Abstract
Congenital anomalies of the kidney and urinary tract are the major cause of ESRD in childhood. Children with a solitary functioning kidney form an important subgroup of congenital anomalies of the kidney and urinary tract patients, and a significant fraction of these children is at risk for progression to CKD. However, challenges remain in distinguishing patients with a high risk for disease progression from those patients without a high risk of disease progression. Although it is hypothesized that glomerular hyperfiltration in the lowered number of nephrons underlies the impaired renal prognosis in the solitary functioning kidney, the high proportion of ipsilateral congenital anomalies of the kidney and urinary tract in these patients may further influence clinical outcome. Pathogenic genetic and environmental factors in renal development have increasingly been identified and may play a crucial role in establishing a correct diagnosis and prognosis for these patients. With fetal ultrasound now enabling prenatal identification of individuals with a solitary functioning kidney, an early evaluation of risk factors for renal injury would allow for differentiation between patients with and without an increased risk for CKD. This review describes the underlying causes and consequences of the solitary functioning kidney from childhood together with its clinical implications. Finally, guidelines for follow-up of solitary functioning kidney patients are recommended.
Introduction
Congenital anomalies of the kidney and urinary tract (CAKUT) are the predominant cause of ESRD in childhood (1). One important condition in the spectrum of CAKUT is the solitary functioning kidney, which can be congenital or acquired after unilateral nephrectomy in childhood. Although both types of solitary functioning kidney are associated with CKD and ESRD (2), early differentiation between patients with and without an increased risk for CKD is challenging (3). Because of the implementation of routine fetal ultrasound screening in most developed countries, patients with a solitary functioning kidney are increasingly identified before birth. This identification not only implies that clinicians will be more often confronted with questions regarding the prognosis of this specific condition, but also that these children can be clinically monitored from birth onward. In this review, we consider causes and consequences of the solitary functioning kidney from childhood. We will specifically focus on the diagnostic and prognostic implications for patients with a solitary functioning kidney.
Causes
Renal Development
Definitive human renal development is initiated at the fifth gestational week and characterized by complex interactions between the outgrowing ureteric bud (UB) of the mesonephric duct (from which the renal pelvis, ureter, and lower urinary tract originate) and the metanephric mesenchyme (MM; from which the renal parenchyma originates) (4). As a result, nephrons are formed until the 34th to 36th gestational week, without the possibility of additional nephron formation later in life (5). This finding implies that the total of number of nephrons at birth, approximately 900,000 nephrons per kidney with a high interindividual variability (6), should last the entire lifespan of an individual.
Failure of interaction between the MM and the UB perturbs normal renal development, resulting in different forms of CAKUT (7). Bilateral absence of functioning renal tissue is considered lethal based on the associated pulmonary hypoplasia (Potter sequence). Unilateral renal agenesis (URA), which defines unilateral nonformation of the kidney, has an estimated worldwide incidence of 1 in ∼2000 births (8). Because differentiation from renal aplasia cannot be made in daily clinical practice, URA is generally used as a term for either clinical entity, although a study has suggested that renal aplasia may be the leading cause of a congenital solitary kidney (1 in ∼1300 births) (9). URA should be differentiated from abnormal or incomplete renal development, which leads to renal hypodysplasia or a nonfunctioning kidney, which can be seen in multicystic dysplastic kidney (MCDK; worldwide incidence of 1 in ∼4300 births) (10). However, it must be noted that the diagnosis URA could derive from the spontaneous (prenatal) involution of MCDK or renal hypodysplasia (8). It is estimated that about 5% of MCDKs show complete involution before birth (10).
In the case of URA or MCDK, the solitary functioning kidney is congenital. However, a solitary functioning kidney can also be acquired after nephrectomy because of various renal diseases, such as CAKUT (e.g., pelviureteric junction obstruction [PUJO], vesicoureteric reflux [VUR], megaureter, and duplex kidney) (11). Renal malignancies, trauma, or renovascular disease may also underlie a solitary functioning kidney, but these causes are outside the scope of this review.
Ipsilateral CAKUT
CAKUT are phenotypically variable and may affect several segments of the urinary tract simultaneously (12). The high proportion of ipsilateral CAKUT (i.e., renal malformations on the side of the solitary functioning kidney) in solitary functioning kidney patients is a clear illustration of this phenomenon. Based on two systematic reviews and meta-analyses of the literature describing over 2000 patients with URA and MCDK (8,10), nearly one in three patients has ipsilateral CAKUT, including VUR, PUJO, a megaureter, or a duplex kidney. Although the prevalence of ipsilateral CAKUT in patients with an acquired solitary functioning kidney has not yet been established, a large cohort study suggests even higher proportions (13).
