Abstract
Background and objectives
Several studies have suggested that activation of the complement system is a contributing pathogenic mechanism in IgA nephropathy (IgAN). C4d staining is an inexpensive and easy-to-perform method for the analysis of renal biopsies. This study aimed to assess the clinical and prognostic implications of C4d staining in IgAN.
Design, setting, participants, & measurements
This retrospective cohort study included 283 patients with IgAN in 11 hospitals in Spain who underwent a renal biopsy between 1979 and 2010. The primary predictor was mesangial C4d staining. Secondary predictors included demographic, clinical, and laboratory characteristics, and Oxford pathologic classification criteria. The primary end point was the cumulative percentage of patients who developed ESRD, defined as onset of chronic dialysis or renal transplantation. C4d was analyzed by immunohistochemical staining using a polyclonal antibody. Kaplan–Meier and Cox proportional hazards analyses were performed to evaluate the effect of C4d staining on renal survival.
Results
There were 109 patients (38.5%) and 174 patients (61.5%) who were classified as C4d positive and C4d negative, respectively. Renal survival at 20 years was 28% in C4d-positive patients versus 85% in C4d-negative patients (P<0.001). Independent risk factors associated with ESRD were as follows: proteinuria (hazard ratio [HR] per every 1 g/d increase. 1.16; 95% confidence interval [95% CI], 1.03 to 1.31; P=0.01), eGFR (HR per every 1 ml/min per 1.73 m2 increase, 0.96; 95% CI, 0.94 to 0.97; P<0.001), T2 Oxford classification (tubular atrophy/interstitial fibrosis, >50%; HR, 4.42; 95% CI, 1.40 to 13.88; P=0.01), and C4d-positive staining (HR, 2.45; 95% CI, 1.30 to 4.64; P=0.01).
Conclusions
C4d-positive staining is an independent risk factor for the development of ESRD in IgAN. This finding is consistent with the possibility that complement activation is involved in the pathogenesis of this disease.
Keywords: complement, IgA nephropathy, survival
Introduction
IgA nephropathy (IgAN) is the most common primary GN worldwide and is defined by the predominant deposition of IgA in the glomerular mesangium (1). It is characterized by a highly variable course ranging from a totally benign condition (2) to ESRD (3,4).
There are several clinical and histologic factors (arterial hypertension, proteinuria, renal function, and Oxford classification score) that strongly determine the final outcome of patients with IgAN (5,6). The identification of new prognostic markers could provide insights into the pathogenesis of IgAN and unveil new therapeutic avenues.
The following processes are involved in the pathogenesis (7) of IgAN: aberrant glycosylation of IgA1 (8), synthesis of antibodies directed against galactose-deficient IgA1, binding of the galactose-deficient IgA1 by antibodies to form immune complexes, and deposition of these complexes in the glomerular mesangium, inducing activation of mesangial cells and glomerular damage (9–12).
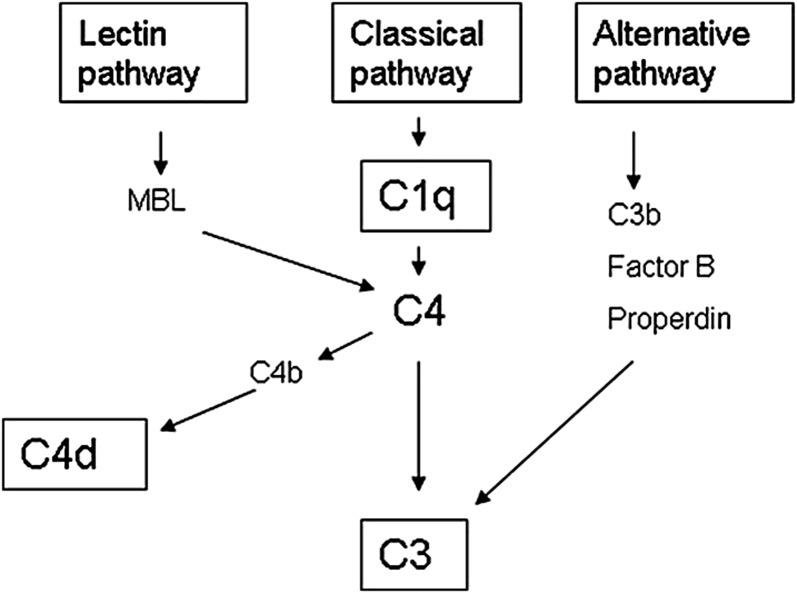
Several studies have demonstrated that activation of the complement system augments the inflammatory cascade and potentiates tissue injury in IgAN (13). Roos et al. (14) showed that those patients with IgAN with glomerular deposition of mannose-binding lectin (MBL) had more severe histologic damage and more proteinuria. In this study, the deposition of MBL was always accompanied by deposits of L-ficolin and C4d, indicating an activation of complement by the lectin pathway. The majority of both MBL-positive and MBL-negative patients showed glomerular deposition of C3, whereas C1q deposits were distinctly absent in all of the patients. These data suggested an activation of complement by the alternative pathway (no deposits of MBL) in a majority of patients with IgAN (75% in this study), and an activation by the lectin pathway (glomerular deposits of C3, C4d, MBL, and L-ficolin) in the remaining 25% of patients (Figure 1). C4d deposition could be a marker of complement activation by the lectin pathway in IgAN. Interestingly, C4d staining is now a routine histologic technique in the study of kidney biopsies, after the discovery of its crucial importance in the diagnosis of humoral rejection in kidney transplantation (15).
