Abstract
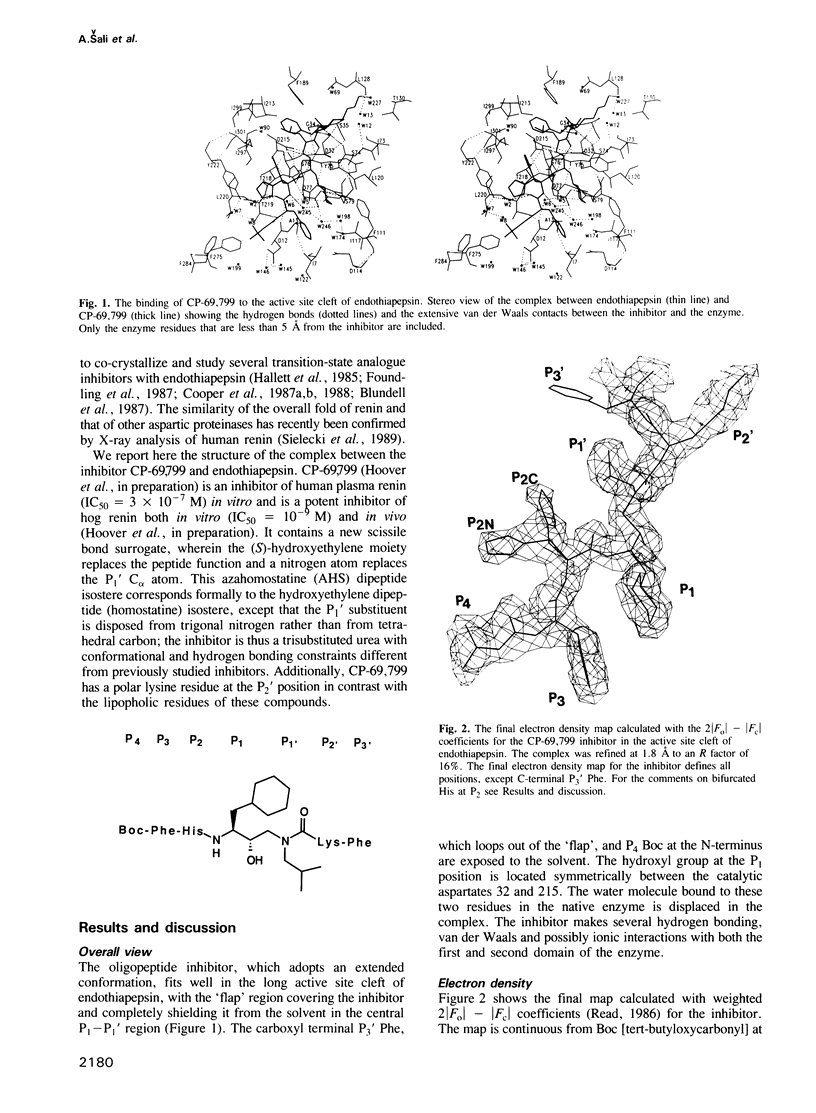
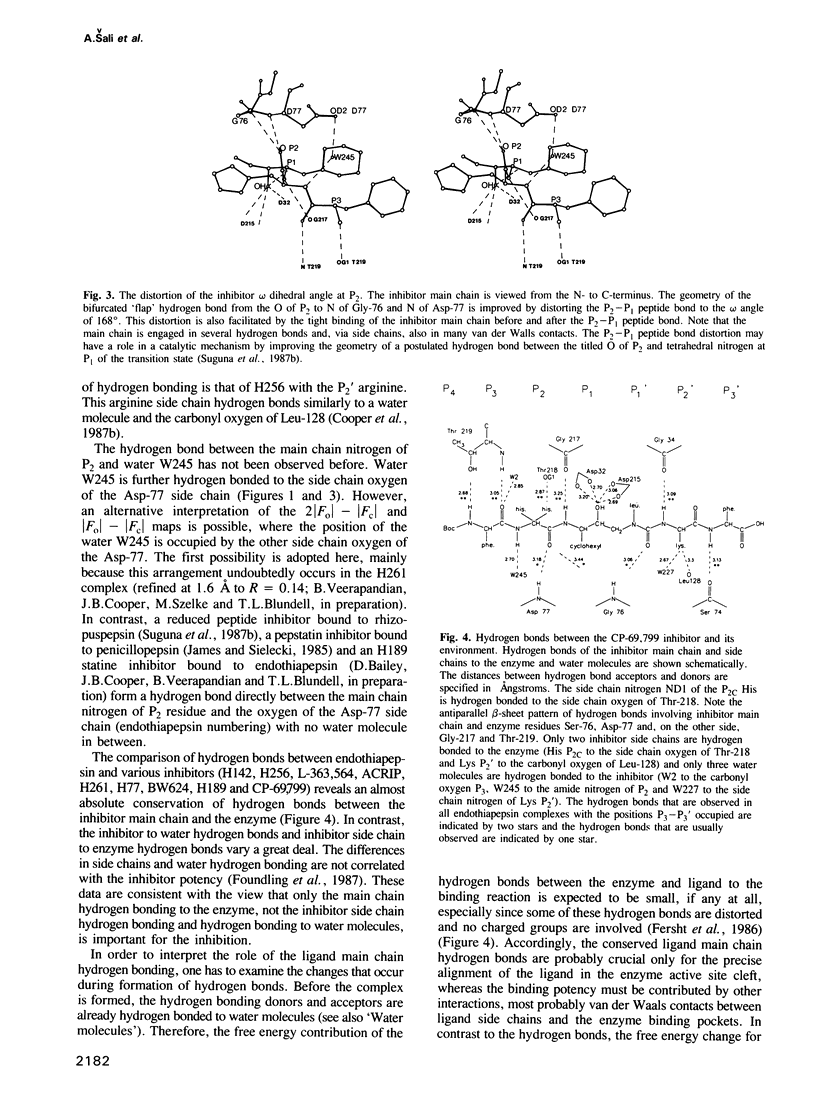
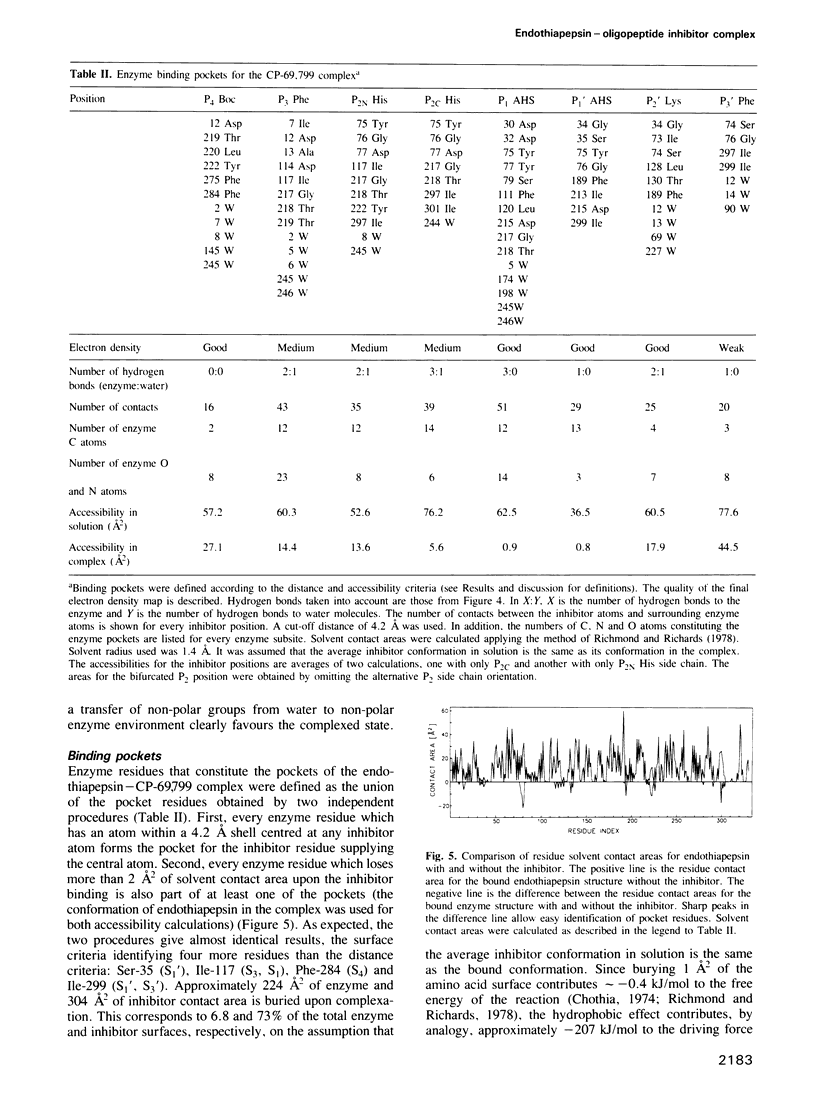
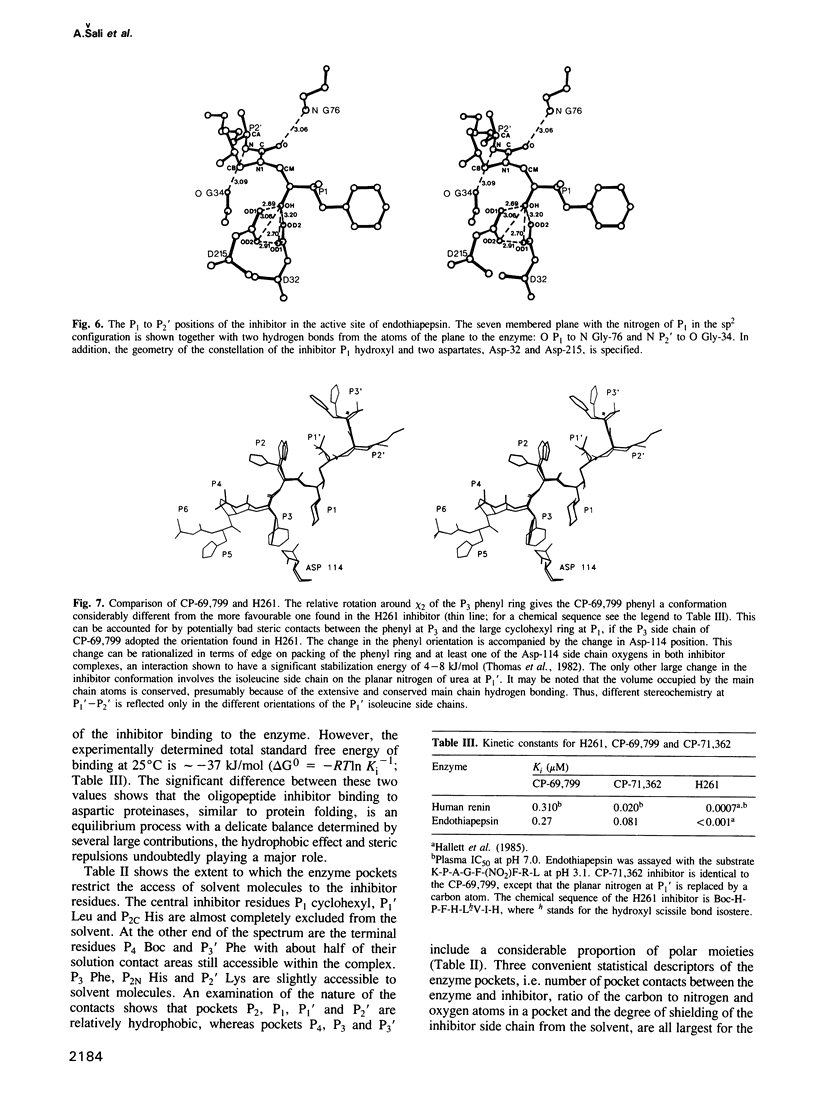
The conformation of the synthetic renin inhibitor CP-69,799, bound to the active site of the fungal aspartic proteinase endothiapepsin (EC 3.4.23.6), has been determined by X-ray diffraction at 1.8 A resolution and refined to the crystallographic R factor of 16%. CP-69,799 is an oligopeptide transition--state analogue inhibitor that contains a new dipeptide isostere at the P1-P1' position. This dipeptide isostere is a nitrogen analogue of the well-explored hydroxyethylene dipeptide isostere, wherein the tetrahedral P1' C alpha atom has been replaced by trigonal nitrogen. The inhibitor binds in the extended conformation, filling S4 to S3' pockets, with hydroxyl group of the P1 residue positioned symmetrically between the two catalytic aspartates of the enzyme. Interactions between the inhibitor and the enzyme include 12 hydrogen bonds and extensive van der Waals contacts in all the pockets, except for S3'. The crystal structure reveals a bifurcated orientation of the P2 histidine side chain and an interesting relative rotation of the P3 phenyl ring to accommodate the cyclohexyl side chain at P1. The binding of the inhibitor to the enzyme, while producing no large distortions in the enzyme active site cleft, results in small but significant change in the relative orientation of the two endothiapepsin domains. This structural change may represent the action effected by the proteinase as it distorts its substrate towards the transition state for proteolytic cleavage.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahane K., Umeyama H., Nakagawa S., Moriguchi I., Hirose S., Iizuka K., Murakami K. Three-dimensional structure of human renin. Hypertension. 1985 Jan-Feb;7(1):3–12. doi: 10.1161/01.hyp.7.1.3. [DOI] [PubMed] [Google Scholar]