Genetic Factors
Normal kidney and urinary tract development requires a temporally and spatially coordinated interaction between the UB and the MM (14). Different molecules expressed in either or both of these compartments have been identified through gene targeting in mice (15). As a result, any insult (genetic or environmental) that disrupts this reciprocal induction can lead to different forms of CAKUT. In fact, this strict cross-talk between the UB and the MM provides the rationale for the pleiotropic effect that genetic mutations or environmental factors can have on the determination of different forms of CAKUT. There is a bulk of evidence that implicates genetic factors in the pathogenesis of the CAKUT phenotypes underlying a solitary functioning kidney. Familial aggregation has been reported in about 10% of cases (more frequently with an autosomal dominant mode of inheritance with incomplete penetrance), but recessive and X-linked families have been reported as well (12). With the exception of rare syndromic forms of CAKUT, the mutations underlying these familial forms are largely unknown (12). The three genes most commonly implicated in nonsyndromic forms of CAKUT are PAX2 (encoding for a nuclear transcription factor involved in early nephrogenesis), HNF1B (encoding for a transcription factor originally implicated in the renal cysts and diabetes syndrome [Online Mendelian Inheritance in Man 137920]), and DSTYK (encoding for a dual specificity serine/threonine and tyrosine kinase; recently identified as a positive regulator of fibroblast growth factor signaling during kidney development) (16). However, many other genes have been implicated in CAKUT phenotypes underlying a solitary functioning kidney (Table 1). Heterozygous mutations in all these genes, nevertheless, account for, at most, 10%–20% of patients with kidney malformations (17,18).
Table 1.
Genes most commonly implicated in congenital anomalies of the kidney and urinary tract phenotypes leading to a solitary functioning kidney
| OMIM | Gene Symbol | Phenotype | Syndrome | Mode of Inheritance |
|---|---|---|---|---|
| Isolated forms of renal hypodysplasia/CAKUT (including solitary functioning kidney) | ||||
| 612666 | DSTYK | RHD, PUJO | — | Dominant |
| 137920 | HNF1B | RHD | Renal cysts and diabetes syndrome | Dominant |
| 120330/191830 | PAX2 | RHD | Papillorenal syndrome | Dominant |
| 219000 | FRAS1, FREM 1 | Renal agenesis, RHD | Fraser syndrome | Dominant/Recessive |
| 611559 | UPK3A | Renal adysplasia/urogenital adysplasia | — | Dominant |
| 112262 | BMP4 | RHD, renal agenesis | — | Dominant |
| 191830 | RET | Renal agenesis | Hirschsprung’s disease | Dominant |
| Syndromic forms of renal hypodysplasia/CAKUT (including solitary functioning kidney) | ||||
| 113650 | EYA1, SIX1, SIX5 | Renal agenesis, RHD | Branchio-oto-renal syndrome | Dominant |
| 107480 | SALL1 | Renal agenesis, RHD, VUR, renal ectopia | Townes–Brocks (branchio-oto-renal–like syndrome) | Dominant |
| 607323 | SALL4 | Renal ectopia, CAKUT | Okihiro syndrome | Dominant |
| 308700 | KALL1, FGFR1 | Renal agenesis, RHD | Kallman’s syndrome | Dominant |
| 610132 | VANGL1 | Renal agenesis, RHD, renal ectopia | VACTERL/caudal regression syndrome | Dominant |
| 142994 | MNX1 | Renal agenesis, RHD, renal ectopia, VUR | VACTERL/caudal regression syndrome/Currarino syndrome | Dominant |
| 118450 | JAG1, NOTCH2 | RHD, MCDK | Alagille syndrome | Dominant |
| 214800 | CHD7 | Renal agenesis, RHD, renal ectopia, VUR | CHARGE syndrome | Dominant |
| 146255 | GATA3 | RHD | Hypothyroidism, sensorial deafness | Dominant |
| 161200 | LMX1B | Renal agenesis | Nail patella syndrome | Dominant |
| 122470 | NIPBL | Renal agenesis, RHD | Cornelia de Lange syndrome | Dominant |
| 180849 | CREBBP | Renal agenesis, RHD, VUR | Rubinstein–Taybi syndrome | Dominant |
| 147920 | MLL2 | VUR, RHD, renal ectopia | Kabuki syndrome (VUR) | Dominant |
| 146510 | GLI3 | Renal agenesis, RHD | Pallister–Hall syndrome | Dominant |
| 130650 | KIP2 | RHD, VUR | Beckwith–Wiedemann syndrome | Dominant |
| 181450 | TBX3 | Renal agenesis, RHD | Ulnar–Mammary’s syndrome | Dominant |
| 270400 | DHCR7 | RHD, cysts, renal agenesis, VUR | Smith–Lemli–Opitz syndrome | Recessive |
| 214100 | PEX family | RHD, cysts | Zellweger syndrome | Recessive |
| 277000 | WNT4 | Renal agenesis, RHD, renal ectopia | Rokitansky syndrome | Dominant |
| 300209 | GPC3 | RHD, cysts, VUR | Simpson–Golabi–Behmel syndrome | X-linked |
OMIM, Online Mendelian Inheritance in Man; CAKUT, congenital anomalies of the kidney and urinary tract; RHD, renal hypodysplasia; PUJO, pelviureteric junction obstruction; VUR, vesicoureteric reflux; VACTERL; MCDK, multicystic dysplastic kidney; CHARGE, coloboma of the eye, heart defects, atresia of the nasal choanae, retardation of growth and/or development, genital and/or urinary abnormalities, and ear abnormalities and deafness.
CAKUT are among the most common birth defects in humans (1 in ∼600 births) (19), and present in over 20% of newborns with chromosomal abnormalities (20), indicating that kidney development is particularly sensitive to gene dosage. Consistently, in a recent study on 522 children with renal hypodysplasia (including solitary functioning kidney), we identified 72 different copy number disorders in 87 patients (∼17%), implicating rare, submicroscopic deletions and duplications that disrupt coding elements as a major cause of renal hypodysplasia and confirming the extreme genetic heterogeneity of this disease (21).