Figure 1.
The complement system and C4d. C4d can be derived from the classic and lectin pathway activation. In patients in whom C1q deposits are not detected, the classic pathway of complement activation can be ruled out. MBL, mannose-binding lectin.
In a previous study, we showed that 32% of patients with IgAN had a positive staining result for glomerular C4d and that their renal survival at 10 years was 43.9% compared with 90.9% in C4d-negative patients (16). Our study aimed to assess the clinical and prognostic implications of C4d staining in a larger cohort of patients with IgAN.
Materials and Methods
Design
This study was an initiative of the Scientific Committee of the Spanish Group for the Study of Glomerular Diseases. Eleven centers in Spain that agreed to participate in the study sent renal tissue to one of the centers (Reina Sofia Hospital, Córdoba, Spain) for C4d study. This study was approved by the ethics committees and research boards of these institutions. Patient information was managed according to applicable data protection regulations.
Patients
Participating centers were required to include all patients with a diagnosis of biopsy-proven IgAN between 1979 and 2010. Patients with Henoch–Schönlein purpura, liver diseases, diabetes, systemic diseases, and any type of secondary IgAN were excluded. Patients with hepatitis C or B infection were also excluded. For each patient, the date of renal biopsy was established as the baseline point. The diagnosis of IgAN was based on histologic assessment of renal biopsy tissue with hematoxylin and eosin, Masson’s trichrome, periodic acid–Schiff, and methenamine silver for light microscopy and staining with antibodies against IgG, IgA, IgM, C1q, and C3 for immunofluorescence. IgAN was defined by the presence of at least 1+ (range, 0–3) IgA mesangial deposits as dominant or codominant Igs on immunofluorescence microscopy performed on frozen tissue.
The medical records were reviewed and the following information at the time of the renal biopsy was recorded: patient age, sex, presence or absence of macroscopic hematuria, hypertension (defined as systolic BP>140 mmHg and/or diastolic BP>90 mmHg or the use of antihypertensive agents), 24-hour urine protein excretion, and serum creatinine level. Mean values of the two latest measurements performed within the month before renal biopsy were selected. Proteinuria was used either as a continuous variable as a category variable (<0.30 g/d, 0.30–0.99 g/d, 1.0–2.99 g/d, and ≥3.00 g/d) or as a dichotomous variable (≥1 g/d versus <1 g/d). We calculated the eGFR in adults by using the abbreviated Modification of Diet in Renal Disease study equation (17): eGFR (ml/min per 1.73 m2)=186.3×(serum creatinine in mg/dl−1.154)×(age−0.203)×(0.742 if female)×(1.21 if black). The Schwartz formula was used to calculate the eGFR in children.
The use of treatment with immunosuppressive agents, corticosteroids or renin-angiotensin system blockers, and either angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor antagonists (ARBs) in the follow-up was also recorded. Onset of renal disease was not recorded and was not analyzed.
C4d Staining and Histologic Studies
C4d staining was performed at Reina Sofía Hospital by one of the investigators (R.O.), who was blinded to the identity of the tissue. Formaldehyde-fixed renal tissue from 437 patients was received for C4d study. Only patients with >3 nonsclerotic glomeruli available for the C4d study were included. We excluded 72 patients who did not fulfill these criteria. In addition, 82 were excluded because of incomplete data on age, proteinuria, renal function, BP, or Oxford pathologic classification criteria. A total of 283 patients comprised the study cohort.
C4d immunohistochemical staining was performed on 3-µm deparaffinized and rehydrated sections of formaldehyde-fixed renal tissue, using rabbit polyclonal anti-human C4d (Biomedica, Vienna, Austria) as the antibody. To block nonspecific staining, antigen retrieval was performed in advance of slide treatment by pressure cooking (5 minutes at +125°C in Tris/EDTA buffer, pH 8.5) and cooling to room temperature. A section of kidney with transplant humoral rejection and a section with minimal change nephropathy served as the positive and negative controls, respectively.