- Andreeva N. S., Zdanov A. S., Gustchina A. E., Fedorov A. A. Structure of ethanol-inhibited porcine pepsin at 2-A resolution and binding of the methyl ester of phenylalanyl-diiodotyrosine to the enzyme. J Biol Chem. 1984 Sep 25;259(18):11353–11365. [PubMed] [Google Scholar]
- Blundell T. L., Cooper J., Foundling S. I., Jones D. M., Atrash B., Szelke M. On the rational design of renin inhibitors: X-ray studies of aspartic proteinases complexed with transition-state analogues. Biochemistry. 1987 Sep 8;26(18):5585–5590. doi: 10.1021/bi00392a001. [DOI] [PubMed] [Google Scholar]
- Blundell T., Sibanda B. L., Pearl L. Three-dimensional structure, specificity and catalytic mechanism of renin. Nature. 1983 Jul 21;304(5923):273–275. doi: 10.1038/304273a0. [DOI] [PubMed] [Google Scholar]
- Bott R., Subramanian E., Davies D. R. Three-dimensional structure of the complex of the Rhizopus chinensis carboxyl proteinase and pepstatin at 2.5-A resolution. Biochemistry. 1982 Dec 21;21(26):6956–6962. doi: 10.1021/bi00269a052. [DOI] [PubMed] [Google Scholar]
- Carlson W., Karplus M., Haber E. Construction of a model for the three-dimensional structure of human renal renin. Hypertension. 1985 Jan-Feb;7(1):13–26. doi: 10.1161/01.hyp.7.1.13. [DOI] [PubMed] [Google Scholar]
- Chen K. C., Tang J. Amino acid sequence around the epoxide-reactive residues in pepsin. J Biol Chem. 1972 Apr 25;247(8):2566–2574. [PubMed] [Google Scholar]
- Chothia C. Hydrophobic bonding and accessible surface area in proteins. Nature. 1974 Mar 22;248(446):338–339. doi: 10.1038/248338a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. B., Foundling S. I., Hemmings A., Watson F. E., Sibanda B. L., Blundell T. L., Jones D. M., Hallett A., Atrash B., Szelke M. Inhibitors of aspartic proteinases and their relevance to the design of antihypertensive agents. Biochem Soc Trans. 1987 Aug;15(4):751–754. doi: 10.1042/bst0150751. [DOI] [PubMed] [Google Scholar]