Environmental Factors
Environmental factors that disturb renal development include medications administered during pregnancy (e.g., angiotensin-converting enzyme [ACE] inhibition, dexamethasone, antiepileptic drugs, and aminoglycosides) (22), intrauterine growth restriction, and maternal diseases, such as diabetes (23). Moreover, drug administration in the prematurely born neonate with a solitary functioning kidney can have detrimental effects on nephrogenesis and GFR, especially when administered before the 28th gestational week (5). The most commonly used drugs that disturb nephrogenesis are aminoglycosides and nonsteroidal anti-inflammatory drugs (22). Finally, observational studies report a boy predominance in solitary functioning kidney patients, as well as a left-side predominance (24). The reasons for these differences are not fully understood. Future studies are needed to clarify the exact mechanisms of renal (mal)development and the factors leading to a solitary functioning kidney.
Consequences
Hyperfiltration Hypothesis
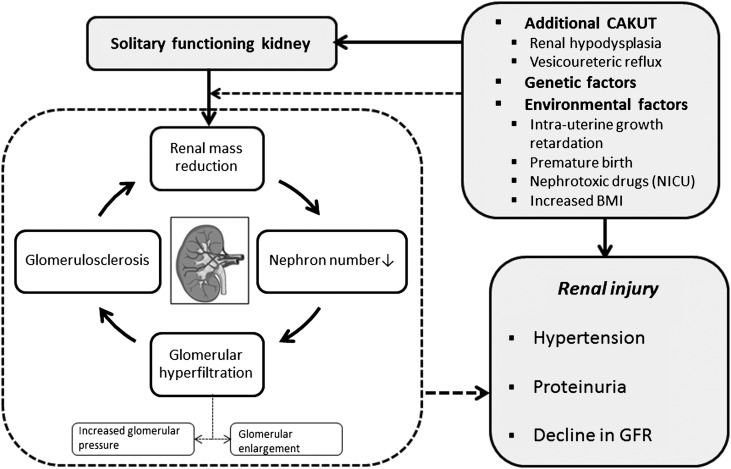
The clinical importance of a reduced nephron number has been described in the hyperfiltration hypothesis by Brenner and coworkers (25,26) more than three decades ago. Based on experiments in rats with five sixths renal mass reduction, these landmark studies showed that a reduced functional nephron number results in compensatory glomerular hypertension and enlargement of remnant nephrons, indicating glomerular hyperfiltration (25,26). Glomerular hyperfiltration may lead to glomerulosclerosis and sets a vicious cycle of additional reduction in nephron number (Figure 1). As a consequence, the nephrectomized animals showed high incidences of hypertension and proteinuria in the early stages and an ongoing decline in GFR in the long run (25).
Figure 1.
Possible mechanisms leading to renal injury in patients with a solitary functioning kidney. Glomerular hyperfiltration caused by insufficient nephron number could be one explanation for this impaired outcome. Nephron number could also be affected by associated congenital anomalies of the kidney and urinary tract as well as genetic and environmental factors. BMI, body mass index; CAKUT, congenital anomalies of the kidney and urinary tract; NICU, neonatal intensive care unit.
Individuals with a solitary functioning kidney from childhood have, by definition, a reduction in renal mass for a prolonged period of time and may, therefore, be at risk for hyperfiltration injury (Figure 1). Animal studies confirmed that a compensatory increase in nephron number is present in congenital solitary functioning kidney (27,28). Nevertheless, the total nephron number identified is still lower than in two kidney controls (Table 2). Compensatory renal hypertrophy of the solitary kidney can be identified in utero in 90% of individuals (29). This hypertrophy indicates renal adaptation to kidney mass reduction and may, therefore, be beneficial at birth. However, a prolonged increase in nephron and kidney size may lead to stretch-induced glomerular cell activation, fibrosis, and vasoconstriction, as well as tubular cell nephrotoxity (30).
Table 2.
Nephron number in different species with a solitary functioning kidney
| Reference | Species | Model | Age at Study | Nephron Number in Percent of Two Kidney Controls | Glomerular Size in Percent of Two Kidney Controls |
|---|---|---|---|---|---|
| Larsson et al. (47) | Rat | Nephrectomy (during postnatal nephrogenesis) | 55–65 d (adulthood) | 52 | NR |
| Bhathena et al. (59) | Human | URA | 34.5 yr (adulthood) | NR | 175 |
| Abellan et al. (60) | Rabbit | Nephrectomy (in utero) | 30 d of gestation (prenatal) | NR | 115a |
| Mesrobian (61) | Mouse | URA | 8 wk (adulthood) | NR | 113 |
| Douglas-Denton et al. (27) | Sheep | Nephrectomy (in utero) | 27–34 d (infancy) | 72 | 74 |
| van Vuuren et al. (28) | Pig | URA | 26 wk (adulthood) | 75 | 152 |
NR, not reported; URA, unilateral renal agenesis.
Measured by total glomerular surface area.
Keller et al. (31) provided indirect evidence of the glomerular hyperfiltration hypothesis in patients with primary hypertension; these patients had fewer nephrons and an increased glomerular volume compared with patients without hypertension (31). Recently, another study showed that urinary albumin is weakly associated with markers of hyperfiltration in solitary functioning kidney patients (32), indicating that factors other than glomerular hyperfiltration likely contribute to the development of CKD.
