Abstract
Background and objectives
Home dialysis creates fewer lifestyle disruptions while providing similar or better outcomes than in-center hemodialysis. Socioeconomically advantaged patients are more likely to commence home dialysis (peritoneal dialysis and home hemodialysis) in many developed countries. This study investigated associations between socioeconomic status and uptake of home dialysis in Australia, a country with universal access to health care and comparatively high rates of home dialysis.
Design, setting, participants, & measurements
This study analyzed 23,281 non-Indigenous adult patients who commenced chronic RRT in Australia from 2000 to 2011 according to the Australia and New Zealand Dialysis and Transplant Registry in a retrospective cohort study. This study investigated the proportion of patients who were ever likely to use home dialysis using nonmixture cure models and followed patients until the end of 2011 (median follow-up time=3.0 years, interquartile range=1.3–5.5 years). The main predictor was area socioeconomic status from postcodes grouped into quartiles using standard indices.
Results
Patients from the most advantaged quartile of areas were less likely to commence peritoneal dialysis (odds ratio, 0.63; 95% confidence interval, 0.58 to 0.69) and more likely to use in-center hemodialysis than patients from the most disadvantaged areas (odds ratio, 1.19; 95% confidence interval, 1.10 to 1.30). Socioeconomic status was not associated with uptake of home hemodialysis. Rural areas were more disadvantaged and had higher rates of peritoneal dialysis, and privately funded hospitals rarely used home dialysis. Patients from the most advantaged quartile of areas were more likely to use private hospitals than patients from the most disadvantaged quartile (odds ratio, 5.9; 95% confidence interval, 4.6 to 7.5).
Conclusion
The lower incidence of peritoneal dialysis among patients from advantaged areas seems to be multifactorial. Identifying and addressing barriers to home dialysis in Australia could improve patient quality of life and reduce costs.
Keywords: peritoneal dialysis, Epidemiology and outcomes, clinical epidemiology
Introduction
Survival and quality of life for patients with end stage kidney disease vary with the mode of dialysis used. Home dialysis (peritoneal dialysis [PD] and home hemodialysis [HHD]) provides advantages for both patients and health care providers. Compared with in-center hemodialysis (ICHD), both PD and HHD are cheaper to deliver (1); additionally, PD provides comparable survival (2,3) and quality of life (4), and HHD is associated with comparable or better survival and quality of life (5,6). Understanding barriers to uptake of home dialysis may enable changes that improve patient wellbeing and reduce delivery costs.
Socioeconomic discrepancies in the uptake of PD exist. In the United States, patients with higher levels of education (4) or income (4,7) and of white race (8) were more likely to use PD. Similar associations have been found in The Netherlands (9) and Taiwan (10). Prakash et al. (11) found no association between area socioeconomic status (SES) and either eligibility for PD or insertion of PD catheter in Canada. HHD was associated with full-time employment and white race in the United States (12). Associations between SES and home dialysis have not been investigated in Australia, a country with comparatively high rates of home dialysis (especially HHD) (13) and universal access to health care.
This work investigates associations between area SES and the proportion of RRT patients who commence home dialysis in Australia, using cure models to estimate the proportion of patients likely to commence home dialysis.
Materials and Methods
Patients
Patients aged 18 years or older commencing chronic RRT in Australia from 2000 to 2011 as recorded in the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) Registry (a complete database of RRT in Australia and New Zealand) were investigated. Indigenous Australians were excluded, because residential postcodes at commencement may not reflect usual place of residence (14).
We used Australian postcodes as area units. In 2006, Australian postcodes had a median population of 3323 (interquartile range=788–11,351). Postcodes were placed into quartiles using the Index of Relative Socio-Economic Advantage and Disadvantage (15), similar to previous studies (16,17). This index was produced from 2006 census data on income, education, employment status, occupation type, housing, internet access, disability status, car ownership and single parent status. Quartiles of postcodes show a linear relationship with employment, income, internet access and unskilled labor (17). Postcodes were also classified as major city or other, based on the Remoteness Areas classification (18).
