Abstract
Background and objectives
In autosomal dominant polycystic kidney disease (ADPKD), progressive kidney cyst formation commonly leads to ESRD. Because important manifestations of ADPKD may be evident in childhood, early intervention may have the largest effect on long-term outcome. Statins are known to slow progressive nephropathy in animal models of ADPKD. This randomized double-blind placebo-controlled phase III clinical trial was conducted from 2007 to 2012 to assess the effect of pravastatin on height-corrected total kidney volume (HtTKV) and left ventricular mass index (LVMI) by magnetic resonance imaging (MRI) and urine microalbumin excretion (UAE) in children and young adults with ADPKD.
Designs, setting, participants, & measurements
There were 110 pediatric participants with ADPKD and normal kidney function receiving lisinopril who were randomized to treatment with pravastatin or placebo for a 3-year period with evaluation at 0, 18, and 36 months. The primary outcome variable was a ≥20% change in HtTKV, LVMI, or UAE over the study period.
Results
Ninety-one participants completed the 3-year study (83%). Fewer participants receiving pravastatin achieved the primary endpoint compared with participants receiving placebo (69% versus 88%; P=0.03). This was due primarily to a lower proportion reaching the increase in HtTKV (46% versus 68%; P=0.03), with similar findings observed between study groups for LVMI (25% versus 38%; P=0.18) and UAE (47% versus 39%; P=0.50). The percent change in HtTKV adjusted for age, sex, and hypertension status over the 3-year period was significantly decreased with pravastatin (23%±3% versus 31%±3%; P=0.02).
Conclusions
Pravastatin is an effective agent to slow progression of structural kidney disease in children and young adults with ADPKD. These findings support a role for early intervention with pravastatin in this condition.
Keywords: statins, polycystic kidney disease, children, kidney volume
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary renal disease, affecting approximately 1 in 1000 people (1). Although it was previously termed “adult” polycystic kidney disease, clinical manifestations may be evident in childhood and even in utero (2,3). ADPKD is characterized by progressive kidney enlargement as a result of tubular cyst expansion and associated compression of adjacent parenchyma. Approximately half of patients with ADPKD manifest ESRD by age 60 years (1). Because ADPKD is genetic, earlier intervention in childhood may have the greatest long-term effect on the natural course of this disease. Our previous randomized studies demonstrated that angiotensin converting enzyme inhibitors (ACEIs) prevented the decrease in creatinine clearance (CrCl) and the increase in left ventricular mass index (LVMI) observed over a 5-year period in children and young adults with ADPKD and high-normal BP. This treatment, however, did not affect kidney volume growth (4).
Recent studies in experimental models of chronic renal insufficiency demonstrate the efficacy of statins to ameliorate progressive nephropathy (5–9). Statins enhance renal blood flow (RBF) (5,10) and GFR (11) and attenuate vascular inflammation through vascular and glomerular nitric oxide production, as reviewed by McFarlane et al. (12). In heterozygous male Han:SPRD rats, an animal model of ADPKD, lovastatin has been shown to reduce the severity of kidney disease as assessed by kidney size, volume density of cysts, and serum urea nitrogen concentration (13). Although the underlying mechanisms are not well understood, these renoprotective effects may be mediated by statin-related inhibition of G proteins with resultant decreased cell proliferation (14). Short-term (4-week) treatment of 10 normocholesterolemic normotensive adults with ADPKD with simvastatin resulted in increased RBF and GFR (10).
On the basis of these findings, we designed a 3-year randomized double-blind placebo-controlled phase III clinical trial to determine the effect of pravastatin treatment on kidney and cardiovascular disease progression in children and young adults with ADPKD who were receiving the ACEI lisinopril (15). Total kidney volume (TKV) and LVMI were assessed by MRI, and urinary microalbumin excretion (UAE) was measured. The results of the clinical trial are presented here.
Materials and Methods
Trial Design
Participants were recruited nationally from July 2007 through October 2009. Eligible participants were aged 8–22 years and had ADPKD defined by kidney imaging demonstrating bilateral cysts in the setting of known family history of ADPKD or multiple cysts clinically consistent with a new diagnosis of ADPKD. Participants had Schwartz CrCl>80 ml/min per 1.73 m2 (16,17). Pravastatin was chosen for this study due to its US Food and Drug Administration (FDA)–approved use in children aged≥8 years for management of hyperlipidemia as well as its minimal drug interactions. Exclusions included allergy to study medications, underlying liver or muscle disease, pregnancy/lactation, inability to cooperate with or clinical contraindication for MRI, or inability to provide informed consent/assent. Participants were prohibited from taking combined ACEIs and angiotensin receptor blockers, mammalian target of rapamycin inhibitors, or vasopressin receptor antagonists during the study. Participants were randomized to either pravastatin or placebo treatment in a double-blind manner with stratification by sex, age group (8–12 or 13–22 years), and hypertensive status (hypertensive or normotensive).