Because the reduction in renal mass in solitary functioning kidney patients is smaller compared with the Brenner animal model, in vivo methods to determine nephron number are necessary to establish the direct association between glomerular numbers and the level of glomerular hyperfiltration in affected individuals. Some of these methods are in a promising developmental phase (33), and when available, they will allow for the direct testing of the hyperfiltration hypothesis in humans.
Clinical Outcome
Human Studies.
The long-term outcome of individuals with a solitary functioning kidney from childhood has been a topic of extensive debate (3) fueled by the conflicting results of observational studies (2,11,34–44). All of these studies vary with respect to type of solitary functioning kidney studied, inclusion criteria (e.g., age at follow-up, age of diagnosis, and normal renal ultrasound), and methods to measure BP, proteinuria, and GFR. Furthermore, observational studies are susceptible to selection and ascertainment bias. The number of longitudinal prospective studies on the clinical outcome of solitary functioning kidney patients is limited because of the decades required for follow-up (45). A longitudinal study on renal outcome in CAKUT showed that 20%–50% of solitary functioning kidney patients were on renal replacement therapy at the age of 30 years (2). Compared with a reference group, the risk for an impaired renal outcome was even higher when VUR was present (hazard ratio, 7.50; 95% confidence interval, 2.72 to 20.68). Results from another retrospective study on URA patients showed that 47% of individuals developed hypertension, 19% of individuals had proteinuria, 13% of individuals had an impaired GFR, and 4% of individuals died of renal failure (36). Similar findings were identified in adults with an acquired solitary functioning kidney (37). Gonzalez et al. (39) showed that obesity is associated with an increase in serum creatinine and the development of proteinuria in adults with a solitary functioning kidney. However, all of the above-mentioned studies describe a selected cohort of patients, which makes generalization of these findings to all patients with a solitary functioning kidney inappropriate. Unfortunately, individuals with a solitary functioning kidney and preserved renal function have traditionally not been followed into adulthood, and, therefore, the availability of long-term data on the clinical outcome in these patients is limited. In this regard, there is a cardinal need for long-term studies of individuals with a solitary functioning kidney with a prenatal diagnosis, because they represent the most unbiased group of patients. When available, these data will strongly assist nephrologists in differentiating patients at risk for CKD from those patients who are not at risk for CKD.
To prospectively study the prognosis of a solitary functioning kidney in childhood, we designed the KIdney of MONofunctional Origin (KIMONO) study (13). This longitudinal follow-up study from The Netherlands includes over 400 children with both types of solitary functioning kidney. Study subjects were routinely screened for markers of renal injury, which were defined as hypertension, (micro)albuminuria, and/or decline of GFR. The use of antihypertensive and antiproteinuric medication during follow-up was monitored. Recent analyses showed that nearly one in three patients with a solitary functioning kidney has signs of renal injury at a mean age of 10 years (11). Furthermore, we showed that GFR in these patients slowly declines from as early as 9 years of age, whereas (micro)albuminuria generally develops around 16 years of age. The median age to develop renal injury in children with either type of solitary functioning kidney was ∼15 years (13). Patients with ipsilateral CAKUT (encompassing 34% of patients) showed higher proportions of renal injury and progressed earlier to renal injury than patients without CAKUT (12.8 versus 15.9 years, respectively; P<0.01) (10). Finally, 6% of children already had CKD stage 3 or higher during follow-up (congenital 4% versus acquired 9%; P=0.05). Other studies that have included solitary functioning kidney patients with a normal renal ultrasound at diagnosis (suggesting absence of ipsilateral CAKUT) report a relatively mild renal outcome (34,40,42,44). Although the exact proportion of individuals with CKD needs to be determined, the increasing number of studies illustrates that a solitary functioning kidney should not be assumed to be harmless.
Differences between Solitary Functioning Kidney Types.
Important differences in renal outcome may exist between congenital and acquired solitary functioning kidneys; the congenital type still has the potential to form new nephrons (Table 2), whereas with the acquired type, nephrogenesis has ceased at the time of the nephrectomy. This finding may imply a higher susceptibility for pronounced glomerular hyperfiltration in acquired solitary functioning kidney patients. Abou Jaoudé et al. (35) reported a lower GFR in children with an acquired solitary functioning kidney than children with a congenital solitary functioning kidney (mean GFR: 95 versus 107 ml/min per 1.73 m2, respectively). Nevertheless, differences between types are generally small and may also be explained by the older age in children with an acquired solitary functioning kidney (13). It must be noted that many of the underlying causes (e.g., CAKUT) leading to an acquired solitary functioning kidney in childhood are already present before birth, whereas some patients with a congenital solitary functioning kidney have hypodysplasia of the remnant kidney. Renal compensation in nephron number, therefore, seems to be strongly overlapping between both types of solitary functioning kidneys. Large longitudinal follow-up studies are needed to differentiate the clinical outcome between solitary functioning kidney types.
Differences with Uninephric Kidney Donors.
In daily clinical practice, the outcome of patients with a solitary functioning kidney is often derived from the excellent prognosis described in adult uninephric kidney donors (46). However, there are important differences between uninephric kidney donors and patients with a solitary functioning kidney that make such a comparison inadequate.
