazard ratio; MPO, myeloperoxidase; PR3, proteinase 3; EUVAS, European Vasculitis Study Group.
CRR was used to determine risk factors associated with ESRD, outcome of interest with competing risks of disease-related mortality, and mortality from other causes.
Disease-related mortality is the outcome of interest competing with ESRD and mortality from other causes.
Conjoined outcomes with ESRD or all-cause mortality were used for Cox proportional hazard model.
For the outcome of disease-related mortality, age (per quartile increase of age), treatment with glucocorticoids alone, no treatment response within 4 months, <10% normal glomeruli, presence of arteriosclerosis, and higher chronicity index score were selected as significant explanatory variables from the univariate analysis (P<0.1). Repeating this analysis using quartiles of chronicity index score gave similar results (data not shown). By multivariate competing risk analysis adjusting for these factors, treatment response at 4 months, older age, and cyclophosphamide use were selected as significant independent risk factors predicting disease-related mortality (P<0.05). In a multivariate Cox regression model with the composite outcome of ESRD or death, treatment response at 4 months along with cyclophosphamide treatment were reaffirmed as significant predictors of dialysis-free survival.
Risk Factors and Probability of Treatment Response
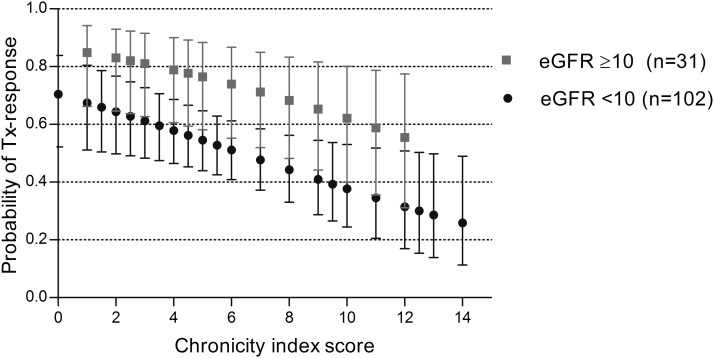
The effect of treatment response at 4 months on long-term patient and renal survival led us to investigate the risk factors influencing this intermediate outcome. By univariate analysis, PR3-ANCA type (versus MPO-ANCA), eGFR>10 ml/min per 1.73 m2, cyclophosphamide treatment, >10% normal glomeruli, low chronicity index score, and the absence of arteriosclerosis on biopsy were significantly associated with treatment response (P<0.1) (Table 3). By multivariate analysis adjusting for these covariates, a baseline eGFR>10 ml/min per 1.73 m2 conferred a 2.8-fold higher likelihood of treatment response (compared with baseline eGFR<10 ml/min per 1.73 m2) and cyclophosphamide treatment was associated with a 4.4-fold higher likelihood of response (compared with treatment with glucocorticoids alone). In addition, the likelihood of treatment response increased by 16% (95% CI, 4% to 30%; P=0.02), with each unit decrease in chronicity index score (Table 3). Using logistic regression analysis, we calculated the probability of treatment response to cyclophosphamide and glucocorticoids for each category of baseline eGFR and chronicity index score (Figure 4). This model predicts a >14% (lowest confidence limit) chance of recovering renal function with cyclophosphamide and glucocorticoid therapy even with a baseline eGFR<10 ml/min per 1.73 m2 and a very high chronicity index score (14 of 16) on biopsy.
Table 3.
Predictors associated with treatment response
| Variable | Treatment Response: Logistic Regression | |
|---|---|---|
| Odds Ratio (95% CI) | P Value | |
| Univariate model | ||
| Age, quartiles | ||
| 54–64 yr (versus <54 yr) | 0.73 (0.29 to 1.82) | 0.50 |
| 65–74 yr (versus <54 yr) | 0.80 (0.33 to 1.97) | 0.63 |
| ≥75 yr (versus <54 yr) | 0.47 (0.18 to 1.22) | 0.12 |
| MPO-ANCA (versus PR3) | 0.40 (0.21 to 0.79) | 0.01 |
| eGFR>10 ml/min per 1.73 m2 (versus ≤10) | 3.16 (1.30 to 7.70) | 0.01 |
| Cyclophosphamide (versus glucocorticoids) | 7.28 (2.03 to 26.09) | 0.003 |
| Lung involvement (versus none | 0.85 (0.41 to 1.64) | 0.62 |
| Plasmapheresis (versus no use) | 1.26 (0.61 to 2.58) | 0.53 |
| Normal glomeruli>10% (versus ≤ 10%) | 2.18 (1.13 to 4.22) | 0.02 |
| Arteriosclerosis (versus none) | 0.25 (0.08 to 0.81) | 0.02 |
| Activity index score (unit decrease) | 1.07 (0.97 to 1.19) | 0.18 |
| Chronicity index score (unit decrease) | 1.18 (1.07 to 1.31) | 0.002 |
| EUVAS classification (13) | ||
| Focal class (versus sclerotic class) | 7.00 (1.22 to 40.0) | 0.03 |
| Mixed class (versus sclerotic class) | 2.63 (1.00 to 6.9) | 0.05 |
| Crescentic class (versus sclerotic class) | 2.06 (0.84 to 5.08) | 0.12 |
| Multivariate modela | ||
| Chronicity index score (unit decrease) | 1.16 (1.04 to 1.30) | 0.02 |
| eGFR>10% (versus ≤10%) | 2.77 (1.09 to 7.01) | 0.04 |
| Cyclophosphamide use (versus steroids) | 4.38 (1.17 to 16.42) | 0.03 |
Activity index score ranges from 0 to 20; chronicity index score ranges from 0 to 16. 95% CI, 95% confidence interval; MPO, myeloperoxidase; PR3, proteinase 3; EUVAS, European Vasculitis Study Group.