Age of RRT (18–59, 60–69, or 70+ years) and body mass index (BMI; <18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], or ≥30 kg/m2 [obese+]) were classified at commencement. Comorbidities at commencement were diabetes, chronic lung disease, coronary artery disease, peripheral vascular disease, cerebrovascular disease, and previous diagnosis of cancer. Patients were considered smokers if they were current smokers at commencement. Primary kidney disease was categorized as diabetes plus hypertension, GN, polycystic diseases, or all others. Late referral was referral to nephrological care within 3 months of commencing RRT. Race was dichotomized as Caucasian or other. Initial treating hospitals were categorized as predominantly privately or publicly funded using Kidney Health Australia's Dialysis Unit Guide (19). eGFR was calculated using the four-variable Modification of Diet in Renal Disease equation (A. Levey, unpublished data). Patient health insurance status was not available.
Analyses
Patient characteristics were compared between quartiles of SES using Wilcoxon rank sum, Pearson’s chi-squared, and Fisher’s exact tests. The proportion of patients ever likely to use home dialysis was investigated using nonmixture cure models. Such models estimate the proportion of patients likely to experience the event of interest separately from the rate of uptake among patients who are likely to experience the event (20). Couchoud et al. (21) used similar mixture cure models to investigate access to the kidney transplant waiting list. Within this framework, we used logistic regression models for the proportion of patients who were likely to commence home dialysis and Weibull models for rate of uptake. The Weibull distribution is common in survival analyses, because it is flexible, simple, and likely to converge (22). Models were repeated, with separate analyses for all home dialysis, PD, and HHD. These models were censored on December 31, 2011.
Analyses of PD uptake were repeated, with the following interaction terms included: SES by remoteness (major city versus other areas), SES by comorbidities (any versus none), and SES by race (Caucasian versus other). Analyses of PD uptake were also stratified by remoteness, comorbidities, and race (if important interactions were found). Additional cure models were run without independent variables to estimate overall uptake of PD and HHD.
To investigate effects of patients’ area SES independently of treatment center, we ran a mixed effects logistic regression for use of PD within 1 year of commencing RRT, with the center nested within jurisdiction as a random effect.
Unless otherwise specified, all models included age group, sex, smoking status, BMI category, comorbidities, state, primary kidney disease, race, residence in a major city, and late referral as covariates, with quartiles of SES as categorical variables. Private hospital use was not included, because it was considered a consequence of SES rather than an independent predictor or confounder (23). Results are generally presented as the odds ratio (OR) of the most advantaged quartile (Q; Q4) versus the most disadvantaged quartile (Q1), with 95% confidence interval (95% CI).
For sensitivity analyses, all survival models were repeated with Indigenous patients included and as competing risk regressions using the method of Fine and Gray (24) (not as cure models). When analyzing PD, HHD, death, and transplantation were competing risks. When analyzing HHD, PD, death, and transplantation were competing risks. HHD use within individual hospitals was investigated by tabulating the number of patients who used HHD within 1 year of commencing RRT among patients who were followed for at least 1 year. All analyses were performed using Stata 13 IC (StataCorp., College Station, TX).
Results
Patients
Of 25,759 adult patients who commenced RRT in Australia from 2000 to 2011, the following patients were excluded: 2401 Indigenous patients, 67 patients who received transplantation overseas, and 10 patients whose residential postcodes did not correspond to those postcodes used to categorize SES and remoteness. In total, 23,281 patients were included in this study; 66% of these patients had one or more comorbidities. Patients were followed for a total of 86,461 person-years and a median follow-up of 3.0 years (interquartile range=1.3–5.5 years).
Patients from disadvantaged areas were less likely to commence RRT in a private hospital (Table 1). Most patients (93.7%) commenced RRT in publicly funded hospitals. No patients commenced RRT in private hospitals in South Australia, Western Australia, Northern Territory, or Tasmania. Private dialysis units exist in South Australia, Western Australia, and Northern Territory, but they all use public hospitals as parent hospitals. Private hospital use was more common among Caucasians than non-Caucasians and more common in Queensland.
Table 1.
