Abstract
Background and objectives
Obese patients encounter barriers to medical care not encountered by lean patients, and inequities in access to care among obese patients may vary by sex. This study aimed to determine the association of body mass index (BMI) with access to kidney transplantation in men and women.
Design, setting, participants, & measurements
In this retrospective analysis of 702,456 incident ESRD patients aged 18–70 years (captured in the US Renal Data System between 1995 and 2007), multivariate time-to-event analyses were used to determine the association of BMI with likelihood of transplantation from any donor source, transplantation from a living donor, and transplantation from a deceased donor, as well the individual steps in obtaining a deceased donor transplant (activation to the waiting list, and transplantation after wait-listing).
Results
Among women, a BMI≥25.0 kg/m2 was associated with a lower likelihood of transplantation from any donor source (hazard ratio [HR], 0.75; 95% confidence interval [95% CI], 0.73 to 0.77), transplantation from a living donor (HR, 0.75; 95% CI, 0.72 to 0.77), and transplantation from a deceased donor (HR, 0.74; 95% CI, 0.72 to 0.77). By contrast, among men, a BMI of 25.0–34.9 kg/m2 was associated with a higher likelihood of the outcomes of transplantation from any donor source (HR, 1.08; 95% CI, 1.06 to 1.11), transplantation from a living donor (HR, 1.18; 95% CI, 1.13 to 1.22), and transplantation from a deceased donor (HR, 1.05; 95% CI, 1.02 to 1.07). Among men, the level beyond which BMI was associated with a lower likelihood of transplantation from any donor source or a living donor was ≥40.0 kg/m2, and ≥35.0 kg/m2 in the case of deceased donor transplantation.
Conclusions
The association of BMI with access to transplantation varies between men and women. The reasons for this difference should be further studied.
Keywords: epidemiology, outcomes, kidney transplantation, obesity
Introduction
Obesity is increasingly prevalent and is associated with an increased risk of comorbid conditions and higher consumption of health care resources (1,2). Therefore, it is important that obese individuals have appropriate access to health care. In the non-ESRD population, obesity has been variably associated with lower access to medical care. For example, some studies have shown that obese patients are less likely to undergo cancer screening (3–6). There has been limited examination of the association of obesity with access to medical care among patients with ESRD. We recently reported that the survival benefit from transplantation compared with treatment with dialysis in most obese patients is similar to that among nonobese patients, and therefore obesity alone should not exclude patients from consideration of transplantation (7).
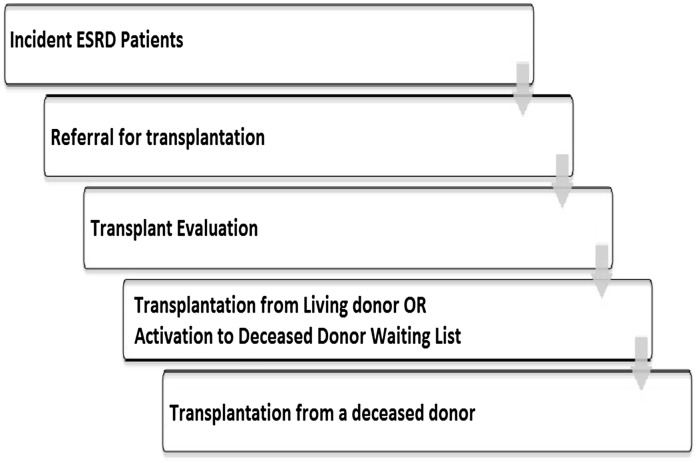
Patients with ESRD must negotiate a number of steps to gain access to kidney transplantation (Figure 1). Incident ESRD patients require education about the health benefits of transplantation, must agree to be considered for transplantation, and must be referred to a transplant specialist. After referral, patients undergo a detailed multidisciplinary medical and psychosocial evaluation. As part of this evaluation, patients may be required to complete any number of specialized diagnostic tests and evaluations by other subspecialists (8). Although there are guidelines for the evaluation of transplant candidates (9), acceptance for transplantation still remains a partially subjective science. Factors at the patient, physician, and health system levels may render obese patients less likely to successfully negotiate these steps compared with nonobese patients. Once accepted for transplantation, patients may receive a living donor transplant (if they have an identified living donor) or may be activated to the deceased donor waiting list. Once activated to the waiting list, access to deceased donor transplantation is determined by organ allocation rules and the likelihood of transplantation should not differ between obese and nonobese patients. However, in a previous study of wait-listed patients by Segev and colleagues (10), obesity was associated with a lower likelihood of deceased donor transplantation. Offers for transplantation among obese wait-listed patients were more likely to be bypassed than offers to nonobese patients, leading the authors to speculate that health care provider bias might contribute to this inequity. That analysis was limited to wait-listed patients, and did not assess the effect of obesity on earlier steps in the transplant evaluation process in which disparities may be more prominent (10). The purpose of the current analysis is to determine the association of obesity with access to transplantation among all incident ESRD patients. Given the literature suggesting that the implications of obesity on access to health care may differ by sex (11), as well as the lower likelihood of transplantation in women with ESRD (12–14), we hypothesized that obese women but not obese men would have lower access to transplantation.