Concordance statistic=0.73.
Figure 4.
Among cyclophosphamide-treated patients, the likelihood of treatment response was >14% (lowest confidence limit) even with highest chronicity index score and eGFR<10 ml/min per 1.73 m2. Chronicity index score (0–16 points) is sum of semiquantitative measurements on four items: glomerular sclerosis, crescent sclerosis, interstitial fibrosis, and tubular atrophy. Each point and error bars represents estimated mean probability and its 95% confidence intervals. eGFR, ml/min per 1.73 m2.
Discussion
Patients with ANCA vasculitis and severe renal dysfunction present special clinical challenges. Their likelihood of response to therapy is diminished compared with that among patients with preserved renal function, and they are at increased risk for adverse effects of immunotherapy. The questions then arise as to whether the potential benefits of treatment justify the increased risks and whether there are patients for whom the risks of therapy outweigh the benefits and have effectively reached a “point of no return.” If immunosuppressive therapy is engaged, the next question pertains to the duration of treatment beyond which no further benefit can be reasonably expected. To answer these questions, we reviewed the outcomes of 155 patients with an eGFR<15 ml/min per 1.73 m2 at presentation.
Despite severe renal failure, half the patients responded to therapy by 4 months and were dialysis independent at the end of the first year of follow-up. By the end of the first year, one third of patients had reached ESRD, and 17% had died of treatment- and disease-related causes. On the basis of the largest cohort of patients with ANCA vasculitis and severe renal failure to date, our results are similar to those in published cohort studies (5,18) and in prospective multicenter clinical trials (19,20).
The major causes of death varied with time. In the first 4 months, deaths were related to vasculitis, cardiovascular events, and treatment-related infections. After 12 months, the major cause of death was cardiovascular disease, presumably related to ESRD. Early cardiovascular events have been previously reported in association with ANCA vasculitis and are postulated to be related to active inflammation as well as underlying atherosclerosis (21–23).
According to our survival analysis accounting for potential competing risks during follow-up, use of cyclophosphamide and treatment response within 4 months were independent predictors for renal and patient survival. The effect of cyclophosphamide therapy on patient survival agrees with the results of earlier studies (4,19,24,25) and emphasizes the importance of assertive immunosuppression in the setting of severe renal failure even on dialysis. Treatment response within 4 months was also the only variable associated with long-term renal survival retained by multivariate analysis after correcting for baseline eGFR, histopathologic chronicity index score, percentage of normal glomeruli, and treatment with cyclophosphamide. Having established response to therapy at 4 months as an important determinant of long-term patient and renal outcomes, we analyzed the risk factors associated with this intermediate endpoint. Compared with treatment with glucocorticoids alone, the use of cyclophosphamide confers a significantly increased likelihood of recovery of renal function. In addition to treatment with cyclophosphamide, a presenting GFR of >10 ml/min per 1.73 m2 and low chronicity index score were also independently associated with response to therapy at 4 months. Therefore, even minor differences in presenting GFR have a marked effect on the long-term outcome of patients with severe renal disease. This finding emphasizes the importance of early diagnosis and prompt initiation of therapy.
The histopathologic chronicity score incorporates semiquantitative measures of glomerular and tubulointerstitial scarring, whereas the EUVAS classification schema (13) is based on scoring of glomerular lesions only. Unlike the EUVAS schema, the chronicity index score was independently and inversely associated with the likelihood of dialysis independence at 4 months. These findings suggest that the tubulointerstitial compartment lesions have a significant effect on the renal outcome of patients with ANCA-associated nephritis (6,26). The difference between our results and those of the EUVAS schema with respect to the prognostic value of histopathologic classification is probably related to the selection of patients with severe renal failure in our patient population, leading to a narrower spectrum of histopathologic findings, and possibly an increased prominence of scarring of both the glomerular and tubulointerstitial compartments.