C4d immunohistochemical staining was scored as negative or positive, with scores of 0 or 1, respectively. Patients were classified as positive when >25% of the nonsclerotic glomeruli were positive for C4d. This positive glomerular staining was predominantly mesangial. No staining was observed in peritubular capillaries, whereas tubular staining was observed irregularly in some patients.
Histologic findings were also grouped according to the Oxford classification (5,6). Four histopathologic variables were scored as follows by the pathologist at the local center by review of the biopsy material: mesangial hypercellularity (M0/M1), endocapillary hypercellularity (E0/E1), segmental glomerulosclerosis (S0/S1), and tubular atrophy/interstitial fibrosis (T0, <25%; T1, 26%–50%; and T2, >50%).
To assess whether immunohistochemistry could be a less sensitive method than immunofluorescence for the detection of C4d, we compared both techniques in a subgroup of 23 patients biopsied after 2008 in one of the participating centers.
Study End Points
For each patient, the date of renal biopsy was established as the baseline point. Follow-up time was considered as the interval between renal biopsy and the last outpatient visit, death, or ESRD (defined as onset of chronic dialysis or renal transplantation). The primary end point of the study was the cumulative percentage of patients who developed ESRD in the course of the study (renal survival). No patient died before the time of ESRD.
Statistical Analyses
Results are reported as the mean±SD when normally distributed or as the median (interquartile range [IQR]) otherwise. Comparisons of continuous variables between two groups were assessed using the unpaired t test or the Mann–Whitney U test as appropriate. The differences in the proportions of different patient groups were compared by the Fisher’s exact test. A P value <0.05 was considered to be statistically significant.
Kaplan–Meier and Cox proportional hazards analyses were performed to evaluate the effect of C4d staining on renal survival. The primary end point was ESRD. Univariate survival comparisons were made using the log-rank test. All univariate tests were two-sided, with a α level of 0.05. The Cox proportional hazards model was used to estimate the adjusted relative risk of each parameter with regard to renal survival. Survival time for each patient was computed from baseline evaluation to the last follow-up or the primary end point (ESRD). Variables previously found to affect renal survival were included in the Cox proportional hazards model. Variables were selected by backward elimination using likelihood ratio tests. Calculations were performed using SPSS statistical software (version 15.0; SPSS, Chicago, IL).
Results
Baseline Clinical and Histologic Findings
Table 1 shows the main baseline clinical and histologic data of the 283 included patients. Participants were predominantly men (73.8%) and the mean age was 39.1±17.2 years. The median proteinuria was 1.9 g/d (IQR, 1.0–3.0) and 55.2% of patients were hypertensive. The mean eGFR was 67±35 ml/min per 1.73 m2. Histologic findings were systematized according to the Oxford classification. We observed mesangial proliferation in >50% of glomeruli (M1) in 183 patients (64%), endocapillary proliferation (E1) in 62 patients (21%), and FSGS (S1) in 57 patients (20%). Regarding the severity of tubulointerstitial fibrosis (T0 to T2), we found T0 in 112 patients (39%), T1 in 90 patients (31%), and T2 in 81 patients (28%). Our results showed that 225 patients (79%) received ACEI or ARB therapy throughout follow-up, 100 patients (35%) received oral corticosteroids, and 60 patients (21%) received other immunosuppressive agents.
Table 1.
