Abstract
Background and objectives
Comorbid major depression is associated with adverse health outcomes in patients with diabetes, but little is known regarding its associations with long-term renal outcomes in this population. Furthermore, the impact of minor depression on renal outcomes is not known. This study evaluated associations between depressive symptoms and risk of incident ESRD in a diabetic cohort.
Design, setting, participants, & measurements
In this prospective, observational cohort study, 3886 ambulatory adults with diabetes were recruited from primary care clinics of a large health maintenance organization in the state of Washington. Demographics, laboratory data, depressive symptoms (based on the Patient Health Questionnaire-9), and patterns of diabetes self-care were collected. Participants were considered depressed if they had the required number of depressive symptoms (≥5 for major or 2–4 for minor depressive symptoms), including depressed mood or anhedonia, >50% of the time for ≥2 weeks and a Patient Health Questionnaire-9 score≥10 for major and ≥5 for minor depressive symptoms. Risk of incident ESRD was estimated using Cox proportional hazards regression, with predialysis death as a competing risk.
Results
During a median follow-up of 8.8 years, 87 patients (2.2%) developed ESRD. Major depressive symptoms were associated with a higher risk of incident ESRD (hazard ratio, 1.85; 95% confidence interval, 1.02 to 3.33) after adjusting for age, sex, race/ethnicity, marital status, education, smoking, body mass index, diabetes duration, hemoglobin A1c, baseline kidney function, microalbuminuria, hypertension, renin-angiotensin system blockers, and adherence to diabetes self-care. Minor depressive symptoms were not significantly associated with incident ESRD (hazard ratio, 1.08; 95% confidence interval, 0.52 to 2.25).
Conclusion
Major depressive symptoms, but not minor depressive symptoms, were associated with a higher risk of incident ESRD over 10 years. Additional studies are needed to determine whether treatment for depression can improve renal outcomes in patients with diabetes.
Introduction
Patients with diabetes mellitus have a high prevalence of clinically important depressive symptoms, with estimates ranging from 11.4% to 31.0% depending on the method of assessment (1). The presence of comorbid depression with diabetes is associated with higher symptom burden (2,3), worse glycemic control (4), nonadherence to recommended self-care and treatments (5–8), and adverse outcomes, including mortality (9–13). Although depression has been shown to be associated with diabetic kidney disease in cross-sectional studies (10,14), its role in the incidence of ESRD in this population is still under investigation.
Studies have yielded conflicting results concerning depression as a risk factor for ESRD. In small prospective cohort studies of persons with known mild to severe CKD not yet on dialysis, depression was a predictor of progression to ESRD after adjustment for demographic variables and comorbidities (15,16). However, these studies were conducted in patients with a high likelihood of progression to ESRD because of preexisting CKD. In contrast, depressive symptoms were not predictive of incident ESRD in the Cardiovascular Health Study, a community-based cohort of individuals with a low likelihood of ESRD incidence (17). These discrepancies in findings may be attributable to differences in the underlying study populations, baseline levels of CKD, participant risk for ESRD, and definitions of ESRD. Although diabetes mellitus is the leading cause of kidney failure (18), information is lacking regarding whether major depression is a risk factor for ESRD in this high-risk population. Moreover, the impact of minor depression on adverse renal outcomes is not known. The primary objective of this study is to evaluate associations between major or minor depressive symptoms and risk of incident ESRD in the Pathways Study, a prospective, population-based observational cohort of primary care patients with diabetes.
Materials and Methods
Study Design
The Pathways Study is a prospective, observational cohort study developed by a multidisciplinary team from the University of Washington and the Group Health (GH) Research Institute to study associations of depression with diabetes outcomes. The study has been described in detail elsewhere (19,20). Briefly, GH is a vertically integrated health maintenance organization with >600,000 enrollees in Washington and Idaho. In 2001 and 2002, surveys were mailed to 9063 potential participants identified from the GH diabetes registry from nine selected primary care clinics; 1222 patients were later determined ineligible because of death, disenrollment, no diabetes, gestational diabetes, severe illness, language/hearing barriers, or cognitive impairment (Figure 1). Of the eligible patients (n=7841), 61.7% (n=4839) returned the baseline epidemiologic survey regarding demographics, diabetes history, and depression, of which 85.3% (n=4128) gave written consent to link survey results with GH automated databases regarding laboratory results and clinical encounters. Participants were followed until the development of ESRD, death, GH disenrollment, or the end of the 10-year study period (August 15, 2012). Participants were excluded from the present study if they had ESRD at baseline or missing depression status. Because baseline kidney function was ascertained using laboratory data up to 6 months after study enrollment, participants were also excluded if they developed incident ESRD, died, or disenrolled from GH within 6 months of study enrollment. The study protocol was approved by the GH and University of Washington institutional review boards.
Figure 1.