Characteristics of adult non-Indigenous patients who commenced RRT in Australia in the period from 2000 to 2011 by area socioeconomic status
| Factor | Quartile 1 (Disadvantaged) | Quartile 2 | Quartile 3 | Quartile 4 (Advantaged) | P Value |
|---|---|---|---|---|---|
| N | 3742 | 4706 | 7521 | 7312 | |
| Men, % | 61.6 | 61.4 | 61.4 | 62.4 | 0.61 |
| Age (yr), median (interquartile range) | 63 (52–72) | 64 (52–73) | 64 (51–73) | 65 (51–75) | <0.01 |
| Body mass index (kg/m2), median (interquartile range) | 26.8 (23.4–31.2) | 26.8 (23.38–31.38) | 26.4 (23.1–30.5) | 25.6 (22.5–29.5) | <0.01 |
| eGFR (ml/min per 1.73 m2), median (interquartile range) | 7.2 (5.5–9.8) | 7.5 (5.6–9.9) | 7.6 (5.6–10.1 | 7.6 (5.6–10.2) | <0.01 |
| Late referral, % | 23.5 | 23.5 | 23.4 | 21.4 | 0.01 |
| Caucasian, % | 86.4 | 90.4 | 88.4 | 85.8 | <0.01 |
| Primary kidney disease, % | <0.01 | ||||
| Diabetic and hypertensive nephropathy | 46.2 | 43.6 | 44.2 | 40.9 | |
| GN | 20.5 | 19.3 | 19.9 | 21.2 | |
| Polycystic kidney disease | 5.9 | 7.8 | 6.7 | 8.3 | |
| Other kidney diseases | 27.5 | 29.3 | 29.2 | 29.6 | |
| Current smoker, % | 56.3 | 55.3 | 52.7 | 48.3 | <0.01 |
| Lung disease as comorbidity, % | 18.6 | 18.3 | 15.8 | 13.7 | <0.01 |
| Coronary disease as comorbidity, % | 42.7 | 42.4 | 41.1 | 39.5 | 0.02 |
| Peripheral vascular disease as comorbidity, % | 29.8 | 26.1 | 27.4 | 22.5 | <0.01 |
| Cerebrovascular disease as comorbidity, % | 16.2 | 16.0 | 16.4 | 14.2 | 0.01 |
| Diabetes as comorbidity, % | 43.7 | 39.0 | 39.1 | 34.5 | <0.01 |
| Major city resident, % | 50.1 | 50.0 | 73.3 | 95.1 | <0.01 |
| Private hospital at commencement, % | 1.9 | 3.8 | 6.2 | 10.2 | <0.01 |
| States, % | <0.01 | ||||
| New South Wales/Australian Capital Territory | 33.8 | 33.0 | 36.7 | 40.0 | |
| Victoria/Tasmania | 30.9 | 25.9 | 24.3 | 26.3 | |
| Queensland | 15.9 | 19.6 | 23.2 | 17.9 | |
| South Australia/Northern Territory | 16.2 | 11.6 | 4.9 | 4.3 | |
| Western Australia | 3.2 | 9.9 | 11.0 | 11.5 |
All details are at the time of commencement of RRT.
Major cities had overall higher SES. Only 4.9% of patients from the most advantaged quartile of postcodes lived outside major cities compared with 49.9% of patients from the most disadvantaged quartile (Table 1). Major cities also contained proportionally more non-Caucasians (7.6% of non-Caucasian [non-Indigenous] patients lived outside major cities compared with 31.1% of Caucasian patients). Patients from disadvantaged areas were slightly younger overall but had higher prevalence of comorbidities, smoking, elevated BMI, and late referral (Table 1).
Dialysis Modality
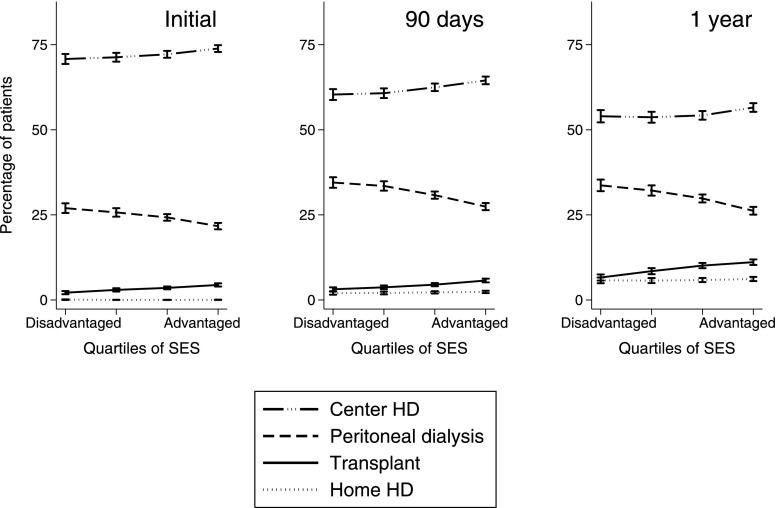
ICHD was the most common modality: 72.3% of all patients commenced RRT using ICHD, and prevalence fell to 62.4% after 90 days and 54.8% after 1 year of RRT (among patients who were followed for at least 90 days and 1 year, respectively). Patients from advantaged areas were less likely to ever commence home dialysis (Table 2) and more likely to use ICHD (Figure 1). Home dialysis was rare among patients starting RRT in private hospitals (Figure 2).