Figure 1.
Steps that incident ESRD patients must negotiate to access deceased or living donor transplantation. Please refer to the text for details.
Materials and Methods
Data Source and Study Population
This study included all patients aged 18–70 years with ESRD captured in the US Renal Data System who initiated their first ESRD treatment (dialysis or preemptive transplantation) between April 1, 1995 and September 31, 2007 (N=702,456). HIV-positive patients (n=12,040) and patients with missing height and weight information (n=11,248) were excluded from the analysis.
Statistical Analyses
Height and weight measurements recorded at the time of first ESRD treatment (dialysis or preemptive transplantation) were used to divide patients into body mass index (BMI) categories defined by the World Health Organization as follows: BMI<18.5 kg/m2 (underweight), 18.5–24.9 kg/m2 (normal weight), 25.0–29.9 kg/m2 (overweight), 30.0–34.9 kg/m2 (class I obesity), 35.0–39.9 kg/m2 (class II obesity), and ≥40.0 kg/m2 (class III obesity). Group characteristics were described using proportions or the mean and SD, and group differences were compared with the chi-squared test for categorical variables or ANOVA for continuous variables.
Access to transplantation was determined using the following outcome measures: transplantation from any donor source, transplantation from a living donor, and transplantation from a deceased donor. To understand the basis for any difference in deceased donor transplantation, we also assessed individual steps in the deceased donor process (i.e., activation to the deceased donor waiting list [excluding patients initially listed as inactive] and transplantation from a deceased donor after activation to the waiting list). The association between BMI and each of the above outcomes was determined from the date of first ESRD treatment (or date of wait-listing for the outcome of deceased donor transplantation after activation to the waiting list), with follow-up through September 30, 2007. Univariate associations were determined using the Kaplan– Meier method and group differences were compared with the log-rank test. Multivariate Cox regression analyses were used to determine the independent association of BMI with each of the study outcomes after adjustment for relevant confounders including age, race, cause of ESRD, history of comorbid conditions (ischemic heart disease, cerebrovascular accident, congestive heart failure, peripheral vascular disease, or cancer), functional status, year of first ESRD treatment, and type of medical insurance. The prevalence of obesity is higher in rural compared with urban areas (15). Because rural residence has been variably associated with a lower likelihood of transplantation in the literature (16,17), we included adjustment for rural or urban place of residence using the rural urban commuting area (RUCA) codes. RUCA codes were assigned to each United States zip code based on markers of population density as previously described (18). We classified each patient in the current analysis as belonging to one of three mutually exclusive RUCA groups: metropolitan (RUCA, 1.0–3.9, cities with population of >50,000 and their associated suburban areas); micropolitan (RUCA, 4.0–6.0, towns or cities with population of 10,000–50,000); and rural (RUCA, >6.0, towns with population of <10,000), and adjusted for differences in RUCA between BMI groups in our models. The Cox models for the outcome of deceased donor transplantation after wait-listing also included adjustment for ABO blood group and panel reactive antibodies. For variables with missing data, a category of missing was created allowing all patients to be included in the models. All analyses to determine the association of BMI with the various transplant outcomes were stratified by sex. In addition for all outcomes, we formally tested for an interaction between sex and BMI using a combined data set that included both men and women. Finally, to determine the extent to which BMI may explain the lower likelihood of transplantation in women compared with men, we also performed Cox multivariate models including both men and women (with and without adjustment for BMI) and compared the models using the likelihood ratio test. In all models, the proportional hazards assumptions were tested using log-negative-log plots of the within-group survivorship probabilities versus log-time. All analyses were performed using Stata Statistical Software (release 12; StataCorp., LP, College Station, TX). This study was conducted with the approval of our local hospital research ethics board.
Results
There were 702,456 incident ESRD patients who met the study criteria. Table 1 shows characteristics of the study population by BMI category. Differences between BMI groups were most evident between patients with a BMI≥40.0 kg/m2 and those with a lower BMI. For example, men, black race, and diabetic ESRD were more prevalent in patients with a BMI≥40.0 kg/m2.
Table 1.