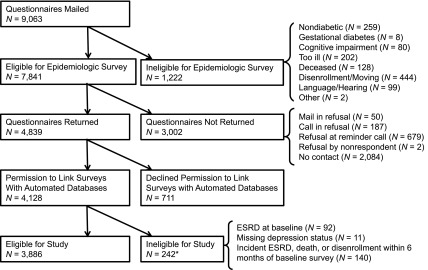
Pathways Study participant recruitment. *Components add up to more than the total N because of overlap.
Primary Predictor
The primary predictor was the presence of depressive symptoms at study entry, determined by the Patient Health Questionnaire-9 (PHQ-9) (21), which is based on the Diagnostic and Statistical Manual (Fourth Edition) criteria for major depression and has been validated in patients with CKD (22). Participants were considered depressed if they had the required number of depressive symptoms (five or more for major depression or two to four for minor depression), including either depressed mood or anhedonia, >50% of the time for at least 2 weeks. Using these criteria, participants with major and minor depressive symptoms had a minimum PHQ-9 score of 10 and 5, respectively (11). Major depression has been shown to often be a chronic illness in patients with diabetes, with >70% of patients stating that they had been ill for over 5 years (23); moreover, in a recent prospective trial, >80% of patients meeting criteria for major depression at 5 years of follow-up had either major or minor depression at baseline interview (24).
Covariates
Demographics and diabetes characteristics were self-reported from the baseline survey. Baseline hemoglobin A1c (HbA1c) and LDL were based on average results in the 12 months before study enrollment. Because of a higher proportion of missing values, baseline creatinine was determined by the average creatinine in the 18 months before or 6 months after study entry if no prior results were available. Chronic Kidney Disease Epidemiology Collaboration equations were used to calculate eGFR (25). Microalbuminuria was defined as a urine albumin to creatinine ratio≥30 mg/g in the 24 months before or 6 months after study entry. Hypertension was identified by International Classification of Diseases, Ninth Revision diagnosis code (ICD-9) 401.x (26). Medication use, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and antidepressants, was obtained from GH pharmacy records. Diabetes self-care was assessed using the modified Summary of Diabetes Self-Care Activities (SDSCA), which is a questionnaire that asks how many days per week an activity was performed (Table 1) (27). Each self-care domain (general diet, special diet, exercise, blood glucose testing, and foot care) was given a score ranging from zero to seven, with higher scores indicating better compliance.
Table 1.
Selected questions from the Summary of Diabetes Self-Care Activities
| Self-Care Domain | Summary of Diabetes Self-Care Activities Question |
|---|---|
| General diet | On how many of the last 7 d have you followed a healthful eating plan? |
| Think about the past month. How many days per week, on average, have you followed your eating plan? | |
| Special diet | On how many of the last 7 d did you eat five or more servings of fruits and vegetables? |
| On how many of the last 7 d did you eat high fat foods, such as red meat or whole-fat dairy products? | |
| Exercise | On how many of the last 7 d did you participate in at least 30 min of physical activity (meaning 30 min of continuous activity, including walking)? |
| On how many of the last 7 d did you participate in a specific exercise session (such as swimming, walking, or biking) other than what you do around the house or as part of your work? | |
| Blood glucose testing | On how many of the last 7 d did you test your blood sugar? |
| On how many of the last 7 d did you test your blood sugar the number of times recommended by your health care provider? | |
| Foot care | On how many of the last 7 d did you check your feet? |
| On how many of the last 7 d did you inspect the inside of your shoes? |
Outcome
The primary outcome was the development of ESRD requiring dialysis or kidney transplant. The date of first dialysis or transplant was determined by GH automated records using the following ICD-9 and procedure codes: ESRD or uremia (585.x and 586.x), kidney transplant (50360, 50365, and 55.69), dialysis (39.95, 54.98, 90935, 90937, 90945, 90947, and G0257), dialysis training (90989 and 90993), or ESRD-related services (90918–90925, 90960–90970, 90999, G0311–G0319, G0323, and G0327).
Statistical Analyses
Statistical analyses were performed using Stata, version 12 (StataCorp, College Station, TX) and R 2.15.2 (R Project for Statistical Computing; http://www.r-project.org/). Baseline characteristics by depression status were determined using one-way ANOVA for continuous variables, chi-squared tests for categorical variables, and Mann–Whitney tests for skewed variables. Cox proportional hazards regression models were used to analyze the associations between depression and the primary outcome (28). A competing risks model was used to incorporate the risk of pre-ESRD death in the hazards estimates for ESRD (29). The primary predictor was included in the model as major, minor, or no depressive symptoms. Multiple imputation by chained equations was used for covariates with missing values (race/ethnicity, marital status, education, salary, body mass index [BMI], diabetes type, diabetes duration, HbA1c, eGFR, microalbuminuria, and diabetes self-care) (30,31). Participants were considered at risk starting 6 months after study enrollment and followed longitudinally for up to 10 years after study entry. Participants were censored at the date of GH disenrollment or the end of the study. Unadjusted analyses were performed for each covariate of interest. In the multivariate analysis, it was decided a priori to adjust for age, sex, race/ethnicity, marital status, education, BMI, diabetes duration, HbA1c, eGFR, hypertension, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use as potential confounders. Based on their associations with depression and ESRD, other covariates of interest were smoking and baseline microalbuminuria (model 1). A second model included additional adjustments for SDSCA scores (model 2) to assess the effect of diabetes self-care on the associations between depression and outcomes of interest. Baseline antidepressant use was not included as a covariate because it is confounded by symptom severity (32,33); however, it was included in a sensitivity analysis that did not show substantially different results from the primary analysis. Other sensitivity analyses included adjustment for obesity instead of BMI and exclusion of baseline eGFR from both models. We also tested interactions between the primary predictor and either sex or age. The primary results are presented in Results.