Each participant was evaluated during a 2-day admission at the Clinical Translational Research Center at Children’s Hospital Colorado in Aurora, Colorado. Participants underwent detailed history and physical examination including growth parameters and Tanner staging (18–20). On the first night of admission, participants fasted for a 10-hour period and then blood was drawn for lipid studies and routine serum chemistries including complete blood count, creatine kinase (CK), aspartate aminotransferase (AST), and alanine aminotransferase (ALT); serum β-human chorionic gonadotropin was also assessed in girls with possible childbearing potential (Tanner stage≥2). Two 24-hour urine collections were obtained to measure creatinine, volume, protein, and microalbumin. Participants underwent abdominal/cardiac MRI scans as previously described (15). Twelve sitting BP measurements were obtained in the right arm (Dinamap Pro 300V2; GE Medical Systems Information Technology, Tampa, FL) and were related to published data for age-, sex-, and height-matched children (21), with participants demonstrating ≥6 BP measurements above the 95th percentile for age, sex, and height defined as hypertensive. Participants were given a digital BP monitor (UA767; A&D Medical, San Jose, CA) with the appropriate size cuff and were instructed on its use for home monitoring. This BP monitor was chosen for use based on its reliability and validity compared with auscultation (22,23).
Medication was provided at the conclusion of the initial visit. The standard dosage of pravastatin was based on age as follows: 20 mg daily (8–12 years) or 40 mg daily (13–22 years). Participants were also treated with the ACEI lisinopril. In normotensive participants, the initial dosage of lisinopril was 2.5 mg/d. Higher doses were started in hypertensive participants at the clinical discretion of the principal investigator. Adjustments of the lisinopril dose were made at least monthly based on home BP reports until at least four of six systolic and diastolic BPs were between the 50th and 75th percentiles for age, sex, and height, or the maximum dose of 0.5 mg/kg body weight (maximum 20 mg twice daily) was reached. Additional antihypertensive medication was provided to hypertensive participants to attain the BP goal if required, with amlodipine utilized as the second antihypertensive agent. If BP was below the 25th percentile, lisinopril was not started unless monthly home BP monitoring demonstrated BP consistently above the 50th percentile (one participant in each study group). Confidential counseling regarding drug safety in pregnancy was provided as appropriate for age/developmental status.
Analyses of Abdominal/Cardiac MRI Scans
Digital Imaging and Communications in Medicine images were deidentified and TKV was measured by stereology (24,25) (Analyze 9.0; Mayo Foundation, Biomedical Imaging Resource, Rochester, MN) by a single analyst (W.W.) who had no knowledge of the participant’s status. Cardiac images were interpreted by a single analyst (J.D.S.) with no knowledge of the participant’s status. To account for normal growth in children, TKV was corrected for height in meters (HtTKV) (26,27).
Follow-Up
Participants submitted home BP reports each month for review (15). Female participants with Tanner stage≥2 were required to perform and report results of home urine pregnancy testing monthly using a kit supplied to them (First Response; Church & Dwight Co. Inc., Ewing, NJ). Medication compliance was assessed by frequent contact and by monitoring of medication supply. Serum CK, AST, and ALT were obtained locally at 1 month and 6 months after the initial visit or adjustment in pravastatin/placebo dose. At 18 and 36 months after the initial visit, participants returned for repeated admission, during which time they underwent the same above-described study procedures. The study was concluded in October 2012.
Trial Oversight
The study protocol was approved and routinely reviewed by the Colorado Multiple Institutional Review Board. A data safety monitoring board reviewed the progress of the study on an annual basis. This clinical trial was registered at ClinicalTrials.gov as NCT00456365 (March 12, 2007). The principles of the Declaration of Helsinki were upheld.
Outcome Measures
The primary outcome variable was considered positive if a study participant had a ≥20% increase in HtTKV, LVMI, or UAE over the 3-year interval (15). Secondary endpoints included a ≥20% increase in the individual variables of HtTKV, LVMI, and UAE and percent change over the study period in these individual variables.
Statistical Analyses
In previous observations of children with ADPKD over a 3-year period, we noted an increase of ≥20% in 80% of study participants for corrected TKV by MRI, 22% of participants for LVMI by echocardiography, and 53% of participants for UAE. None of these children were treated with statins. With a significance level of 0.05, we anticipated 80% power to detect a difference of 30% in the percentage of participants reaching the primary endpoint with 40 participants completing the study in each group. With an anticipated dropout rate of up to 25%, we planned to recruit a minimum of 50 participants for each study group.