Patient characteristics by BMI (N=702,456)
| Characteristic | BMI (kg/m2) | P Value | |||||
|---|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25–29.9 | 30–34.9 | 35–39.9 | ≥40 | ||
| Patients (n) | 54,398 | 222,237 | 194,436 | 116,968 | 60,700 | 53,717 | |
| Age (yr), mean±SD | 61±19 | 64±17 | 63±15 | 61±14 | 59±14 | 57±13 | <0.001 |
| Men | 49 | 58 | 58 | 51 | 56 | 63 | <0.001 |
| Race | <0.001 | ||||||
| White | 62 | 66 | 67 | 65 | 62 | 59 | |
| Black | 31 | 26 | 28 | 30 | 34 | 37 | |
| Other | 7 | 8 | 5 | 5 | 4 | 4 | |
| Inability to ambulate | 6 | 5 | 4 | 4 | 5 | 7 | <0.001 |
| Insurance | <0.001 | ||||||
| Medicare/Medicaid | 85 | 82 | 81 | 79 | 78 | 78 | |
| Private | 2 | 4 | 5 | 6 | 7 | 8 | |
| Other | 3 | 6 | 6 | 7 | 7 | 6 | |
| No insurance | 10 | 8 | 8 | 8 | 8 | 8 | |
| Cause of ESRD | <0.001 | ||||||
| Diabetes | 32 | 36 | 47 | 55 | 60 | 60 | |
| Hypertension | 33 | 32 | 26 | 22 | 19 | 19 | |
| Glomerular | 10 | 9 | 9 | 8 | 8 | 9 | |
| Polycystic disease | 2 | 3 | 3 | 2 | 2 | 2 | |
| Other | 23 | 20 | 15 | 13 | 11 | 10 | |
| History of comorbid conditions | |||||||
| Ischemic heart disease | 21 | 24 | 25 | 24 | 23 | 20 | <0.001 |
| Cerebrovascular disease | 10 | 10 | 9 | 9 | 8 | 7 | <0.001 |
| Congestive heart failure | 30 | 31 | 32 | 34 | 35 | 37 | <0.001 |
| Peripheral vascular disease | 15 | 15 | 14 | 14 | 14 | 13 | <0.001 |
| Cancer | 6 | 7 | 6 | 5 | 5 | 4 | <0.001 |
| Year of first ESRD treatment | <0.001 | ||||||
| 1995–1999 | 60 | 34 | 29 | 25 | 23 | 20 | |
| 2000–2003 | 22 | 35 | 36 | 36 | 35 | 35 | |
| 2004–2007 | 18 | 31 | 35 | 39 | 42 | 45 | |
| RUCA group | <0.001 | ||||||
| Metropolitan | 80 | 81 | 80 | 78 | 77 | 77 | |
| Micropolitan | 10 | 11 | 10 | 12 | 12 | 12 | |
| Rural | 10 | 9 | 10 | 11 | 11 | 11 | |
Data are given in percentages unless otherwise indicated. The frequency of missing data was as follows: data on age, race, cause of ESRD, ambulatory status, and comorbid conditions were missing in <1%; data on insurance were missing in 2%. BMI, body mass index; RUCA, rural urban commuting area codes.
Univariate Analyses
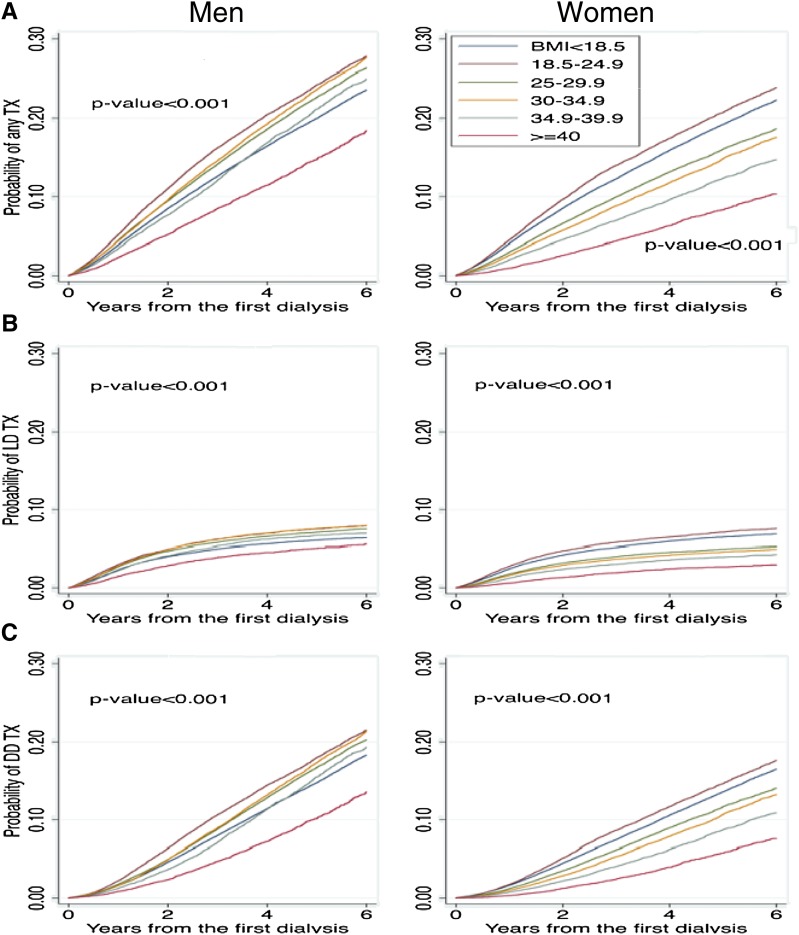
Figure 2 shows Kaplan–Meier curves stratified by sex for the outcomes of transplantation from any donor source, living donor transplantation, and deceased donor transplantation. The time to transplantation from any donor source was shorter in men compared with women. A higher BMI was also associated with a longer time to transplantation from any donor source and this was consistent in both men and women. However, the separation between BMI groups was more evident in women, with the separation in men most evident when the BMI was ≥40.0 kg/m2. Similar patterns were evident for the outcomes of living donor transplantation (Figure 2B) and deceased donor transplantation (Figure 2C).