Results
Baseline Characteristics
Of the 3886 participants in this study, 448 (11.5%) participants had major and 327 (8.4%) participants had minor depressive symptoms. Mean PHQ-9 scores were 17.2±3.8 for major depressive symptoms, 9.6±2.2 for minor depressive symptoms, and 3.7±3.4 for no depressive symptoms. Participants with major depressive symptoms tended to be younger, women, and non-Hispanic black, and they had lower levels of education and salary compared with those participants without depressive symptoms (Table 2). Participants with depressive symptoms were more likely to be smokers and have a higher BMI than participants without depressive symptoms. The prevalence of hypertension, dyslipidemia, and type 1 diabetes was similar between groups. Participants with major depressive symptoms had the poorest baseline metabolic control (mean HbA1c=8.1±1.6 versus 7.9±1.6 for minor and 7.7±1.5 for no depressive symptoms) and highest baseline eGFR (77.3±23.5 ml/min per 1.73 m2 versus 71.6±24.8 ml/min per 1.73 m2 for minor and 73.6±21.7 ml/min per 1.73 m2 for no depressive symptoms), and they also had the highest proportion of microalbuminuria (39.6% versus 36.2% for minor and 30.5% for no depressive symptoms) compared with the other groups. Adherence to recommended diet and exercise was lowest in participants with major depressive symptoms and highest in participants with no depressive symptoms; adherence to blood glucose testing and self–foot care was comparable between groups. A greater proportion of participants with major depressive symptoms was prescribed antidepressants at baseline (52.2% versus 27.5% for minor and 15.4% for no depressive symptoms). Renin-angiotensin system blocker use was similar between groups.
Table 2.
Baseline characteristics of Pathways Study cohort by depression status (n=3886)
| Variable | Major Depressive Symptomsa (n=448) | Minor Depressive Symptomsa (n=327) | No Depressive Symptoms (N=3111) |
|---|---|---|---|
| Patient Health Questionnaire-9 score | 17.2 (3.8) | 9.6 (2.2) | 3.7 (3.4) |
| Age (yr) | 59.3 (13.4) | 64.4 (13.6) | 64.1 (13.1) |
| Men (%) | 185 (41.2) | 169 (51.7) | 1663 (53.5) |
| Race/ethnicity (%) | |||
| Non-Hispanic white | 347 (77.6) | 243 (74.5) | 2483 (80.2) |
| Non-Hispanic black | 49 (11.0) | 27 (8.3) | 249 (8.0) |
| Asian | 28 (6.3) | 34 (10.5) | 248 (8.0) |
| Other | 23 (5.2) | 22 (6.8) | 118 (3.8) |
| Married (%) | 243 (54.9) | 199 (61.8) | 2015 (65.4) |
| Education beyond high school (%) | 323 (72.9) | 219 (68.0) | 2379 (77.3) |
| Salary≥$20,000/yr (%) | 201 (53.2) | 129 (50.4) | 1468 (59.0) |
| Smoker (%) | 65 (14.5) | 33 (10.1) | 227 (7.3) |
| Body mass index (kg/m2) | 35.1 (9.2) | 32.1 (7.1) | 31.0 (6.8) |
| Hypertension (%) | 203 (45.3) | 158 (48.3) | 1334 (42.9) |
| LDL (mg/dl) | 114.3 (36.5) | 108.0 (32.1) | 111.8 (34.7) |
| Type 1 diabetes (%) | 13 (2.9) | 8 (2.5) | 132 (4.3) |
| Duration of diabetes (yr) | 7 (3–13) | 8 (4–15) | 6 (3–12) |
| Hemoglobin A1c (%) | 8.1 (1.6) | 7.9 (1.6) | 7.7 (1.5) |
| Creatinine (mg/dl) | 1.0 (0.4) | 1.1 (0.4) | 1.0 (0.4) |
| eGFR (ml/min per 1.73 m2) | 77.3 (23.5) | 71.6 (24.8) | 73.6 (21.7) |