Table 2.
Proportion of patients likely to commence home dialysis, peritoneal dialysis, and home hemodialysis from adjusted nonmixture cure models
| Dialysis Type | Quartile 1 (Disadvantaged) | Quartile 2 | Quartile 3 | Quartile 4 (Advantaged) | P Value |
|---|---|---|---|---|---|
| All home dialysis | 72.8% (68.6–76.8) | 70.8% (66.4–74.9) | 67.7% (63.5–71.6) | 63.2% (58.6–67.7) | <0.001 |
| Peritoneal dialysis | 53.7% (48.6–58.7) | 51.1% (46.2–56.1) | 48.1% (43.6–52.7) | 42.2% (37.5–47.0) | <0.001 |
| Home hemodialysis | 30.8% (21.5–41.9) | 30.4% (21.3–41.4) | 27.6% (20.1–36.5) | 29.3% (20.6–39.8) | 0.75 |
Values presented are fitted values for Caucasian men ages 18–59 years living in major cities with no comorbidities and a normal body mass index (18.5–24.9 km/m2).
Figure 1.
Percentages of patients using in-center hemodialysis (center HD), peritoneal dialysis, and home hemodialysis (home HD) versus area socioeconomic status (SES; unadjusted). These analyses were performed for commencement of RRT (left panel), after 90 days of RRT (center panel), and after 1 year of RRT (right panel). Patients were only included if they were followed for at least 90 days (center panel) and 1 year (right panel). Deceased and censored patients were included when calculating percentages, but they are not shown. Error bars show 95% confidence intervals calculated using binomial distributions.
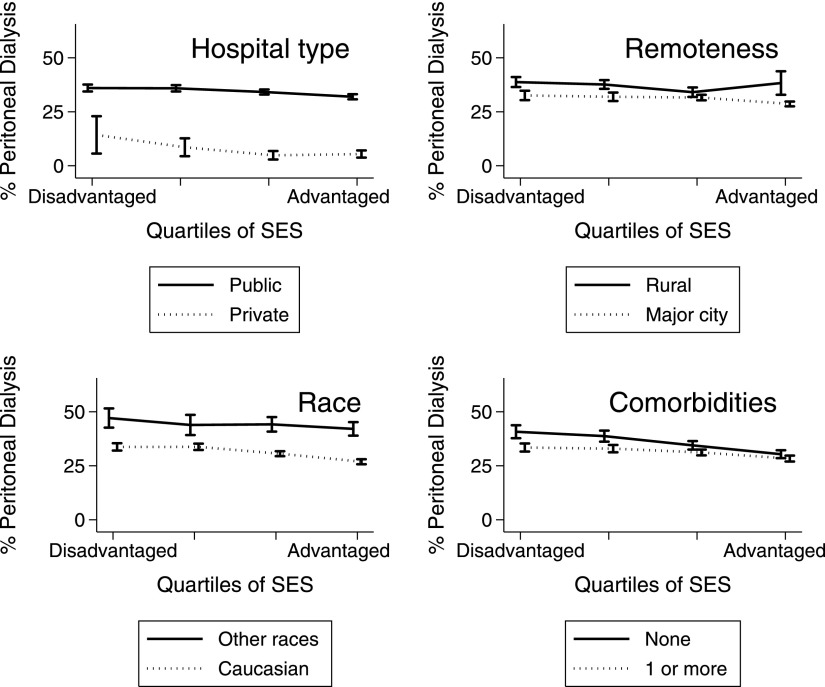
Figure 2.
Proportion of dialysis patients using peritoneal dialysis 90 days after commencing RRT versus area SES (unadjusted with transplanted patients excluded). Analyses are stratified by primary funding source of initial hospital (upper left panel), patient residence (upper right panel), race (lower left panel), and presence of comorbidities (lower right panel). Error bars show 95% confidence intervals calculated using binomial distributions. Error bars are staggered slightly on the x axes for clarity.