Figure 2.
Association of BMI with access to transplantation was most evident in women. Time to transplantation from any donor source (A), transplantation from a living donor (B), and transplantation from a deceased donor (C) (N=702,456). Any TX, transplantation from any donor source; BMI, body mass index; DDTX, deceased donor transplantation; LDTX, living donor transplantation.
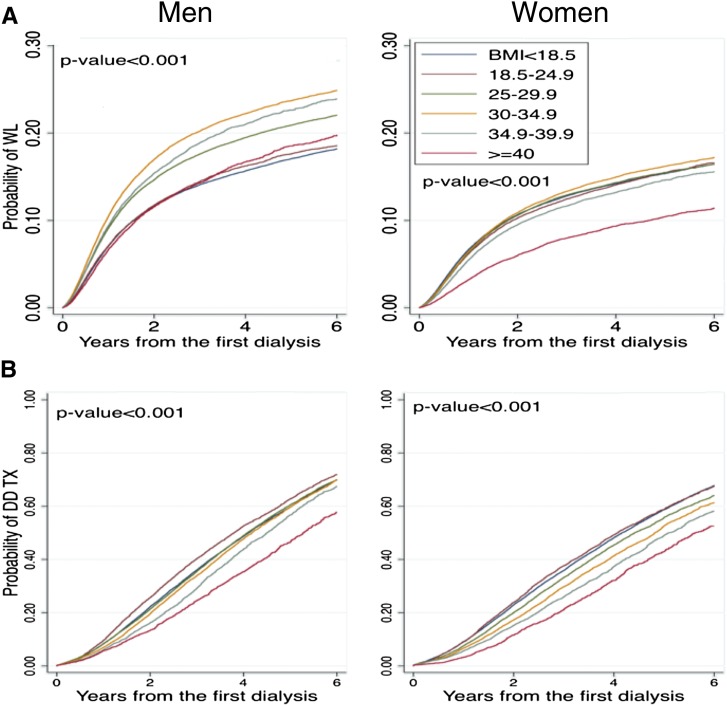
Figure 3 divides the process of deceased donor transplantation into activation to the waiting list and transplantation from a deceased donor after wait-listing. The cumulative incidence of activation to the waiting list was higher in men compared with women. Among men, patients with BMIs of 30.0–34.9 kg/m2 and 35.0–39.9 kg/m2 had the shortest time to wait-listing, and patients with BMIs<18.5, 18.5–24.9, and ≥40.0 kg/m2 had the longest time to wait-listing. Among women, there was little separation between BMI groups, with the exception of patients with a BMI≥40.0 kg/m2 who had the longest time to wait-listing (Figure 3A). The time to transplantation from a deceased donor after wait-listing was shorter in men (Figure 3B). In both men and women, patients with a normal BMI of 18.5–24.9 kg/m2 had the shortest time to deceased donor transplantation after wait-listing, whereas patients with a BMI≥40.0 kg/m2 had the longest time.
Figure 3.
Higher BMI was associated with a progressively lower likelihood of deceased donor transplantation after wait-listing. (A) Time to activation to the deceased donor waiting list in men (n=395,812) and women (n=306,644). (B) Time to transplantation from a deceased donor after activation to the waiting list in men (n=84,939), and women (n=57,662). BMI, body mass index; DDTX, deceased donor transplantation; WL, wait-listing.
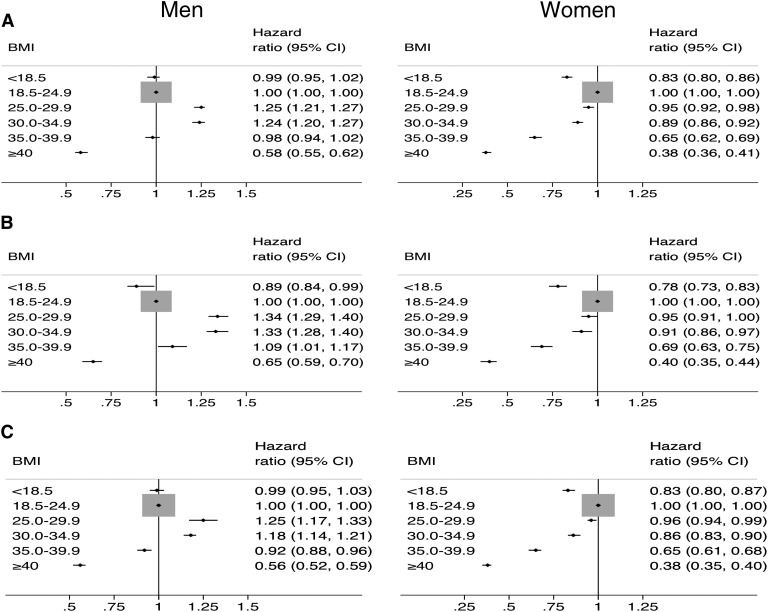
Figure 4 summarizes the results from separate Cox regression models performed in men and women to determine the association of BMI with transplantation from any donor source, transplantation from a living donor, and transplantation from a deceased donor. The association of BMI with these outcomes differed between men and women. Among women, a higher BMI≥25.0 kg/m2 was associated with a progressively lower likelihood of all three outcomes. By contrast, among men, a lower likelihood of these measures of transplant access was observed at much higher levels of BMI (usually BMI≥40.0 kg/m2). Furthermore, overweight (BMI 25.0–29.9 kg/m2) and class I obesity (BMI 30.0–34.9 kg/m2) in men were associated with a higher likelihood of these outcomes compared with normal weight men (BMI 18.5–24.9 kg/m2).