| Microalbuminuria (UACR≥30 mg/g) | 134 (39.6) | 83 (36.2) | 687 (30.5) |
| Summary of Diabetes Self-Care Activities scoreb | |||
| General diet | 3.7 (2.2) | 4.3 (2.2) | 4.9 (2.0) |
| Special diet | 3.4 (1.6) | 3.6 (1.7) | 4.0 (1.6) |
| Exercise | 2.0 (1.9) | 2.1 (2.0) | 2.9 (2.2) |
| Blood glucose testing | 4.3 (2.7) | 4.1 (2.8) | 4.2 (2.8) |
| Foot care | 3.2 (2.3) | 3.4 (2.2) | 3.3 (2.4) |
| Antidepressant use (%) | 234 (52.2) | 90 (27.5) | 479 (15.4) |
| ACEI/ARB use (%) | 263 (61.6) | 186 (59.6) | 1735 (59.1) |
Data are mean (SD), N (%), or median (interquartile range). Data are missing in less than 1% for race/ethnicity, marital status, body mass index, type 1 diabetes, duration of diabetes, exercise, and blood glucose testing; 1%–2% for education, hemoglobin A1c, general diet, special diet, and foot care; 3%–10% for creatinine and eGFR; and >10% for salary (20%), LDL (28%), and microalbuminuria (27%). UACR, urine albumin to creatinine ratio; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Participants with major depressive symptoms had Patient Health Questionnaire-9 scores≥10 and at least five depressive symptoms (including depressed mood or anhedonia) >50% of the time for at least 2 weeks. Participants with minor depressive symptoms had Patient Health Questionnaire-9 scores≥5 and two to four depressive symptoms (including depressed mood or anhedonia) >50% of the time for at least 2 weeks.
Self-care scores correspond with how many days per week that the self-care activity was performed.
Incident ESRD by Depression Status
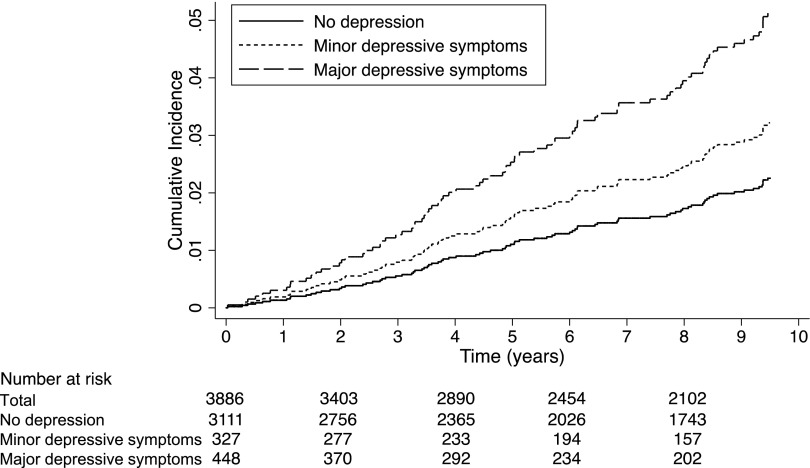
In total, 87 participants developed ESRD over 26,397 patient-years at risk, for an incidence rate of 3.30 cases per 1000 patient-years (95% confidence interval [95% CI], 2.66 to 4.05) (Table 3). The incidence of ESRD was 6.65 per 1000 patient-years for participants with major depression symptoms (95% CI, 4.07 to 10.31), 4.30 per 1000 patient-years for participants with minor depression symptoms (95% CI, 2.10 to 7.89), and 2.78 per 1000 patient-years for participants without depressive symptoms (95% CI, 2.14 to 3.55). The cumulative incidence of ESRD by depression status is shown in Figure 2.
Table 3.
Incident rates of ESRD by depression status
| Events | Median Years of Follow-Up (IQR) | Total Patient-Years | Incidence Rate/1,000 Patient-Years (95% CI) | |
| Total | 87 | 8.84 (3.88, 9.50) | 26,397 | 3.30 (2.66–4.05) |
| Major depressive symptomsa | 18 | 6.47 (2.78, 9.50) | 2707 | 6.65 (4.07–10.31) |
| Minor depressive symptomsa | 9 | 7.76 (3.27, 9.50) | 2094 | 4.30 (2.10–7.89) |
| No depression | 60 | 9.24 (4.24, 9.50) | 21,596 | 2.78 (2.14–3.55) |
IQR, interquartile range; 95% CI, 95% confidence interval.
Participants with major depressive symptoms had Patient Health Questionnaire-9 scores≥10 and at least five depressive symptoms (including depressed mood or anhedonia) >50% of the time for at least 2 weeks. Participants with minor depressive symptoms had Patient Health Questionnaire-9 scores≥5 and two to four depressive symptoms (including depressed mood or anhedonia) >50% of the time for at least 2 weeks.
Figure 2.