Clinical and histologic characteristics of the patients at baseline and during the follow-up period according to C4d staining
| Variable | Total (N=283) | C4d-Positive (n=109) | C4d-Negative (n=174) | P Value |
|---|---|---|---|---|
| Mean age (yr) | 39.1±17.2 | 38.6±15.0 | 39.4±18.5 | 0.72 |
| Male sex | 208 (73.8) | 81 (74.3) | 127 (73.4) | 0.89 |
| Macroscopic hematuria | 129 (45.6) | 43 (39.4) | 86 (49.4) | 0.06 |
| Hypertension | 155 (55.2) | 78 (71.6) | 77 (44.8) | <0.001 |
| eGFR (ml/min per 1.73 m2) | 67.7±35.9 | 58.9±34.1 | 73.2±36.0 | 0.001 |
| Stage (eGFR in ml/min/1.73 m2) | 0.02 | |||
| 1 (≥90) | 84 (29.7) | 22 (20.2) | 62 (35.8) | |
| 2 (60–89) | 69 (24.4) | 26 (23.9) | 43 (24.9) | |
| 3 (30–59) | 82 (29.0) | 38 (34.9) | 44 (25.4) | |
| 4 (15–29) | 29 (10.2) | 16 (14.7) | 13 (7.5) | |
| 5 (<15) | 18 (6.4) | 7 (6.4) | 11 (6.4) | |
| Median urinary protein excretion (g/d) | 1.9 (1.0–3.0) | 2.2 (1.4–3.6) | 1.7 (1.0–2.6) | <0.001 |
| Urinary protein excretion (g/d) | 0.01 | |||
| <0.30 | 13 (4.6) | 1 (0.9) | 12 (6.9) | |
| 0.30–0.99 | 42 (14.8) | 14 (12.8) | 28 (16.1) | |
| 1–2.99 | 150 (53.0) | 53 (48.6) | 97 (55.7) | |
| ≥3.0 | 78 (27.6) | 41 (37.6) | 37 (21.3) | |
| Proteinuria≥1 g/d | 228 (80.6) | 94 (86.2) | 134 (77.0) | 0.03 |
| Oxford classification | ||||
| M1 | 183 (64.7) | 77 (73.3) | 106 (66.3) | 0.13 |
| E1 | 62 (21.9) | 28 (25.7) | 34 (19.5) | 0.14 |
| S1 | 57 (20.1) | 35 (32.4) | 22 (12.6) | <0.001 |
| Tubular atrophy/interstitial fibrosis (%) | <0.001 | |||
| T0 (<25) | 112 (39.6) | 15 (13.8) | 97 (55.7) | |
| T1 (25–50) | 90 (31.8) | 41 (37.6) | 49 (28.2) | |
| T2 (>50) | 81 (28.6) | 53 (48.6) | 28 (16.1) | |
| Use of ACEI/ARB | 225 (79.5) | 90 (89.1) | 135 (87.1) | 0.39 |
| Use of prednisone | 100 (35.3) | 35 (33.7) | 65 (38.2) | 0.51 |
| Any other immunosuppressive treatmenta | 60 (21.2) | 23 (23.2) | 37 (23.9) | >0.99 |
| Median follow-up period (yr) | 6.0 (3.0–11.7) | 5.3 (2.9–8.3) | 6.6 (3.0–13.1) | 0.03 |
| Evolution to ESRD in the follow-upb | 58 (20.5) | 41 (37.6) | 17 (9.8) | <0.001 |
For quantitative variables, values are expressed as the mean±SD or the median (interquartile range) as appropriate. For qualitative variables, values are expressed as n (%). M, mesangial hypercellularity; E, endocapillary hypercellularity; S, segmental glomerulosclerosis; T, tubular atrophy/interstitial fibrosis; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Immunosuppressive agents include cyclophosphamide, mycophenolate, or others.
ESRD: chronic dialysis or renal transplantation.
Complement Staining
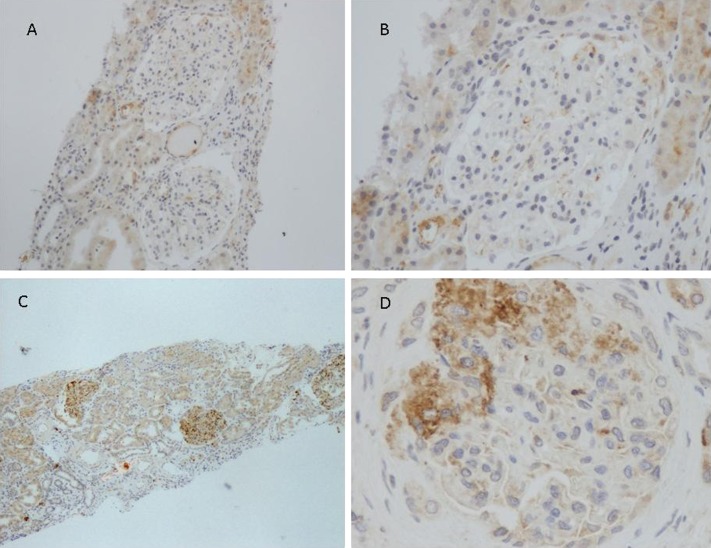
Glomerular staining for C4d (>25% of glomeruli) was observed, in a mesangial pattern, in 109 biopsies (38.5%). In 174 biopsies (61.5%), ≤25% of glomeruli were positive for C4d and these patients were considered as negative. The proportion of patients who stained positive for C3 and IgG was similar among C4d-positive patients (71% and 17%, respectively) and C4d-negative patients (72% and 22%, respectively). C1q staining was negative in all of the patients. Figure 2 shows a patient with a negative staining and a representative patient with a C4d-positive staining. A renal biopsy was repeated in only one patient, 12 years after the first one, because of progressive renal failure. Mesangial C4d staining was evident in both renal biopsies.