- Cooper J., Foundling S., Hemmings A., Blundell T., Jones D. M., Hallett A., Szelke M. The structure of a synthetic pepsin inhibitor complexed with endothiapepsin. Eur J Biochem. 1987 Nov 16;169(1):215–221. doi: 10.1111/j.1432-1033.1987.tb13600.x. [DOI] [PubMed] [Google Scholar]
- Foundling S. I., Cooper J., Watson F. E., Cleasby A., Pearl L. H., Sibanda B. L., Hemmings A., Wood S. P., Blundell T. L., Valler M. J. High resolution X-ray analyses of renin inhibitor-aspartic proteinase complexes. 1987 May 28-Jun 3Nature. 327(6120):349–352. doi: 10.1038/327349a0. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A. Structural dynamics of liganded myoglobin. Biophys J. 1980 Oct;32(1):465–483. doi: 10.1016/S0006-3495(80)84984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruton J. S. The mechanism of the catalytic action of pepsin and related acid proteinases. Adv Enzymol Relat Areas Mol Biol. 1976;44:1–36. doi: 10.1002/9780470122891.ch1. [DOI] [PubMed] [Google Scholar]
- Haber E., Burton J. Inhibitors of renin and their utility in physiologic studies. Fed Proc. 1979 Dec;38(13):2768–2773. [PubMed] [Google Scholar]
- Haber E. The first Sir George Pickering memorial lecture. Which inhibitors will give us true insight into what renin really does? J Hypertens. 1984 Jun;2(3):223–230. [PubMed] [Google Scholar]
- Hartsuck J. A., Tang J. The carboxylate ion in the active center of pepsin. J Biol Chem. 1972 Apr 25;247(8):2575–2580. [PubMed] [Google Scholar]