Cumulative incidence of ESRD by depression status. Participants with major depressive symptoms had Patient Health Questionnaire-9 scores≥10 and at least five depressive symptoms (including depressed mood or anhedonia) >50% of the time for at least 2 weeks. Participants with minor depressive symptoms had Patient Health Questionnaire-9 scores≥5 and two to four depressive symptoms (including depressed mood or anhedonia) >50% of the time for at least 2 weeks.
Using Cox proportional hazards models with predialysis death as a competing risk, the presence of major, but not minor, depressive symptoms was associated with incident ESRD (Table 4). In model 1, persons with major depressive symptoms at baseline had a 1.90-fold higher risk of incident ESRD (95% CI, 1.09 to 3.32). After additional adjustment for diabetes self-care variables (model 2), the association between major depression symptoms and incident ESRD persisted (hazard ratio [HR], 1.85; 95% CI, 1.02 to 3.33). Minor depressive symptoms were not significantly associated with ESRD risk in either model 1 (HR, 1.08; 95% CI, 0.52 to 2.24) or model 2 (HR, 1.08; 95% CI, 0.52 to 2.25). In both models, other risk factors for incident ESRD included younger age, longer duration of diabetes, higher baseline HbA1c, lower baseline eGFR, and the presence of baseline microalbuminuria. None of the diabetes self-care variables were predictive of incident ESRD. The associations between major depressive symptoms and incident ESRD were robust in each of the sensitivity analyses. Interactions between depressive status and either sex or age were not significant (P>0.05).
Table 4.
Competing risks regression for time to ESRD in the Pathways Study
| Variable | Unadjusted HR (95% CI) | Model 1 Adjusted HR (95% CI) | Model 2 Adjusted HR (95% CI)b |
|---|---|---|---|
| Major depressive symptomsa | 2.31 (1.37 to 3.92) | 1.90 (1.09 to 3.32) | 1.85 (1.02 to 3.33) |
| Minor depressive symptomsa | 1.43 (0.71 to 2.89) | 1.08 (0.52 to 2.24) | 1.08 (0.52 to 2.25) |
| Age (yr) | 0.99 (0.98 to 1.01) | 0.94 (0.92 to 0.96) | 0.94 (0.92 to 0.96) |
| Men | 0.95 (0.63 to 1.45) | 1.02 (0.65 to 1.61) | 1.01 (0.63 to 1.64) |
| Race/ethnicity | |||
| Non-Hispanic white | Reference | Reference | Reference |
| Non-Hispanic black | 2.04 (1.10 to 3.78) | 1.71 (0.88 to 3.34) | 1.74 (0.90 to 3.37) |
| Asian | 1.66 (0.85 to 3.24) | 1.73 (0.90 to 3.33) | 1.64 (0.85 to 3.18) |
| Other | 0.67 (0.16 to 2.72) | 0.67 (0.15 to 2.97) | 0.66 (0.15 to 2.98) |
| Married | 1.04 (0.67 to 1.62) | 1.20 (0.73 to 1.97) | 1.22 (0.74 to 2.01) |
| Education beyond high school | 0.69 (0.44 to 1.09) | 0.62 (0.38 to 1.04) | 0.61 (0.37 to 1.02) |
| Smoker | 0.58 (0.21 to 1.58) | 0.57 (0.20 to 1.62) | 0.56 (0.20 to 1.62) |
| Body mass index (kg/m2) | 1.02 (1.00 to 1.05) | 1.01 (0.97 to 1.05) | 1.01 (0.97 to 1.05) |
| Duration of diabetes (yr) | 1.04 (1.02 to 1.05) | 1.02 (1.00 to 1.04) | 1.03 (1.01 to 1.05) |
| Hemoglobin A1c (%) | 1.21 (1.07 to 1.36) | 1.17 (1.03 to 1.33) | 1.18 (1.04 to 1.35) |
| eGFR (ml/min per 1.73 m2) | 0.96 (0.95 to 0.97) | 0.95 (0.94 to 0.96) | 0.95 (0.94 to 0.96) |
| Microalbuminuria | 4.10 (2.23 to 7.53) | 2.36 (1.25 to 4.46) | 2.36 (1.24 to 4.49) |
| Hypertension | 1.52 (1.00 to 2.33) | 0.91 (0.58 to 1.42) | 0.91 (0.58 to 1.43) |
| ACEI/ARB use | 1.75 (1.07 to 2.87) | 1.52 (0.90 to 2.58) | 1.54 (0.92 to 2.60) |
| General diet (d/wk) | 0.93 (0.85 to 1.02) | 0.98 (0.88 to 1.10) | |
| Special diet (d/wk) | 0.96 (0.84 to 1.09) | 1.01 (0.88 to 1.16) | |
| Exercise (d/wk) | 0.92 (0.85 to 1.01) | 1.00 (0.90 to 1.10) | |
| Blood glucose testing (d/wk) | 1.01 (0.93 to 1.10) | 0.93 (0.85 to 1.02) | |
| Foot care (d/wk) | 1.00 (0.91 to 1.10) | 0.97 (0.88 to 1.08) |
Survival models for continuous variables are hazard ratios for an increase by one unit. Adjusted for age, sex, race/ethnicity, marital status, education level, smoking status, body mass index, duration of diabetes, hemoglobin A1c, eGFR, presence of baseline microalbuminuria, hypertension, and ACEI/ARB use. HR, hazard ratio.