Among cyclophosphamide-treated patients, we provide graded estimates of dialysis independence at 4 months for each category of baseline eGFR and for the range of chronicity index scores (Figure 4). We also aimed to identify a threshold of severity of renal disease below which treatment would be considered futile. However, even among patients with a baseline eGFR<10 ml/min per 1.73 m2 and near-maximal chronicity score (14 of 16), the likelihood of response remains >14%. We therefore conclude that all patients presenting with severe renal failure can benefit from an initial therapy trial with glucocorticoids and cyclophosphamide for 4 months. Conversely, the likelihood of attaining sustained dialysis independence beyond 4 months of therapy is very low (5%). In the absence of extrarenal active vasculitis, we therefore suggest that treatment with cyclophosphamide should be discontinued after 4 months if dialysis independence is not attained by that time. The previous study of histologic prognostic factors based on 68 dialysis-dependent patients had similarly identified the extent of tubular atrophy and glomerular injury as important prognostic indicators of poor renal outcome (24). In that study, investigators estimated that in patients treated with plasma exchange, the risk of death exceeded the likelihood of renal recovery only in patients with the most extensive glomerular lesions (≤2% normal glomeruli). Our use of stepwise regression modeling may have overestimated the effect size of the selected variable on response to treatment. It is possible that factors other than baseline eGFR, chronicity index, and cyclophosphamide use may also influence treatment response. Future validation of these findings in other datasets is warranted.
The strong association between renal recovery at 4 months and improved long-term renal and patient survival differs somewhat with the long-term follow-up results of the methylprednisolone versus plasmapheresis (MEPEX) trial (18). In that study, the association between plasmapheresis and renal recovery at 3 and 12 months did not confer a strong long-term patient or renal survival benefit.
In our study, only 43 patients received adjunctive plasmapheresis. By univariate analysis, we did not detect an association of plasmapheresis with any of the clinical outcomes of interest. This result differs from those of the MEPEX study (27), wherein therapeutic plasma exchange was associated with a significantly improved likelihood of renal recovery at 3 and 12 months after start of treatment. This difference in results may be attributable to several factors. Our study was not set up to specifically assess the effect of plasmapheresis. Before the publication of the MEPEX trial results, patients with severe renal dysfunction were not systematically treated with plasmapheresis, which was primarily restricted to patients with severe pulmonary hemorrhage. It is therefore likely that plasmapheresis was preferentially used in patients presenting with more severe illness. Finally, the total number of plasmapheresis-treated patients is too small to allow us to confidently detect an association between this treatment modality and outcomes. Similarly, only two patients received adjunctive treatment with rituximab. Therefore, our results and conclusions cannot be extrapolated to induction therapy with rituximab and glucocorticoids alone. It is noteworthy that the randomized controlled trial of rituximab versus cyclophosphamide in ANCA vasculitis excluded patients with severe renal failure (28). In the Rituximab versus Cyclophosphamide in ANCA-Associated Vasculitis trial, five of eight dialysis-dependent patients treated with a combination of glucocorticoids, rituximab, and cyclophosphamide with or without plasmapheresis recovered renal function (29). Because of the importance of prompt recovery of renal function on long-term patient and renal survival, it will be important to study the comparative efficacy and time to response of new treatment modalities (e.g., rituximab), specifically among patients with low GFR at presentation.
In summary, although patients with ANCA vasculitis and severe renal failure have an increased risk for death or ESRD, 50% of them achieve dialysis independence in response to glucocorticoids and cyclophosphamide. Our study confirms the importance of prompt diagnosis because even small increments of GFR at the time of treatment initiation have a substantial effect on the likelihood of recovery. Finally, we could not identify a threshold at which treatment is deemed futile and recommend that all patients be considered for immunosuppressive therapy. However, in the absence of active extrarenal vasculitis, continued immunosuppressive therapy beyond 4 months is very unlikely to benefit patients who remain dialysis dependent.
Disclosures
None.
Acknowledgments
We are indebted to the contribution of the nephrologists of the Glomerular Disease Collaborative Network.
T.L. was supported by the UNC Kidney Center.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08290813/-/DCSupplemental.