Figure 4.
Differential association of BMI with access to transplantation in men and women. The adjusted likelihood of transplantation from any donor source (A), transplantation from a living donor (B), and transplantation from a deceased donor (C). Results were derived from separate multivariate models in men (n=395,812) and women (n=306,644). The reference group in each model was individuals with normal BMI (18.0–24.9 kg/m2). Women with a BMI≥25.0 kg/m2 had a lower likelihood of all three outcomes. By contrast, a decrease likelihood of all outcomes among men was only evident when the BMI was ≥40 kg/m2. 95% CI, 95% confidence interval.
Table 2 shows the results from Cox regression models performed to determine the association of BMI with individual steps in the deceased donor transplant process. A higher BMI was consistently associated with a lower likelihood of deceased donor transplantation after wait-listing in both women and men. However, the association of BMI with activation to the waiting list was more variable: a higher BMI was not associated with a lower likelihood of activation until the BMI was ≥40.0 kg/m2 in men and ≥35.0 kg/m2 in women. Table 3 shows the outcomes of wait-listed patients 3 years after activation to the waiting list. The frequency of being placed on hold on the waiting list was higher in obese patients. Among obese patients with a BMI≥35 kg/m2, the frequency of being placed on hold was higher in women compared with men.
Table 2.
Independent association of BMI with individual steps in deceased donor transplant process
| Step in Deceased Donor Transplant Process | Men | Women |
|---|---|---|
| Hazard Ratio (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) | |
| Activation to the waiting list | ||
| Patients (n) | 395,812 | 306,644 |
| BMI (kg/m2) | ||
| <18.5 | 0.92 (0.88 to 0.96) | 0.90 (0.87 to 0.93) |
| 18.5–24.9 | 1.00 | 1.00 |
| 25.0–29.9 | 1.26 (1.23 to 1.29) | 1.07 (1.04 to 1.10) |
| 30.0–34.9 | 1.36 (1.32 to 1.39) | 1.10 (1.06 to 1.13) |
| 35.0–39.9 | 1.12 (1.08 to 1.17) | 0.84 (0.81 to 0.88) |
| ≥40.0 | 0.74 (0.71 to 0.78) | 0.50 (0.48 to 0.53) |
| Transplantation from a deceased donor after wait-listing | ||
| Patients (n) | 84,939 | 57,662 |
| BMI (kg/m2) | ||
| <18.5 | 1.04 (0.99 to 1.10) | 0.98 (0.92 to 1.04) |
| 18.5–24.9 | 1.00 | 1.00 |
| 25.0–29.9 | 1.00 (0.97 to 1.03) | 0.92 (0.88 to 0.96) |
| 30.0–34.9 | 0.99 (0.95 to 1.03) | 0.87 (0.83 to 0.92) |
| 35.0–39.9 | 0.88 (0.83 to 0.93) | 0.77 (0.72 to 0.82) |
| ≥40.0 | 0.72 (0.66 to 0.78) | 0.65 (0.59 to 0.71) |
Separate multivariate Cox regression models were performed in men and women for the outcome of activation to the waiting list, and transplantation from a deceased donor after wait-listing. All models included adjustment for the following factors: age, race, cause of ESRD, history of comorbid conditions (ischemic heart disease, cerebrovascular accident, congestive heart failure, peripheral vascular disease, cancer), functional status, year of first ESRD treatment, type of medical insurance, and RUCA codes. The Cox models for the outcome of deceased donor transplantation after wait-listing also included adjustment for ABO blood group and panel reactive antibodies.
Table 3.
Patient status 3 years after activation to the waiting list by BMI group
| BMI Group (kg/m2) | Deceased | Removed from Wait List | Transplanted | On Hold | Active on Wait List |
|---|---|---|---|---|---|
| Male wait-list candidates (n=84,939) | |||||
| <18.5 | 13 | 5 | 48 | 6 | 27 |
| 18.5–24.9 | 10 | 5 | 48 | 8 | 30 |
| 25.0–29.9 | 9 | 5 | 47 | 8 | 31 |
| 30.0–34.9 | 10 | 4 | 45 | 10 | 32 |
| 35.0–39.9 | 10 | 4 | 41 | 11 | 33 |
| ≥40 | 11 | 4 | 36 | 14 | 35 |
| Female wait-list candidates (n=57,662) | |||||
| <18.5 | 12 | 5 | 48 | 6 | 29 |
| 18.5–24.9 | 9 | 5 | 50 | 7 | 29 |
| 25.0–29.9 | 10 | 5 | 44 | 9 | 33 |
| 30.0–34.9 | 10 | 4 | 41 | 10 | 35 |
| 35.0–39.9 | 10 | 4 | 37 | 13 | 36 |
| ≥40 | 10 | 5 | 32 | 16 | 37 |
Data are given in percentages.