Participants with major depressive symptoms had Patient Health Questionnaire-9 scores≥10 and at least five depressive symptoms (including depressed mood or anhedonia) >50% of the time for at least 2 weeks. Participants with minor depressive symptoms had Patient Health Questionnaire-9 scores≥5 and two to four depressive symptoms (including depressed mood or anhedonia) >50% of the time for at least 2 weeks.
Additionally adjusted for Summary of Diabetes Self-Care Activities scores for general diet, special diet, exercise, blood glucose testing, and foot care.
Discussion
The current study found that, in this primary care cohort of patients with diabetes, major depressive symptoms were associated with nearly two times the risk of incident ESRD at up to 10 years of follow-up, and this association persisted after adjustment for multiple diabetes self-care variables. In this study, the association between minor depressive symptoms and ESRD risk was not statistically significant. Although patients with diabetes mellitus are at high risk for ESRD, to our knowledge, this study is the first study to show an association between major depressive symptoms and long-term ESRD risk in this population.
Our study observed an association between major depressive symptoms and long-term ESRD risk among primary care diabetic patients. These results are congruent with findings in CKD cohorts, which are also at high risk for ESRD. In a prospective cohort study of predominantly male veterans with CKD stages 2–5, Hedayati et al. (15) found that a history of major depression was associated with a 3.5-fold higher risk of progression to chronic dialysis at 1 year after adjustment for age, race, and baseline kidney function. Similarly, Tsai et al. (16) found that high levels of depressive symptoms in patients with CKD were associated with more rapid decline in kidney function and a higher risk of the combined outcome of incident ESRD or death. In the largest prospective CKD cohort to date, Fischer et al. (34) found that depressive symptoms in the Chronic Renal Insufficiency Cohort Study were associated with a 21% higher risk of CKD progression over 5 years. However, the Cardiovascular Health Study (CHS) did not find an association between depressive symptoms and incident ESRD in their community-based cohort of elderly participants (17). This discrepancy may be related to the low prevalence of CKD in the study population in the CHS and, therefore, a lower incidence of ESRD (<2%) compared with the CKD cohorts in the other studies. Our study contributes to existing literature by showing an association between major depressive symptoms and incident ESRD in a non-CKD primary care population-based cohort. This association was evident after up to 10 years of follow-up, which is a longer duration than any of the previous studies.
Another novel aspect of this study was our ability to adjust for multiple diabetes self-care variables. Although depression is associated with poorer diabetes self-care (5,7,8), and adherence to self-care is associated with reductions in chronic complications of diabetes (35–39), we found that self-reported diabetes self-care was not associated with long-term ESRD risk, and adjustment for self-care did not substantially alter the association between major depressive symptoms and incident ESRD. There are several potential reasons for these negative findings. Although the SDSCA has been shown to be a valid measure of diabetes self-management (27), it is still self-reported rather than objectively measured, and it may incompletely or imprecisely capture diabetes self-care. Our study ascertained diabetes self-care at study entry and does not account for behavioral changes over time. Finally, diabetes self-care may affect chronic diabetic complications through metabolic and cardiovascular risk factor control, which was already adjusted for in our models; this finding may explain why additional adjustment for diabetes self-care did not have an appreciable impact on the association between major depressive symptoms and ESRD risk.
We did not find a statistically significant association between minor depressive symptoms and long-term ESRD risk. Our analysis is limited by the low event rate in this group. Although minor depression is common in patients with ESRD (40), it is not currently known whether minor depression is associated with CKD incidence or progression. Minor depression has not been found to be associated with other diabetic micro- or macrovascular complications, but it is associated with increased mortality in patients with diabetes (9,11,41). Additional research on its associations with adverse renal outcomes is needed.
Major depression may be associated with ESRD risk through several potential mechanisms. In patients with diabetes, comorbid depression is associated with a higher number of cardiovascular risk factors compared with those patients without depression (42). Although we attempted to control for common cardiovascular risk factors, we were unable to account for actual BP measurements or changes in cardiovascular risk factors over time. Depression is associated with medication nonadherence (5,7,8), which was not measured in this analysis. Depression is also associated with poorer diabetes self-care (5,7,8), which might, to a small extent, mediate the association between depression and ESRD, despite our study’s negative findings. Depression is associated with a proinflammatory state (43), which has been linked with a higher risk of diabetic complications, including diabetic kidney disease (44). Lastly, depression is associated with hyperactivity of the hypothalamic–pituitary–adrenocortical axis and sympathetic nervous system, resulting in reduced insulin sensitivity and potentially poorer glycemic control (45).