Figure 2.
Renal tissue from patients with IgAN was stained for the presence of C4d. Representative images are shown. (A and B) A patient with a negative C4d staining. (C and D) A representative patient with a positive C4d staining in mesangial areas. No staining is detected in peritubular capillaries. Tubular staining is observed. IgAN, IgA nephropathy. Original magnification, ×100 in A; ×200 in B; ×40 in C; ×400 in D.
C4d staining was analyzed by immunofluorescence and immunohistochemistry in 23 patients from one of the participating centers. Both techniques were concordant in 20 patients (87%), showing positive staining in 14 patients and negative staining in 6 patients. Disagreement between both techniques was observed in three patients, in whom immunofluorescence was positive and immunohistochemistry was negative.
Differences between C4d-Positive and C4d-Negative Patients
Table 1 shows the clinical and pathologic data of the patients at the time of renal biopsy according to their positive or negative staining for C4d. The proportion of participants with hypertension was significantly higher (71.6% versus 44.8%; P<0.001) in C4d-positive patients. C4d-positive patients also had a lower eGFR (59±34 versus 73±36 ml/min per 1.73 m2; P<0.001) and a higher amount of proteinuria compared with C4d-negative patients (median 2.2 versus 1.7 g/d; P<0.001).
The proportion of patients treated in the follow-up with ACEI/ARB, prednisone, or immunosuppressive agents was similar in both groups. The proportion of patients with segmental glomerulosclerosis (S1) and tubulointerstitial fibrosis (T1 and T2) was higher in C4d-positive patients. On the contrary, the proportion of patients without tubulointerstitial fibrosis (T0) was significantly lower in C4d-positive patients. Finally, a higher proportion of C4d-positive patients developed ESRD (37.6% versus 9.8%; P<0.001). The median follow-up was 5.3 years (IQR, 2.9–8.3) for C4d-positive patients and 6.6 years (IQR, 3.0–13.1) for C4d-negative patients.
Primary End Point
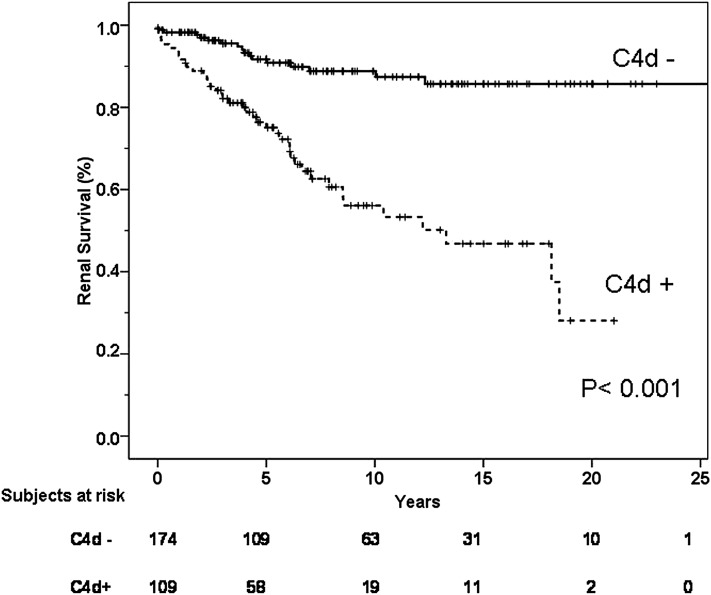
Fifty-eight patients (20.5%) reached the primary end point (ESRD). As shown in Figure 3, the Kaplan–Meier total renal survival at 10, 15, and 20 years was 75%, 70%, and 64%, respectively. Table 2 presents unadjusted and multivariable adjusted hazard ratios (HRs) for the end point of ESRD. Univariate analysis showed that age, hypertension, proteinuria, and eGFR among clinical data, and tubulointerstitial fibrosis (T1, T2), segmental glomerulosclerosis (S1), and positive C4d staining among histologic data, were associated with the appearance of ESRD. Only the amount of proteinuria (HR per every 1 g/d increase, 1.16; 95% confidence interval [95% CI], 1.03 to 1.31; P=0.01), eGFR (HR per every 1 ml/min per 1.73 m2 increase, 0.96; 95% CI, 0.94 to 0.97; P<0.001), presence of T2 tubulointerstitial fibrosis (HR, 4.42; 95% CI, 1.40 to 13.88; P=0.01) and a positive C4d staining (HR, 2.45; 95% CI, 1.30 to 4.64; P=0.01) remained as significant predictors of ESRD by multivariate analysis.
Figure 3.
Renal survival according to C4d staining.
Table 2.