Table 4 shows the results of a multivariate Cox model for the outcome of transplantation from any donor source that included formal testing for the interaction of sex and BMI. In this model, the hazard ratios for subgroups of men and women in different BMI categories were determined in comparison with the reference group of men with BMI in the normal range of 18.1–24.9 kg/m2. Women in all BMI groups ≥25.0 had a lower likelihood of transplantation from any donor source (with the likelihood progressively lower in higher BMI groups), whereas a lower likelihood of transplantation from any donor source was observed in men when the BMI was <18.5 or >35.0 kg/m2. In similar models for the outcomes of transplantation from a living donor, transplantation from a deceased donor, activation to the waiting list, and transplantation from a deceased donor after wait-listing, the interaction terms for sex and BMI were highly significant with P<0.001 in all models (individual model results not shown).
Table 4.
Association of sex and BMI with transplantation from any donor source
| Factor | Hazard Ratio (95% Confidence Interval) |
|---|---|
| Sex and BMI (kg/m2) | |
| Men | |
| <18.5 | 0.95 (0.91 to 0.99) |
| 18.5–24.9 | 1.00 |
| 25.0–29.9 | 1.21 (1.18 to 1.24) |
| 30.0–34.9 | 1.18 (1.14 to 1.22) |
| 35.0–39.9 | 0.93 (0.89 to 0.97) |
| ≥40.0 | 0.56 (0.52 to 0.59) |
| Women | |
| <18.5 | 0.91 (0.87 to 0.95) |
| 18.5–24.9 | 1.06 (1.03 to 1.08) |
| 25.0–29.9 | 0.94 (0.92 to 0.97) |
| 30.0–34.9 | 0.85 (0.82 to 0.88) |
| 35.0–39.9 | 0.61 (0.58 to 0.64) |
| ≥40.0 | 0.35 (0.32 to 0.37) |
| Age (per year older) | 0.95 (0.94 to 0.95) |
| Race | |
| White | 1.00 |
| Black | 0.43 (0.42 to 0.44) |
| Other | 0.60 (0.58 to 0.62) |
| Inability to ambulate | 0.33 (0.30 to 0.36) |
| Insurance | |
| Medicare/Medicaid | 1.00 |
| Private | 1.75 (1.65 to 1.86) |
| Other | 2.42 (2.33 to 2.51) |
| No insurance | 0.72 (0.71 to 0.73) |
| Cause of ESRD | |
| Diabetes | 0.60 (0.58 to 0.62) |
| Hypertension | 0.64 (0.62 to 0.65) |
| Glomerular | 1.00 |
| Polycystic disease | 1.47 (1.42 to 1.53) |
| Other | 0.60 (0.57 to 0.61) |
| History of comorbid conditionsa | |
| Ischemic heart disease | 0.88 (0.86 to 0.91) |
| Cerebrovascular disease | 0.76 (0.73 to 0.79) |
| Congestive heart failure | 0.64 (0.63 to 0.66) |
| Peripheral vascular disease | 0.74 (0.72 to 0.77) |
| Cancer | 0.64 (0.61 to 0.69) |
| Year of first ESRD treatment | |
| 1995–1999 | 1.00 |
| 2000–2003 | 0.89 (0.87 to 0.90) |
| 2004–2007 | 0.72 (0.70 to 0.74) |
Cox multivariate regression including interaction terms of sex×BMI (N=702,456).
Reference: condition not present.
In multivariate Cox models performed to determine the association of sex with the outcome of transplantation from any donor source, the hazard ratios for women compared with men with and without adjustment for BMI were 0.82 (95% confidence interval, 0.80 to 0.83) and 0.84 (95% confidence interval, 0.82 to 0.86). The log likelihood in the model without adjustment for BMI was −844,101.7 compared with −843,080.9 in the model with adjustment for BMI. The increase in the log likelihood statistics was statistically significant (P<0.001) by the log likelihood ratio test, indicating improved model fit with inclusion of BMI in the model.
Discussion
This study demonstrates that BMI has a differential association with access to transplantation in men and women. Among women, being classified as overweight and obese was associated with lower access to transplantation as measured by a number of different metrics including access to transplantation from any donor source, transplantation from a living donor, and transplantation from a deceased donor. By contrast, overweight men and men with class I obesity had a higher likelihood of all outcomes, and it was only when BMI was ≥40.0 kg/m2 that men had consistently lower access to transplantation. We also found that most of the BMI-associated differences in access to deceased donor transplantation were because of a lower likelihood of deceased donor transplantation after wait-listing, rather than differences in activation to the waiting list. Finally, this study identified BMI as a potentially modifiable factor contributing to the sex-based inequity in access to transplantation. These findings indicate the need for further research to understand the basis for the differential association of BMI with access to transplantation in men and women.