The results from this study lend support to the current American Diabetes Association guidelines, which recommend screening for depression in patients with diabetes and poor self-management (grade B recommendation) (46). Moreover, the expert opinion is that psychological assessment is a reasonable component of comprehensive diabetes management. Nonetheless, additional studies are needed to determine whether routine screening and treatment of depression in diabetic patients can reduce the risk of ESRD.
There are several notable limitations of this study to consider. Depression was ascertained by a self-rated questionnaire rather than a clinical interview; although the PHQ-9 has been validated against the gold standard clinical interview (22), a recent meta-analysis found that self-reported depression scales may overestimate the presence of major depression compared with the clinical interview (47). This study only used depression status at study entry rather than multiple measures over time. However, our group previously found that, in this cohort of primary care diabetic patients, over 70% of those patients with major depressive symptoms had a history of chronic depression lasting over 2 years (23), and over 80% of patients with major depressive symptoms after 5 years of follow-up had major or minor depressive symptoms at study entry (24). We did not adjust for BP, because these values were unavailable; although we did adjust for hypertension based on ICD-9 codes, these values do not discriminate between treated and untreated hypertension or optimal versus suboptimal BP control. However, a prior prospective study showed that patients with comorbid major depression and diabetes had similar BP control over a 5-year period as patients with diabetes alone (48). Although we adjusted for prescription of renin-angiotensin system inhibitors, we did not have a measure of medication adherence, which differs by depression status. Because this population was insured, generalizability of results may be limited to other patient populations with comparable access to health care. Furthermore, although the study had an acceptable response rate, our estimated incidence of ESRD may be biased, because the incidence rate of ESRD is not known for the nonrespondents. Finally, as an observational study, unmeasured and residual confounding are always possible. However, our study has several strengths, including its large sample size, prospective design, primary care population base, long length of follow-up, and ability to adjust for multiple covariates, including diabetes self-care variables.
Major depressive symptoms were associated with a higher risk of development of ESRD in a primary care population with diabetes, even after adjustment for the quality of diabetes self-care. Additional studies are needed to determine whether screening and treatment of depression reduce the incidence of ESRD in patients with diabetes.
Disclosures
W.J.K. reports board membership with Eli Lilly and Wyeth and honoraria for lectures from Eli Lilly, Wyeth, Pfizer, and Forest. No other disclosures are reported.
Acknowledgments
We thank Dr. Michael Von Korff, Dr. Elizabeth Lin, Dr. Evette Ludman, and Malia Oliver for their roles in study design, data acquisition, and obtainment of funding for the Pathways Study, which was supported by National Institute of Mental Health Services Grants MH41739 and MH01643.
M.K.Y. was supported, in part, by the American Kidney Fund. In addition, this material is the result of work supported by resources from the Veterans Affairs Puget Sound Health Care System (M.K.Y. and B.A.Y.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Veterans Affairs Puget Sound.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ: The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care 24: 1069–1078, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski P, Lin E, Bush T, Walker E, Young B: Depression and diabetes symptom burden. Gen Hosp Psychiatry 26: 430–436, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Lustman PJ, Clouse RE, Carney RM: Depression and the reporting of diabetes symptoms. Int J Psychiatry Med 18: 295–303, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE: Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care 23: 934–942, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Ciechanowski PS, Katon WJ, Russo JE: Depression and diabetes: Impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 160: 3278–3285, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Katon W, Ciechanowski P: Impact of major depression on chronic medical illness. J Psychosom Res 53: 859–863, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, Ciechanowski P, Ludman EJ, Bush T, Young B: Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care 27: 2154–2160, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, Blais MA, Meigs JB, Grant RW: Depression, self-care, and medication adherence in type 2 diabetes: Relationships across the full range of symptom severity. Diabetes Care 30: 2222–2227, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin EH, Rutter CM, Katon W, Heckbert SR, Ciechanowski P, Oliver MM, Ludman EJ, Young BA, Williams LH, McCulloch DK, Von Korff M: Depression and advanced complications of diabetes: A prospective cohort study. Diabetes Care 33: 264–269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ: Association of depression and diabetes complications: A meta-analysis. Psychosom Med 63: 619–630, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Katon WJ, Rutter C, Simon G, Lin EH, Ludman E, Ciechanowski P, Kinder L, Young B, Von Korff M: The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care 28: 2668–2672, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Egede LE, Nietert PJ, Zheng D: Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care 28: 1339–1345, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Lin EH, Heckbert SR, Rutter CM, Katon WJ, Ciechanowski P, Ludman EJ, Oliver M, Young BA, McCulloch DK, Von Korff M: Depression and increased mortality in diabetes: Unexpected causes of death. Ann Fam Med 7: 414–421, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu MK, Katon W, Young BA: Diabetes self-care, major depression, and chronic kidney