Cure models without covariates estimated that 46.4% (95% CI, 45.1% to 45.7%) of patients were likely to ever commence home dialysis. Among those patients likely to commence home dialysis, the median time between commencing RRT and first home dialysis was 25.6 days (95% CI, 24.6 to 26.7 days). There was no association between SES and the rate of uptake among patients who were likely to ever commence any home dialysis (P=0.27). Among patients who were observed for at least 1 year, 75 initial hospitals were recorded. All hospitals that treated more than 35 incident patients during the study had at least 1 patient using HHD within 1 year of RRT. The median number per hospital was 100 patients (interquartile range=31–294 patients). Including Indigenous patients or using competing risk regression made little difference to any results presented.
PD
Univariate cure models estimated that 38.2% (95% CI, 37.6% to 38.9%) of patients were likely to ever commence PD. The median time to commencement was 16 days (95% CI, 15 to 16 days) of RRT, and this finding was not associated with SES (P=0.28). Patients from advantaged areas were less likely to commence PD in adjusted cure models (OR Q4 versus OR Q1, 0.63; 95% CI, 0.58 to 0.69) (Figure 2 and Table 2), unadjusted cure models (OR Q4 versus OR Q1, 0.72; 95% CI, 0.66 to 0.78), and competing risk regression (subhazard ratio Q4 versus subhazard Q1, 0.80; 95% CI, 0.75 to 0.85).
Patients from advantaged areas were more likely to use private hospitals, which rarely used PD (Figure 2). PD was also more common among patients who lived outside major cities (P<0.001) (Figure 3) and much less common among patients who commenced RRT at a private hospital (Figure 2).
Figure 3.
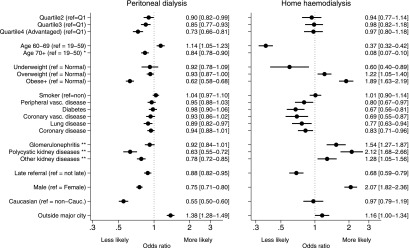
Odds ratios for the likelihood of commencing peritoneal dialysis and home HD. Results are from cure models, and they are adjusted for all other factors presented and states (not shown). *Results for patients 70+ years old are not graphed for home HD for clarity. **Reference group for kidney diseases is diabetic nephropathy and hypertension (combined). Values in brackets are 95% confidence intervals. Cauc, Caucasian; Q, quartile; ref, reference; vasc, vascular.
SES gradients in PD use were largest within major cities (P=0.004 for remoteness–SES interaction); however, SES gradients still existed for patients outside of major cities (OR Q4 versus OR Q1, 0.87; 95% CI, 0.77 to 0.99; P<0.001). SES had the strongest association with PD use for patients with no comorbidities (P=0.01 for comorbidity–SES interaction for likelihood of ever commencing PD); however, SES gradients existed even among patients with comorbidities (OR Q4 versus OR Q1, 0.77; 95% CI, 0.69 to 0.87; P<0.001). Peripheral vascular disease, obesity, advanced age, and GN were associated with lower uptake of PD (Figure 3). Caucasians were less likely to use PD (Figure 3). There was no interaction between SES and race (P=0.74) for uptake of PD (Figure 2). Men were less likely to commence PD than women (Figure 3).
After accounting for states, individual hospital funding, and hospital as random effects and adjusting for patient factors in a mixed effects logistic regression, patients from advantaged areas were less likely to use PD (OR Q4 versus OR Q1, 0.89; 95% CI, 0.80 to 1.00; P=0.04).
HHD
We estimated that 9.5% of patients (95% CI, 9.0% to 9.9%) would ever use HHD, and of those patients who do use HHD, 50% of patients commenced within 283 days (95% CI, 264 to 303 days). Rate of uptake of HHD was not associated with SES (P=0.46). The proportion of patients likely to commence HHD did not vary with SES in adjusted (Table 2) or unadjusted (P=0.85) (Figure 3) models. Patients outside major cities were more likely to commence HHD (P=0.05) (Figure 3).
HHD patients were younger, had higher BMI, and had fewer comorbidities than patients using ICHD. Age and comorbidities were stronger predictors of uptake of HHD than PD (Figure 3).
Discussion
Patients from advantaged areas are overall less likely to commence home dialysis, which is driven by an SES gradient in PD uptake, because there was no association between SES and HHD use.