The primary analysis was the comparison of the percentage of children reaching the primary endpoint between the two study groups by chi-squared analysis. A P value <0.05 for a two-sided test was considered significant. Analysis of covariance (ANCOVA) was used to test for differences between groups after adjusting for age, sex, and hypertension status. Mixed-model, longitudinal data analysis was utilized to examine the change in endpoints over time after adjustment for covariates. These analyses allowed us to model a random slope and intercept for each participant. In addition, the mixed model is robust to missing data. Given the modest sample size, secondary endpoint analyses were considered exploratory and no correction for multiple tests was used. Correlations between each of the three endpoints and LDL cholesterol concentration or BP were assessed using Pearson correlation coefficients. Descriptive statistics were reported as the mean±SD or as the median with 25th and 75th percentiles for variables that were not normally distributed, whereas results of ANCOVA and mixed models are presented as the least-squares mean±SEM or β±SEM.
Results
A total of 110 participants were enrolled in this clinical trial, including 56 randomized to pravastatin and 54 randomized to placebo (Figure 1). Baseline characteristics of enrolled participants were similar between the study groups except for serum creatinine and hematocrit (Table 1). These were not clinically significant differences, and 24-hour urine CrCl was similar between study groups. Ninety-one participants completed the 3-year study, resulting in an overall completion rate of 83%. Evaluation at the final visit showed similar demographic and laboratory characteristics between pravastatin and placebo groups with the exception of total and LDL cholesterol, which were significantly lower in the pravastatin group (Table 2). Comparison of baseline demographic variables between those participants who completed the study and those who did not were similar except for hematocrit (40%±3% versus 42%±4%; P=0.03); this was not a clinically significant difference.
Figure 1.
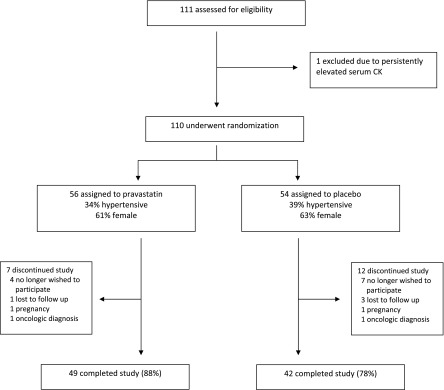
Course of study participants. A total of 111 potential participants were screened, including 110 participants who were randomly assigned in a double-blind manner to receive either pravastatin or placebo. A total of 91 participants (83%) completed the 3-year trial. CK, creatine kinase.
Table 1.
Characteristics of the study groups at the initial visit (N=110)
| Variable | Pravastatin (n=56) | Placebo (n=54) | P Value |
|---|---|---|---|
| Age (yr) | 16±4 | 16±4 | 0.50 |
| Height (cm) | 165±15 | 162±13 | 0.31 |
| Weight (kg) | 66±21 | 59±19 | 0.13 |
| Urine microalbumin excretion (μg/min) | |||
| Mean | 27±37 | 51±123 | 0.20 |
| Median | 12 [7, 28] | 11 [7, 21] | 0.90 |
| Left ventricular mass index (g/m2) | 55±12 | 53±10 | 0.39 |
| Total kidney volume (ml) | |||
| Mean | 571±315 | 522±306 | 0.41 |
| Median | 495 [349, 661] | 432 [329, 629] | 0.25 |
| Total kidney volume corrected for height (ml/m) | |||
| Mean | 336±187 | 315±175 | 0.55 |
| Median | 287 [213, 379] | 273 [204, 358] | 0.40 |
| Serum creatinine (mg/dl) | |||
| Mean | 0.7±0.2 | 0.6±0.1 | 0.04 |
| Median | 0.7 [0.6, 0.8] | 0.6 [0.5, 0.7] | 0.07 |
| 24-h urine creatinine clearance (ml/min per 1.73 m2) | 135±28 | 138±27 | 0.66 |
| BP (mmHg) | |||
| Systolic | 123±10 | 122±10 | 0.44 |
| Diastolic | 73±9 | 73±7 | 0.75 |
| Hematocrit (%) | 42±4 | 40±4 | 0.03 |
| 24-h urine protein (g/d) | |||
| Mean | 0.1±0.1 | 0.2±0.2 | 0.31 |
| Median | 0.11 [0.08, 0.15] | 0.10 [0.08, 0.16] | 0.68 |
| Cholesterol (mg/dl) | |||
| HDL | 49±12 | 48±11 | 0.67 |
| LDL | 84±23 | 90±21 | 0.23 |
| Total | 145±30 | 151±27 | 0.26 |
Values are the mean±SD or median [25th percentile, 75th percentile]. Non-normally distributed variables were also tested with the Wilcoxon rank sum test.
Table 2.