- Hemmings A. M., Foundling S. I., Sibanda B. L., Wood S. P., Pearl L. H., Blundell T. Energy calculations on aspartic proteinases: human renin, endothiapepsin and its complex with an angiotensinogen fragment analogue, H-142. Biochem Soc Trans. 1985 Dec;13(6):1036–1041. doi: 10.1042/bst0131036. [DOI] [PubMed] [Google Scholar]
- Hofmann T., Allen B., Bendiner M., Blum M., Cunningham A. Effect of secondary substrate binding in penicillopepsin: contributions of subsites S3 and S2' to kcat. Biochemistry. 1988 Feb 23;27(4):1140–1146. doi: 10.1021/bi00404a010. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R. Molecular structure of an aspartic proteinase zymogen, porcine pepsinogen, at 1.8 A resolution. Nature. 1986 Jan 2;319(6048):33–38. doi: 10.1038/319033a0. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R. Stereochemical analysis of peptide bond hydrolysis catalyzed by the aspartic proteinase penicillopepsin. Biochemistry. 1985 Jul 2;24(14):3701–3713. doi: 10.1021/bi00335a045. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R. Structure and refinement of penicillopepsin at 1.8 A resolution. J Mol Biol. 1983 Jan 15;163(2):299–361. doi: 10.1016/0022-2836(83)90008-6. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A., Salituro F., Rich D. H., Hofmann T. Conformational flexibility in the active sites of aspartyl proteinases revealed by a pepstatin fragment binding to penicillopepsin. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6137–6141. doi: 10.1073/pnas.79.20.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Wodak S. Conformation of amino acid side-chains in proteins. J Mol Biol. 1978 Nov 5;125(3):357–386. doi: 10.1016/0022-2836(78)90408-4. [DOI] [PubMed] [Google Scholar]