Larsson et al. (47) showed that the level of glomerular hyperfiltration in rats is doubled when renal mass reduction is performed during active nephrogenesis compared with later in life. Similar findings have been identified in a study with rabbits (48). Furthermore, healthy uninephric kidney donors undergo stringent screening to ensure that a healthy kidney will remain, whereas children with a solitary functioning kidney frequently show additional anomalies and may have hypodysplasia of the solitary functioning kidney (8,10). This finding is further underlined by the fact that not all children with a solitary functioning kidney have renal compensatory hypertrophy (11). On the basis of the above-mentioned differences between uninephric kidney donors and patients with a solitary functioning kidney, it is not justified to aggregate conclusions on outcome in both groups.
Clinical Implications
Subsequent to the ongoing debate on the clinical outcome of patients with a solitary functioning kidney from childhood, guidelines for the follow-up of these patients have not been established. However, the need for regular clinical follow-up in these patients is increasingly recognized (11,45,49).
Based on findings of the KIMONO study (13), a differentiation between patients with and without a high risk for CKD should be made at diagnosis. This evaluation should focus on identified risk factors, such as ipsilateral CAKUT, small renal size, low birth weight, prematurity, and history of urinary tract infection. In addition, the Chronic Kidney Disease in Children cohort study has identified clinical (anemia, hyperphospatemia, and short stature) (50) and socioeconomic (lower income and lower maternal education) (51) risk factors that are associated with the development of CKD in children, including solitary functioning kidney patients. Risk assessment also requires imaging techniques, such as renal ultrasound, in all patients and on indication, renal scintigraphy and/or a micturating cystourethrogram (for additional CAKUT including VUR) together with repeated follow-up of renal length. If imaging studies identify associated CAKUT, such as high-grade VUR or PUJO, an endoscopic or surgical approach may be indicated. Finally, identification of genetic factors to establish a molecular diagnosis and screening for CAKUT in relatives may provide additional information on the clinical outcome.
Corbani et al. (45) suggest regular clinical follow-up for hypertension, (micro)albuminuria, and GFR every 3–5 years in a patient with a solitary functioning kidney without ipsilateral CAKUT. For patients with ipsilateral CAKUT, clinical follow-up should be conducted annually, and surgical correction of CAKUT must be performed when indicated.
On the basis of all available data, we present recommendations for the clinical monitoring of children with a solitary functioning kidney (Table 3). Because we have a limited number of factors at hand in clinical practice, it is not yet feasible to provide an individualized risk assessment and follow-up scheme. However, we feel that the available data do not allow for exclusion of any group of children with a solitary functioning kidney for clinical monitoring. We have, therefore, suggested a follow-up based on two factors (i.e., the presence of ipsilateral CAKUT and the presence of [signs of] renal injury). Based on these factors, follow-up for all is proposed, with a differentiation in the frequency of follow-up. No unequivocal evidence exists for any follow-up frequency, which will, therefore, always result in an arbitrary proposal. However, because analyses of the Kaplan–Meier curves on renal injury in children with a solitary functioning kidney show that, each year, ±4% of children additionally develop signs of renal injury (13), we feel that a yearly follow-up is justified. The proposed time intervals allow timely intervention, which mainly encompasses medications, such as ACE inhibitors or angiotensin II receptor blockers. ACE inhibitors have been shown to slow down disease progression in children with renal hypodysplasia (52). Patients with a solitary functioning kidney who were diagnosed prenatally and do not show risk factors for CKD at diagnosis can be monitored less stringently. Clinicians should be specifically aware of the administration of medication that may cause AKI, such as nonsteroidal anti-inflammatory drugs and aminoglycosides. Limiting the use of drugs that disturb nephrogenesis is of particular importance in premature infants with a solitary functioning kidney.
Table 3.
Opinion-based recommendation for clinical follow-up intervals of children with a solitary functioning kidney
| Clinical Parameter/Modality | No Renal Injury | GFR<60 ml/min per 1.73 m2 or Medication for Proteinuria/Hypertension | |
|---|---|---|---|
| CAKUT − | CAKUT + | ||
| BP | One time per year | Two times per year | Two to four times per year |
| (Micro)albuminuria | One time per year | Two times per year | Two to four times per year |
| Serum creatinine/GFR | Every 5 years | Every 5 years | Two to four times per year |
| Ultrasound | Every 5 yearsa | As indicated | As indicated |
Guidelines for the clinical follow-up of children with a solitary functioning kidney. The presented follow-up intervals are based on risk assessment at diagnosis; 24-hour ambulatory BP measurement is preferred in children and adults. Microalbuminuria should be determined in a first fresh morning sample (urinary albumin cutoff value>30 mg/24 h). GFR can be estimated using the commonly used Schwartz formula.
Last ultrasound to be performed at 15–16 years of age.
The impact of early dietary management in infants with a solitary functioning kidney remains unclear. Rapid postnatal catch-up growth is associated with obesity in adulthood (53), which, in turn, may deteriorate renal function in the solitary functioning kidney patient (39). Additionally, studies by Brenner and coworkers (25,26) showed that protein restriction has a protective effect on glomerular hyperfiltration in rats (26). Interestingly, a combination of the Brenner hypothesis and the developmental origin of health and disease hypothesis has been proposed to cause the impaired renal outcome of very low birth weight infants (54). One can, therefore, speculate that rapid catch-up growth in low birth weight infants with a solitary functioning kidney could negatively impact renal outcome and should be discouraged. Nevertheless, this relationship between postnatal feedings strategies and renal outcome needs additional investigation.