Characteristics of the study groups at the final visit (n=91)
| Variable | Pravastatin (n=49) | Placebo (n=42) | P Value |
|---|---|---|---|
| Age (yr) | 19±4 | 18±4 | 0.55 |
| Height (cm) | 171±11 | 170±11 | 0.73 |
| Weight (kg) | 74±20 | 68±20 | 0.20 |
| Urine microalbumin excretion (μg/min) | |||
| Mean | 29±39 | 49±99 | 0.21 |
| Median | 14 [9, 32] | 14 [8, 31] | 0.81 |
| Left ventricular mass index (g/m2) | 60±15 | 58±13 | 0.69 |
| Total kidney volume (ml) | |||
| Mean | 739±433 | 756±426 | 0.85 |
| Median | 604 [428, 878] | 622 [479, 907] | 0.67 |
| Percent change (per 3 yr) | 27±18 | 38±25 | 0.04 |
| Total kidney volume corrected for height (ml/m) | |||
| Mean | 429±244 | 442±244 | 0.80 |
| Median | 344 [251, 496] | 372 [271, 514] | 0.56 |
| Percent change (per 3 yr) | 21±16 | 30±20 | 0.02 |
| Serum creatinine (mg/dl) | |||
| Mean | 0.8±0.2 | 0.7±0.1 | 0.29 |
| Median | 0.7 [0.7, 0.8] | 0.7 [0.6, 0.8] | 0.26 |
| 24-h urine creatinine clearance (ml/min per 1.73 m2) | 126±24 | 126±25 | 0.98 |
| BP (mmHg) | |||
| Systolic | 123±10 | 123±9 | 0.96 |
| Diastolic | 74±9 | 75±8 | 0.59 |
| Hematocrit (%) | 42±4 | 40±7 | 0.17 |
| 24-h urine protein (g/d) | |||
| Mean | 0.2±0.1 | 0.2±0.2 | 0.38 |
| Median | 0.12 [0.09, 0.17] | 0.14 [0.09, 0.19] | 0.58 |
| Cholesterol (mg/dl) | |||
| HDL | 48±16 | 49±10 | 0.92 |
| LDL | 71±25 | 87±25 | 0.003 |
| Total | 141±32 | 156±29 | 0.02 |
Values are the mean±SD or median [25th percentile, 75th percentile]. Non-normally distributed variables were also tested with the Wilcoxon rank sum test.
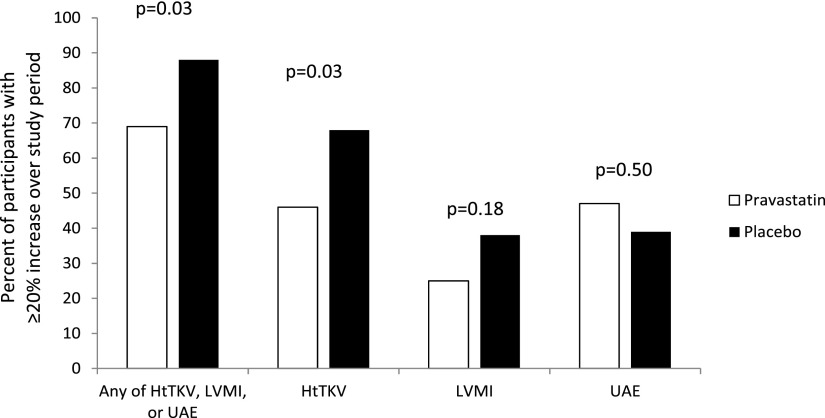
There was a significant difference between study groups for the primary endpoint. Upon conclusion of the study, 34 participants (69%) in the statin group demonstrated a ≥20% increase in UAE, LVMI, or HtTKV compared with 37 participants (88%) in the placebo group (P=0.03) (Figure 2, Table 2). Evaluation of individual variables showed that this finding was primarily related to change in HtTKV, with 46% of the statin group and 68% of the placebo group demonstrating a ≥20% increase (P=0.03) over the course of the study. However, there were no significant differences in the percentage of participants demonstrating a ≥20% increase in LVMI (25% versus 38%; P=0.18) or UAE (47% versus 39%; P=0.50).
Figure 2.
Study endpoints. The primary end point was the percent of participants demonstrating ≥20% increase over the three-year study period in any of the total kidney volume corrected for height (HtTKV), left ventricular mass index (LVMI), or urine microalbumin excretion (UAE) between pravastatin and placebo study groups. Secondary end points included ≥20% increase in these individual variables.