Patients who started RRT in private hospitals are unlikely to use PD, and patients from advantaged areas are more likely to use private hospitals. Registry data cannot determine the direction of causality. It is possible that patients with private health insurance preferentially commence dialysis at private hospitals (where available). Alternatively, many advantaged patients may first choose to use ICHD and then choose a private hospital to receive ICHD. Private hospital use generally reflects private health insurance in Australia. Dialysis in private hospitals would be prohibitively expensive for uninsured patients.
Private hospitals in Australia are reimbursed per treatment and rarely use PD (25). The prevalence of PD has been previously associated with reimbursement patterns (26). Some countries and states provide incentives for increasing the proportion of patients who use PD within publicly funded systems (25,27). Medicare in the United States essentially reimburses a flat price per week per patient, regardless of modality, whereas in Germany, more is paid for PD (28). Funding structures used by private providers have received little attention, although similar approaches may address some disparities. Access to HHD was possible for all patients who commenced RRT in all larger hospitals (those hospitals with more than 35 incident RRT patients over 12 years).
PD is more common outside of major cities (29), and rural areas have lower SES. Patients from remote areas may be unable to travel to clinics on a regular basis. Similarly, remote PD patients are less likely to change to HD after experiencing peritonitis (29). The SES gradient in PD uptake was strongest within major cities, which generally offer more choices of hospitals.
Patients without comorbidities are more likely to use PD, which was found in the United States (7). Sicker patients may be better suited to assisted dialysis in ICHD. This finding would be consistent with our findings of a stronger SES gradient for patients without comorbidities.
Non-Caucasians are more likely to use PD than Caucasians, despite being more likely to live in a major city. We suggest that this finding is caused by non-Caucasians using public hospitals more commonly than Caucasians. Conversely, in the United States, white patients were more likely to use PD than African Americans (30).
Three quarters of Australians with stage 5 CKD are educated about home dialysis (31). Few patients do not receive such education because of SES-related factors (late referral to nephrological care, unsuitable living conditions, psychosocial contraindications, and literacy). Education is generally withheld for medical reasons. Predialysis education has been shown to increase patients’ intentions to commence home dialysis (32). ANZDATA does not record any data on education provided to patients about dialysis modalities.
The lower rates of PD in advantaged areas seem to be unique to Australia. PD was more common among patients with more education in Taiwan (10) and The Netherlands (9) and higher income in the United States (4,7). PD was less common among socioeconomically disadvantaged people in New Jersey in the United States (33) and US patients who did not own their home (30). PD use was not associated with area SES in Canada (11), which has a mix of government and private health care providers that is similar to the mix in Australia. Gradients in health literacy (9), comorbidities (34), and out-of-pocket expenses related to PD (35) would be expected to result in higher rates of PD among advantaged patients. Government rebates for water and electricity vary between states, and many rebates are only available to concession card holders (36), who are typically on low incomes.
The likelihood of commencing HHD is not associated with area SES in Australia, contrary to findings from other countries. HHD is more common among advantaged areas (37) in the United Kingdom and white and employed patients in the United States (12). The small number of HHD patients in our registry limits the power of our analyses and the generalizability of our results. HHD is a relatively uncommon mode of RRT, despite being suited to frequent and extended hour dialysis, which is associated with excellent outcomes (5,6,38).
Observational studies cannot prove direction of causality. Patients on RRT may stop or reduce work and move to less advantaged areas, although previous work has found no net shift to less advantaged areas after commencement (16). Unmeasured or incompletely measured covariates may contribute to effects of SES. Possible determinants of RRT modality (e.g., health literacy, patient motivation, medical suitability for home dialysis, distance from home to treating center, and fear of self-care) are not recorded in ANZDATA, and additionally, education given to patients and patient preferences are not recorded. There is also potential for ecological fallacy—some relatively advantaged patients may live in disadvantaged areas (e.g., 1.9% of patients from disadvantaged areas used private hospitals), and individual SES may predict health independently of area SES (39,40). Registry data are subject to potential reporting bias, although ANZDATA covers all treating centers in Australia and may be more accurate than some other renal registries (e.g., 94% of hemodialysis commencement dates in ANZDATA were within 1 month of dates derived independently from medical records) (41).