Univariate and multivariate Cox regression analyses with ESRDa as the end point in 283 patients with IgA nephropathy
| Analysis | β | P Value | Hazard Ratio (95% Confidence Interval) |
|---|---|---|---|
| Univariate | |||
| Age (yr) | 0.02 | 0.001 | 1.02 (1.01 to 1.04) |
| Positive C4d staining | 1.53 | <0.001 | 4.65 (2.63 to 8.22) |
| Hypertension | 1.84 | <0.001 | 6.30 (2.98 to 13.31) |
| Proteinuria (g/d) | 0.25 | <0.001 | 1.28 (1.16 to 1.43) |
| eGFR (ml/min per 1.73 m2) | −0.04 | <0.001 | 0.95 (0.94 to 0.96) |
| Oxford classification | |||
| S1 | 1.10 | <0.001 | 3.02 (1.76 to 5.16) |
| T0 | 1 (reference) | ||
| T1 | 1.93 | <0.001 | 6.89 (2.31 to 20.5) |
| T2 | 3.27 | <0.001 | 26.23 (9.23 to 75.43) |
| Multivariate | |||
| Positive C4d staining | 0.89 | 0.01 | 2.45 (1.30 to 4.64) |
| Proteinuria (g/d) | 0.15 | 0.01 | 1.16 (1.03 to 1.31) |
| eGFR (ml/min per 1.73 m2) | −0.04 | <0.001 | 0.96 (0.94 to 0.97) |
| Oxford classification | |||
| T0 | 1 (reference) | ||
| T2 | 1.48 | 0.01 | 4.42 (1.40 to 13.88) |
ESRD: chronic dialysis or renal transplantation.
Renal survival at 5 and 20 years was 76% and 28%, respectively, in C4d-positive patients compared with 91% and 85% in C4d-negative patients (log-rank, P<0.001) (Figure 3).
There were 24 patients aged<18 years: 6 (5.5%) in the C4d-positive group and 18 (10.3%) in the C4d-negative group. When these patients were excluded, the results did not change. C4d-positive staining was an independent risk factor for the development of ESRD (HR, 2.38; 95% CI, 1.26 to 4.48; P=0.01). In this analysis, proteinuria, eGFR, and T2 Oxford classification score were also independent risk factors.
When patients with an eGFR<15ml/min per 1.73 m2 were excluded the results were similar. C4d-positive staining was an independent risk factor for the development of ESRD (HR, 3.06; 95% CI, 1.49 to 6.29; P=0.002). In this analysis, the other independent risk factors were proteinuria, eGFR, and S1 Oxford classification score.
Discussion
IgAN is characterized by a highly variable course ranging from a totally benign condition to progressive loss of renal function leading to ESRD. As demonstrated by several studies, serum creatinine, proteinuria, and arterial hypertension at presentation are the strongest predictors of an unfavorable outcome (18–24). Among pathologic data, mesangial hypercellularity, glomerular sclerosis, interstitial fibrosis, and tubular atrophy have been recognized as independent predictors of renal failure (6).
Our study shows by the first time that mesangial C4d staining is an independent risk factor for the development of ESRD in IgAN. As shown in Figure 3, renal survival was significantly higher in C4d-negative patients compared with C4d-positive patients. This fact is relevant because in addition to identifying a new prognostic factor, it supports the notion that the activation of the complement system may play a crucial role in the pathogenesis of IgAN (25).
Mesangial deposition of IgA is considered to be the initiating event that precipitates renal damage in IgAN, inducing mesangial cell proliferation and expansion of extracellular matrix components. The contribution of the complement system to amplify tissue injury in IgAN has been suggested by several studies, but the precise pathways of complement activation remain largely unknown. However, as strongly suggested by the study by Roos et al. (14) as well as our own data (16), activation of the lectin pathway (indicated by C4d deposition) is associated with more severe histologic damage and significantly reduced renal survival.
It is unknown whether mesangial C4d deposition might change during the course of the disease (26). In our study, a second renal biopsy was performed in a C4d-positive patient, 12 years after the first one. Mesangial C4d staining, similar to that observed in the first renal biopsy, was found.
The pathogenic mechanisms explaining why C4d-positive patients with IgAN have more serious histologic injury and a worse prognosis are presently unknown. The pattern of IgA1 glycosylation and the molecular mass of IgA1-containing immune complexes are important factors that determine the ability of IgA1 to activate the different complement pathways (27). It was recently shown that elevated serum levels of galactose-deficient IgA1 are associated with a poor prognosis in IgAN (28). Whether the IgA1 glycosylation pattern of patients who show a positive staining for C4d is different from that of C4d negative patients is unknown. On the other hand, it is possible that IgA1-induced activation of the lectin pathway could specifically recruit other amplifying mechanisms, rendering the complement system activation more aggressive. Thus, positive costaining for C4-binding protein has been reported in patients with IgAN with a C4d-positive staining, but not in those with a C4d-negative staining (14,29). C4-binding protein behaves as a bridge between the complement system and coagulation (30), and this pathway could play a role in the formation of thrombotic microangiopathy lesions that some studies have reported (31,32) as well as in the pathogenesis of accelerated or malignant hypertension that some patients with IgAN develop (31,33).