- Moews P. C., Bunn C. W. An x-ray crystallographic study of the rennin-like enzyme of Endothia parasitica. J Mol Biol. 1970 Dec 14;54(2):395–397. doi: 10.1016/0022-2836(70)90439-0. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Cushman D. W. Enzymes of the renin-angiotensin system and their inhibitors. Annu Rev Biochem. 1982;51:283–308. doi: 10.1146/annurev.bi.51.070182.001435. [DOI] [PubMed] [Google Scholar]
- Pearl L., Blundell T. The active site of aspartic proteinases. FEBS Lett. 1984 Aug 20;174(1):96–101. doi: 10.1016/0014-5793(84)81085-6. [DOI] [PubMed] [Google Scholar]
- Ponder J. W., Richards F. M. Tertiary templates for proteins. Use of packing criteria in the enumeration of allowed sequences for different structural classes. J Mol Biol. 1987 Feb 20;193(4):775–791. doi: 10.1016/0022-2836(87)90358-5. [DOI] [PubMed] [Google Scholar]
- Richards F. M., Kundrot C. E. Identification of structural motifs from protein coordinate data: secondary structure and first-level supersecondary structure. Proteins. 1988;3(2):71–84. doi: 10.1002/prot.340030202. [DOI] [PubMed] [Google Scholar]
- Richmond T. J., Richards F. M. Packing of alpha-helices: geometrical constraints and contact areas. J Mol Biol. 1978 Mar 15;119(4):537–555. doi: 10.1016/0022-2836(78)90201-2. [DOI] [PubMed] [Google Scholar]
- Shipman L. L., Christoffersen R. E. Ab initio calculations on large molecules using molecular fragments. Model peptide studies. J Am Chem Soc. 1973 Mar 7;95(5):1408–1416. doi: 10.1021/ja00786a009. [DOI] [PubMed] [Google Scholar]
- Sibanda B. L., Blundell T., Hobart P. M., Fogliano M., Bindra J. S., Dominy B. W., Chirgwin J. M. Computer graphics modelling of human renin. Specificity, catalytic activity and intron-exon junctions. FEBS Lett. 1984 Aug 20;174(1):102–111. doi: 10.1016/0014-5793(84)81086-8. [DOI] [PubMed] [Google Scholar]