Patients from advantaged areas are less likely to use home dialysis, despite PD causing fewer lifestyle disruptions, HHD being associated with optimal outcomes, and both being generally cheaper than ICHD. Current funding structures within the private health care system may not always encourage the use of home dialysis for many suitable patients. Identifying and addressing barriers to home dialysis will enable changes within the Australian health care system that will improve patient quality of life and reduce costs.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Do Socioeconomic Factors Affect Dialysis Modality Selection?,” on pages 837–839.
References
- 1.Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, Donaldson C: Cost analysis of ongoing care of patients with end-stage renal disease: The impact of dialysis modality and dialysis access. Am J Kidney Dis 40: 611–622, 2002 [DOI] [PubMed] [Google Scholar]
- 2.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR: Relationship between dialysis modality and mortality. J Am Soc Nephrol 20: 155–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu YW, Jiwakanon S, Lukowsky L, Duong U, Kalantar-Zadeh K, Mehrotra R: An update on the comparisons of mortality outcomes of hemodialysis and peritoneal dialysis patients. Semin Nephrol 31: 152–158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutner NG, Zhang R, Barnhart H, Collins AJ: Health status and quality of life reported by incident patients after 1 year on haemodialysis or peritoneal dialysis. Nephrol Dial Transplant 20: 2159–2167, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Mowatt G, Vale L, MacLeod A: Systematic review of the effectiveness of home versus hospital or satellite unit hemodialysis for people with end-stage renal failure. Int J Technol Assess Health Care 20: 258–268, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Stokes JB: Nocturnal hemodialysis: Analysis following the Frequent Hemodialysis Network trial. Semin Dial 24: 614–620, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Stack AG: Determinants of modality selection among incident US dialysis patients: Results from a national study. J Am Soc Nephrol 13: 1279–1287, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Gadegbeku C, Freeman M, Agodoa L: Racial disparities in renal replacement therapy. J Natl Med Assoc 94[Suppl]: 45S–54S, 2002 [PMC free article] [PubMed] [Google Scholar]
- 9.Jager KJ, Korevaar JC, Dekker FW, Krediet RT, Boeschoten EW, Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group : The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in The Netherlands. Am J Kidney Dis 43: 891–899, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Huang HC, Wang JY, Chang CC, Chiu PF, Chiang MC, Yang Y: Nonclinical factors associated with treatment with peritoneal dialysis in ESRD patients in Taiwan. Perit Dial Int 30: 638–643, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Prakash S, Perzynski AT, Austin PC, Wu CF, Lawless ME, Paterson JM, Quinn RR, Sehgal AR, Oliver MJ: Neighborhood socioeconomic status and barriers to peritoneal dialysis: A mixed methods study. Clin J Am Soc Nephrol 8: 1741–1749, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker DR, Inglese GW, Sloand JA, Just PM: Dialysis facility and patient characteristics associated with utilization of home dialysis. Clin J Am Soc Nephrol 5: 1649–1654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Renal Data System : International comparisons. In: USRDS: 2010 Annual Report, Bethesda, MD, National Insitutes of Health, National Insitute of Diabetes and Digestive and Kidney Diseases, 2010, pp 385–397 [Google Scholar]
- 14.Preston-Thomas A, Cass A, O’Rourke P: Trends in the incidence of treated end-stage kidney disease among Indigenous Australians and access to treatment. Aust N Z J Public Health 31: 419–421, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Australian Bureau of Statistics: Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia. ABS Catalogue 2033.0.55, Canberra, Australia, Australian Bureau of Statistics, 2008
- 16.Grace BS, Clayton P, Cass A, McDonald SP: Socio-economic status and incidence of renal replacement therapy: A registry study of Australian patients. Nephrol Dial Transplant 27: 4173–4180, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Grace BS, Clayton PA, Cass A, McDonald SP: Transplantation rates for living- but not deceased-donor kidneys vary with socioeconomic status in Australia. Kidney Int 83: 138–145, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Australian Bureau of Statistics: Australian Standard Geographical Classification. ABS Catalogue No. 1216.0, Canberra, Australia, Australian Bureau of Statistics, 2010