Patient preference, physician bias, and health system–related factors may contribute to obesity-related differences in access to transplantation. With regard to living donor transplantation, it is possible that overweight and obese women may have different perceptions about pursuing living donor transplantation compared with men with a similar BMI. Alternatively, physicians and potential living donors may perceive obesity differently in men versus women.
The finding that obesity is associated with a lower likelihood of deceased donor transplantation after wait-listing was anticipated from recent work (10). However, the finding that higher BMI was associated with a higher likelihood of activation to the waiting list until the BMI was ≥40.0 kg/m2 in men and ≥35.0 kg/m2 in women was surprising. This contradicts our hypothesis that referral bias and increased medical complexity of overweight and obese patients would result in a lower likelihood of obese patients being activated to the waiting list. Instead, these findings suggest that these issues may only be relevant for those with class II or III obesity. Consistent with previous work, we found that obese patients were less likely to receive a deceased donor transplant after activation to the waiting list (10). The finding that obese patients had a higher frequency of being place on hold (Table 3) suggests that the lower likelihood of transplantation after activation to the wait list is in part due to increased comorbid disease burden and medical complications in patients with a high BMI. Other potential reasons for the lower likelihood of deceased donor transplantation in wait-listed obese patients include increased medical surveillance, or physician bias, and increased bypassing of offers for transplantation in patients with a high BMI (10). There are a number of reasons for potential bias in obese patients: obesity is associated with an increased risk for delayed graft function (19), wound complications (20), and longer hospital admissions for deceased donor transplantation (21), hospital readmission (22), and new-onset diabetes after transplantation (23) and therefore may be associated with higher transplant costs.
This study also identified obesity as a factor contributing to the long-known disparity in access to both deceased (12) and living donor (14) transplantation between men and women that has been reported in both United States and Canadian (24) transplant systems. Relatively few recent studies have examined sex-based differences in access to transplantation (25). Our findings may be useful in ensuring access to transplantation among women in the current era in which the prevalence of obesity among patients with ESRD is rapidly increasing. Our approach to stratify our analysis based on sex was informed by literature suggesting that BMI may have a differential effect in men and women (3). The differential association of BMI with the various metrics of access to transplantation suggested in our stratified models was confirmed with formal testing for an interaction between sex and BMI in combined models that included men and women.
Readers of this study should consider the inherent limitations of observational studies including residual confounding, and should understand that the associations identified in this analysis cannot be used to prove a causal relationship between BMI and transplantation. BMI is an imperfect metric to assess the clinical implications of body habitus on the selection of patients for transplantation (26,27). At a given BMI, women typically have more fat than men, whereas men tend to have more muscle and muscle weight. Fat distribution, rather than BMI, may be a more relevant predictor of medical and surgical risk. Abdominal obesity, which can be measured by the waist to hip ratio, waist circumference, and waist to height ratio, may be more predictive of cardiovascular morbidity and mortality compared with BMI (28–31). The waist to hip ratio is associated with both medical and surgical complications after elective abdominal surgery (32). Estrogen causes fat to be stored in the buttocks, thighs, and hips in women, whereas men have a higher tendency to accumulate abdominal fat. These sex-related differences in fat distribution are not accounted for by BMI. Therefore, some of the sex-related differences observed in our study might be due to limitations of BMI as a metric of the body habitus–related factors that might affect patient selection for transplantation. In addition, BMI groups were defined based on information collected at the time of first treatment for ESRD. Because of incomplete information regarding longitudinal changes in BMI, this study cannot determine whether changes in BMI over time are associated with access to transplantation. Although our analysis is adjusted for known confounders, there is potential for residual confounding from unmeasured factors as well as potential for the covariates in our models to inadequately account for factors that might confound the associations between BMI and access to transplantation in this study. For example, the RUCA categories may inadequately reflect differences in access to transplantation between individuals in large metropolitan areas (i.e., inner city populations).
In summary, a higher BMI is associated with a lower likelihood of transplantation primarily in women. Given the increasing prevalence of obesity among patients with ESRD, further studies are needed to understand the factors contributing to the differential association of BMI with access to transplantation in men and women.
Disclosures
None.