- Sielecki A. R., Hayakawa K., Fujinaga M., Murphy M. E., Fraser M., Muir A. K., Carilli C. T., Lewicki J. A., Baxter J. D., James M. N. Structure of recombinant human renin, a target for cardiovascular-active drugs, at 2.5 A resolution. Science. 1989 Mar 10;243(4896):1346–1351. doi: 10.1126/science.2493678. [DOI] [PubMed] [Google Scholar]
- Suguna K., Bott R. R., Padlan E. A., Subramanian E., Sheriff S., Cohen G. H., Davies D. R. Structure and refinement at 1.8 A resolution of the aspartic proteinase from Rhizopus chinensis. J Mol Biol. 1987 Aug 20;196(4):877–900. doi: 10.1016/0022-2836(87)90411-6. [DOI] [PubMed] [Google Scholar]
- Suguna K., Padlan E. A., Smith C. W., Carlson W. D., Davies D. R. Binding of a reduced peptide inhibitor to the aspartic proteinase from Rhizopus chinensis: implications for a mechanism of action. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7009–7013. doi: 10.1073/pnas.84.20.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelke M., Leckie B., Hallett A., Jones D. M., Sueiras J., Atrash B., Lever A. F. Potent new inhibitors of human renin. Nature. 1982 Oct 7;299(5883):555–557. doi: 10.1038/299555a0. [DOI] [PubMed] [Google Scholar]
- Tang J., James M. N., Hsu I. N., Jenkins J. A., Blundell T. L. Structural evidence for gene duplication in the evolution of the acid proteases. Nature. 1978 Feb 16;271(5646):618–621. doi: 10.1038/271618a0. [DOI] [PubMed] [Google Scholar]
- Thomas K. A., Smith G. M., Thomas T. B., Feldmann R. J. Electronic distributions within protein phenylalanine aromatic rings are reflected by the three-dimensional oxygen atom environments. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4843–4847. doi: 10.1073/pnas.79.16.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Morishima H., Matsuzaki M., Hamada M. Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J Antibiot (Tokyo) 1970 May;23(5):259–262. doi: 10.7164/antibiotics.23.259. [DOI] [PubMed] [Google Scholar]