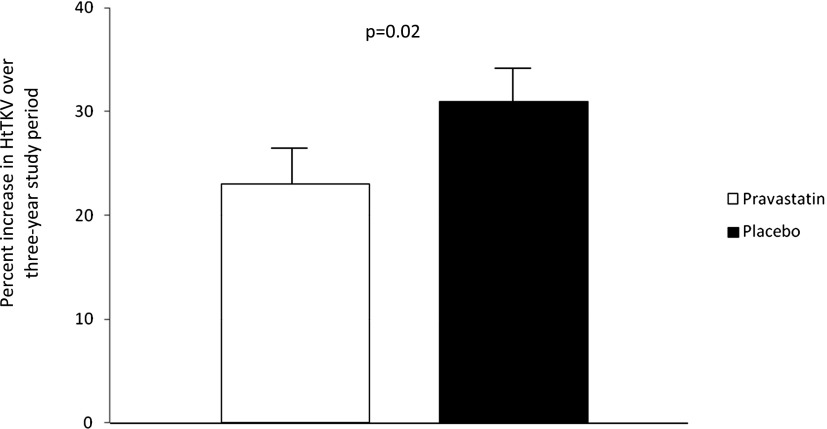
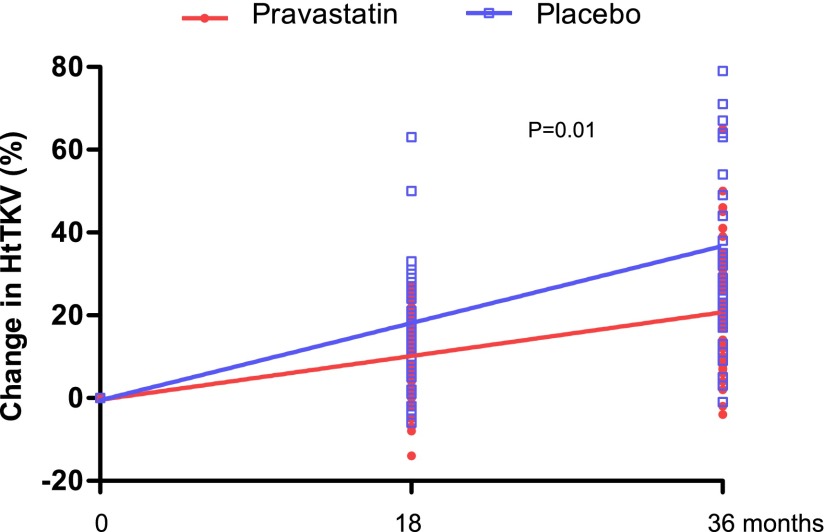
Using ANCOVA adjusted for age (P=0.14), sex (P=0.002), and hypertension status at baseline (P=0.84), there was a significant difference in the percent change in HtTKV over the study period between groups (23%±3% for statin versus 31%±3% for placebo per 3 years; P=0.02) (Figure 3). In a mixed-model longitudinal data analysis, the increase in percent growth over 3 years was significant between the statin and placebo groups (P=0.01) (Figure 4).The magnitude of the effect of pravastatin on mean HtTKV was not immediately apparent between groups at the study conclusion (Table 2) despite these findings.
Figure 3.
Percent increase in HtTKV over the course of the study.
Figure 4.
Effect of pravastatin on change in HtTKV.
There was no correlation between 24-hour urine CrCl and either TKV or HtTKV at baseline or over the course of the study. There was no significant change in CrCl or urine protein excretion over time in either group. There was no correlation between LDL or total cholesterol or pravastatin dose and any of TKV, HtTKV, UAE, LVMI, or CrCl. There was no significant difference in LDL cholesterol between participants who experienced a <20% change in HtTKV compared with those with a ≥20% change (P=0.73).
Safety Considerations
No participant required discontinuation of pravastatin/placebo because of side effects. There were no significant differences between the study groups for serum CK, AST, or ALT at any time point. No participant demonstrated a significant elevation in serum CK. One participant in each group had an elevation in AST exceeding 2 times the upper limit of normal (maximum 2.3 times the upper limit). Further evaluation of these participants raised concern for underlying fatty liver associated with obesity. Three participants became pregnant during the study. A noncompleting participant taking lisinopril and pravastatin reported a positive pregnancy test 4 weeks after a negative test and immediately discontinued the study medications. A noncompleting placebo participant never started lisinopril due to low BP and was taking no active medications at the time of conception. Both women delivered healthy term infants with no medical problems during infancy. A completing participant in the placebo group admitted to >1 year of noncompliance with study medications but had resumed lisinopril and placebo 3 weeks before her final visit. A positive pregnancy test was documented 2 weeks before the final visit but a local obstetrical ultrasound could not identify an embryo. Lisinopril/placebo was discontinued but she was believed to have suffered a miscarriage.
Discussion
ADPKD is the fourth most common cause of ESRD in adults in the United States and Europe. Evidence is emerging regarding early occurrence and expansion of renal cysts in ADPKD. Therefore, early intervention in children and young adults has potential advantages in slowing the progression of ADPKD and minimizing long-term complications.