An impaired GFR is a relatively late phenomenon in children with a solitary functioning kidney, because the first signs of renal injury generally include hypertension and (micro)albuminuria (5). Monitoring for renal injury in children with a solitary functioning kidney and a normal initial estimated GFR, therefore, should focus on BP and determination of (micro)albuminuria, which can both be done noninvasively. Ambulatory BP measurement to monitor for hypertension is the preferred method in children and adults (55), whereas microalbuminuria should be measured in a first morning void (56). GFR in children can be estimated by the commonly used Schwartz equation, which has been shown to be precise in children with a solitary functioning kidney (57). The follow-up of children with a solitary functioning kidney, but without signs of renal injury, can be performed by the general pediatrician, whereas patients with renal injury and early CKD may require clinical visits by a pediatric nephrologist. Clinical visits by a pediatric nephrologist also apply to children with associated CAKUT. In the follow-up of children with a solitary functioning kidney and complex CAKUT, a multidisciplinary approach, involving general pediatricians, pediatric nephrologists, and pediatric urologists, is mandatory to optimize renal outcome, particularly when surgical intervention is necessary.
Puberty seems to be a critical time in renal injury development (13), when growth spurt and associated increases in metabolic demands may drive glomerular hyperfiltration to maintain normal GFR. Other explanations for the increase in renal injury may be found in sex hormones that could affect renal outcome (58). After this critical time period, there is a strong need for appropriate transition of care from a pediatric to an adult nephrology setting. Ineffective transition to adult care and loss to follow-up may increase the risk for CKD in adults with a solitary functioning kidney (49). Because CKD can be relatively symptom-free until a late stage, we emphasize the need for clinical follow-up programs of adult solitary functioning kidney patients. During these regular clinical visits, it seems mandatory to also evaluate body mass index, because obesity is an additional risk factor for the development of CKD (39).
Finally, we recognize that our recommendations are limited because of the lack of consensus for long-term follow-up and the fact that longitudinal data on the clinical outcomes of individuals with a solitary functioning kidney are absent. However, we feel that these recommendations can support pediatric and adult nephrologists in the monitoring of this specific patient group.
Conclusion
A solitary functioning kidney from childhood implies a substantial risk for hypertension, proteinuria, and progression to CKD, a statement that is supported by a well defined hypothesis, animal studies, and retrospective and longitudinal human studies. Possible explanations for this increased risk for renal injury are glomerular hyperfiltration and impaired renal development caused by genetic and/or environmental factors. Although not yet widely established, regular clinical monitoring of these patients is necessary and requires a multidisciplinary approach. In these clinical visits, it is important to differentiate between patients with and without a high risk for CKD. Future genetic studies and the development of new techniques to determine nephron number will further contribute to making this differentiation, optimizing the clinical approach for all solitary functioning kidney patients.
Disclosures
None.
Acknowledgments
R.W. is funded by a grant from Fonds NutsOhra Zorgsubsidies (Project Number 1101-058) and a stipend from the Ter Meulen Fund, Royal Netherlands Academy for Arts and Sciences (2012/225). S.S.-C. is supported by American Heart Association Grant-in-Aid 13GRNT14680075.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Wühl E, van Stralen KJ, Verrina E, Bjerre A, Wanner C, Heaf JG, Zurriaga O, Hoitsma A, Niaudet P, Palsson R, Ravani P, Jager KJ, Schaefer F: Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol 8: 67–74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, Izzi C, Scolari F, Mattioli G, Allegri L, Ghiggeri GM: Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int 76: 528–533, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Hegde S, Coulthard MG: Renal agenesis and unilateral nephrectomy: What are the risks of living with a single kidney? Pediatr Nephrol 24: 439–446, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Woolf AS, Davies JA: Cell biology of ureter development. J Am Soc Nephrol 24: 19–25, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Schreuder MF: Safety in glomerular numbers. Pediatr Nephrol 27: 1881–1887, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE: Human nephron number: Implications for health and disease. Pediatr Nephrol 26: 1529–1533, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Schedl A: Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Westland R, Schreuder MF, Ket JC, van Wijk JA: Unilateral renal agenesis: A systematic review on associated anomalies and renal injury. Nephrol Dial Transplant 28: 1844–1855, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Hiraoka M, Tsukahara H, Ohshima Y, Kasuga K, Ishihara Y, Mayumi M: Renal aplasia is the predominant cause of congenital solitary kidneys. Kidney Int 61: 1840–1844, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Schreuder MF, Westland R, van Wijk JA: Unilateral multicystic dysplastic kidney: A meta-analysis of observational studies on the incidence, associated urinary tract malformations and the contralateral kidney. Nephrol Dial Transplant 24: 