We showed in a previous study that renal survival at 10 years was 43.9% in C4d-positive patients compared with 90.9% in C4d-negative patients (16). However, C4d staining was not an independent factor for renal survival, probably because of the low number of patients analyzed. In this study, which included a large cohort of patients with biopsy-proven IgAN, a positive staining for C4d was a conclusive independent risk factor for renal survival. This finding implies important practical applications because C4d staining is a relatively inexpensive and easy-to-perform test in the routine study of renal biopsies. In fact, C4d staining is routinely performed in renal transplant biopsies as a marker of antibody-mediated humoral rejection. Interpretation of C4d staining is simple and relatively specific and has a low possibility of false positive results. Immunofluorescence studies on frozen tissues are a more sensitive technique than immunohistochemistry for detecting C4d deposition in renal transplantation. Data regarding sensitivity of both techniques in glomerular diseases are scarce. Suzuki et al. (34) showed similar sensitivity of immunofluorescence and immunohistochemistry to detect C4d glomerular deposits, although staining was apparently weaker with immunohistochemistry. In our experience (35), which is confirmed by others (36), there were no differences between both techniques to detect C4d deposits in patients with membranous nephropathy and minimal change disease (C4d staining was positive in all of the patients with membranous nephropathy and negative in all of the patients with minimal change disease). For this study, we compared both techniques in a subgroup of 23 patients and we found that both techniques yielded the same results in 87% of patients.
Our study has the inherent limitations of a retrospective observational investigation. The relationship between C4d staining and progression of renal damage must be interpreted cautiously in terms of association rather than causality. We selected the date of renal biopsy as the baseline time. The onset of renal disease and proteinuria in the follow-up were not analyzed. In our study, MBL staining was also not analyzed. However, it was previously shown that deposition of C4d was accompanied by deposits of MBL in 100% of patients (14). C4d in the absence of C1q is a marker for lectin pathway activation (Figure 1).
Our analysis has important strengths, mainly the number of biopsy-proven patients included, their careful and long-term follow-up, and the use of hard clinical end points such as ESRD.
In conclusion, our study shows for the first time that the deposition of C4d is an independent risk factor for renal survival in IgAN. Staining for C4d could be incorporated into the routine analysis of renal biopsies in patients with this disease, given its prognostic importance. These findings are consistent with the possibility that complement activation is involved in the pathogenesis of IgAN.
Disclosures
None.
Acknowledgments
This study was supported by grants from the Fundación Nefrológica, Fondo de Investigaciones Sanitarias (10/02668), Community of Madrid (S2010/BMD-2316), and REDinREN (RD012/0021).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09710913/-/DCSupplemental.
References
- 1.Wyatt R, Julian B: IgA nephropathy. N Engl J Med 368: 2402–2414, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Gutiérrez E, Zamora I, Ballarín JA, Arce Y, Jiménez S, Quereda C, Olea T, Martínez-Ara J, Segarra A, Bernis C, García A, Goicoechea M, García de Vinuesa S, Rojas-Rivera J, Praga M, Grupo de Estudio de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN) : Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol 23: 1753–1760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donadio JV, Grande JP: IgA nephropathy. N Engl J Med 347: 738–748, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, Liu Z: Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 27: 1479–1485, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannakakis K, Feriozzi S, Perez M, Faraggiana T, Muda AO: Aberrantly glycosylated IgA1 in glomerular immune deposits of IgA nephropathy. J Am Soc Nephrol 18: 3139–3146, 2007 [DOI] [PubMed] [Google Scholar]
- 9.van den Dobbelsteen MEA, van der Woude FJ, Schroeijers WEM, van den Wall Bake AWL, van Es LA, Daha MR: Binding of dimeric and polymeric IgA to rat renal mesangial cells enhances the release of interleukin 6. Kidney Int 46: 512–519, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Duque N, Gómez-Guerrero C, Egido J: Interaction of IgA with Fc alpha receptors of human mesangial cells activates transcription factor nuclear factor-kappa B and induces expression and synthesis of monocyte chemoattractant protein-1, IL-8, and IFN-inducible protein 10. J Immunol 159: 3474–3482, 1997 [PubMed] [Google Scholar]