Acknowledgments
The data reported here were supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official government policy or interpretation of the US government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “BMI, Sex, and Access to Transplantation,” on pages 843–844.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR: Prevalence and trends in obesity among US adults, 1999-2008. JAMA 303: 235–241, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Ellis RP, Fiebig DG, Johar M, Jones G, Savage E: Explaining health care expenditure variation: Large-sample evidence using linked survey and health administrative data. Health Econ 22: 1093–1110, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Fagan HB, Wender R, Myers RE, Petrelli N: Obesity and cancer screening according to race and gender. J Obes 2011: 218250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wee CC, McCarthy EP, Davis RB, Phillips RS: Screening for cervical and breast cancer: Is obesity an unrecognized barrier to preventive care? Ann Intern Med 132: 697–704, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Quinn VP, Jacobsen SJ, Slezak JM, Van Den Eeden SK, Caan B, Sternfeld B, Haque R: Preventive care and health behaviors among overweight/obese men in HMOs. Am J Manag Care 18: 25–32, 2012 [PubMed] [Google Scholar]
- 6.Hernandez-Boussard T, Ahmed SM, Morton JM: Obesity disparities in preventive care: Findings from the National Ambulatory Medical Care Survey, 2005-2007. Obesity (Silver Spring) 20: 1639–1644, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Gill JS, Lan J, Dong J, Rose C, Hendren E, Johnston O, Gill J: The survival benefit of kidney transplantation in obese patients. Am J Transplant 13: 2083–2090, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Knoll G, Cockfield S, Blydt-Hansen T, Baran D, Kiberd B, Landsberg D, Rush D, Cole E, Kidney Transplant Working Group of the Canadian Society of Transplantation : Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ 173: 1181–1184, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segev DL, Simpkins CE, Thompson RE, Locke JE, Warren DS, Montgomery RA: Obesity impacts access to kidney transplantation. J Am Soc Nephrol 19: 349–355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontaine KR, Heo M, Allison DB: Body weight and cancer screening among women. J Womens Health Gend Based Med 10: 463–470, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Bloembergen WE, Mauger EA, Wolfe RA, Port FK: Association of gender and access to cadaveric renal transplantation. Am J Kidney Dis 30: 733–738, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Wolfe RA, Ashby VB, Milford EL, Bloembergen WE, Agodoa LY, Held PJ, Port FK: Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis 36: 1025–1033, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Bloembergen WE, Port FK, Mauger EA, Briggs JP, Leichtman AB: Gender discrepancies in living related renal transplant donors and recipients. J Am Soc Nephrol 7: 1139–1144, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Befort CA, Nazir N, Perri MG: Prevalence of obesity among adults from rural and urban areas of the United States: Findings from NHANES (2005-2008). J Rural Health 28: 392–397, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonelli M, Klarenbach S, Rose C, Wiebe N, Gill J: Access to kidney transplantation among remote- and rural-dwelling patients with kidney failure in the United States. JAMA 301: 1681–1690, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Axelrod DA, Guidinger MK, Finlayson S, Schaubel DE, Goodman DC, Chobanian M, Merion RM: Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA 299: 202–207, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Gill J, Dong J, Rose C, Johnston O, Landsberg D, Gill J: The effect of race and income on living kidney donation in the United States. J Am Soc Nephrol 24: 1872–1879, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S, Streja E, Krishnan M, Kalantar-Zadeh K: Higher recipient body mass index is associated with post-transplant delayed kidney graft function. Kidney Int 80: 218–224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo JH, Wong MS, Perez RV, Li CS, Lin TC, Troppmann C: Renal transplant wound complications in the modern era of obesity. J Surg Res 173: 216–223, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Bardonnaud N, Pillot P, Lillaz J, Delorme G, Chabannes E, Bernardini S, Guichard G, Bittard H, Kleinclauss F: Outcomes of renal transplantation in obese recipients. Transplant Proc 44: 2787–2791, 2012 [DOI] [PubMed] [Google Scholar]
- 22.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL: Early hospital readmission after kidney transplantation: Patient and center-level associations. Am J Transplant 12: 3283–3288, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Schaubel DE, Stewart DE, Morrison HI, Zimmerman DL, Cameron JI, Jeffery JJ, Fenton SS: Sex inequality in kidney transplantation rates. Arch Intern Med 160: 2349–2354, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Couchoud C, Bayat S, Villar E, Jacquelinet C, Ecochard R, REIN registry : A new approach for measuring gender disparity in access to renal transplantation waiting lists. Transplantation 94: 513–519, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Postorino M, Marino C, Tripepi G, Zoccali C, CREDIT (Calabria Registry of Dialysis and Transplantation) Working Group : Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol 53: 1265–1272, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Streja E, Molnar MZ, Kovesdy CP, Bunnapradist S, Jing J, Nissenson AR, Mucsi I, Danovitch GM, Kalantar-Zadeh K: Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol 6: 1463–1473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CM, Huxley RR, Wildman RP, Woodward M: Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J Clin Epidemiol 61: 646–653, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS, INTERHEART Study Investigators : Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet 366: 1640–1649, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD: Body mass index, waist circumference and waist-hip ratio: Which is the better discriminator of cardiovascular disease mortality risk?: Evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev 12: 680–687, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutinho T, Goel K, Corrêa de Sá D, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F: Central obesity and survival in subjects with coronary artery disease: A systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol 57: 1877–1886, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Kartheuser AH, Leonard DF, Penninckx F, Paterson HM, Brandt D, Remue C, Bugli C, Dozois E, Mortensen N, Ris F, Tiret E, Waist Circumference Study Group : Waist circumference and waist/hip ratio are better predictive risk factors for mortality and morbidity after colorectal surgery than body mass index and body surface area. Ann Surg 258: 722–730, 2013 [DOI] [PubMed] [Google Scholar]