disease in an outpatient diabetic population. Nephron Clin Pract 124: 106–112, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ: Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA 303: 1946–1953, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai YC, Chiu YW, Hung CC, Hwang SJ, Tsai JC, Wang SL, Lin MY, Chen HC: Association of symptoms of depression with progression of CKD. Am J Kidney Dis 60: 54–61, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Kop WJ, Seliger SL, Fink JC, Katz R, Odden MC, Fried LF, Rifkin DE, Sarnak MJ, Gottdiener JS: Longitudinal association of depressive symptoms with rapid kidney function decline and adverse clinical renal disease outcomes. Clin J Am Soc Nephrol 6: 834–844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 19.Katon W, Von Korff M, Lin E, Simon G, Ludman E, Bush T, Walker E, Ciechanowski P, Rutter C: Improving primary care treatment of depression among patients with diabetes mellitus: The design of the pathways study. Gen Hosp Psychiatry 25: 158–168, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Katon WJ, Lin EH, Williams LH, Ciechanowski P, Heckbert SR, Ludman E, Rutter C, Crane PK, Oliver M, Von Korff M: Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: A prospective cohort study. J Gen Intern Med 25: 423–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB: The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 16: 606–613, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watnick S, Wang PL, Demadura T, Ganzini L: Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis 46: 919–924, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T: The Pathways Study: A randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 61: 1042–1049, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Katon W, Russo J, Lin EH, Heckbert SR, Ciechanowski P, Ludman EJ, Von Korff M: Depression and diabetes: Factors associated with major depression at five-year follow-up. Psychosomatics 50: 570–579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Physician ICD-9-CM, Salt Lake City, UT, Medicode Publications, 1999 [Google Scholar]
- 27.Toobert DJ, Hampson SE, Glasgow RE: The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care 23: 943–950, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Cox D: Regression models and life tables (with discussion). J R Stat Soc Series B Stat Methodol 34: 187–220, 1972 [Google Scholar]
- 29.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 30.White IR, Royston P: Imputing missing covariate values for the Cox model. Stat Med 28: 1982–1998, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall A, Altman DG, Holder RL: Comparison of imputation methods for handling missing covariate data when fitting a Cox proportional hazards model: A resampling study. BMC Med Res Methodol 10: 112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patten SB: Confounding by severity and indication in observational studies of antidepressant effectiveness. Can J Clin Pharmacol 15: e367–e371, 2008 [PubMed] [Google Scholar]
- 33.Simon GE, VonKorff M: Recognition, management, and outcomes of depression in primary care. Arch Fam Med 4: 99–105, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Fischer MJ, Ricardo AC, Jordan N, Diamantidis CJ, Xie D, Teal VL, Anderson AH, Bazzano L, Chen J, Kusek JW, Yaffe K, Powe NR, Lash JP: Depressive Symptoms and Health Outcomes among Patients with Chronic Kidney Disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study, San Diego, CA, American Society of Nephrology Kidney Week, 2012 [Google Scholar]
- 35.Hu FB, Stampfer MJ, Solomon C, Liu S, Colditz GA, Speizer FE, Willett WC, Manson JE: Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med 134: 96–105, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM: Relationship of walking to mortality among US adults with diabetes. Arch Intern Med 163: 1440–1447, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Wing RR, Look AHEAD Research Group : Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch Intern Med 170: 1566–1575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin S, Schneider B, Heinemann L, Lodwig V, Kurth HJ, Kolb H, Scherbaum WA: Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: An epidemiological cohort study. Diabetologia 49: 271–278, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Singh N, Armstrong DG, Lipsky BA: Preventing foot ulcers in patients with diabetes. JAMA 293: 217–228, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Hedayati SS, Finkelstein FO: Epidemiology, diagnosis, and management of depression in patients with CKD. Am J Kidney Dis 54: 741–752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman SM, Katon W, Lin E, Von Korff M: Depression and death in diabetes; 10-year follow-up of all-cause and cause-specific mortality in a diabetic cohort. Psychosomatics 54: 428–436, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katon WJ, Lin EH, Russo J, Von Korff M, Ciechanowski P, Simon G, Ludman E, Bush T, Young B: Cardiac risk factors in patients with diabetes mellitus and major depression. J Gen Intern Med 19: 1192–1199, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart JC, Rand KL, Muldoon MF, Kamarck TW: A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun 23: 936–944, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldberg RB: Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab 94: 3171–3182, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Musselman DL, Betan E, Larsen H, Phillips LS: Relationship of depression to diabetes types 1 and 2: Epidemiology, biology, and treatment. Biol Psychiatry 54: 317–329, 2003 [DOI] [PubMed] [Google Scholar]
- 46.American Diabetes Association : Standards of medical care in diabetes—2013. Diabetes Care 36[Suppl 1]: S11–S66, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Fishbane S, Strippoli GF: Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int 84: 179–191, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Heckbert SR, Rutter CM, Oliver M, Williams LH, Ciechanowski P, Lin EH, Katon WJ, Von Korff M: Depression in relation to long-term control of glycemia, blood pressure, and lipids in patients with diabetes. J Gen Intern Med 25: 524–529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]