Experimental evidence in rats has shown that statins decrease the severity of functional and structural disease in ADPKD (13,14). Moreover, statins are of benefit for endothelial dysfunction (28–30), which is known to occur early in patients with ADPKD, even before the onset of hypertension (31). Hypertension and increased LVMI occur early in children with ADPKD (32), and statins are known to reduce left ventricular mass in ischemic heart disease (33). Finally, short-term (4-week) administration of simvastatin is known to improve RBF and GFR in adults with ADPKD (10). A 3-year randomized study was therefore undertaken to examine the effect of statin treatment on kidney volume growth, LVMI, and UAE in children and young adults with ADPKD. Here we report the first randomized controlled interventional trial to demonstrate the efficacy of a pharmacologic agent to slow the progression of structural kidney disease in children and young adults with ADPKD. Significantly fewer participants treated with pravastatin achieved the primary endpoint of a ≥20% change in HtTKV, UAE, or LVMI over the 3-year study period compared with controls. This effect was primarily due to diminished growth in TKV in the pravastatin group. There is evidence that kidney growth is associated with declining kidney function over time in children (4) and adults (25,34) with ADPKD.
Routine sonographic screening has not been advocated for children at risk for ADPKD in the United States. In most European countries, sonographic screening is routinely performed because of the prevalence of complications such as hypertension, microalbuminuria, or proteinuria in otherwise asymptomatic children with ADPKD (35). Although our referral population is likely biased toward more severe disease, the efficacy of pravastatin in this cohort of pediatric participants with ADPKD suggests that routine diagnostic screening should be considered in this age-defined subset of at-risk children whenever such screening is not the standard of care. In considering this approach, however, a detailed discussion between the provider and patient/family is required and should address not only the potential benefits of diagnosis and pravastatin treatment but also the potential risks, including implications for future insurability and psychosocial effects on the individual and family.
Pravastatin was well tolerated in this study, with no participant requiring discontinuation of the medication as a result of adverse effects. However, the occurrence of three pregnancies during the study highlights the importance of appropriate counseling and follow-up when prescribing potentially teratogenic medications to female patients of childbearing age. Recent studies suggest minimal teratogenicity of statins during pregnancy (36,37), although statins remain pregnancy category X drugs as labeled by the US FDA. ACEIs and angiotensin receptor blockers are associated with an increased risk of fetal birth defects (38), although risk with use in early pregnancy is comparable to that of other antihypertensive medications (39,40).
The mechanisms underlying the effect of pravastatin on structural kidney disease in pediatric ADPKD require further elucidation. The prevalence of hypercholesterolemia in this study was low (3% overall) and as anticipated, there was no significant correlation between either total or LDL cholesterol and HtTKV, LVMI, UAE, or CrCl. Independent of lipid-lowering effects, inhibition of the conversion of hepatic hydroxymethyl glutaryl–CoA to mevalonate prevents the synthesis of certain isoprenoids that provide lipid attachments for post-translational modification of proteins including Ras and Rho. These effects would be anticipated to alter signal transduction, cell proliferation, and cell polarity, as reviewed by Kostapanos et al. (41). These pleiotropic effects of statins may be critical in the formation and expansion of kidney cysts in ADPKD. High-dose statins are also known to inhibit angiogenesis (42), which may have an important role in the development and progression of renal cystic disease. In this regard, we previously observed that serum vascular endothelial growth factor and angiopoietin 1 concentrations are positively correlated with kidney and cardiac structure but negatively correlated with CrCl in children and young adults with ADPKD (43).
Statins induce regression of left ventricular hypertrophy in animals and humans with ischemic heart disease (33,44), an effect that may be mediated via reduction of Ras membrane targeting and activation (45). Although there was a trend for pravastatin treatment to mitigate the increase in LVMI in this study, this was not statistically significant. Likely confounding factors included the use of ACEIs in nearly all participants in both study groups and the low frequency of left ventricular hypertrophy at baseline. There has been a marked decrease in the prevalence of left ventricular hypertrophy in adults with ADPKD in the current era with improved control of hypertension and widespread use of ACEIs (46,47). There was no effect of pravastatin on UAE; with repeated study, this marker appears to have minimal predictive value in children with ADPKD compared with affected adults (4,48). There was no effect of pravastatin on CrCl or proteinuria in this research study.
In summary, pravastatin appears to be a beneficial treatment to slow progression of structural kidney disease in children and young adults aged 8–22 years with ADPKD. We strongly recommend consideration of its use in this subset of children with ADPKD who have no contraindication to statin treatment. It should be noted that pravastatin is not FDA-approved for this indication; therefore, appropriate discussion with patients and families regarding potential risks and benefits, including possible psychosocial and financial implications, is mandatory. Further study is needed to clarify the molecular mechanisms contributing to these findings. Finally, our findings in this and previous studies confirm that early intervention before the development of established hypertension and/or severe structural kidney disease may be warranted.