- 11.Lai KN, Tang SC, Guh JY, Chuang TD, Lam MF, Chan LY, Tsang AW, Leung JC: Polymeric IgA1 from patients with IgA nephropathy upregulates transforming growth factor-beta synthesis and signal transduction in human mesangial cells via the renin-angiotensin system. J Am Soc Nephrol 14: 3127–3137, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J: Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 23: 1579–1587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogers WM, Stad RK, van Es LA, Daha MR: Immunoglobulin A: Interaction with complement, phagocytic cells and endothelial cells. Complement Inflamm 8: 347–358, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, Matsushita M, Fujita T, van Kooten C, Daha MR: Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol 17: 1724–1734, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Watschinger B, Pascual M: Capillary C4d deposition as a marker of humoral immunity in renal allograft rejection. J Am Soc Nephrol 13: 2420–2423, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Espinosa M, Ortega R, Gómez-Carrasco JM, López-Rubio F, López-Andreu M, López-Oliva MO, Aljama P: Mesangial C4d deposition: A new prognostic factor in IgA nephropathy. Nephrol Dial Transplant 24: 886–891, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 18.D’Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F: Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am J Kidney Dis 18: 12–19, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Koyama A, Igarashi M, Kobayashi M, Research Group on Progressive Renal Diseases : Natural history and risk factors for immunoglobulin A nephropathy in Japan. Am J Kidney Dis 29: 526–532, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Haas M: Histologic subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis 29: 829–842, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Bartosik LP, Lajoie G, Sugar L, Cattran DC: Predicting progression in IgA nephropathy. Am J Kidney Dis 38: 728–735, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Li PK, Ho KK, Szeto CC, Yu L, Lai FM: Prognostic indicators of IgA nephropathy in the Chinese—clinical and pathological perspectives. Nephrol Dial Transplant 17: 64–69, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen D, Colvin RB, Daha MR, Drachenberg CB, Haas M, Nickeleit V, Salmon JE, Sis B, Zhao MH, Bruijn JA, Bajema IM: Pros and cons for C4d as a biomarker. Kidney Int 81: 628–639, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zwirner J, Felber E, Herzog V, Riethmüller G, Feucht HE: Classical pathway of complement activation in normal and diseased human glomeruli. Kidney Int 36: 1069–1077, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Lachmann PJ: Glycosylation of IgA is required for optimal activation of the alternative complement pathway by immune complexes. Immunology 81: 137–141, 1994 [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H: The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int 82: 790–796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endo M, Ohi H, Satomura A, Hidaka M, Ohsawa I, Fujita T, Kanmatsuse K, Matsushita M, Fujita T: Regulation of in situ complement activation via the lectin pathway in patients with IgA nephropathy. Clin Nephrol 55: 185–191, 2001 [PubMed] [Google Scholar]
- 30.Hessing M: The interaction between complement component C4b-binding protein and the vitamin K-dependent protein S forms a link between blood coagulation and the complement system. Biochem J 277: 581–592, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang A, Kowalewska J, Smith KD, Nicosia RF, Alpers CE: A clinicopathologic study of thrombotic microangiopathy in the setting of IgA nephropathy. Clin Nephrol 66: 397–404, 2006 [DOI] [PubMed] [Google Scholar]
- 32.El Karoui K, Hill GS, Karras A, Jacquot C, Moulonguet L, Kourilsky O, Frémeaux-Bacchi V, Delahousse M, Duong Van Huyen JP, Loupy A, Bruneval P, Nochy D: A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol 23: 137–148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang L, Zhang JJ, Lv JC, Liu G, Zou WZ, Zhao MH, Zhang H: Malignant hypertension in IgA nephropathy was not associated with background pathological phenotypes of glomerular lesions. Nephrol Dial Transplant 23: 3921–3927, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Horita S, Kadoya K, Mitsuiki K, Aita K, Harada A, Nitta K, Nagata M: C4d Immunohistochemistry in glomerulonephritis with different antibodies. Clin Exp Nephrol 11: 287–291, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Espinosa-Hernández M, Ortega-Salas R, López-Andreu M, Gómez-Carrasco JM, Pérez-Sáez MJ, Pérez-Seoane C, Aljama-García P: C4d as a diagnostic tool in membranous nephropathy. Nefrologia 32: 295–299, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Val-Bernal JF, Garijo MF, Val D, Rodrigo E, Arias M: C4d immunohistochemical staining is a sensitive method to confirm immunoreactant deposition in formalin-fixed paraffin-embedded tissue in membranous glomerulonephritis. Histol Histopathol 26: 1391–1397, 2011 [DOI] [PubMed] [Google Scholar]