References
- 1.Hedger N, Stevens J, Drey N, Walker S, Roderick P: Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: A 10-year retrospective study. Nephrol Dial Transplant 15: 1593–1599, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Sinico RA, Di Toma L, Radice A: Renal involvement in anti-neutrophil cytoplasmic autoantibody associated vasculitis. Autoimmun Rev 12: 477–482, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Weidner S, Geuss S, Hafezi-Rachti S, Wonka A, Rupprecht HD: ANCA-associated vasculitis with renal involvement: aAn outcome analysis. Nephrol Dial Transplant 19: 1403–1411, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Day CJ, Howie AJ, Nightingale P, Shabir S, Adu D, Savage CO, Hewins P: Prediction of ESRD in pauci-immune necrotizing glomerulonephritis: Quantitative histomorphometric assessment and serum creatinine. Am J Kidney Dis 55: 250–258, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, Höglund P, Jayne D, Luqmani R, Mahr A, Mukhtyar C, Pusey C, Rasmussen N, Stegeman C, Walsh M, Westman K, European Vasculitis Study Group : Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 70: 488–494, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ: Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 7: 23–32, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Lionaki S, Hogan SL, Jennette CE, Hu Y, Hamra JB, Jennette JC, Falk RJ, Nachman PH: The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int 76: 644–651, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidanz F, Day CJ, Hewins P, Savage CO, Harper L: Recurrences and infections during continuous immunosuppressive therapy after beginning dialysis in ANCA-associated vasculitis. Am J Kidney Dis 50: 36–46, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, Hauser T, Hellmich B, Jayne D, Kallenberg CG, Merkel PA, Raspe H, Salvarani C, Scott DG, Stegeman C, Watts R, Westman K, Witter J, Yazici H, Luqmani R, European Vasculitis Study Group : EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 68: 310–317, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Nachman PH, Reisner HM, Yang JJ, Jennette JC, Falk RJ: Shared idiotypy among patients with myeloperoxidase-anti-neutrophil cytoplasmic autoantibody associated glomerulonephritis and vasculitis. Lab Invest 74: 519–527, 1996 [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Kim HT: Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res 13: 559–565, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Gaynor JJ, Feuer EJ, Tan CC, Wu DH, Little CR, Straus DJ, Clarkson BD, Brennan MF: On the use of cause-specific failure and conditional failure probabilities — examples from clinical oncology data. J Am Stat Assoc 88: 400–409, 1993 [Google Scholar]
- 16.Gray RJ: A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16: 1141–1154, 1988 [Google Scholar]
- 17.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 18.Walsh M, Casian A, Flossmann O, Westman K, Höglund P, Pusey C, Jayne DR, European Vasculitis Study Group (EUVAS) : Long-term follow-up of patients with severe ANCA-associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclear. Kidney Int 84: 397–402, 2013 [DOI] [PubMed] [Google Scholar]
- 19.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, Noël LH, Ferrario F, Waldherr R, Hagen EC, Bruijn JA, Bajema IM: Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 17: 2264–2274, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hauer HA, Bajema IM, Van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC, European Vasculitis Study Group (EUVAS) : Determinants of outcome in ANCA-associated glomerulonephritis: A prospective clinico-histopathological analysis of 96 patients. Kidney Int 62: 1732–1742, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B: Increased morbidity from ischemic heart disease in patients with Wegener’s granulomatosis. Arthritis Rheum 60: 1187–1192, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Morgan MD, Turnbull J, Selamet U, Kaur-Hayer M, Nightingale P, Ferro CJ, Savage CO, Harper L: Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: A matched-pair cohort study. Arthritis Rheum 60: 3493–3500, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Suppiah R, Judge A, Batra R, Flossmann O, Harper L, Höglund P, Javaid MK, Jayne D, Mukhtyar C, Westman K, Davis JC, Jr, Hoffman GS, McCune WJ, Merkel PA, St Clair EW, Seo P, Spiera R, Stone JH, Luqmani R: A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 63: 588–596, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, Noël LH, Ferrario F, Waldherr R, Bruijn JA, Bajema IM, Hagen EC, Pusey CD, EUVAS : Chances of renal recovery for dialysis-dependent ANCA-associated glomerulonephritis. J Am Soc Nephrol 18: 2189–2197, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, Nachman PH: Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 143: 621–631, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Berden AE, Jones RB, Erasmus DD, Walsh M, Noël LH, Ferrario F, Waldherr R, Bruijn JA, Jayne DR, Bajema IM, European Vasculitis Society : Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol 23: 313–321, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, Mirapeix E, Savage CO, Sinico RA, Stegeman CA, Westman KW, van der Woude FJ, de Lind van Wijngaarden RA, Pusey CD, European Vasculitis Study Group : Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 18: 2180–2188, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U, RAVE-ITN Research Group : Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen P, Walsh D, Walsh M, Westman K, Jayne DR, European Vasculitis Study Group : Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363: 211–220, 2010 [DOI] [PubMed] [Google Scholar]