Disclosures
None.
Acknowledgments
We thank the dedicated research nurses of the Clinical Translational Research Center at Children’s Hospital Colorado for many years of excellent care for our research participants and the individuals and families who have committed their time and effort to improving medical care for all children with ADPKD.
This research was supported by the National Institutes of Health, the National Center for Research Resources, and the Zell Family Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Novel Treatments of Autosomal Dominant Polycystic Kidney Disease,” on pages 831–836.
References
- 1.Somlo S, Chapman AB: Autosomal dominant polycystic kidney disease. In: Schrier's Diseases of the Kidney, edited by Coffman TM, Falk RJ, Molitoris BA, Neilson EG, Schrier RW, 9th Ed., Philadelphia, Lippincott Williams & Wilkins, 2012, pp 519–563 [Google Scholar]
- 2.MacDermot KD, Saggar-Malik AK, Economides DL, Jeffery S: Prenatal diagnosis of autosomal dominant polycystic kidney disease (PKD1) presenting in utero and prognosis for very early onset disease. J Med Genet 35: 13–16, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pretorius DH, Lee ME, Manco-Johnson ML, Weingast GR, Sedman AB, Gabow PA: Diagnosis of autosomal dominant polycystic kidney disease in utero and in the young infant. J Ultrasound Med 6: 249–255, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW: Prospective change in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol 4: 820–829, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hafez KS, Inman SR, Stowe NT, Novick AC: Renal hemodynamic effects of lovastatin in a renal ablation model. Urology 48: 862–867, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Glazer AA, Inman SR, Stowe NT, Novick AC: Renal microcirculatory effects of lovastatin in a rat model of reduced renal mass. Urology 50: 812–817, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Inman SR, Stowe NT, Cressman MD, Brouhard BH, Nally JV, Jr, Satoh S, Satodate R, Vidt DG: Lovastatin preserves renal function in experimental diabetes. Am J Med Sci 317: 215–221, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Joyce M, Kelly C, Winter D, Chen G, Leahy A, Bouchier-Hayes D: Pravastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, attenuates renal injury in an experimental model of ischemia-reperfusion. J Surg Res 101: 79–84, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Bianchi S, Bigazzi R, Caiazza A, Campese VM: A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease. Am J Kidney Dis 41: 565–570, 2003 [DOI] [PubMed] [Google Scholar]
- 10.van Dijk MA, Kamper AM, van Veen S, Souverijn JH, Blauw GJ: Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 16: 2152–2157, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Stowe NT, Inman SR, Tapolyai M, Brouhard BH, Hodge EE, Novick AC: Lovastatin has direct renal hemodynamic effects in a rodent model. J Urol 156: 249–252, 1996 [PubMed] [Google Scholar]
- 12.McFarlane SI, Muniyappa R, Francisco R, Sowers JR: Clinical review 145: Pleiotropic effects of statins: Lipid reduction and beyond. J Clin Endocrinol Metab 87: 1451–1458, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Gile RD, Cowley BD, Jr, Gattone VH, 2nd, O’Donnell MP, Swan SK, Grantham JJ: Effect of lovastatin on the development of polycystic kidney disease in the Han:SPRD rat. Am J Kidney Dis 26: 501–507, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Zafar I, Tao Y, Falk S, McFann K, Schrier RW, Edelstein CL: Effect of statin and angiotensin-converting enzyme inhibition on structural and hemodynamic alterations in autosomal dominant polycystic kidney disease model. Am J Physiol Renal Physiol 293: F854–F859, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Cadnapaphornchai MA, George DM, Masoumi A, McFann K, Strain JD, Schrier RW: Effect of statin therapy on disease progression in pediatric ADPKD: Design and baseline characteristics of participants. Contemp Clin Trials 32: 437–445, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 17.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall WA, Tanner JM: Growth and physiological development during adolescence. Annu Rev Med 19: 283–300, 1968 [DOI] [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM: Variations in pattern of pubertal changes in girls. Arch Dis Child 44: 291–303, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM: Variations in the pattern of pubertal changes in boys. Arch Dis Child 45: 13–23, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 22.Artigao LM, Llavador JJ, Puras A, López Abril J, Rubio MM, Torres C, Vidal A, Sanchis C, Divisón JA, Naharro F, Caldevilla D, Fuentes G: [Evaluation and validation of Omron Hem 705 CP and Hem 706/711 monitors for self-measurement of blood pressure]. Aten Primaria 25: 96–102, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien E, Waeber B, Parati G, Staessen J, Myers MG: Blood pressure measuring devices: Recommendations of the European Society of Hypertension. BMJ 322: 531–536, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW: Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol 6: 369–376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF, Jr, Glockner JF, Wetzel LH, Brummer