1810–1818, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Westland R, Schreuder MF, Bökenkamp A, Spreeuwenberg MD, van Wijk JA: Renal injury in children with a solitary functioning kidney—the KIMONO study. Nephrol Dial Transplant 26: 1533–1541, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Sanna-Cherchi S, Caridi G, Weng PL, Scolari F, Perfumo F, Gharavi AG, Ghiggeri GM: Genetic approaches to human renal agenesis/hypoplasia and dysplasia. Pediatr Nephrol 22: 1675–1684, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westland R, Kurvers RA, van Wijk JA, Schreuder MF: Risk factors for renal injury in children with a solitary functioning kidney. Pediatrics 131: e478–e485, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Costantini F, Kopan R: Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18: 698–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vainio S, Lin Y: Coordinating early kidney development: Lessons from gene targeting. Nat Rev Genet 3: 533–543, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Sanna-Cherchi S, Sampogna RV, Papeta N, Burgess KE, Nees SN, Perry BJ, Choi M, Bodria M, Liu Y, Weng PL, Lozanovski VJ, Verbitsky M, Lugani F, Sterken R, Paragas N, Caridi G, Carrea A, Dagnino M, Materna-Kiryluk A, Santamaria G, Murtas C, Ristoska-Bojkovska N, Izzi C, Kacak N, Bianco B, Giberti S, Gigante M, Piaggio G, Gesualdo L, Kosuljandic Vukic D, Vukojevic K, Saraga-Babic M, Saraga M, Gucev Z, Allegri L, Latos-Bielenska A, Casu D, State M, Scolari F, Ravazzolo R, Kiryluk K, Al-Awqati Q, D’Agati VD, Drummond IA, Tasic V, Lifton RP, Ghiggeri GM, Gharavi AG: Mutations in DSTYK and dominant urinary tract malformations. N Engl J Med 369: 621–629, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber S, Moriniere V, Knüppel T, Charbit M, Dusek J, Ghiggeri GM, Jankauskiené A, Mir S, Montini G, Peco-Antic A, Wühl E, Zurowska AM, Mehls O, Antignac C, Schaefer F, Salomon R: Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: Results of the ESCAPE study. J Am Soc Nephrol 17: 2864–2870, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Thomas R, Sanna-Cherchi S, Warady BA, Furth SL, Kaskel FJ, Gharavi AG: HNF1B and PAX2 mutations are a common cause of renal hypodysplasia in the CKiD cohort. Pediatr Nephrol 26: 897–903, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiesel A, Queisser-Luft A, Clementi M, Bianca S, Stoll C, EUROSCAN Study Group : Prenatal detection of congenital renal malformations by fetal ultrasonographic examination: An analysis of 709,030 births in 12 European countries. Eur J Med Genet 48: 131–144, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Garne E, Dolk H, Loane M, Boyd PA, EUROCAT : EUROCAT website data on prenatal detection rates of congenital anomalies. J Med Screen 17: 97–98, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Sanna-Cherchi S, Kiryluk K, Burgess KE, Bodria M, Sampson MG, Hadley D, Nees SN, Verbitsky M, Perry BJ, Sterken R, Lozanovski VJ, Materna-Kiryluk A, Barlassina C, Kini A, Corbani V, Carrea A, Somenzi D, Murtas C, Ristoska-Bojkovska N, Izzi C, Bianco B, Zaniew M, Flogelova H, Weng PL, Kacak N, Giberti S, Gigante M, Arapovic A, Drnasin K, Caridi G, Curioni S, Allegri F, Ammenti A, Ferretti S, Goj V, Bernardo L, Jobanputra V, Chung WK, Lifton RP, Sanders S, State M, Clark LN, Saraga M, Padmanabhan S, Dominiczak AF, Foroud T, Gesualdo L, Gucev Z, Allegri L, Latos-Bielenska A, Cusi D, Scolari F, Tasic V, Hakonarson H, Ghiggeri GM, Gharavi AG: Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet 91: 987–997, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreuder MF, Bueters RR, Huigen MC, Russel FG, Masereeuw R, van den Heuvel LP: Effect of drugs on renal development. Clin J Am Soc Nephrol 6: 212–217, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Tran S, Chen YW, Chenier I, Chan JS, Quaggin S, Hébert MJ, Ingelfinger JR, Zhang SL: Maternal diabetes modulates renal morphogenesis in offspring. J Am Soc Nephrol 19: 943–952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreuder MF: Unilateral anomalies of kidney development: Why is left not right? Kidney Int 80: 740–745, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM: Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. Am J Physiol 241: F85–F93, 1981 [DOI] [PubMed] [Google Scholar]
- 26.Brenner BM, Lawler EV, Mackenzie HS: The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int 49: 1774–1777, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Douglas-Denton R, Moritz KM, Bertram JF, Wintour EM: Compensatory renal growth after unilateral nephrectomy in the ovine fetus. J Am Soc Nephrol 13: 406–410, 2002 [DOI] [PubMed] [Google Scholar]
- 28.van Vuuren SH, Sol CM, Broekhuizen R, Lilien MR, Oosterveld MJ, Nguyen TQ, Goldschmeding R, de Jong TP: Compensatory growth of congenital solitary kidneys in pigs reflects increased nephron numbers rather than hypertrophy. PLoS One 7: e49735, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Vuuren SH, van der Doef R, Cohen-Overbeek TE, Goldschmeding R, Pistorius LR, de Jong TP: Compensatory enlargement of a solitary functioning kidney during fetal development. Ultrasound Obstet Gynecol 40: 665–668, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Cleper R: Mechanisms of compensatory renal growth. Pediatr Endocrinol Rev 10: 152–163, 2012 [PubMed] [Google Scholar]