- 19.Kidney Health Australia: Dialysis Unit Guide, Adelaide, SA, Australia, Kidney Health Australia, 2012
- 20.Maller RA, Zhou X: Survival Analysis with Long-Term Survivors, New York, Wiley, 2001 [Google Scholar]
- 21.Couchoud C, Bayat S, Villar E, Jacquelinet C, Ecochard R, REIN registry : A new approach for measuring gender disparity in access to renal transplantation waiting lists. Transplantation 94: 513–519, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Cleves M, Gurierrez RG, Gould W, Marchenko YV: An Introduction to Survival Analysis Using Stata, College Station, TX, Stata Press, 2010 [Google Scholar]
- 23.Baker SG: Causal inference, probability theory, and graphical insights [published online ahead of print May 10, 2013]. Stat Med 32: 4310–4330, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine J, Gray R: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 25.Gray NA, Dent H, McDonald SP: Dialysis in public and private hospitals in Queensland. Intern Med J 42: 887–893, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Lameire N, Van Biesen W: Epidemiology of peritoneal dialysis: A story of believers and nonbelievers. Nat Rev Nephrol 6: 75–82, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Cleemput I, De Laet C: Analysis of the costs of dialysis and the effects of an incentive mechanism for low-cost dialysis modalities. Health Policy 110: 172–179, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Vanholder R, Davenport A, Hannedouche T, Kooman J, Kribben A, Lameire N, Lonnemann G, Magner P, Mendelssohn D, Saggi SJ, Shaffer RN, Moe SM, Van Biesen W, van der Sande F, Mehrotra R, Dialysis Advisory Group of American Society of Nephrology : Reimbursement of dialysis: A comparison of seven countries. J Am Soc Nephrol 23: 1291–1298, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Gray NA, Grace BS, McDonald SP: Peritoneal dialysis in rural Australia. BMC Nephrol 14: 278, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker-Cummings C, McClellan W, Soucie JM, Krisher J: Ethnic differences in the use of peritoneal dialysis as initial treatment for end-stage renal disease. JAMA 274: 1858–1862, 1995 [PubMed] [Google Scholar]
- 31.Morton RL, Howard K, Webster AC, Snelling P: Patient INformation about Options for Treatment (PINOT): Prospective national study of information given to incident CKD Stage 5 patients. Nephrol Dial Transplant 26: 1266–1274, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Manns BJ, Taub K, Vanderstraeten C, Jones H, Mills C, Visser M, McLaughlin K: The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: A randomized trial. Kidney Int 68: 1777–1783, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Winkelmayer WC, Glynn RJ, Levin R, Owen W, Jr., Avorn J: Late referral and modality choice in end-stage renal disease. Kidney Int 60: 1547–1554, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Zhang AH, Bargman JM, Lok CE, Porter E, Mendez M, Oreopoulos DG, Chan CT: Dialysis modality choices among chronic kidney disease patients: Identifying the gaps to support patients on home-based therapies. Int Urol Nephrol 42: 759–764, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Ludlow MJ, George CRP, Hawley CM, Mathew TH, Agar JWM, Kerr PG, Lauder LA: How Australian nephrologists view home dialysis: Results of a national survey. Nephrology (Carlton) 16: 446–452, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Kidney Health Australia : Financial Support for Home Dialysis Patients in Australia, Adelaide, SA, Australia, KHA, 2012 [Google Scholar]
- 37.Nitsch D, Steenkamp R, Tomson CRV, Roderick P, Ansell D, MacGregor MS: Outcomes in patients on home haemodialysis in England and Wales, 1997–2005: A comparative cohort analysis. Nephrol Dial Transplant 26: 1670–1677, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Lowrie EG, Laird NM, Parker TF, Sargent JA: Effect of the hemodialysis prescription of patient morbidity: Report from the National Cooperative Dialysis Study. N Engl J Med 305: 1176–1181, 1981 [DOI] [PubMed] [Google Scholar]
- 39.Link BG, Phelan J: Social conditions as fundamental causes of disease. J Health Soc Behav Spec No: 80–94, 1995 [PubMed] [Google Scholar]
- 40.Bentley R, Kavanagh AM, Subramanian SV, Turrell G: Area disadvantage, individual socio-economic position, and premature cancer mortality in Australia 1998 to 2000: A multilevel analysis. Cancer Causes Control 19: 183–193, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Gray NA, Mahadevan K, Campbell VK, Noble EP, Anstey CM: Data quality of the Australia and New Zealand Dialysis and Transplant Registry: A pilot audit. Nephrology (Carlton) 18: 665–670, 2013 [DOI] [PubMed] [Google Scholar]