ME, O’Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort : Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64: 1035–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Torres VE, Chapman AB, Perrone RD, Bae KT, Abebe KZ, Bost JE, Miskulin DC, Steinman TI, Braun WE, Winklhofer FT, Hogan MC, Oskoui FR, Kelleher C, Masoumi A, Glockner J, Halin NJ, Martin DR, Remer E, Patel N, Pedrosa I, Wetzel LH, Thompson PA, Miller JP, Meyers CM, Schrier RW, HALT PKD Study Group : Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int 81: 577–585, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Driscoll G, Green D, Taylor RR: Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 95: 1126–1131, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Laufs U, La Fata V, Plutzky J, Liao JK: Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 97: 1129–1135, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Laufs U: Beyond lipid-lowering: Effects of statins on endothelial nitric oxide. Eur J Clin Pharmacol 58: 719–731, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Kocaman O, Oflaz H, Yekeler E, Dursun M, Erdogan D, Demirel S, Alisir S, Turgut F, Mercanoglu F, Ecder TE: Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 43: 854–860, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW: Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int 74: 1192–1196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishikawa H, Miura S, Zhang B, Shimomura H, Arai H, Tsuchiya Y, Matsuo K, Saku K: Statins induce the regression of left ventricular mass in patients with angina. Circ J 68: 121–125, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Fick-Brosnahan GM, Belz MM, McFann KK, Johnson AM, Schrier RW: Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: A longitudinal study. Am J Kidney Dis 39: 1127–1134, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Fencl F, Janda J, Bláhová K, Hríbal Z, Stekrová J, Puchmajerová A, Seeman T: Genotype-phenotype correlation in children with autosomal dominant polycystic kidney disease. Pediatr Nephrol 24: 983–989, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Winterfeld U, Allignol A, Panchaud A, Rothuizen LE, Merlob P, Cuppers-Maarschalkerweerd B, Vial T, Stephens S, Clementi M, De Santis M, Pistelli A, Berlin M, Eleftheriou G, Maňáková E, Buclin T: Pregnancy outcome following maternal exposure to statins: A multicentre prospective study. BJOG 120: 463–471, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Costantine MM, Cleary K, Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric--Fetal Pharmacology Research Units Network : Pravastatin for the prevention of preeclampsia in high-risk pregnant women. Obstet Gynecol 121: 349–353, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullo M, Tschumi S, Bucher BS, Bianchetti MG, Simonetti GD: Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: A systematic review. Hypertension 60: 444–450, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Walfisch A, Al-maawali A, Moretti ME, Nickel C, Koren G: Teratogenicity of angiotensin converting enzyme inhibitors or receptor blockers. J Obstet Gynaecol 31: 465–472, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Diav-Citrin O, Shechtman S, Halberstadt Y, Finkel-Pekarsky V, Wajnberg R, Arnon J, Di Gianantonio E, Clementi M, Ornoy A: Pregnancy outcome after in utero exposure to angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Reprod Toxicol 31: 540–545, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Kostapanos MS, Liberopoulos EN, Elisaf MS: Statin pleiotropy against renal injury. J Cardiometab Syndr 4: E4–E9, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO: Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB J 20: 1706–1708, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Reed BY, Masoumi A, Elhassan E, McFann K, Cadnapaphornchai MA, Maahs DM, Snell-Bergeon JK, Schrier RW: Angiogenic growth factors correlate with disease severity in young patients with autosomal dominant polycystic kidney disease. Kidney Int 79: 128–134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauersachs J, Galuppo P, Fraccarollo D, Christ M, Ertl G: Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation 104: 982–985, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Luo JD, Zhang WW, Zhang GP, Guan JX, Chen X: Simvastatin inhibits cardiac hypertrophy and angiotensin-converting enzyme activity in rats with aortic stenosis. Clin Exp Pharmacol Physiol 26: 903–908, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Chapman AB, Johnson AM, Rainguet S, Hossack K, Gabow P, Schrier RW: Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 8: 1292–1297, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Perrone RD, Abebe KZ, Schrier RW, Chapman AB, Torres VE, Bost J, Kaya D, Miskulin DC, Steinman TI, Braun W, Winklhofer FT, Hogan MC, Rahbari-Oskoui F, Kelleher C, Masoumi A, Glockner J, Halin NJ, Martin D, Remer E, Patel N, Pedrosa I, Wetzel LH, Thompson PA, Miller JP, Bae KT, Meyers CM, HALT PKD Study Group : Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6: 2508–2515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chapman AB, Johnson AM, Gabow PA, Schrier RW: Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 1349–1354, 1994 [DOI] [PubMed] [Google Scholar]