- 31.Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Cachat F, Combescure C, Chehade H, Zeier G, Mosig D, Meyrat B, Frey P, Girardin E: Microalbuminuria and hyperfiltration in subjects with nephro-urological disorders. Nephrol Dial Transplant 28: 386–391, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Beeman SC, Zhang M, Gubhaju L, Wu T, Bertram JF, Frakes DH, Cherry BR, Bennett KM: Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol 300: F1454–F1457, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Wikstad I, Celsi G, Larsson L, Herin P, Aperia A: Kidney function in adults born with unilateral renal agenesis or nephrectomized in childhood. Pediatr Nephrol 2: 177–182, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Abou Jaoudé P, Dubourg L, Bacchetta J, Berthiller J, Ranchin B, Cochat P: Congenital versus acquired solitary kidney: Is the difference relevant? Nephrol Dial Transplant 26: 2188–2194, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Argueso LR, Ritchey ML, Boyle ET, Jr., Milliner DS, Bergstralh EJ, Kramer SA: Prognosis of patients with unilateral renal agenesis. Pediatr Nephrol 6: 412–416, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Argueso LR, Ritchey ML, Boyle ET, Jr., Milliner DS, Bergstralh EJ, Kramer SA: Prognosis of children with solitary kidney after unilateral nephrectomy. J Urol 148: 747–751, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Dursun H, Bayazit AK, Cengiz N, Seydaoglu G, Buyukcelik M, Soran M, Noyan A, Anarat A: Ambulatory blood pressure monitoring and renal functions in children with a solitary kidney. Pediatr Nephrol 22: 559–564, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González E, Gutiérrez E, Morales E, Hernández E, Andres A, Bello I, Díaz-González R, Leiva O, Praga M: Factors influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney Int 68: 263–270, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Seeman T, Patzer L, John U, Dusek J, Vondrák K, Janda J, Misselwitz J: Blood pressure, renal function, and proteinuria in children with unilateral renal agenesis. Kidney Blood Press Res 29: 210–215, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Wasilewska A, Zoch-Zwierz W, Jadeszko I, Porowski T, Biernacka A, Niewiarowska A, Korzeniecka-Kozerska A: Assessment of serum cystatin C in children with congenital solitary kidney. Pediatr Nephrol 21: 688–693, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Vu KH, Van Dyck M, Daniels H, Proesmans W: Renal outcome of children with one functioning kidney from birth. A study of 99 patients and a review of the literature. Eur J Pediatr 167: 885–890, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Peco-Antić A, Paripović D, Kotur-Stevuljević J, Stefanović A, Sćekić G, Miloševski-Lomić G: Renal functional reserve in children with apparently normal congenital solitary functioning kidney. Clin Biochem 45: 1173–1177, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Godbole PP, Wilcox DT, Mushtaq I: Follow-up after unilateral nephrectomy in children: Is an estimate of glomerular filtration rate necessary? BJU Int 95: 635–637, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Corbani V, Ghiggeri GM, Sanna-Cherchi S: ‘Congenital solitary functioning kidneys: Which ones warrant follow-up into adult life?’. Nephrol Dial Transplant 26: 1458–1460, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ: Long-term consequences of kidney donation. N Engl J Med 360: 459–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsson L, Aperia A, Wilton P: Effect of normal development on compensatory renal growth. Kidney Int 18: 29–35, 1980 [DOI] [PubMed] [Google Scholar]
- 48.Chou YH, Hsu CP: Age factor in compensatory hypertrophy of the kidney evaluated by 99mTc-DTPA renography. Urol Int 46: 126–128, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Chevalier RL: When is one kidney not enough? Kidney Int 76: 475–477, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA: Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 2132–2140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hidalgo G, Ng DK, Moxey-Mims M, Minnick ML, Blydt-Hansen T, Warady BA, Furth SL: Association of income level with kidney disease severity and progression among children and adolescents with CKD: a report from the Chronic Kidney Disease in Children (CKiD) Study. Am J Kidney Dis 62: 1087–1094, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F, ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A: Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 301: 2234–2242, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE: Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382: 273–283, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S, American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee : Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment: A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension 52: 433–451, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R: First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol 20: 436–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westland R, Abraham Y, Bökenkamp A, Stoffel-Wagner B, Schreuder MF, van Wijk JA: Precision of estimating equations for GFR in children with a solitary functioning kidney: The KIMONO study. Clin J Am Soc Nephrol 8: 764–772, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westland R, Schreuder MF: Gender differences in solitary functioning kidney: Do they affect renal outcome? [published online ahead of print April 11, 2013]. Pediatr Nephrol doi:10.1007/s00467-013-2474-z [DOI] [PubMed] [Google Scholar]
- 59.Bhathena DB, Julian BA, McMorrow RG, Baehler RW: Focal sclerosis of hypertrophied glomeruli in solitary functioning kidneys of humans. Am J Kidney Dis 5: 226–232, 1985 [DOI] [PubMed] [Google Scholar]
- 60.Abellan MC, Chehade A, Grignon Y, Galloy MA, Fabre B, Schmitt M: Compensatory renal growth post fetal nephrectomy in the rabbit. Eur J Pediatr Surg 7: 282–285, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Mesrobian HG: Compensatory renal growth in the solitary kidneys of Danforth mice with genetic renal agenesis. J Urol 160: 146–149, 1998 [PubMed] [Google Scholar]